Abstract
This study aimed to characterize hypoxic, but salvageable, tissue imaged by 18F-fluoromisonidazole (18F-FMISO), combining with perfusion-computed tomography (PCT) for regional cerebral blood flow (rCBF) measurement and metabolism by microdialysis (MD) in aneurysmal subarachnoidal hemorrhage (SAH) patients. 18F-FMISO positron-emission tomography (PET)/CT was performed within the period of possible vasospasm (day 6.8±3 after SAH) in seven SAH patients. In parallel, rCBF was determined within the MD region of interest (MD-ROI) (n=5). The MD catheter was inserted into the brain parenchyma with highest risk for ischemia; extracellular levels of glutamate and energy metabolites were registered at time of PET and hourly for 10 days. Twelve-month outcome was evaluated. In asymptomatic patients (n=3) no hypoxia was detected and glutamate levels were low (<10 mmol/L), whereas symptomatic patients had higher glutamate concentrations (P<0.001). Increased 18F-FMISO uptake within the MD-ROI (n=3) was related to higher glutamate levels, while rCBF was above the ischemic range. Hypoxia (increased 18F-FMISO uptake) was present in symptomatic patients and associated with relevant metabolic derangement of extracellular glutamate levels, whereas energy metabolism and rCBF were preserved. This technique has the potential to improve our understanding of the role of cellular hypoxia in aneurysmal SAH.
Keywords: penumbra, PET (positron-emission tomography), subarachnoid hemorrhage
Introduction
Despite recent advances in the management of aneurysmal subarachnoid hemorrhage (SAH), morbidity and mortality remain high. Many factors contribute to this poor outcome, such as infarction after vasospasm.
The pathophysiological cascade of events (for example, release of excitotoxic neurotransmitters) during ischemia may further deteriorate the neurological condition of the patient. Studies using microdialysis (MD) have shown that experimentally induced vasoconstriction after SAH occurs independently of changes in intracranial pressure and cerebral perfusion pressure, but is associated with persistent elevations of extracellular glutamate and poor outcome (Schirmer et al, 2007). Furthermore, cerebral edema formation, an independent risk factor for mortality and poor outcome after SAH, is discussed as a consequence of glutamate-mediated excitotoxicity (Bullock et al, 1998).
Several hypoxia tracers for positron-emission tomography (PET) were synthesized; the most extensively investigated and validated group of hypoxia markers to date are nitroimidazole derivates like 18F-fluoromisonodazole (18F-FMISO). 18F-FMISO has been used to image hypoxia in tumors, ischemic myocardium, and stroke (Markus et al, 2004; Martin et al, 1992; Nunn et al, 1995; Read et al, 2000; Read et al, 1998; Saita et al, 2004). When this lipophilic tracer enters a cell the molecule undergoes a single-electron reduction and forms a radical anion RN-O2-, which quickly gets converted back in the presence of intracellular O2. Thus, the intracellular retention of 18F-FMISO is inversely related to the oxygen content inside the cell. This theoretically allows early imaging of the target of therapy being the severely hypoxic, but salvageable, brain tissue, also called penumbra (Markus et al, 2004). Animal studies (Saita et al, 2004; Takasawa et al, 2007) and few studies on stroke patients (Markus et al, 2004; Read et al, 2000; Read et al, 1998) had demonstrated the ability of 18F-FMISO PET to detect the penumbra around the ischemic core. Importantly, the proportion of bound tissue progressing to salvage correlates with improvements in clinical severity as measured for example, by modified Rankin score (Markus et al, 2004; Read et al, 2000). Therapeutic strategies are aimed to limit the size of infarction and improve functional outcome to rescue this potentially reversible ischemic reaction (Fisher, 1997). Nevertheless, due to the limited data in patients so far, the correlation of 18F-MISO uptake with hypoxic, but salvageable (penumbra), tissue is a hypothesis that remains to be tested and the prognosis of the tissue that takes up 18F-MISO depends on the efficacy and kind of treatment.
In this prospective pilot study, we sought to investigate ischemia-related hypoxia in seven patients with aneurysmal SAH, characterizing the relation of hypoxia (18F-FMISO-PET), regional cerebral blood flow (rCBF), measured by perfusion-computed tomography (PCT), and tissue metabolites (cerebral MD), which were monitored within the critical phase of possible vasospasm.
Materials and methods
Patient Population
This study was approved by the Local Research Ethics in accordance with the Declaration of Helsinki Principles as revised in Edinburgh in October 2000. Written informed consent was obtained from the patient or nearest family relative.
Patient Characteristics and Management
During the study period (November 2006 to August 2007), seven consecutive patients with aneurysmal SAH, enrolled in a prospective study on cerebral metabolism monitored by bedside MD, were studied by 18F-FMISO-PET/CT. Inclusion criteria were: (1) SAH confirmed by CT; (2) cerebral angiogram and/or CT angiography demonstrating intracranial aneurysm(s); (3) patients underwent surgical therapy with clipping of the aneurysm; and (4) patients were fully stable for transportation. The aneurysm location was assessed using four-vessel angiography or CT angiography on the day of admission. Clinical presentation was graded according to the WFNS Scale (Drake, 1988). The distribution and pattern of the hemorrhage was graded as proposed by Fisher et al (1980). Patients were excluded if they were hemodynamically unstable, presented with fixed and dilated pupils on admission, or died within 24 h after admission. In case of suspicion of contrast-agent allergy or thyroid hormone disturbance, PCT was not performed.
All patients were categorized according to their clinical course without knowledge of the PET, MD, and CBF data, but using angiography/TCD, for confirming a suspicion of symptomatic vasospasm (DIND) into the following three groups:
Asymptomatic patients presenting with only minor symptoms such as headache and no neurological deficits on admission and after aneurysm occlusion during the complete ICU/clinical stay.
