Abstract
Inflammatory mechanisms are known to contribute to the pathophysiology of traumatic brain injury (TBI). Since bradykinin is one of the first mediators activated during inflammation, we investigated the role of bradykinin and its receptors in posttraumatic secondary brain damage. We subjected wild-type (WT), B1-, and B2-receptor-knockout mice to controlled cortical impact (CCI) and analyzed tissue bradykinin as well as kinin receptor mRNA and protein expression up to 48 h thereafter. Brain edema, contusion volume, and functional outcome were assessed 24 h and 7 days after CCI. Tissue bradykinin was maximally increased 2 h after trauma (P<0.01 versus sham). Kinin B1 receptor mRNA was upregulated up to four-fold 24 h after CCI. Immunohistochemistry showed that B1 and B2 receptors were expressed in the brain and were significantly upregulated in the traumatic penumbra 1 to 24 h after CCI. B2R−/− mice had significantly less brain edema (−51% versus WT, 24 h; P<0.001), smaller contusion volumes (∼50% versus WT 24 h and 7 d after CCI; P<0.05), and better functional outcome 7 days after TBI as compared with WT mice (P<0.05). The present results show that bradykinin and its B2 receptors play a causal role for brain edema formation and cell death after TBI.
Keywords: Traumatic brain injury, brain edema, inflammation, bradykinin, Kallikerin-kinin system, neuroptotection
Introduction
Traumatic brain injury (TBI) is the leading cause of death among young adults (<45 years) and one of the major causes of disability in the overall population in industrialized countries (Jennett, 1998). The initial mechanical impact on the brain leads to an almost immediate parenchymal damage for which there is no possible therapy. This primary damage leads to blood–brain barrier disruption, brain edema formation, and subsequent increase of intracranial pressure (ICP). These events cause a multitude of delayed secondary mechanisms on the cellular and molecular level, which result in additional cell death, finally leading to secondary expansion of the primary lesion (Gennarelli, 1993).
The kallikrein–kinin system is believed to be one of the first inflammatory pathways activated after tissue damage. Bradykinin, a nonapeptide found in blood and tissue, is cleaved from its pro-form kininogen by the protease kallikrein. It has a half-life of less than 30 secs and binds predominantly to kinin B2 receptors, whereas its degradation product, des-Arg9-bradykinin, binds mainly to B1 receptors. Kinin B2 receptors are constitutively and ubiquitously expressed on mammalian cells, including neurons in rodents and humans (Raidoo et al, 1996; Chen et al, 2000; Groger et al, 2005). Binding of bradykinin to B2 receptors leads to vasodilatation and increased vessel permeability (Wahl et al, 1983; Whalley and Wahl, 1983a), resulting in brain edema formation, as shown, after cerebral ischemia (Relton et al, 1997; Groger et al, 2005) or TBI (Maier-Hauff et al, 1984; Unterberg et al, 1986; Pruneau et al, 1999; Stover et al, 2000; Plesnila et al, 2001; Hellal et al, 2003). So far, however, many aspects of the kallikrein–kinin system have not been investigated after TBI. To date, it is still unknown whether bradykinin is released in the traumatized brain, whether bradykinin receptors may not only be involved in brain edema formation but also in neuronal cell death, and hence in the development of morphological brain damage, whether inhibition of B2 or B1 receptors promotes long-term functional recovery after TBI, and whether B1 receptors may have similar neuroprotective properties to B2 receptors. The aim of the current study was to investigate the time-course of the production of bradykinin and the expression of kinin B1 and B2 receptor mRNA and protein after experimental TBI, and to clarify the role of B1 and B2 receptors in brain edema formation, secondary contusion volume expansion, and neurological function in a kinetic model of experimental TBI (CCI) by using B2- and B1-receptor-knockout mice.
Materials and methods
Controlled Cortical Impact
Male C57/BL6 (Charles River), bradykinin B1-receptor-knockout (B1R−/−; kind gift from Professor Michael Bader), and B2-receptor-knockout mice (B2R−/−; kind gift from Dr Didier Pruneau) with a body weight of 25 to 28 g were used for the current experiments. Both knockout mouse strains were back-crossed more than 10 times with C57/BL6 mice to avoid any difference between the genetic background of the genetically engineered mice and control animals (C57/BL6). Mice were subjected to controlled cortical impact (CCI) as previously described (Zweckberger et al, 2003). Briefly, animals were anaesthetized in a halothane chamber (4%) and anesthesia was maintained with a face mask using 1.2% halothane, 30% O2, and 69% N2O. Body temperature during anesthesia was kept constant at 37°C by a feedback-controlled heating pad. After induction of anesthesia, the skull was fixed in a stereotactic frame and a large craniotomy was performed above the right parietal cortex between the sagittal, the lambdoid, and the coronal sutures, and the insertion of the temporal muscle with a high-speed drill under continuous cooling with saline. Special attention was paid to leave the dura mater intact. CCI was performed perpendicular to the surface of the brain. The impactor tip had a diameter of 3 mm, the final impact velocity being 8 m/s, the impact duration was 150 ms, and the displacement of the brain was 1.0 mm. After trauma, the craniotomy was closed with the initially removed bone flap to allow development of intracranial hypertension. The skin over the craniotomy was carefully closed and animals were transferred to an incubator heated to 35°C until recovery of spontaneous motor activity. Sham-operated animals were subjected to the same procedure without trauma application.
All procedures described are in concordance with local laws and were approved by the animal protection committee of the Government of Upper Bavaria (protocol no. 48-03).
Measurements of Tissue Bradykinin Levels
Brains from sham-operated, C57/BL6 mice and those subjected to CCI were sampled and snap frozen in 2 ml ethanol pre-cooled to –80°C 2, 6, 12, 24, or 48 h after CCI (n=5 per group). Measurements were performed as previously described (Groger et al, 2005). Briefly, ethanol extracts were evaporated under nitrogen at 37°C after homogenization. The residues were dissolved in 140 μL of 10 mmol/L acetic acid. Bradykinin was separated by isocratic high-performance liquid chromatography from precursors and metabolites, which may cross-react with the antiserum (HewlettPackard 1090A; Agilent Technologies, Palo Alto, CA, USA). For bradykinin measurement by radioimmunoassay, rabbit antiserum 1881 (final dilution 1:35,000) and 125iodine-labelled bradykinin (New England Nuclear, Herts, UK) were administered to the samples or to known amounts of bradykinin (0.06 to 125 fmol) (Peninsula Labs, St Helens, UK). After incubation of the samples for 24 h at 4°C, antibody-bound and free bradykinin were separated by addition of 0.2 ml dextran-coated charcoal (1% suspension in water). Bradykinin was quantified by comparison of the percentage of bound radioactivity to that of unknown samples with that of standards.
