Abstract
Glycogen is a hallmark of mature astrocytes, but its emergence during astrocytic differentiation is unclear. Differentiation of E14 mouse neurospheres into astrocytes was induced with fetal bovine serum (FBS), Leukemia Inhibitory Factor (LIF), or Ciliary Neurotrophic Factor (CNTF). Cytochemical and enzymatic analyses showed that glycogen is present in FBS- or LIF- but not in CNTF-differentiated astrocytes. Glycogenolysis was induced in FBS- and LIF-differentiated astrocytes but glycogen resynthesis was observed only with FBS. Protein targeting to glycogen mRNA expression appeared with glial fibrillary acidic protein and S100β in FBS and LIF conditions but not with CNTF. These results show that glycogen metabolism constitutes a useful marker of astrocyte differentiation.
Keywords: astrocyte differentiation, brain energy metabolism, glycogen, Leukemia Inhibitory Factor
Introduction
Generation of astrocytes during development occurs in stages that depend on a complex set of cell-intrinsic factors and environmental cues (Sauvageot and Stiles, 2002). Identification of specific markers for all steps of astrocytic differentiation remains a key issue for understanding the process at work. Glial fibrillary acidic protein (GFAP) is the classic marker used to identify differentiated astrocytes (Eng et al, 2000). However, functional features of mature astrocytes may represent better markers of terminal differentiation for astrocytes. Glycogen is the major energy reserve of the brain and it is exclusively localized in astrocytes (Cataldo and Broadwell, 1986). Glycogen levels reflect a dynamic equilibrium between glycogenolysis and glycogen synthesis with the glycogen shunt having a significant role in astrocyte energy metabolism (Walls et al, 2009). Moreover, characteristics of its metabolism have been well studied both in vitro and in vivo. (Brown and Ransom, 2007). Nevertheless, no information exists regarding the appearance of glycogen metabolism during astrocytic differentiation. In this study, we took advantage of multipotent stem cells to study the emergence of glycogen metabolism as cells were induced to differentiate into astrocytes by different factors.
Materials and methods
Neurosphere Cultures
The experiments were approved by the Animal Care and Use committee of the University of Lausanne. Neurospheres were prepared essentially as described previously (Brunet et al, 2004). For details, see Supplementary information. Three independent preparations of striato-pallidal neurospheres were used for our study and cultures obtained from each produced similar results.
Treatment with Differentiation Factors
Neurospheres were mechanically dissociated and plated on 12-mm diameter glass coverslips (Assistent n° 1001, Munich, Germany) precoated with poly-ornithine and placed in 24-well tissue culture plates (Costar 3524, Corning, Amsterdam, The Netherlands) for immunocytochemistry or seeded in 12-well tissue culture plates (Nunc N° 150629, Nalge Nunc International, Kamstrup, Denmark) for metabolic tests or RNA extraction. All immunocytochemical labelings, quantitative reverse transcription-PCR measurements, and metabolic tests were conducted at day 7 in vitro after dissociation and plating. Factors such as Leukemia Inhibitory Factor (LIF) or Ciliary Neurotrophic Factor (CNTF) were added at 5 ng/mL twice to the culture, 3 days and 1 day before immunocytochemical and metabolic assessment. Epidermal Growth Factor (EGF) at 5 ng/mL and fetal calf serum (FCS) at 10% were added once, 2 days before analysis. All experiments were conducted on three independent neurosphere preparations.