Symptomatic patients presenting with ‘symptomatic vasospasm,' also called DIND. DIND was defined as development of new focal neurological signs, deterioration in level of consciousness, or both, when the cause was thought to be ischemia attributable to vasospasm after other possible causes of worsening (for example, hydrocephalus) had been excluded. The secondary deterioration had to be attributable to DIND and was confirmed by control angiography (vessel narrowing) or TCD. The presence of symptomatic vasospasm was defined according to Lanzino and co-workers (Lanzino and Kassell, 1999) and confirmed in control angiography and/or TCD. An increase in mean blood flow velocity in TCD of more than 50% within 24 h or a mean blood flow velocity of more than 200 cm/s was regarded as pathological.
Symptomatic patients presenting with acute focal neurological deficits (AFNDs). The diagnosis of AFNDs was determined on a clinical basis, with the use of the following criteria: (1) symptoms of neurological deficits with onset of SAH related to the initial hemorrhage or directly after surgery (vessel clip occlusion, thromboembolic events, or early edema); (2) symptoms developing immediately after the insult or after surgery within a few hours; (3) CT findings to rule out a pre-existing neurological disorder or hydrocephalus as the cause of the acute neurological deterioration; and (4) no other identifiable cause of neurological deterioration such as electrolyte disturbances, seizure, and symptomatic vasospasm. (Sarrafzadeh et al, 2003). These AFND patients are mostly high-WFNS-grade patients, frequently difficult to evaluate neurologically as they are comatose or with reduced vigilance, and generally with poor outcome. In our view, these patients are the most important to monitor invasively. Of course AFND patients can develop additionally symptomatic vasospasm as a secondary complication, which can be difficult to detect. AFND patients have the highest derangements of cerebral parameters, making this patient categorization necessary for a detailed interpretation of metabolic parameters. All patients with AFNDs even with possible DIND are classified as AFNDs.
Global handicap was assessed with the Glasgow Outcome Scale, both at 6 and 12 months (Jennett and Bond, 1975).
Bedside MD
An MD catheter (CMA 70; CMA, Stockholm, Sweden; length 10 mm, molecular weight limit 100.000) was inserted immediately after aneurysm clipping into the brain parenchyma of the corresponding vascular territory of the aneurysm, for example, the right frontal lobe in patients with an anterior-communicating-artery aneurysm. Insertion depth was approximately 10 to 15 mm from dura level. Care was taken to avoid insertion into macroscopically damaged brain tissue or an intracerebral hemorrhage. The correct positioning of the catheter tip within the vascular territory of the occluded aneurysm was verified postoperatively by CT. Catheters were perfused with sterile Ringer's solution at a flow rate of 0.3 μL/min. On the outlet tube, perfusates were collected in microvials and analyzed on an hourly basis at bedside with a mobile photometric, enzyme-kinetic analyzer (CMA 600; CMA). The estimated recovery fraction for the system is 0.65 to 0.72 (Hutchinson et al, 2000). MD data are presented as microdialysate concentrations. MD data at time of 18F-FMISO-PET and 24-h median values for each MD variable for each patient of the first 10 days after SAH were recorded.
Imaging Protocol
Patients were studied within the critical phase of possible vasospasm day 4 to 8 after initial bleeding. 18F-FMISO was provided by IASON GmbH (Graz, Austria) and transported to Berlin on the day of investigation. Two hours after intravenous administration of 18F-FMISO at a dose of 0.05 mCi/kg, CT and PET brain scans were acquired on with PET/CT scanner Biograph 16 (Siemens, Erlangen, Germany). First, an initial unenhanced cranial CT was performed, which served for attenuation correction, detection of infarcted areas, to exclude acute hemorrhage, and localization of the MD catheter. After unenhanced CT of the whole brain, two adjacent 12-mm sections (detector collimation 12 × 1.5 mm) were selected, one covering the MD-catheter tip and the neighboring section below or above the MD-catheter tip, depending on the location, surrounding bone and metallic implants such as coils or clips, which were to be excluded in the second section as far as possible to reduce artifacts. Forty milliliters of a nonionic contrast agent (Ultravist 370; Bayer/Schering, Berlin, Germany) were injected at a rate of 7 mL/s. With a delay of 7 s from initiation of the injection, a dynamic CT scan was initiated (scan duration: 40 secs; number of images, 80 with two adjacent images per second; tube voltage, 80 kV; tube current, 209 mA; rotation time, 1 s; reconstructed field of view, 20 cm). Perfusion maps showing CBF were calculated using the maximum slope method as provided by Siemens. Briefly, this method is based on analysis of the maximal slope of the time–density curve deriving from serial CT scans, obtained using rapid injection (10 to 20 mL/s) of a sharp contrast medium bolus (Siemens, Erlangen, Germany) (Mayer et al, 2000).
On the CT scan, a circular region of interest (ROI) of 15 mm diameter was defined around the tip of the MD catheter; identical ROIs were placed just above and below the MD-ROI on the neighbouring CT slices, resulting in a total volume of interest (MD-VOI) of 2.65 mL. These three ROIs were mirrored to the contralateral (CL) hemisphere. For all patients, rCBF in the MD-VOI (average of the three MD-ROIs) and in the CL-VOI was determined by perfusion-CT.