Brain Edema
Brain water content was determined as previously described (Zweckberger et al, 2006). Sham-operated animals, wild type (WT), and B2R−/− (n=6 per group) were killed at the point of maximal brain edema formation, that is, 24 h after trauma (Zweckberger et al, 2006). After removing of the pons and the olfactory bulbs, brains were weighed (wet weight (ww)) and dried at 110°C for 24 h. After calculation of dry weight (dw), brain water content was quantified by the following formula: ((ww−dw)/ww) × 100.
Contusion Volume
Mice were killed in deep halothane anesthesia. Thereafter brains were carefully removed, frozen in powdered dry ice, and stored at −80°C until further use. Coronal sections measuring 10 μm were prepared every 500 μm on a cryostat (CryoStar HM 560; Microm, Walldorf, Germany) and stained with cresyl violet. Sections were photographed with a digital camera system and the contusion area was determined on the digitized images with a standard image analysis software (DP-Soft; Olympus, Hamburg, Germany) by an investigator blinded to the treatment of the animals. Contusion volume was calculated based on the contusion areas (after correction for brain edema) obtained from 15 sections as previously described (Zweckberger et al, 2003, 2006).
To determine primary damage, contusion volume was measured 15 mins after trauma in WT mice (n=8). To assess secondary damage, contusion volumes of WT, B1R−/−, and B2R−/− (n=8 per group) and WT and B2R−/− (n=7 per group) mice were quantified 24 h and 7 days after trauma, respectively (Zweckberger et al, 2006).
Quantification of Functional Outcome
Functional outcome of the animals was determined over a period of 7 days after trauma by measuring the body weight as a parameter of food intake and general physical activity, and by counting misplacements of the hind paw contralateral to the traumatized brain hemisphere during a beam walking task, as previously described (Zweckberger et al, 2003). These tests were chosen based on our previous experience gained from experiments where we evaluated the usefulness of different functional tests for reliable and sensitive detection of functional deficits in our model.
Quantification of Kinin B1 and B2 Receptor mRNA by Real-Time PCR
Brains from naive, sham-operated, or C57/BL6 mice subjected to CCI were quickly removed 6, 24, or 48 h after trauma, or 24 h after sham surgery, and placed in ice-cold phosphate-buffered saline (n=4 to 6 per group). Coronal sections of 2 mm thickness were cut through the contusion and quartered by a midline horizontal and vertical cut, and total RNA was immediately extracted and prepared from both brain hemispheres using Qiagen's RNA Lipid Tissue Mini Kit (Qiagen, Hilden, Germany). Residual genomic DNA was eliminated by the Rnase-Free Dnase Set (Qiagen) and RNA was finally reverse-transcribed into cDNA. A 1-μg weight of RNA was mixed with 100 ng oligo(dT)15 and incubated for 5 mins at 65°C. 1 mmol/L dNTPs, 60 mmol/L KCl, 15 mmol/L Tris–Cl, pH 8.4, 3 mmol/L MgCl2, 0.3% Tween 20, 10 mmol/-β-mercaptoethanol, 10 U Rnasin (Promega, Madison, WI, USA), and 100 U Mo-MLVRT (Invitrogen, Karlsruhe, Germany) were added. The mix was incubated for 55 mins at 37°C followed by enzyme inactivation for 5 mins at 95°C. Real-time PCR was performed using a Lightcycler in a reaction volume of 10 μL using SYBR Green I (LC-FastStart DNA MasterPlus; Roche, Mannheim, Germany) according to the manufacturer's protocol. Primers were designed using the Beacon Designer software (BioRad, Hercules, CA, USA) based on the following GenBank accession numbers:
Mouse β-actin (X03672): CCACTGCCGCATCCTCTTCC and CGCTCGTTGCCAATAGTGATGAC.
Mouse kinin B1 receptor (NM_007539): GGGTTCGTCATCACTGTCTGTT and GCCAGGTAGATTTCTGCTATGGT.
Mouse kinin B2 receptor (NM_009747): CACTGTGGCCGAGATCTACCT and GGCACAACACCTCTCCAAACA.
Primers were used at a final concentration of 0.5 μmol/L each. Cycling conditions were as follows: after an initial denaturation step at 95°C for 10 mins, amplification was performed by using 45 cycles of denaturation (95°C, 1 s), annealing (65°C, 10 s), and extension (72°C, 20 s).
Immunohistochemistry for Kinin B1 and B2 Receptors
C57/BL6 mice subjected to CCI and sham-operated animals were killed 15 mins, 1, 6, and 24 h after trauma (n=5 per group) by transcardial perfusion with 4% paraformaldehyde solution in deep halothane anesthesia. Brains were postfixed in 4% paraformaldehyde overnight, dehydrated, and embedded in paraffin. Coronal sections (4 μm thick) through the contusion were prepared on conventional glass slides coated with aminopropyl-triethoxy-silane (Merck, Darmstadt, Germany). Nonspecific binding was blocked for 20 mins with horse serum (Vectastain Elite ABC kit PK-6200; Vector Laboratories, Burlingame, CA, USA). Endogenous peroxidase activity was blocked with 3% H2O2 in methanol for 10 mins. A mouse monoclonal antibody raised against a peptide mapping the N-terminus of human bradykinin B1 receptor (1:250; BD Biosciences/Transduction Laboratories, San Jose, CA, USA) and rabbit polyclonal anti-B2 receptor antibody (kind gift from Professor Mueller-Esterl) were used as primary antibodies for the two receptors and were applied in phosphate-buffered saline containing 2% bovine serum albumin and 0.3% Triton X-100 overnight at 4°C. The sections were then incubated with 100 μL of a horse anti-mouse/rabbit biotinylated secondary antibody (BA-1400; Vector Laboratories) together with 100 μL universal blocking solution, according to the manufacturer's instructions (Vectastain Elite ABC kit, PK-6200; Vector Laboratories), for 60 mins at room temperature. Visualization was performed using amino-9-ethylcarbazole (AEC-Kit; Vector Laboratories). To exclude unspecific binding of the secondary antibody, sections (negative controls) were subjected to the same procedure except incubation with the primary antibody.