Quantitative Real-Time Reverse Transcriptase-PCR
Cells grown in poly-ornithine precoated 12-well plates were lysed in Trizol (Life Technology, Gaithersburg, MD, USA) after differentiation or under control conditions at day 7 in vitro. Total RNA from three wells per condition was extracted as described by the supplier and quantified at 260 nm. Reverse transcription was performed using 500 ng total RNA in 50 μL final volume using Taqman Reverse Transcription Kit (Applied Biosystem, Warrington, UK) as described by the supplier. Controls were processed with similar RT conditions but without the reverse transcriptase. Quantitative PCR was performed with SYBR Green PCR Master Mix (part number 4309155, Applied Biosystem) using the ABI Prism 7000 Sequence Detection System (Applied Biosystem). Optimal concentration of primers was determined for each primer couple with a RT product made from normal brain total RNA. Quantitative real-time RT-PCR experiments were conducted on three independent cultures prepared from the same neurosphere preparation with similar results. The results were expressed in relative unit of RNA copies normalized to actin and EGF condition. For primer sequences, see Supplementary information.
Cytologic Reaction for Glycogen and Immunocytochemistry
Cytologic localization of glycogen was performed using the periodic acid-Schiff method (Rosenberg and Dichter, 1985). For details, see Supplementary information. For cytologic counterstaining, cells were dehydrated in absolute ethanol and counterstained with accustain hematoxylin solution Gill No. 2 (Sigma, Buchs, SG, CH). Cells were then rinsed in water, dehydrated in absolute ethanol, and mounted in Eukitt (Merck, Fontenay sous Bois, France). For immunohistochemistry, cells were fixed with methanol for 5 mins. Immunolabeling was performed by incubating fixed cells overnight at 4°C with an antibody directed against the phenotypic marker GFAP (polyclonal rabbit Z0334 Dako, Glostrup, Denmark) diluted at 1:500 in phosphate-buffered saline with 0.3% bovine serum albumin. After washing in phosphate-buffered saline, cultures were treated with anti-rabbit Igs conjugated to fluorescein isothiocyanate (diluted 1/500, number 111-095-144, Jackson Immunoresearch Laboratories, Suffolk, UK) to reveal all antibodies (30 mins, room temperature). DAPI staining was used to visualize nuclei. Preparations were mounted with fluoromount (Vectashield, Vector Laboratories, Burlingame, CA, USA) and examined using an epifluorescence microscope (Zeiss Axioplan, Feldbach, ZH, CH) with appropriate filters. Glial fibrillary acidic protein and nestin immunostainings were performed on each culture as a control of the astrocyte differentiation state.
Glycogen Assay
After appropriate time of incubation, cells were washed thrice with ice-cold phosphate-buffered saline and sonicated in HCl 30 mmol/L. The suspension was used to measure glycogen as described previously (Sorg and Magistretti, 1992). For details, see Supplementary information.
Results
After mechanical dissociation of neurospheres and seeding on poly-ornithine-coated dishes in control DN1 medium, cells in all conditions were observed at 7 days in vitro (DIV). Neural stem cells formed a monolayer of small flat cells after addition of EGF at 5 ng/mL during the last 48 h (5 to 7 DIV). If 10% of FCS was added instead during the last 48 h (5 to 7 DIV) or if LIF or CNTF was added twice (4 and 6 DIV) at 5 ng/mL during the last 72 h (4 to 7 DIV), cells adopted a characteristic, cultured astrocyte morphology with large polygonal cytoplasm and small processes, as revealed with GFAP immunostaining (Figure 1A). The periodic acid-Schiff reaction showed the presence of high glycogen content (pink) in cells from FCS and LIF conditions but much lower levels in the CNTF condition. Glial fibrillary acidic protein immunofluorescence confirmed that astrocytic differentiation occurred after FCS, LIF, or CNTF stimulation. Strong colocalization between GFAP immunolabeling and glycogen staining was observed, with high intensity in FCS and LIF conditions but much lower intensity in the CNTF condition (Figure 1A).
Figure 1.