Image Analysis
18F-FMISO images were first evaluated visually to identify areas of increased activity. Using ROI techniques, the mean activity (±s.d.) was determined in the MD-ROIs on the side of the clipped aneurysm and compared with that of the mirror ROI site in the normal CL hemisphere; ROIs were transferred from the inherently fused CT scan as the MD tip is not visible on PET images (Sarrafzadeh et al, 2004). Furthermore, 18F-FMISO trapping within the vascular territory of the vessel of the clipped aneurysm was visually determined in knowledge of the location of the MD catheter. As the PET/CT scanner was used for acquisition of 18F-FMISO PET data, the position of the catheter/trephination area was visible in the corresponded CT scan. The evaluation of PET data alone (without the corresponded CT scan) would be misleading, because the unspecific increase in tracer activity in the trepanation area (adjacent to brain tissue) could only be reliably differentiated from the hypoxic brain areas in the fused PET/CT data. The visually determined trapping of 18F-MISO was blinded to the clinical symptoms and the MD data.
Statistical Analysis
Summary data for metric variables are expressed as mean±s.d. if normally distributed or median and quartiles if the distribution was not normal. Comparisons between groups were performed using t-tests for normally distributed variables, Mann–Whitney U-tests for not normally distributed metric variables, and χ2-tests or in case of expected cell frequencies <5, Fisher's exact tests for ordinal or nominal data. Statistical analyses were conducted using SPSS, version 15.0 (SPSS Inc., Chicago, IL, USA) and SAS (version 8.0 (SAS Institute Inc., Cary, NC, USA). Differences were considered statistically significant at P<0.05.
Results
Patient Characteristics
Seven aneurysmal SAH patients (one male, six female; mean age 51. 6±11.3 years) were recruited to the study. All patients underwent early aneurysm clipping within 24 h after initial symptoms. The decision for clipping and not coiling was previously discussed in accordance by a vascular neuroradiologist and neurosurgeon. An MD catheter was inserted directly after aneurysm clipping in the vascular territory related to the location of the aneurysm, as described previously by others and our group (Nilsson et al, 1999; Sarrafzadeh et al, 2002). The duration of the MD monitoring was on average 8.5 days.
Patients were aimed to be studied by combined PET/PCT within the critical phase of possible vasospasm day 4 to 8 after initial bleeding (6.8±2.9 days). In five of seven patients, the rCBF (PCT) could be measured. Patients had an initial WFNS grade of 2±1.5.
Three patients presented with symptoms of AFND, two of them developed additionally symptomatic vasospasm and one patient developed DIND. The cause of the AFND was an intracerebral hemorrhage within the middle cerebral artery (MCA) region in all the cases. Three patients were categorized as asymptomatic. Mean-outcome Glasgow Outcome Scale (Jennett and Bond, 1975) after 12 months was 4.6±0.8 (Table 1).
Table 1. Patient details.
| Patient ID/ age/sex | Aneurysm location | Symptoms | Day of PET (SAH=0) | rCBF (PCT) left/right (mL/100 g/min) | FMISO trapping in MD-ROI (close to) | Trapping within the vascular territory of the aneurysm | GOS (12 months) |
|---|---|---|---|---|---|---|---|
| 185/41/F | L MCA | Aphasia, centralis facialis paresis | 4 | 20.9/19.8 | + | + | 5 |
| 186/62/F | L MCA | R hemiparesis, aphasia, somnolent | 5 | − | + | + | 3 |
| 189/64/F | R MCA | Asymptomatic | 12 | 48.4/33.2 | − | − | 5 |
| 190/49/F | R MCA | L hemiparesis, aphasia | 7 | 28.4/22.6 | (+) | + | 4 |
| 192/63/F | L PCA | Aphasia | 8 | (198.2/144.9)a | − | − | 5 |
| 197/45/F | L MCA | Asymptomatic | 4 | – | − | − | 5 |
| 199/37/M | L MCA | Asymptomatic | 8 | 27.5/28.5 | − | − | 5 |
F, female; FMISO, fluoromisonidazole; GOS, Glasgow Outcome Scale; L, left; M, male; MCA, middle cerebral artery; MD-ROI, microdialysis-region of interest; PCT, perfusion-computed tomography; PET, positron emission tomography; R, right; rCBF, regional cerebral blood flow; ROI, region of interest; SAH, subarachnoidal hemorrhage.
Data are expressed as absolute numbers.
Unexplained extremely high rCBF values (rather a bug than a combination of cortical hyperemia and partial volume effects).
18F-FMISO Trapping
18F-FMISO trapping in the affected vascular territory was found in two patients (Table 1 and Figures 1 and 2). Furthermore, in all but one patient areas of increased tracer activity were detected within the M. temporalis of the trepanation side.
Figure 1.
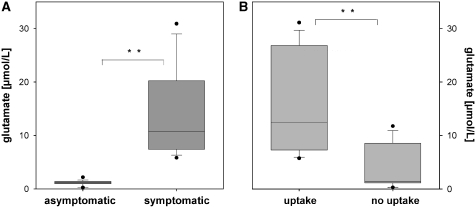
Glutamate levels in relation to symptoms (A) and 18F-FMISO uptake (B). Data are expressed as median and quartiles of the 24-h glutamate values on the day of PET. *, significance P<0.05 and **, significance P<0.01.
Figure 2.