Experimental Groups
The following experimental groups were investigated: (1) Measurement of bradykinin in brain tissue at five different time points (2, 6, 12, 24, and 24μh after TBI)+sham-operated controls (six groups with five animals each (total 30 mice)); (2) Quantitative PCR of B1 and B2 receptor expression at three different time points (6, 24, and 48 h after TBI)+naïve animals+sham-operated controls (five groups with four to six animals each (total 28 mice)); (3) Immunohistochemistry for B1 and B2 receptors at three time points (1, 6, and 24 h after TBI)+sham-operated controls (four groups with four animals each (total 16 mice)); (4) Contusion volume in WT, B1−/−, and B2−/− mice 15 mins and 24 h after TBI (four groups with eight animals each (total 32 mice)); (5) Brain water content in WT and B2−/− mice 24 h after TBI (three groups with six animals each (total 18 mice)); and (6) Contusion volume, body weight, and motor function for up to 7 days in WT and B2−/− mice (two groups with seven mice each (total 14 mice)). Accordingly, a total of 138 mice were used for the current study.
Statistical Analysis
All data are expressed as means±s.d. unless otherwise indicated. For statistical comparisons between groups, the Mann–Whitney Rank-Sum test was used. Measurements over time (body weight and beam walk) were tested versus baseline with Friedman Repeated-Measures analysis of variance on Ranks followed by Dunnett's All Pairwise Multiple Comparison Procedure as post hoc test. Calculations were performed with a standard statistical software package (SigmaStat 3.0; Jandel Scientific, Erkrath, Germany). P<0.05 at a power of >0.8 was considered statistically significant.
Results
Tissue Bradykinin
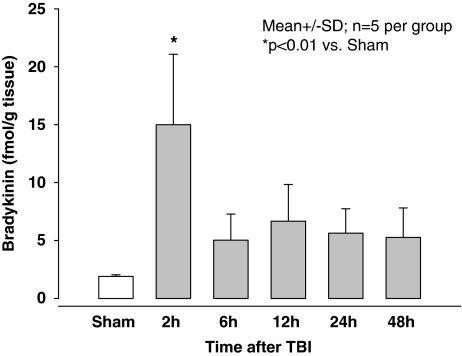
With the technique used we were able to detect basal tissue bradykinin levels in the brains of sham-operated WT (Figure 1, white bar, 1.9±0.1 fmol/g). After CCI bradykinin increased significantly to a maximum of 15.0±6.1 fmol/g 2 h after trauma (P<0.01 versus sham-operated mice). Thereafter the level decreased (5.0±2.3 fmol/g 6 h after CCI) and remained at this level up to 48 h after TBI.
Figure 1.
Brain bradykinin concentration in sham-operated (open bar) and traumatized (closed bars) C57/BL6 mice (n=5 per group) at different time points after experimental TBI.
Kinin B1 and B2 Receptor mRNA Expression
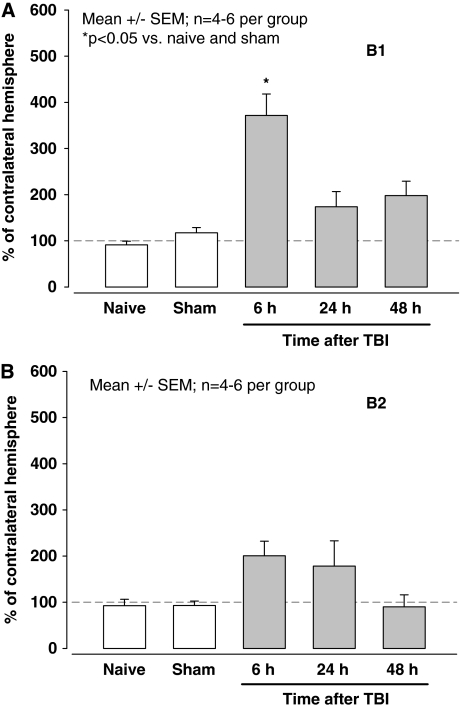
Kinin B1 and B2 mRNA expression was measured in naïve, sham-operated, and traumatized animals at different time points by quantitative polymerase chain reaction (PCR; n=4 to 6 per group). Surgery and anesthesia had no influence on the expression of B1 or B2 receptor mRNA (Figure 2A and 2B, naïve versus sham). Trauma led to significant (P<0.05) fourfold upregulation of B1 receptor mRNA expression after 6 h. Thereafter B1 receptor mRNA expression remained elevated; however, the increase did not reach statistical significance (Figure 2A). Expression of B2 receptor mRNA was not significantly increased at any investigated time point after CCI (Figure 2B).
Figure 2.
Expression of kinin B1 (A) and B2 (B) receptors in healthy and sham-operated C57/BL6 mice (open bars) and at different time points after experimental TBI (closed bars; n=4 to 6 per group). Data are corrected for β-actin expression and presented as % of B1 or B2 receptor expression in healthy tissue, that is, in the contralateral hemisphere.
Kinin B1 and B2 Receptor Protein Expression and Localization
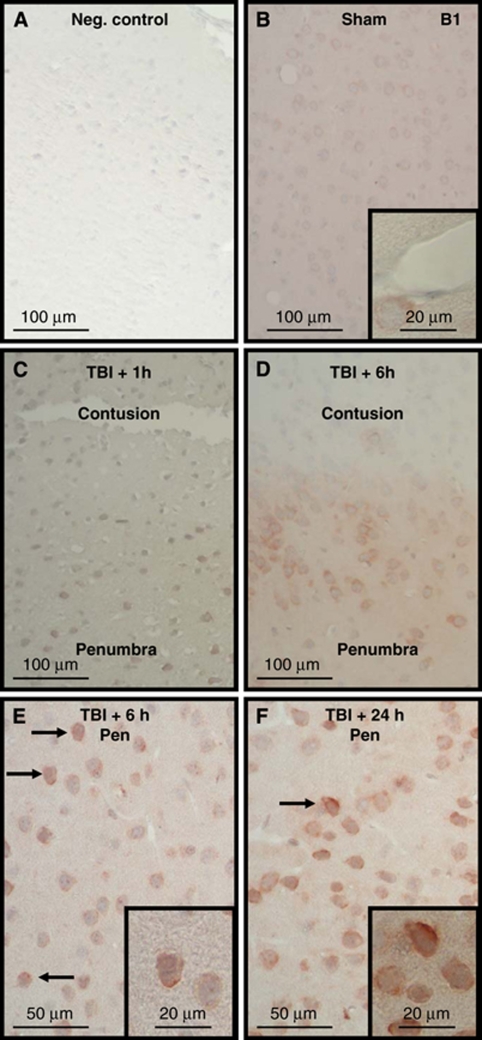
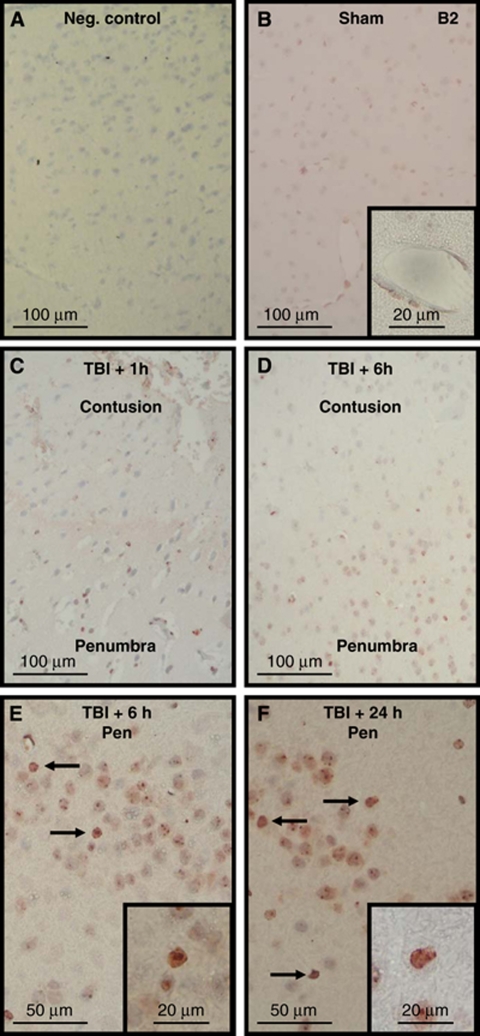