Glycogen content and levels in neural stem cells induced to differentiate into astrocytes. (A) Cytochemical and immunocytochemical labeling of mouse neural stem cells treated to induce differentiation into astrocytes. Mouse neural stem cells cultured for 7 days on poly-ornithine-coated coverslips received either EGF (5 ng/mL) or FCS (10%) at 5 DIV or either LIF or CNTF at 5 ng/mL applied at 4 and 6 DIV. Cultures were stained for glycogen with periodic acid and basic fuschin (in pink) and counterstained with hematoxylin (in blue) (lane PAS hematoxylin), or immunocounterstained with GFAP antibody (in green) (lane fluorescent green filter), glycogen PAS-staining (in red) (lane PAS fluorescent red filter), merged image with DAPI-counterstained nuclei (in blue). Bar=100 μm. Data shown from one representative experiment were repeated 5 times with similar results. (B) Effect of forskolin on glycogen levels in mouse neural stem cells treated to induce differentiation into astrocytes. After treatment as mentioned above to differentiate mouse neural stem cells into astrocytes, forskolin (50 μmol/L) was added and glycogen levels were determined for each time indicated. Data represent mean±s.e.m. of n=4 determinations. Data shown from one representative experiment were repeated 5 times with similar results. The color reproduction of this figure is available on the html full text version of the manuscript.
Glycogen levels measured in the EGF condition were very low (∼3 nmol of glycosyl unit per mg of protein). They were much higher in FCS-differentiated astrocytes (150 nmol/mg protein) and further in LIF-differentiated astrocytes (600 nmol/mg protein) but they remained low in CNTF-differentiated astrocytes (15 nmol/mg protein; Figure 1B, time 0). Stimulation with forskolin had no effect on the very low level of glycogen in EGF-treated cells and in CNTF-differentiated astrocytes. In FCS-differentiated astrocytes, a significant decrease in glycogen levels was observed after 30 mins and 1 h with forskolin. After 2 h, glycogen levels increased above control values with a maximum after 6 h. Despite a small decrease, they remained above control values after 24 and 32 h. For LIF-differentiated astrocytes, glycogen levels remained elevated for 2 h and decreased progressively between 6 and 32 h after forskolin treatment (Figure 1B).
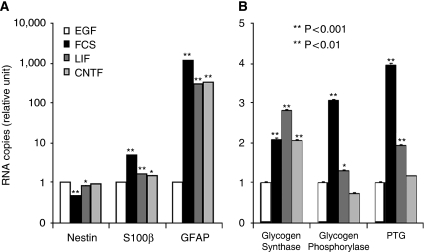
Quantitative assessment of mRNA expression for several phenotypic and metabolic markers was performed in mouse neural stem cell cultures after treatment with EGF, FCS (10%), LIF, or CNTF. Real-time quantitative RT-PCR data confirmed that an enhancement of GFAP and S100β mRNA expression was induced by FCS, LIF, and CNTF (Figure 2A). A significant reduction in nestin mRNA was also observed with FCS and LIF but not with CNTF. In parallel, FCS caused an increase in glycogen synthase, glycogen phosphorylase, and protein targeting to glycogen (PTG) mRNA levels (Figure 2B). A significant increase was also observed after LIF-induced differentiation, although the effect on glycogen phosphorylase and PTG mRNAs was not as strong as in FCS-differentiated astrocytes. In contrast, CNTF only induced glycogen synthase mRNA expression.
Figure 2.
Quantitative gene expression analysis by real-time RT-PCR of various phenotypic and metabolic markers in mouse neural stem cells induced to differentiate into astrocytes. Mouse neural stem cells cultured for 7 days on coverslips received either EGF (5 ng/mL) or FCS 10% at day 5 or either LIF or CNTF (5 ng/mL) at DIV 4 and 6 before total RNA extraction and quantitative real-time RT-PCR analysis. Data are mean±s.e.m. (n=3) for each condition. Statistical analysis was performed using ANOVA followed by Dunnett's test. *P<0.01 versus EGF treatment; **P<0.001 versus EGF treatment. One representative experiment shown repeated thrice with similar results.