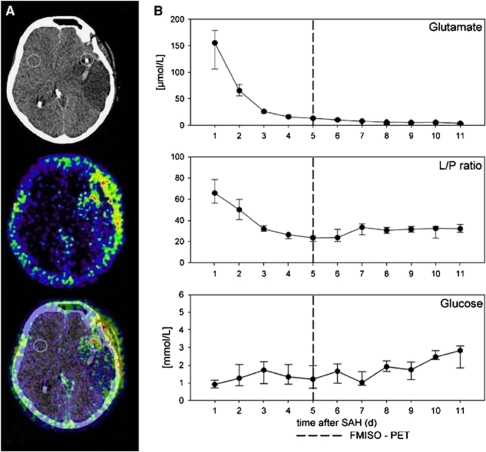
Clinical case I. Individual course of parameters of cerebral metabolism (in hours after SAH) in a patient (female, 62 years, WNFS grade 1, left MCA aneurysm) on day of PET and during the whole clinical stay (B). Especially glutamate showed distinct cerebral metabolic derangement while the lactate/pyruvate ratio (L/P) ratio was within normal range. The patient later developed middle cerebral infarction within the monitored region explaining the high glutamate levels. Please note that the metabolism (daily medians) at day of PET is marked with a line (PET/CT was performed 5 days after initial bleeding). Hourly measured samples at time of PET (A) did not differ from the median levels shown.
Two patients showed 18F-FMISO trapping on PET scan within and one patient very close (<3 mm) to the MD-ROI (Table 1 and Figures 1, 2 and 3). The co-registered CT detected hypodense areas, confirmed in later CT as infarcted brain tissue within the affected vascular territory in one case. PET showed no 18F-FMISO trapping inside the infarct core, but showed areas of increased tracer uptake in the adjacent peri-infarct tissue.
Figure 3.
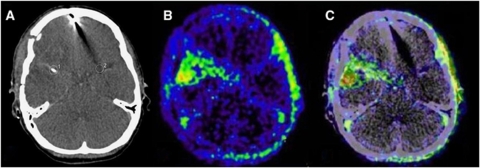
Clinical case II. A second case showing 18F-MISO uptake (B, C) in a symptomatic patient (female, 49 years, WFNS grade 3, right MCA aneurysm). The patient developed left hemiparesis and aphasia. PET/CT was performed 7 days after SAH. At time of PET the MD values were as follows: glutamate, 20.7 μmol/L; glycerol and 28.5 μmol/L. L/P ratio was 29.3. In panel A (CT scan) the MD catheter is visible on the right side (ROI, 1).
18F-FMISO Trapping and Brain Metabolism
In asymptomatic patients (n=3), no hypoxic region was found by PET in the MD-ROI (and elsewhere apart from the trephination area). Related glutamate levels, measured in the MD-ROI were low (<10 mmol/L). Symptomatic patients (n=4) had significantly higher glutamate concentrations (P<0.001; Figure 1). In regions with increased 18F-FMISO uptake (n=3), glutamate levels were significantly higher compared with levels measured in non-hypoxic areas. Interestingly, parameters of energy metabolism (lactate/pyruvate ratio, glucose), shown to be valuable markers of compromised or anaerobic metabolism (Schlenk et al, 2008; Vespa et al, 2005), were within normal range, indicating still no anaerobic metabolism within the hypoxic region (Figure 2 and Table 2).
Table 2. Cerebral metabolism on the day of PET.
| Patient ID | Glucose (mmol/L) | Lactate (mmol/L) | Pyruvate (μmol/L) | L/P ratio | Glutamate (μmol/L) |
|---|---|---|---|---|---|
| 185 | 1.8 (1.7–2.0) | 5.6 (5.1–5.3) | 278.9 (262.9–296.4) | 19.2 (18.7–20.2) | 7.1 (6.6–7.5) |
| 186 | 1.2 (0.8–1.9) | 5.4 (5.1–5.8) | 242.8 (217.4–250.8) | 22.4 (20.0–25.2) | 13.2 (12.0–14.3) |
| 189 | 1.4 (1.3–1.7) | 2.2 (2.1–2.5) | 110.7 (106.8–122.8) | 20.9 (19–22.0) | 0.3 (0.3–0.5) |
| 190 | 0.6 (0.5–0.7) | 11.2 (10.3–11.5) | 281.2 (233.7–295.6) | 39.8 (35.7–46.8) | 28.5 (26.9–29.9) |
| 192 | 1.6 (1.0–1.9) | 3.4 (3.0–3.7) | 175.0 (161.4–183.2) | 18.3 (17.9–20.0) | 9.1 (8.1–10.7) |
| 197 | 0.7 (0.7–1.0) | 2.2 (2.0–2.8) | 87.2 (83.1–107.1) | 24.3 (22.8–26.0) | 1.4 (1.1–1.5) |
| 199 | 1.6 (1.3–1.7) | 3.4 (2.7–3.7) | 140.7 (132.1–153.5) | 22.2 (20.5–26.1) | 1.3 (1.2–1.5) |
L/P ratio, lactate/pyruvate ratio; PET, positron-emission tomography.
Microdialysates are expressed as medians (quartiles) on the day of PET.
Relation of rCBF and 18F-FMISO Trapping
There was a co-registration of rCBF (PCT) within the MD-ROI in five patients in whom the PCT could be acquired. For one patient with known hyperthyroidism, the PCT could not be performed because the injection of the iodinated contrast media was contraindicated. For another patient, the PCT was not possible due to technical reasons.
In the regions of 18F-FMISO uptake (n=2) rCBF was relatively low (20 to 23 mL/100 g/min) compared with that in the mirrored non-hypoxic areas (27 to 33 mL/100 g/min), although the rCBF values were always above the threshold of ischemia (17 to 22 mL/100 g/min) (Furlan et al, 1996; Marchal et al, 1996, 1999) (Table 1). A correlation between rCBF and intensity of 18F-FMISO-uptake was not observed (NS).