Staining for both bradykinin receptors was identified throughout the healthy brain, including all layers of the cerebral cortex, striatum, and the hippocampus. Omission of the primary antibody completely abolished staining for B1 and B2 receptors (Figures 3A and 4A), indicating that the secondary antibody used did not show any unspecific binding. Both receptors were identified in cells showing neuronal morphology. The B1 receptor was nearly exclusively located on the cell membrane, whereas the B2 receptor showed a more ubiquitous cellular staining pattern, that is, in addition to the cell membrane intracellular staining was also observed, as previously described (Groger et al, 2005). Differently to the B1 receptor, the B2 receptor was also found on cerebral vessels (Figure 4B versus Figure 3B, insets). After CCI, a change of both receptors' immunoreactivity was observed in cells showing signs of traumatic damage: the staining intensity for the B1 and B2 receptors increased significantly (Figures 3C to F and 4C to F). Dead cells in the contusion core did not show any staining at any time for either receptor, showing no unspecific staining of damaged cells by the antibodies (Figures 3C and D and 4C and D). In contrast to these dead cells and to cells from normal tissue, cells in the border zone of the contusion, that is, in the traumatic penumbra, showed strong immunoreactivity of the cell membrane for the B1 receptor (Figure 3E to 3F) and of the cell membrane and intracellular structures (including the nucleus) for B2 receptors (Figure 4E to 4F). These findings suggest that most of the cells that undergo delayed cell death in the traumatic penumbra upregulate their kinin B1 and B2 receptors. As expected based on their known biological functions, B1 receptors did not change their staining pattern, that is, staining remained confined to the cell membrane, whereas B2 receptors showed increased immunoreactivity of the cell membrane, indicating its translocation and upregulation on the cell surface, as previously reported, after ischemic brain injury (Groger et al, 2005).
Figure 3.
Immunohistochemistry for kinin B1 receptors in sham-operated and traumatized C57/BL6 mice. The primary antibody was omitted to test for unspecific binding of the secondary antibody (A, negative control). Staining was performed in sham-operated mice (B) or in mice killed 1 (C), 6 (D, E), or 24 h (F) after experimental TBI (C57/BL6; n=4 per group). No staining was observed within the contused tissue (C, D), whereas cell membrane staining (arrows) increased in the traumatic penumbra with time (C to F).
Figure 4.
Immunohistochemistry for kinin B2 receptors in sham-operated and traumatized C57/BL6 mice. Negative control by omission of the primary antibody (A, negative control). Staining was performed in sham-operated mice (B) or in mice killed 1 (C), 6 (D, E), or 24 h (F) after experimental TBI (C57/BL6; n=4 per group). In contrast to the B1 receptor the B2 receptor is also found in cerebrovascular vessels (B, inset). Again, no staining was observed within the contused tissue (C, D), whereas cellular staining (arrows) became apparent in the traumatic penumbra with time (C to F).
Contusion Volume
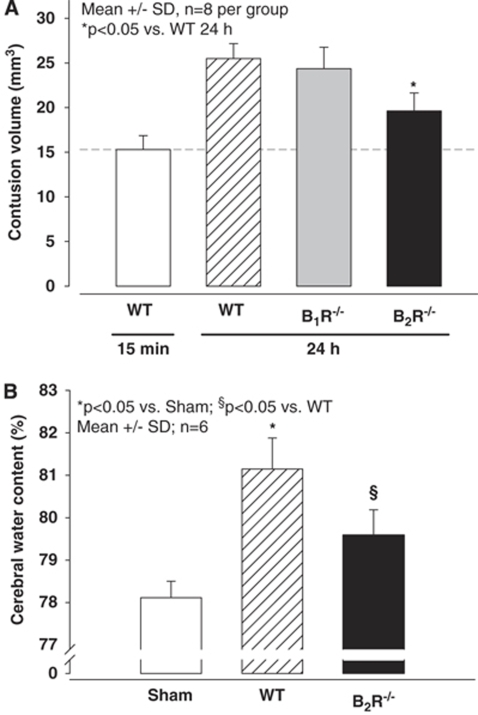
The primary damage after CCI was evaluated as early as possible, that is, 15 mins, after trauma. At this time point, WT animals had a contusion volume of 15.3 mm3 (n=8 per group). Previous results showed that the primary damage is not different between different strains of mice, including knockout animals, since the primary damage is purely mechanical and not influenced by secondary changes on the physiological or molecular level. Twenty-four hours after TBI, WT, B1R−/−, and B2R−/− animals had contusion volumes of 25.5, 24.4, and 19.6 mm3 (P<0.05 versus WT 24 h), respectively (Figure 5A). Compared with primary damage, contusion volume expanded by 67% in the WT mice, a value well in line with previous results (Zweckberger et al, 2006). In B1R−/− mice the expansion was similar (61%), whereas in B2R−/− mice secondary contusion expansion was only 28%. This represents a reduction of almost 60% as compared with WT mice.
Figure 5.