Discussion
In a previous study, it was shown that EGF maintains stem cells in an undifferentiated state, enhancing nestin expression and preventing them from spontaneously expressing characteristic markers of astrocytes (such as GFAP) or displaying metabolic features (such as glutamate uptake) (Brunet et al, 2004). Accordingly, glycogen levels found in neural stem cells treated with EGF were barely detectable, indicating that glycogen metabolism is not associated with such an undifferentiated stage. Exposure to FCS is a classic mean to obtain differentiated astrocytes from neural stem cells. Fetal calf serum was found to induce the expression of glutamine synthetase (Loo et al, 1995), an enzyme typically associated with mature astrocytes as it participates to glutamate recycling, which is a major astrocytic function (Ereciñska and Silver, 1990). Our previous data also showed dramatic effects of FCS on a number of specific astroglial proteins and/or mRNAs such as GFAP, vimentin, or S100β, and on expression of key metabolic proteins such as the glutamate aspartate transporter (GLAST), the monocarboxylate transporter 1 (MCT1), and the α2-subunit of the Na+/K+ ATPase (Brunet et al, 2004). Moreover, metabolic features of mature astrocytes such as glutamate uptake or glutamate-induced activation of glycolysis emerged after treatment with FCS. Glycogen metabolism also seems to be associated with maturation of astrocytes. Thus, the cellular glycogen content increased dramatically after FCS exposure. Cells also responded to forskolin treatment by exhibiting both a short-term glycogenolysis and a long-term, overcompensated glycogen resynthesis, two phenomena previously described both in vitro and in vivo (Sorg and Magistretti, 1991, 1992; Swanson et al, 1992; Oz et al, 2009). In parallel, FCS-treated cells had enhanced mRNA expression of three key proteins involved in glycogen metabolism, namely glycogen synthase, glycogen phosphorylase, and PTG. The observation regarding PTG is particularly interesting as this protein was found to be critical for the regulation of glycogen metabolism in astrocytes (Allaman et al, 2000). On this basis, it is proposed that PTG expression could be a valuable marker to identify mature astrocytes both in vitro and in vivo (Lovatt et al, 2007).
Specific growth factors have been identified as key elements in gliogenesis. Among them, the family of interleukin-6 type cytokines which includes CNTF and LIF, occupies a central role (Lee et al, 2000) Numerous reports have suggested that CNTF and LIF can induce the differentiation of stem cells isolated at different embryonic ages into astrocytes, as determined by the expression of GFAP (Rajan and McKay, 1998). Indeed, both CNTF and LIF were found to enhance GFAP expression in our stem cell cultures. Interestingly, the impact of each factor on glycogen metabolism was different. Ciliary Neurotrophic Factor modestly enhanced glycogen levels, whereas LIF led to a massive increase, thrice as much as for FCS. Moreover, CNTF-treated cells did not respond to forskolin, whereas a delayed but marked glycogenolysis was observed in cells differentiated with LIF. In contrast to FCS treatment, no glycogen resynthesis was observed. In parallel, these cytokines also differentially affected the expressions of glycogen synthase, glycogen phosphorylase, and PTG. Thus, the presence of FCS seems to be the only condition in which the full complement of glycogen metabolism is expressed in a manner that matches mature astrocytes. These data suggest that each trophic factor, as exemplified by LIF, although participating to a certain degree in differentiation, is not sufficient to reach the final stage of mature astrocytes. In this regard, glycogen metabolism seems to represent an exquisite and sensitive marker to assess the degree of astrocytic differentiation. In the search for understanding the precise sequence of events in gliogenesis, glycogen metabolism and its associated proteins could become very useful tools.
Acknowledgments
We express our gratitude to Laurence Grollimund for her expert technical assistance.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Conflict of interest
The authors declare no conflict of interest.