Discussion
18F-FMISO, the best validated PET radiotracer of hypoxia, allowing early imaging of hypoxic tissue, was used to characterize the target of therapy in aneurysmal SAH patients possibly having ischemic but viable brain tissue. To our knowledge, 18F-FMISO was used for the first time for SAH patients, especially in combination with perfusion CT (PCT), measuring rCBF and regional cerebral metabolism monitored with MD.
The results presented have shown that in asymptomatic patients no hypoxic tissue binding was observed; whereas symptomatic patients had increased 18F-FMISO uptake within the afflicted vascular territory. In the monitored ROI, glutamate levels were low in asymptomatic patients (<10 mmol/L) and significantly higher in symptomatic patients. In regions of increased 18F-FMISO uptake, glutamate level was significantly higher whereas rCBF was slightly lower compared with that in non-hypoxic areas, but always above the threshold of ischemia (15 mL/100 g/min). In regions of increased 18F-FMISO uptake, relevant metabolic derangement of the excitotoxic neurotransmitter, glutamate, occurred already above the ischemic threshold. The viability of tissue was reflected in stable levels of markers of energy metabolism (MD monitoring), indicating no anaerobic metabolism and a favorable outcome after 12 months in all but one patients.
18F-FMISO-PET in Experimental and Clinical Stroke Studies
In vivo measurement of hypoxia in individual patients is of major clinical interest. It can provide insight into the natural course and the pathophysiology of ischemia, possibly assisting in optimizing anti-ischemic therapies and in the evaluation of treatment response. Invasive techniques such as measurement of regional tissue PO2 with oxygen probes are available for patients but may miss the target of therapy being the penumbra, which is the severely hypoxic but potentially salvageable region surrounding the ischemic score (Baron, 2001).
Misonidazole is a derivate, which is selectively retained in hypoxic tissue after reduction by cellular reductases and binding to cellular components (Chapman et al, 1983). This tracer is not retained in normoxic cells in which the molecule is immediately reoxidized and is not available for further reduction and trapping or in irreversibly injured cells in which the enzymes responsible for reduction and binding of the tracer are compromised (Markus et al, 2003). Furthermore, 18F-FMISO binding did not occur after effective reperfusion, despite histological injury from the preceding MCA occlusion (MCAo), and is, therefore, seen to be indicative of ongoing tissue hypoxia, not merely recent tissue injury (Spratt et al, 2006a). The implication for human studies is that if patients effectively reperfuse before study, they will have a negative 18F-FMISO PET scan (Spratt et al, 2006a). It has still to be demonstrated in studies of early reperfusion whether 18F-FMISO-binding tissue reliably can be saved.
Interestingly, the increased 18F-FMISO binding was observed in animals after reperfusion, reflecting the ongoing ischemia from vasospasm after subarachnoid hemorrhage (Spratt et al, 2006b).
18F-FMISO Trapping in Hypoxic Brain Areas
An experimental study with permanent temporary MCAo in seven rats has confirmed the lack of 18F-FMISO trapping both when ischemic necrosis has fully developed 48 h after permanent MCAo and when tissue is not necrotic and has been reperfused after brief MCAo, that is, when hypoxia is not expected (Takasawa et al, 2007 and Table 3). Some 18F-FMISO accumulation was also seen in the temporalis muscle early after MCAo, which is transsected during the surgical exposure of the MCA and is, therefore, potentially hypoxic acutely. Also in the present study, in almost all patients areas of increased tracer activity were seen close to the trephination area. In contrast to the above mentioned experimental study, the timing of PET after surgery was clearly later (6.8±2.9 days after SAH). Interestingly tracer activity within trepination area was observed also in asymptomatic patients, therefore in our view the regional uptake might be of less relevance.
Table 3. Literature on 18F-FMISO-PET of patients and experimental data.
| Reference | No. of FMISO- PET | Type of lesion | Neuromonitoring | Study design | Conclusion |
|---|---|---|---|---|---|
| Takasawa et al, 2007 (J Cereb Blood Flow Metab) | 7 (rats) | MCA occlusion | — | Experimental | Elevated 18F-FMISO uptake in the stroke area only in the early phase of MCAo, but neither after early reperfusion nor when tissue necrosis has developed. Validity of 18F-FMISO as a marker of viable hypoxic tissue/penumbra after stroke. |
| Bruehlmeier et al, 2004 (J Nucl Med) | 11 | Various brain tumors | — | Clinical | Late 18F-FMISO-PET images provide a spatial description of hypoxia in brain tumors that is independent of BBB disruption and tumor perfusion. The distribution volume is an appropriate measure to quantify 18F-FMISO uptake. The perfusion-hypoxia patterns described in glioblastoma suggest that hypoxia in these tumors may develop irrespective of the magnitude of perfusion. |
| Saita et al, 2004 (Stroke) | 38 (rats) | Transient MCA occlusion | — | Experimental | The pattern of 18F-FMISO-binding rats reproduced the pattern seen in humans, consistent with this tracer being a marker of the ischemic penumbra in both species. This technique may have application in studying the ischemic penumbra in animal models, and correlating this with similar studies in humans. |
| Markus et al, 2003(Stroke) | 19 | Acute MCA territory stroke | — | Clinical | Infarct expansion might occur at the expense of hypoxic tissue from the center to the periphery of the ischemic region in humans, similar to that seen in experimental animal models. |
| Read et al, 1998 (Neurology) | 15 | Acute hemispheric stroke | — | Clinical | FMISO-PET can detect peri-infarct hypoxic tissue after acute ischemic stroke. The distribution of hypoxic tissue may represent the ischemic penumbra. Hypoxic tissues do not persist to the subacute phase of stroke (6 to 11 days). |
Abbreviations: FMISO, fluoromisonidazole; MCAo, middle cerebral artery occlusion; PET, positron emission tomography.