Contusion volume (A) and cerebral water content (B) in C57/BL6 (WT), B1 (B1R−/−), and B2 (B2R−/−)-receptor-knockout mice 24 h after experimental TBI. Contusion volume increased in WT mice between 15 mins and 24 h after trauma, indicating development of secondary brain damage. The contusion volume increase was not altered in B1R−/− mice, whereas in B2R−/− mice secondary brain damage was significantly (P<0.05) reduced (n=8 per group). Similarly, WT mice showed an ∼3% increase in brain water content, indicating formation of brain edema, whereas in B2R−/− mice brain edema formation was reduced by ∼50% (P<0.05; n=6 per group).
Brain Edema
To determine whether the protection observed in B2R−/− mice also affected brain edema formation, brain water content was measured 24 h after trauma, that is, at the maximum stage of brain edema formation in the current model (Zweckberger et al, 2006). Sham-operated animals had a brain water content of 78.1%. In WT animals, brain water content increased by 3.3% to 81.4% 24 h after CCI (P<0.001 versus sham; Figure 5). In contrast to WT littermates B2R−/− animals had significantly less pronounced increase in brain water content to only 79.1%. This represented a reduction of brain edema formation by 70% (n=6 per group).
Long-Term Morphological and Functional Outcome
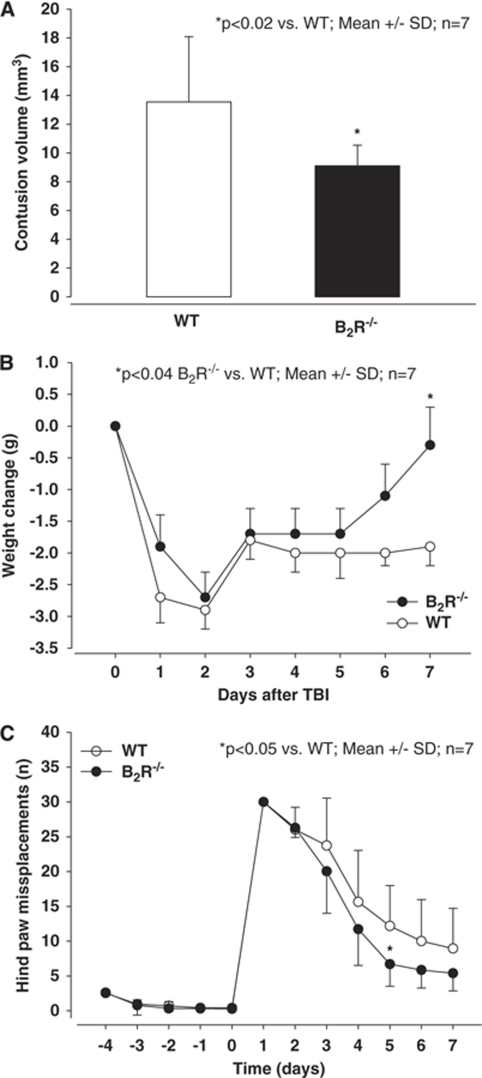
To show that the neuroprotection of B2R−/− mice observed at 24 h was not a temporary phenomenon, we also assessed contusion volumes 7 days after TBI (Figure 6A). Contusion volumes at this late time point after trauma are significantly smaller than those observed after 24 h, due to removal of dead tissue by invading phagocytes, as also described previously (Zweckberger et al, 2006). In these long-term experiments, contusion volume was significantly smaller in B2R−/− mice as compared with their WT littermates (P<0.02; n=7 per group).
Figure 6.
Contusion volume (A), body weight (B), and motor function (C) in WT and B2-receptor-knockout mice (B2R−/−) at or during the first 7 days after experimental TBI, respectively. B2R−/− mice had significantly smaller contusion volumes (P<0.02), recovered almost completely from post-traumatic weight loss (P<0.04), and showed better motor performance (P<0.05) as compared with WT mice (n=7 per group).
The functional outcome of WT and B2R−/− mice was evaluated using the body weight and a beam walking task up to 7 days after trauma. Body weight of WT and B2R−/− mice was comparable under baseline conditions (∼25g; data not shown). One day after trauma, all mice lost almost 3 g, that is, 12%, of their body weight. After day two following trauma, both mouse strains started to gain weight; however, whereas WT did not reach their pre-traumatic body weight, B2R−/− almost completely recovered on day 7 after trauma (P<0.04).
In the beam walking task, which mainly tests balance and motor performance, comparable values were observed before trauma for WT and B2R−/− mice (Figure 6C). One day after trauma, all investigated mice showed the most severe motor deficits. Although all mice gradually recovered during the following 7 days, B2R−/− mice recovered significantly faster and better than the control animals (P<0.05 versus WT).
Discussion
The results of the present study show that following experimental TBI in mice, brain tissue bradykinin increases, B1 receptor mRNA is significantly upregulated by four-fold, and kinin receptor expression is markedly enhanced in the cells of the traumatic penumbra. Using B1R−/− and B2R−/− mice we show that only B2 receptors are involved in the development of secondary brain damage, brain edema formation, and functional recovery after experimental TBI.
Release of Bradykinin in Traumatized Brain Tissue
All components necessary for generation of bradykinin are present in the central nervous system (CNS) and traumatic and ischemic brain damage are both associated with activation of the kallikrein–kinin system (Francel, 1992; Plesnila and Relton, 2001). So far bradykinin, the most active component of the kallikrein–kinin system, was detected in normal (Kariya et al, 1985) as well as in ischemic brain tissue (Kamiya et al, 1993; Groger et al, 2005). As bradykinin has a very short half-life (T1/2<30 secs) and is expressed at very low concentrations under normal as well as pathological conditions, measurement of bradykinin serum or tissue concentrations are technically demanding. This may explain why data on tissue concentrations of bradykinin after TBI have, so far, not been published. In sham-operated animals, tissue bradykinin was 1.9±0.1 fmol/g, a value comparable to our previous measurements (Groger et al, 2005). Values from the literature are much higher than those currently and previously reported by our laboratory (Perry and Snyder, 1984; Kariya et al, 1985), however, this may be well explained by more specific and sensitive techniques for measurement of bradykinin, which became available only recently, as also discussed previously (Groger et al, 2005).
Bradykinin reached its maximal tissue concentration 2 h after trauma, that is, 15.0±6.1 fmol/g or 7.5-fold higher than in sham-operated or naïve animals. This time course differs significantly from the time course we reported after ischemic brain damage, where the maximum bradykinin tissue concentration was three-fold higher than that in naïve animals and was found 12 h after injury (Groger et al, 2005). This difference is certainly a result of the different injury kinetics observed in the two models. While cell death starts gradually after focal cerebral ischemia and develops slowly thereafter, over 60% of the contusion volume found 24 h after TBI is already present 15 mins after the traumatic impact (Zweckberger et al, 2006). Therefore, it is not surprising that bradykinin production, which is triggered by tissue and vascular damage (Francel, 1992; Plesnila and Relton, 2001), increases significantly earlier after contusional TBI than after focal cerebral ischemia.