Supplementary Material
References
- Allaman I, Pellerin L, Magistretti PJ. Protein targeting to glycogen mRNA expression is stimulated by noradrenaline in mouse cortical astrocytes. Glia. 2000;30:382–391. [PubMed] [Google Scholar]
- Brown AM, Ransom BR. Astrocyte glycogen and brain energy metabolism. Glia. 2007;55:1263–1271. doi: 10.1002/glia.20557. [DOI] [PubMed] [Google Scholar]
- Brunet JF, Grollimund L, Chatton J-Y, Lengacher S, Magistretti PJ, Villemure J-G, Pellerin L. Early acquisition of typical metabolic features upon differentiation of mouse neural stem cells into astrocytes. Glia. 2004;46:8–17. doi: 10.1002/glia.10348. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, Broadwell RD. Cytochemical identification of cerebral glycogen and glucose-6-phosphatase activity under normal and experimental conditions. II. Choroid plexus and ependymal epithelia, endothelia and pericytes. J Neurocytol. 1986;15:511–524. doi: 10.1007/BF01611733. [DOI] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- Erecińska M, Silver IA. Metabolism and role of glutamate in mammalian brain. Prog Neurobiol. 1990;35:245–296. doi: 10.1016/0301-0082(90)90013-7. [DOI] [PubMed] [Google Scholar]
- Lee JC, Mayer-Proschel M, Rao MS. Gliogenesis in the central nervous system. Glia. 2000;30:105–121. doi: 10.1002/(sici)1098-1136(200004)30:2<105::aid-glia1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Loo DT, Althoen MC, Cotman CW. Differentiation of serum-free mouse embryo cells into astrocytes is accompanied by induction of glutamine synthetase activity. J Neurosci Res. 1995;42:184–191. doi: 10.1002/jnr.490420205. [DOI] [PubMed] [Google Scholar]
- Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH, Han X, Takano T, Wang S, Sim FJ, Goldman SA, Nedergaard M. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci. 2007;27:12255–12266. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz G, Kumar A, Rao JP, Kodl CT, Chow L, Eberly LE, Seaquist ER.2009Human brain glycogen metabolism during and following hypoglycemia Diabetes(e-pub ahead of print, 5 June) [DOI] [PMC free article] [PubMed]
- Rajan P, McKay RD. Multiple routes to astrocytic differentiation in the CNS. J Neurosci. 1998;18:3620–3629. doi: 10.1523/JNEUROSCI.18-10-03620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg PA, Dichter MA. Glycogen accumulation in rat cerebral cortex in dissociated cell culture. J Neurosci Methods. 1985;15:101–112. doi: 10.1016/0165-0270(85)90048-2. [DOI] [PubMed] [Google Scholar]
- Sauvageot CM, Stiles CD. Molecular mechanisms controlling cortical gliogenesis. Curr Opin Neurobiol. 2002;12:244–249. doi: 10.1016/s0959-4388(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Sorg O, Magistretti PJ. Characterization of the glycogenolysis elicited by vasoactive intestinal peptide, noradrenaline and adenosine in primary cultures of mouse cerebral cortical astrocytes. Brain Res. 1991;563:227–233. doi: 10.1016/0006-8993(91)91538-c. [DOI] [PubMed] [Google Scholar]
- Sorg O, Magistretti PJ. Vasoactive intestinal peptide and noradrenaline exert long-term control on glycogen levels in astrocytes: blockade by protein synthesis inhibition. J Neurosci. 1992;12:4923–4231. doi: 10.1523/JNEUROSCI.12-12-04923.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Morton MM, Sagar SM, Sharp FR. Sensory stimulation induces local cerebral glycogenolysis: demonstration by autoradiography. Neuroscience. 1992;51:451–461. doi: 10.1016/0306-4522(92)90329-z. [DOI] [PubMed] [Google Scholar]
- Walls AB, Heimbürger CM, Bouman SD, Schousboe A, Waagepetersen HS. Robust glycogen shunt activity in astrocytes: effects of glutamatergic and adrenergic agents. Neurosci. 2009;158:284–292. doi: 10.1016/j.neuroscience.2008.09.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.