Abstract
A major challenge associated with the determination of the unbound brain-to-plasma concentration ratio of a drug (Kp,uu,brain), is the error associated with correction for the drug in various vascular spaces of the brain, i.e., in residual blood. The apparent brain vascular spaces of plasma water (Vwater, 10.3 μL/g brain), plasma proteins (Vprotein, 7.99 μL/g brain), and the volume of erythrocytes (Ver, 2.13 μL/g brain) were determined and incorporated into a novel, drug-specific correction model that took the drug-unbound fraction in the plasma (fu,p) into account. The correction model was successfully applied for the determination of Kp,uu,brain for indomethacin, loperamide, and moxalactam, which had potential problems associated with correction. The influence on correction of the drug associated with erythrocytes was shown to be minimal. Therefore, it is proposed that correction for residual blood can be performed using an effective plasma space in the brain (Veff), which is calculated from the measured fu,p of the particular drug as well as from the estimates of Vwater and Vprotein, which are provided in this study. Furthermore, the results highlight the value of determining Kp,uu,brain with statistical precision to enable appropriate interpretation of brain exposure for drugs that appear to be restricted to the brain vascular spaces.
Keywords: BBB (blood–brain barrier), brain distribution (of neuroactive substances), CBV (cerebral blood volume)
Introduction
The extent of drug transport across the blood–brain barrier (BBB) determines the in vivo potency of centrally acting drugs, and it can also affect the incidence of central side effects associated with drugs intended to act on peripheral targets. The latter is most relevant when the target protein is expressed in both peripheral and brain tissues. The second-generation nonsedating antihistamines, such as cetirizine, provide examples of the successful separation of primary antiallergic effects from central sedative side effects by efficient efflux of the drug at the BBB (Gupta et al, 2007). Similarly, and for the same reason, there are no observed central nervous system (CNS) effects associated with the opioid antimotility agent loperamide at the effective clinical dose (Heykants et al, 1974). Therefore, drug discovery programs for drugs with a peripheral site of action strategically aim at minimizing drug exposure in the brain to reduce the potential for CNS-driven side effects. The chemical structure of the drug and its interaction with drug transporters determine the balance of the CNS to systemic drug exposure. Although the design of drugs restricted to peripheral tissues is an issue for medicinal chemistry, drug efflux at the BBB also needs to be studied in vivo using reliable models (Hammarlund-Udenaes et al, 2009). This is generally done in rats by estimating the steady-state unbound brain-to-plasma concentration ratio Kp,uu,brain (Cu,brainISF/Cu,p) (Fridén et al, 2009, 2007; Gupta et al, 2006). The Kp,uu,brain parameter describes the difference between the concentration of pharmacologically active unbound drug in the brain interstitial fluid (Cu,brainISF) and that in the plasma (Cu,p). A Kp,uu,brain value less than or greater than 1 indicates dominating drug efflux and influx, respectively. A Kp,uu,brain value close to 1 indicates the dominance of unrestricted and unfacilitated (passive) transport to the brain. The lower the value of Kp,uu,brain, the larger is the therapeutic window for peripherally acting drugs with potential CNS side effects.
Although microdialysis has been regarded as the ‘gold standard' for measuring Cu,brainISF, it has its limitations. It is technically demanding and laborious, and may not work for compounds that are very lipophilic and ‘sticky.' Therefore, alternative methods for estimating Cu,brainISF have been developed; these methods combine measuring the amount of drugs in the whole brain, excluding the vascular spaces, (Abrain) with in vitro estimates of the unbound brain volume of distribution (Vu,brain). Cu,brainISF is obtained by dividing Abrain (μmol/g brain) by Vu,brain (mL/g brain) for the drug (Fridén et al, 2009, 2007). Similarly, the unbound drug concentration in the plasma is obtained by multiplying the measured total plasma concentration by the estimated unbound fraction in the plasma (fu,p). It has been shown that in vitro measurements of fu,p are predictive of BBB transport in vivo (Mandula et al, 2006). In practice, the total brain-to-plasma concentration ratio of the drug (Kp,brain; Equation (1)) is determined in vivo in individual animals. These values are subsequently converted to values for Kp,uu,brain (Equation (2)), which reflects drug efflux at the BBB and relates to the therapeutic window. The product of Vu,brain and fu,p, which appears in the denominator of Equation (2), can be understood as the ratio of unbound ‘fractions' in the brain and plasma, with higher or lower values representing greater or lesser binding, respectively, to the brain tissue than to plasma proteins.