Expression of Kinin B1 and B2 Receptors in Normal and Traumatized Brain
Kinin B2 receptors are constitutively expressed in most tissues, whereas B1 receptors, with few exceptions, are not expressed under normal conditions and need to be upregulated by certain stimuli, for example, tissue damage (Plesnila and Relton, 2001). In the healthy CNS, B2 receptors have been described in the spinal cord, cortex, hippocampus, and medulla oblongata (Fujiwara et al, 1989; Cholewinski et al, 1991; Privitera et al, 1992; Groger et al, 2005). Initial functional studies suggested that B2 receptor-binding sites were primarily located on cerebral blood vessels (Wahl et al, 1983), but subsequent work by us and others also described kinin B2 receptor expression on neurons (Raidoo et al, 1996; Groger et al, 2005), on cerebrovascular endothelial, and smooth-muscle cells (Raidoo et al, 1997), as well as on cultured astrocytes and microglia (Cholewinski et al, 1991; Noda et al, 2003). Our current findings are well in agreement with these previous studies showing expression of B2 receptor mRNA in brain lysates and staining of B2 receptors on cortical neurons and on cerebral vessels (Figure 4B). The constitutive expression of kinin B2 receptors on the cerebral vasculature is well in line with a possible functional role for these receptors for vessel permeability and brain edema formation (Wahl et al, 1983; Unterberg and Baethmann, 1984; Unterberg et al, 1984). The functional role of these receptors on neurons is, however, still unknown. Although it was suggested that B2 receptors may serve as an important signaling system between astrocytes and neurons (Parpura et al, 1994), the significance of this finding was challenged by the lack of any CNS-related phenotype in B2R−/− mice (Borkowski et al, 1995). Accordingly, additional research will be necessary to clarify the role of kinin B2 receptors in the healthy brain.
There is general agreement that in contrast to B2 receptors B1 receptors are not constitutively expressed on healthy cerebral vessels (Whalley and Wahl, 1983a; Unterberg et al, 1984), and we confirmed this finding in the current study (Figure 3B). Only two studies reported constitutive B1 receptor expression in the brain parenchyma so far. One study of human brain samples found B1 receptors only in the striatum (Raidoo and Bhoola, 1997; Lee, 2004a, 2004b, 2004c), whereas another study performed on primates reported expression in all investigated brain regions, including the cerebral cortex and the cerebellum (Shughrue et al, 2003). Our results using highly specific quantitative PCR clearly show that B1 receptor mRNA is constitutively expressed in rodent brain lysates (Figure 2). This constitutive expression was confirmed by the detection of the respective protein on the membrane of cells with neuronal morphology using an antibody directed towards the N-terminus of the bradykinin B1 receptor. Accordingly, our current findings confirm that B1 receptors are indeed constitutively expressed in the healthy brain. The physiological role of B1 receptors in the brain, however, seems to be of minor relevance since bradykinin B1-receptor-knockout mice do not show any CNS-related phenotype.
The regulation of kinin receptors after TBI has, to our knowledge, not been reported so far. As expected from previous findings showing that only B1 receptor expression is induced by tissue damage (see above), B1 but not B2 receptor mRNA was upregulated after experimental TBI (Figure 2). B1 receptor mRNA upregulation was accompanied by concomitant upregulation of B1 receptor protein on the cell membrane of neuronal cells (Figure 3), suggesting expression of a functional receptor. Interestingly, despite the lack of upregulation on the mRNA level, staining for the B2 receptor protein also increased after TBI (Figure 4). This suggests that B2 receptors are stored in an inactive form in intracellular vesicles and may translocate to the cell membrane or the nucleus and become functional only upon tissue damage, as also suggested previously (Groger et al, 2005).
The Role of B1 and B2 Kinin Receptors in the Development of Brain Injury
Despite the presence of both kinin receptors in the injured brain, there is ample evidence that B2 receptors play the main role in the development of delayed brain damage. Pharmacological blockade of kinin B2 receptors with highly selective receptor antagonists protects against ischemic brain injury after middle-cerebral-artery occlusion in the rat (Relton et al, 1997) and B2R−/− mice show less severe brain damage after focal cerebral ischemia (Groger et al, 2005). Application of a selective B2 bradykinin-receptor antagonist reduced brain edema in a diffuse-closed and in an open-focal brain injury model in rats (Pruneau et al, 1999; Stover et al, 2000). Moreover, B2-receptor-knockout mice were protected from diffuse axonal injury (Hellal et al, 2003). Accordingly, despite a recent report claiming that the exact opposite may be the case (Austinat et al, 2009), there is general agreement that B2 receptors mediate brain injury whereas B1 receptors may not play a major role. In line with these arguments, only the B2-receptor antagonist was used for clinical evaluation (Narotam et al, 1998; Marmarou et al, 1999).
Mechanisms of B2 Kinin Receptor-Induced Brain Injury
Until now the mechanisms of bradykinin-induced brain damage have been explained mainly by the formation of brain edema through increase of cerebrovascular permeability (Unterberg et al, 1984). Bradykinin opened the blood–brain barrier upon perivascular as well as intracarotid application (Unterberg et al, 1984). The opening of the blood–brain barrier occurred for small as well as with high-molecular-weight markers (Unterberg et al, 1984). These effects were inhibited by B2-receptor antagonists (Sarker et al, 2000) and could be mimicked by application of B2-receptor agonists (Bartus et al, 1996).
Since intracranial hypertension may not only be caused by brain edema formation, but also by dilatation of cerebral vessels and subsequent increase of intracranial blood volume, some authors investigated whether bradykinin may also influence cerebrovascular reactivity. Incubation with bradykinin in vitro dose dependently relaxed the ring segments of human cerebral arteries (Toda, 1977). Perivascular microapplication of bradykinin dilated cat pial arterioles by 45% (Wahl et al, 1983). A similar, but somewhat lesser, dilation was seen after superfusion of the brain with bradykinin (Unterberg et al, 1984). Bradykinin-induced vasodilatation could not be inhibited by B1-receptor antagonists (Whalley and Wahl, 1983b), but could be reduced by B2-receptor inhibition and was mediated by nitric oxide (Gorlach and Wahl, 1996).
Our present results support the hypothesis that the detrimental effect of bradykinin after TBI is mainly mediated through B2 receptors on cerebral vessels, that is, through a cerebrovascular effect (formation of brain edema, vasodilatation, and/or changes of cerebral blood flow). However, it remains unclear why the number of these receptors increases also in parenchymal cells. Since a similar finding was also observed after focal cerebral ischemia (Groger et al, 2005), further experiments are necessary to clarify the question as to how intraparenchymal cells may mediate the detrimental effects of bradykinin.