18F-FMISO-PET, rCBF, and Brain Metabolism
An important aspect of this study was to investigate the relation of cerebral perfusion and 18F-FMISO uptake. There are only few studies analyzing 18F-FMISO and perfusion so far. Bruehlmeier et al (2004) have shown that the initially positive correlation between early 18F-FMISO uptake and perfusion is completely lost at 60 to 90 min after injection (Table 3). In our study, the 18F-FMISO images were obtained at 120 min after injection. Therefore, it can be excluded that 18F-FMISO uptake simply reflects perfusion.
One interesting finding of the present study was that patients who presented with increased 18F-FMISO uptake had normal rCBF values (>15 mL/100 g/min) within the monitored region of interest. Interestingly, these rCBF levels observed within the hypoxic tissue by PET were in the range defined for the penumbra for PCT (<25 mL/100 g/min) (Murphy et al, 2006, 2008).
For humans, studies using multi-tracer PET have identified hypoperfused tissue with preserved energy metabolism, compatible with penumbra in the acute stages after stroke. Survival of this tissue was associated with better neurological outcome (Furlan et al, 1996). The metabolic results of the present study similarly suggest that although glutamate levels were increased in the ROI of 18F-FMISO uptake, cerebral energy metabolism was still preserved. The elevated glutamate levels, known to reflect impending or relevant ischemia (SARR PETstroke), did surpass the critical threshold of ischemia (>25 mmol/L) (Vespa et al, 2005) at the time of PET only in one patient (Table 2). This finding is in accordance to the normal rCBF values measured simultaneously.
Clinical Implications
Imaging of the viable hypoxic (penumbra) tissue after SAH allows visualizing a brain region at high risk for ischemia and permanent neurological deficits. The most interesting finding of the present study is that 18F-FMISO-PET images hypoxia even when rCBF still normal. This approach could allow studying the pathophysiology of DIND, especially when combining 18F-FMISO-PET with other neuromonitoring techniques. Since the PET technology is highly time-, labor-, and cost-intensive, in our view, it is reserved for research purpose only—so far. There are some indications that cerebral metabolism, mainly of glutamate, meliorates with triple-h therapy (Sarrafzadeh et al, 2001). In the case of symptomatic vasospasm, it would be relevant to know which treatment (such as high mean arterial blood pressure, angioplasty, and drugs) is sufficient to reduce the hypoxic penumbra shown by 18F-FMISO-PET.
Limitations of this Study
This study was consecutive and prospective, but a larger patient population would be desirable. This is, however, associated with considerably high effort 6as the patients are monitored by MD, have to be clinically stable to allow transport to the inhouse PET, and the tracer has to be produced in time. Furthermore, cerebral MD is a regional method of brain monitoring, with the catheter capturing only metabolic processes within a few millimeters around the membrane. For this reason, only brain metabolism within the vascular territory of the aneurysm's parent vessel was recorded, 6which is not necessarily representative for the whole brain. Combining regional (MD), CBF (PCT), and global monitoring (18F-FMISO) obviously are different apporaches to monitor the brain.
It should be noted that the calculation of tissue perfusion from contrast bolus CT has some profound limitations regarding the validity of perfusion values derived from ischemic brain areas. The maximum-slope model (Gillard et al, 2000; Koenig et al, 1998) used in the present study only provides approximately accurate values for CBF if the maximum slope of the arterial input is attained before venous output starts. Consequently, this method requires theoretically very high injection speeds. If this condition is not fulfilled, the CBF is underestimated and only relative CBF values can be used for evaluation. In everyday clinical practice, however, this disadvantage for stroke diagnosis is of no importance (König et al, 2000).
More studies are needed to show that the vital brain tissue, which takes up 18F-MISO and is characterized by low redox potential, reflects the salvageable (penumbra) tissue. Furthermore 18F-MISO uptake reflects only a snapshot and does not indicate if this tissue will survive with optimal treatment. Additionally, the area of 18F-MISO uptake may not always be related to the neurological symptoms present in the patient.
The Glasgow Outcome Scale, used to asses neurological outcome, is a highly global measure for clinical recovery. It can reliably be used in phone interviews, but is not designed to detect subtle cognitive deficits, which can also cause severe impairment in the quality of life.
Conclusion
18F-FMISO-PET allows detection of the metabolically compromised brain tissue and may identify the salvageable tissue for targeted therapy even when rCBF is within normal range. Increased cerebral 18F-FMISO-PET uptake was only present in symptomatic patients and was associated with increased glutamate levels and preserved energy metabolism. This technique has the potential to improve our understanding of the role of cellular hypoxia in aneurysmal SAH.