Taken together our results show that bradykinin is produced in the brain after experimental TBI and that inhibition of kinin B2 receptors reduces secondary brain damage and neurological deficits long term. The main mechanism of bradykinin-mediated brain damage seems to be the involvement of B2 receptors in the formation of brain edema. These findings broaden our, so far limited, understanding of the kallikrein–kinin system after TBI and may have a clinical potential for the development of novel, more specific, and so far missing strategies for the treatment of TBI and brain edema formation.
Acknowledgments
We thank Eric Whalley for constant encouragement to work on the kallikrein–kinin system; Alke Schropp, Mathias Semisch, Mei-Ping Wu, and Veronika Bischoff for excellent technical assistance; and Gayle Kenney for proof reading the paper. We apologize to all co-workers whose work could not be cited due to space limitations.
Footnotes
Disclosure/conflict of interest
The authors declare that they do not have any conflict of interests related to the current study.
References
- Austinat M, Braeuninger S, Pesquero JB, Brede M, Bader M, Stoll G, Renne T, Kleinschnitz C. Blockade of bradykinin receptor B1 but not bradykinin receptor B2 provides protection from cerebral infarction and brain edema. Stroke. 2009;40:285–293. doi: 10.1161/STROKEAHA.108.526673. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Elliott P, Hayward N, Dean R, McEwen EL, Fisher SK. Permeability of the blood brain barrier by the bradykinin agonist, RMP-7: evidence for a sensitive, auto-regulated, receptor-mediated system. Immunopharmacology. 1996;33:270–278. doi: 10.1016/0162-3109(96)00070-7. [DOI] [PubMed] [Google Scholar]
- Borkowski JA, Ransom RW, Seabrook GR, Trumbauer M, Chen H, Hill RG, Strader CD, Hess JF. Targeted disruption of a B2 bradykinin receptor gene in mice eliminates bradykinin action in smooth muscle and neurons. J Biol Chem. 1995;270:13706–13710. doi: 10.1074/jbc.270.23.13706. [DOI] [PubMed] [Google Scholar]
- Chen EY, Emerich DF, Bartus RT, Kordower JH. B2 bradykinin receptor immunoreactivity in rat brain. J Comp Neurol. 2000;427:1–18. [PubMed] [Google Scholar]
- Cholewinski AJ, Stevens G, McDermott AM, Wilkin GP. Identification of B2 bradykinin binding sites on cultured cortical astrocytes. J Neurochem. 1991;57:1456–1458. doi: 10.1111/j.1471-4159.1991.tb08314.x. [DOI] [PubMed] [Google Scholar]
- Francel PC. Bradykinin and neuronal injury. J Neurotrauma. 1992;9 (Suppl 1:S27–S45. [PubMed] [Google Scholar]
- Fujiwara Y, Mantione CR, Vavrek RJ, Stewart JM, Yamamura HI. Characterization of [3H]bradykinin binding sites in guinea-pig central nervous system: possible existence of B2 subtypes. Life Sci. 1989;44:1645–1653. doi: 10.1016/0024-3205(89)90481-5. [DOI] [PubMed] [Google Scholar]
- Gennarelli TA. Mechanisms of brain injury. J Emerg Med. 1993;11 (Suppl 1:5–11. [PubMed] [Google Scholar]
- Gorlach C, Wahl M. Bradykinin dilates rat middle cerebral artery and its large branches via endothelial B2 receptors and release of nitric oxide. Peptides. 1996;17:1373–1378. doi: 10.1016/s0196-9781(96)00223-9. [DOI] [PubMed] [Google Scholar]
- Groger M, Lebesgue D, Pruneau D, Relton J, Kim SW, Nussberger J, Plesnila N. Release of bradykinin and expression of kinin B2 receptors in the brain: role for cell death and brain edema formation after focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005;25:978–989. doi: 10.1038/sj.jcbfm.9600096. [DOI] [PubMed] [Google Scholar]
- Hellal F, Pruneau D, Palmier B, Faye P, Croci N, Plotkine M, Marchand-Verrecchia C. Detrimental role of bradykinin B2 receptor in a murine model of diffuse brain injury. J Neurotrauma. 2003;20:841–851. doi: 10.1089/089771503322385773. [DOI] [PubMed] [Google Scholar]
- Jennett B. Epidemiology of head injury. Arch Dis Child. 1998;78:403–406. doi: 10.1136/adc.78.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya T, Katayama Y, Kashiwagi F, Terashi A. The role of bradykinin in mediating ischemic brain edema in rats. Stroke. 1993;24:571–575. doi: 10.1161/01.str.24.4.571. [DOI] [PubMed] [Google Scholar]
- Kariya K, Yamauchi A, Sasaki T. Regional distribution and characterization of kinin in the CNS of the rat. J Neurochem. 1985;44:1892–1897. doi: 10.1111/j.1471-4159.1985.tb07185.x. [DOI] [PubMed] [Google Scholar]
- Lee DK. Agonist-independent nuclear localization of the Apelin, angiotensin AT1, and bradykinin B2 receptors. J Biol chem. 2004;279:7901–7908. doi: 10.1074/jbc.M306377200. [DOI] [PubMed] [Google Scholar]
- Maier-Hauff K, Baethmann AJ, Lange M, Schurer L, Unterberg A. The kallikrein-kinin system as mediator in vasogenic brain edema. Part 2: Studies on kinin formation in focal and perifocal brain tissue. J Neurosurg. 1984;61:97–106. doi: 10.3171/jns.1984.61.1.0097. [DOI] [PubMed] [Google Scholar]
- Marmarou A, Nichols J, Burgess J, Newell D, Troha J, Burnham D, Pitts L. Effects of the bradykinin antagonist Bradycor (deltibant, CP-1027) in severe traumatic brain injury: results of a multi-center, randomized, placebo-controlled trial. American Brain Injury Consortium Study Group. J Neurotrauma. 1999;16:431–444. doi: 10.1089/neu.1999.16.431. [DOI] [PubMed] [Google Scholar]
- Narotam PK, Rodell TC, Nadvi SS, Bhoola KD, Troha JM, Parbhoosingh R, van DJ. Traumatic brain contusions: a clinical role for the kinin antagonist CP-0127. Acta Neurochir (Wien) 1998;140:793–802. doi: 10.1007/s007010050181. [DOI] [PubMed] [Google Scholar]
- Noda M, Kariura Y, Amano T, Manago Y, Nishikawa K, Aoki S, Wada K. Expression and function of bradykinin receptors in microglia. Life Sci. 2003;72:1573–1581. doi: 10.1016/s0024-3205(02)02449-9. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Perry DC, Snyder SH. Identification of bradykinin in mammalian brain. J Neurochem. 1984;43:1072–1080. doi: 10.1111/j.1471-4159.1984.tb12846.x. [DOI] [PubMed] [Google Scholar]
- Plesnila N, Relton J.2001The kallikrein-kinin system in ischemic and traumatic brain injury Inflammation and Stroke(Feuerstein GZ, ed),Basel: Birkhäuser Verlag; 333–342. [Google Scholar]
- Plesnila N, Schulz J, Stoffel M, Eriskat J, Pruneau D, Baethmann A. Role of bradykinin B2 receptors in the formation of vasogenic brain edema in rats. J Neurotrauma. 2001;18:1049–1058. doi: 10.1089/08977150152693746. [DOI] [PubMed] [Google Scholar]
- Privitera PJ, Daum PR, Hill DR, Hiley CR. Autoradiographic visualization and characteristics of [125I]bradykinin binding sites in guinea pig brain. Brain Res. 1992;577:73–79. doi: 10.1016/0006-8993(92)90539-l. [DOI] [PubMed] [Google Scholar]
- Pruneau D, Chorny I, Benkovitz V, Artru A, Roitblat L, Shapira Y. Effect of LF 16-0687MS, a new nonpeptide bradykinin B2 receptor antagonist, in a rat model of closed head trauma. J Neurotrauma. 1999;16:1057–1065. doi: 10.1089/neu.1999.16.1057. [DOI] [PubMed] [Google Scholar]
- Raidoo DM, Bhoola KD. Kinin receptors on human neurones. J Neuroimmunol. 1997;77:39–44. doi: 10.1016/s0165-5728(97)00048-9. [DOI] [PubMed] [Google Scholar]
- Raidoo DM, Ramchurren N, Naidoo Y, Naidoo S, Muller-Esterl W, Bhoola KD. Visualisation of bradykinin B2 receptors on human brain neurons. Immunopharmacology. 1996;33:104–107. doi: 10.1016/0162-3109(96)00021-5. [DOI] [PubMed] [Google Scholar]
- Raidoo DM, Ramsaroop R, Naidoo S, Muller-Esterl W, Bhoola KD. Kinin receptors in human vascular tissue: their role in atheromatous disease. Immunopharmacology. 1997;36:153–160. doi: 10.1016/s0162-3109(97)00015-5. [DOI] [PubMed] [Google Scholar]
- Relton JK, Beckey VE, Hanson WL, Whalley ET. CP-0597, a selective bradykinin B2 receptor antagonist, inhibits brain injury in a rat model of reversible middle cerebral artery occlusion. Stroke. 1997;28:1430–1436. doi: 10.1161/01.str.28.7.1430. [DOI] [PubMed] [Google Scholar]
- Sarker MH, Hu D, Fraser PA. Acute effects of bradykinin on cerebral microvascular permeability in the anaesthetized rat. J Physiol. 2000;528 (Pt 1:177–187. doi: 10.1111/j.1469-7793.2000.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Ky B, Austin CP. Localization of B1 bradykinin receptor mRNA in the primate brain and spinal cord: an in situ hybridization study. J Comp Neurol. 2003;465:372–384. doi: 10.1002/cne.10846. [DOI] [PubMed] [Google Scholar]
- Stover JF, Dohse NK, Unterberg AW. Significant reduction in brain swelling by administration of nonpeptide kinin B2 receptor antagonist LF 16-0687Ms after controlled cortical impact injury in rats. J Neurosurg. 2000;92:853–859. doi: 10.3171/jns.2000.92.5.0853. [DOI] [PubMed] [Google Scholar]
- Toda N. Actions of bradykinin on isolated cerebral and peripheral arteries. Am J Physiol. 1977;232:H267–H274. doi: 10.1152/ajpheart.1977.232.3.H267. [DOI] [PubMed] [Google Scholar]
- Unterberg A, Baethmann AJ. The kallikrein-kinin system as mediator in vasogenic brain edema. Part 1: Cerebral exposure to bradykinin and plasma. J Neurosurg. 1984;61:87–96. doi: 10.3171/jns.1984.61.1.0087. [DOI] [PubMed] [Google Scholar]
- Unterberg A, Dautermann C, Baethmann A, Muller-Esterl W. The kallikrein-kinin system as mediator in vasogenic brain edema. Part 3: Inhibition of the kallikrein-kinin system in traumatic brain swelling. J Neurosurg. 1986;64:269–276. doi: 10.3171/jns.1986.64.2.0269. [DOI] [PubMed] [Google Scholar]
- Unterberg A, Wahl M, Baethmann A. Effects of bradykinin on permeability and diameter of pial vessels in vivo. J Cereb Blood Flow Metab. 1984;4:574–585. doi: 10.1038/jcbfm.1984.82. [DOI] [PubMed] [Google Scholar]
- Wahl M, Young AR, Edvinsson L, Wagner F. Effects of bradykinin on pial arteries and arterioles in vitro and in situ. J Cereb Blood Flow Metab. 1983;3:231–237. doi: 10.1038/jcbfm.1983.31. [DOI] [PubMed] [Google Scholar]
- Whalley ET, Wahl M. Analysis of bradykinin receptor mediating relaxation of cat cerebral arteries in vivo and in vitro. Naunyn-Schmiedeberg's Arch Pharmacol. 1983a;323:66–71. doi: 10.1007/BF00498830. [DOI] [PubMed] [Google Scholar]
- Whalley ET, Wahl M. The effect of kininase II inhibitors on the response of feline cerebral arteries to bradykinin and angiotensin. Pflugers Arch. 1983b;398:175–177. doi: 10.1007/BF00581069. [DOI] [PubMed] [Google Scholar]
- Zweckberger K, Eros C, Zimmermann R, Kim SW, Engel D, Plesnila N. Effect of early and delayed decompressive craniectomy on secondary brain damage after controlled cortical impact in mice. J Neurotrauma. 2006;23:1083–1093. doi: 10.1089/neu.2006.23.1083. [DOI] [PubMed] [Google Scholar]
- Zweckberger K, Stoffel M, Baethmann A, Plesnila N. Effect of decompression craniotomy on increase of contusion volume and functional outcome after controlled cortical impact in mice. J Neurotrauma. 2003;20:1307–1314. doi: 10.1089/089771503322686102. [DOI] [PubMed] [Google Scholar]