Acknowledgments
We acknowledge the technical assistance of Sabine Seidlitz and Jasmin Kopetzki and the support of the ICU team.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Baron JC. Mapping the ischaemic penumbra with PET: a new approach. Brain. 2001;124:2–4. doi: 10.1093/brain/124.1.2. [DOI] [PubMed] [Google Scholar]
- Bruehlmeier M, Roelcke U, Schubiger PA, Ametamey SM. Assessment of hypoxia and perfusion in human brain tumors using PET with 18F-fluoromisonidazole and 15O-H2O. J Nucl Med. 2004;45:1851–1859. [PubMed] [Google Scholar]
- Bullock R, Zauner A, Woodward JJ, Myseros J, Choi SC, Ward JD, Marmarou A, Young HF. Factors affecting excitatory amino acid release followingsevere human head injury. J Neurosurg. 1998;89:507–518. doi: 10.3171/jns.1998.89.4.0507. [DOI] [PubMed] [Google Scholar]
- Chapman JD, Baer K, Lee J. Characteristics of the metabolism-induced binding of misonidazole to hypoxic mammalian cells. Cancer Res. 1983;43:1523–1528. [PubMed] [Google Scholar]
- Drake C. Report of World Federation of Neurological Surgeons Committee on a Universal Subarachnoid Hemorrhage Grading Scale. J Neurosurg. 1988;68:985–986. doi: 10.3171/jns.1988.68.6.0985. [DOI] [PubMed] [Google Scholar]
- Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- Fisher M. Characterizing the target of acute stroke therapy. Stroke. 1997;28:866–872. doi: 10.1161/01.str.28.4.866. [DOI] [PubMed] [Google Scholar]
- Furlan M, Marchal G, Viader F, Derlon JM, Baron JC. Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Ann Neurol. 1996;40:216–226. doi: 10.1002/ana.410400213. [DOI] [PubMed] [Google Scholar]
- Gillard JH, Minhas PS, Hayball MP, Bearcroft PW, Antoun NM, Freer CE, Mathews JC, Miles KA, Pickard JD. Assessment of quantitative computed tomographic cerebral perfusion imaging with H2(15)O positron emission tomography. Neurol Res. 2000;22:457–464. doi: 10.1080/01616412.2000.11740700. [DOI] [PubMed] [Google Scholar]
- Hutchinson PJ, O'Connell MT, Al-Rawi PG, Maskell LB, Kett-White R, Gupta AK, Richards HK, Hutchinson DB, Kirkpatrick PJ, Pickard JD. Clinical cerebral microdialysis: a methodological study. J Neurosurg. 2000;93:37–43. doi: 10.3171/jns.2000.93.1.0037. [DOI] [PubMed] [Google Scholar]
- Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- König M, Klotz E, Heuser L. Cerebral perfusion CT: theoretical aspects, methodical implementation and clinical experience in the diagnosis of ischemic cerebral infarction. Rofo. 2000;172:210–218. doi: 10.1055/s-2000-109. [DOI] [PubMed] [Google Scholar]
- Koenig M, Klotz E, Luka B, Venderink DJ, Spittler JF, Heuser L. Perfusion CT of the brain: diagnostic approach for early detection of ischemic stroke. Radiology. 1998;209:85–93. doi: 10.1148/radiology.209.1.9769817. [DOI] [PubMed] [Google Scholar]
- Lanzino G, Kassell NF. Double-blind, randomized, vehicle-controlled study of high-dosetirilazad mesylate in women with aneurysmal subarachnoid hemorrhage. Part II. A cooperative study in North America. J Neurosurg. 1999;90:1018–1024. doi: 10.3171/jns.1999.90.6.1018. [DOI] [PubMed] [Google Scholar]
- Marchal G, Beaudouin V, Rioux P, de la Sayette V, Le Doze F, Viader F, Derlon JM, Baron JC. Prolonged persistence of substantial volumes of potentially viable brain tissue after stroke: a correlative PET–CT study with voxel-based data analysis. Stroke. 1996;27:599–606. doi: 10.1161/01.str.27.4.599. [DOI] [PubMed] [Google Scholar]
- Marchal G, Young AR, Baron JC. Early postischemic hyperperfusion: pathophysiologic insights from positron emission tomography. J Cereb Blood Flow Metab. 1999;19:467–482. doi: 10.1097/00004647-199905000-00001. [DOI] [PubMed] [Google Scholar]
- Markus R, Reutens DC, Kazui S, Read S, Wright P, Chambers BR, Sachinidis JI, Tochon-Danguy HJ, Donnan GA. Topography and temporal evolution of hypoxic viable tissue identified by 18F-fluoromisonidazole positron emission tomography in humans after ischemic stroke. Stroke. 2003;34:2646–2652. doi: 10.1161/01.STR.0000094422.74023.FF. [DOI] [PubMed] [Google Scholar]
- Markus R, Reutens DC, Kazui S, Read S, Wright P, Pearce DC, Tochon-Danguy HJ, Sachinidis JI, Donnan GA. Hypoxic tissue in ischaemic stroke: persistence and clinical consequences of spontaneous survival. Brain. 2004;127:1427–1436. doi: 10.1093/brain/awh162. [DOI] [PubMed] [Google Scholar]
- Martin GV, Caldwell JH, Graham MM, Grierson JR, Kroll K, Cowan MJ, Lewellen TK, Rasey JS, Casciari JJ, Krohn KA. Noninvasive detection of hypoxic myocardium using fluorine-18-fluoromisonidazole and positron emission tomography. J Nucl Med. 1992;33:2202–2208. [PubMed] [Google Scholar]
- Mayer TE, Hamann GF, Baranczyk J, Rosengarten B, Klotz E, Wiesmann M, Missler U, Schulte-Altedorneburg G, Brueckmann HJ. Dynamic CT perfusion imaging of acute stroke. Am J Neuroradiol. 2000;21:1441–1449. [PMC free article] [PubMed] [Google Scholar]
- Murphy BD, Fox AJ, Lee DH, Sahlas DJ, Black SE, Hogan MJ, Coutts SB, Demchuk AM, Goyal M, Aviv RI, Symons S, Gulka IB, Beletsky V, Pelz D, Hachinski V, Chan R, Lee TY. Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke. 2006;37:1771–1777. doi: 10.1161/01.STR.0000227243.96808.53. [DOI] [PubMed] [Google Scholar]
- Murphy BD, Fox AJ, Lee DH, Sahlas DJ, Black SE, Hogan MJ, Coutts SB, Demchuk AM, Goyal M, Aviv RI, Symons S, Gulka IB, Beletsky V, Pelz D, Chan RK, Lee TY. White matter thresholds for ischemic penumbra and infarct core in patients with acute stroke: CT perfusion study. Radiology. 2008;247:818–825. doi: 10.1148/radiol.2473070551. [DOI] [PubMed] [Google Scholar]
- Nilsson OG, Brandt L, Ungerstedt U, Saveland H.1999Bedside detection of brain ischemia using intracerebral microdialysis: subarachnoid hemorrhage and delayed ischemic deterioration Neurosurgery 451176–1184.discussion 1184–1175 [DOI] [PubMed] [Google Scholar]
- Nunn A, Linder K, Strauss HW. Nitroimidazoles and imaging hypoxia. Eur J Nucl Med. 1995;22:265–280. doi: 10.1007/BF01081524. [DOI] [PubMed] [Google Scholar]
- Read SJ, Hirano T, Abbott DF, Markus R, Sachinidis JI, Tochon-Danguy HJ, Chan JG, Egan GF, Scott AM, Bladin CF, McKay WJ, Donnan GA. The fate of hypoxic tissue on 18F-fluoromisonidazole positron emission tomography after ischemic stroke. Ann Neurol. 2000;48:228–235. [PubMed] [Google Scholar]
- Read SJ, Hirano T, Abbott DF, Sachinidis JI, Tochon-Danguy HJ, Chan JG, Egan GF, Scott AM, Bladin CF, McKay WJ, Donnan GA. Identifying hypoxic tissue after acute ischemic stroke using PET and 18F-fluoromisonidazole. Neurology. 1998;51:1617–1621. doi: 10.1212/wnl.51.6.1617. [DOI] [PubMed] [Google Scholar]
- Saita K, Chen M, Spratt NJ, Porritt MJ, Liberatore GT, Read SJ, Levi CR, Donnan GA, Ackermann U, Tochon-Danguy HJ, Sachinidis JI, Howells DW. Imaging the ischemic penumbra with 18F-fluoromisonidazole in a rat model of ischemic stroke. Stroke. 2004;35:975–980. doi: 10.1161/01.STR.0000121647.01941.ba. [DOI] [PubMed] [Google Scholar]
- Sarrafzadeh AS, Haux D, Ludemann L, Amthauer H, Plotkin M, Kuchler I, Unterberg AW. Cerebral ischemia in aneurysmal subarachnoid hemorrhage: a correlative microdialysis–PET study. Stroke. 2004;35:638–643. doi: 10.1161/01.STR.0000116101.66624.F1. [DOI] [PubMed] [Google Scholar]
- Sarrafzadeh A, Haux D, Sakowitz O, Benndorf G, Herzog H, Kuechler I, Unterberg A. Acute focal neurological deficits in aneurysmal subarachnoid hemorrhage: relation of clinical course, CT findings, and metabolite abnormalities monitored with bedside microdialysis. Stroke. 2003;34:1382–1388. doi: 10.1161/01.STR.0000074036.97859.02. [DOI] [PubMed] [Google Scholar]
- Sarrafzadeh AS, Peltonen EE, Kaisers U, Kuchler I, Lanksch WR, Unterberg AW. Secondary insults in severe head injury—do multiply injured patients do worse. Crit Care Med. 2001;29:1116–1123. doi: 10.1097/00003246-200106000-00004. [DOI] [PubMed] [Google Scholar]
- Sarrafzadeh AS, Sakowitz OW, Kiening KL, Benndorf G, Lanksch WR, Unterberg AW. Bedside microdialysis: a tool to monitor cerebral metabolism in subarachnoid hemorrhage patients. Crit Care Med. 2002;30:1062–1070. doi: 10.1097/00003246-200205000-00018. [DOI] [PubMed] [Google Scholar]
- Schirmer CM, Hoit DA, Malek AM. Decompressive hemicraniectomy for the treatment of intractable intracranial hypertension after aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:987–992. doi: 10.1161/01.STR.0000257962.58269.e2. [DOI] [PubMed] [Google Scholar]
- Schlenk F, Nagel A, Graetz D, Sarrafzadeh Hyperglycemia and cerebral glucose in aneurysmal subarachnoid hemorrhage. Intensive Care Med. 2008;34:1200–1207. doi: 10.1007/s00134-008-1044-5. [DOI] [PubMed] [Google Scholar]
- Spratt NJ, Ackerman U, Tochon-Danguy HJ, Donnan GA, Howells DW. Characterization of fluoromisonidazole binding in stroke. Stroke. 2006a;37:1862–1867. doi: 10.1161/01.STR.0000226908.93295.9d. [DOI] [PubMed] [Google Scholar]
- Spratt NJ, Fernandez J, Chen M, Rewell S, Cox S, van Raay L, Hogan L, Howells DW. Modification of the method of thread manufacture improves stroke induction rate and reduces mortality after thread-occlusion of the middle cerebral artery in young or aged rats. J Neurosci Methods. 2006b;155:285–290. doi: 10.1016/j.jneumeth.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Takasawa M, Beech JS, Fryer TD, Hong YT, Hughes JL, Igase K, Jones PS, Smith R, Aigbirhio FI, Menon DK, Clark JC, Baron JC. Imaging of brain hypoxia in permanent and temporary middle cerebral artery occlusion in the rat using (18)F-fluoromisonidazole and positron emission tomography: a pilot study. J Cereb Blood Flow Metab. 2007;27:679–89. doi: 10.1038/sj.jcbfm.9600405. [DOI] [PubMed] [Google Scholar]
- Vespa P, Bergsneider M, Hattori N, Wu HM, Huang SC, Martin NA, Glenn TC, McArthur DL, Hovda DA. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab. 2005;25:763–774. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]