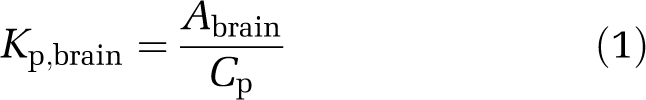 |
 |
This methodology adapts well to typical CNS-active drugs with moderate-to-high values of Kp,brain. For drug compounds with very low values of Kp,brain, irrespective of CNS activity, the drug in the residual blood of the brain tissue capillaries can cause major misinterpretations. Drugs obtained from these sources can contribute substantially to the measured drug concentration in the homogenized brain sample and must be corrected to obtain a value for Abrain, which represents a drug that has actually crossed the BBB. The correction is generally made by estimating the amount of intravascular drug as the product of the brain residual blood volume and the drug concentration in a systemic blood sample and then subtracting this from the total sample concentration (Khor et al, 1991; Preston and Haas, 1986). The subtraction of one value, estimated with some uncertainty, from another value of similar magnitude, also estimated with some uncertainty, will often result in unacceptably imprecise estimates. This statistical problem is commonly experienced when subtracting the amount of drug in residual blood, but it may only become apparent to the experimenter when negative values appear, i.e., when the estimated amount of drug in the residual blood actually exceeds the total amount found in the brain sample.
In addition to precise and accurate measurements of drug concentrations, it is an essential requirement that the standard value used for capillary volume is correct. The residual blood volume can be measured using radiolabeled vascular tracers. However, estimates of brain vascular space volumes vary substantially in the literature and seem to be dependent on the method used to kill the experimental animals (Table 1). The estimated volume of the plasma vascular space also seems to be dependent on the size of the vascular tracer used, suggesting that plasma water has a larger volume of distribution than do plasma proteins (Smith et al, 1988). Furthermore, the hematocrit of brain residual blood, i.e., erythrocyte volume fraction, is substantially lower than that of arterial blood (Preston and Haas, 1986; Smith et al, 1988; Todd et al, 1992). Taken together, these factors imply that the composition of brain residual blood is quite different from that of arterial blood. This should be taken into account when correcting for intravascular drug concentrations (Preston and Haas, 1986).
Table 1. Collated information from the literature on brain vascular spacesa.
| Measured property | Value (μL/g) | Tracer | Euthanasia method | Reference |
|---|---|---|---|---|
| Brain erythrocyte volume | 2.2–2.8 | 55Fe-erythrocytes | Decapitation | Lin et al (1990) |
| 2.73±0.25 | 51Cr-erythrocytes | Decapitation | Preston and Haas (1986) | |
| 2.28b | 51Cr-erythrocytes | Decapitation | Cremer and Seville (1983) | |
| 5.0±0.5 | 99mTc-erythrocytes | Decapitation | Todd et al (1993) | |
| 8.0 | 99mTc-erythrocytes | In situ fixationc | Todd et al (1992) | |
| 8.8 | 59Fe-erythrocytes | In situ fixationc | Everett et al (1956) | |
| 9.3 | 99mTc-erythrocytes | In situ fixationc | Todd et al (1993) | |
| Brain plasma volume | 4.83±0.02 | 125I-albumin | Decapitation | Preston and Haas (1986) |
| 5.47b | 125I-albumin | Decapitation | Cremer and Seville (1983) | |
| 6.0 | 131I-albumin | ––d | Reed and Woodbury (1963) | |
| 6.7b | 3H-dextran (40–200 kDa) | Severing the heart | Smith et al (1988) | |
| 11.9b | 3H-inulin,3H-dextran (10–18 kDa) | Severing the heart | Smith et al (1988) | |
| 11.4±2.9 | 14C-dextran (70 kDa) | Decapitation | Todd et al (1993) | |
| 12.0 | 14C-inulin | ––d | Reed and Woodbury (1963) | |
| 14±1 | 14C-sucrose | Decapitation | Bickel et al (1996) | |
| 15.6±1.9 | 14C-dextran (70 kDa) | Decapitation | Todd et al (1992) | |
| 17.4±2.5 | 14C-dextran (70 kDa) | In situ fixationc | Todd et al (1993) | |
| 19.2±1.7 | 14C-dextran (70 kDa) | In situ fixationc | Todd et al (1992) | |
| 20.8±1.4 | 14C-dextran (70 kDa) | In situ fixatione | Todd et al (1992) | |
| 21.0±2.6 | 14C-dextran (70 kDa) | In situ fixatione | Todd et al (1992) | |
| 21.2 | 131I-albumin | In situ fixatione | Everett et al (1956) | |
| 34±4 | 14C-dextran (40 kDa) | In vivo | Shockley and LaManna (1988) | |
| Brain blood volume | 47.7±1.3 | Photoelectric method | In vivo | Sandor et al (1986) |
Values are presented as means±s.d.
Calculated average value from different regions.
Microwave irradiation.
Euthanasia method is not specified.
Liquid nitrogen freezing.
There is obviously a limit for Kp,brain below which it cannot be accurately quantified using the existing methods for correction. This problem has so far been overlooked because of the perception that a value for Kp,brain that is too low to quantify translates into a Kp,uu,brain value of similar magnitude. However, with the recently increased use of Kp,uu,brain measurements, it is becoming increasingly evident that a low value for Kp,brain can appear not only when the drug is effluxed (in which case Kp,uu,brain would also be low) but also when the drug binds to plasma proteins to an extent that greatly exceeds that of the nonspecific binding of the drug in brain tissue. The latter situation is very common for acidic compounds for which the product of Vu,brain and fu,p is small and hence, the value for Kp,uu,brain is much larger than that of the measured Kp,brain (Equation (2)). When estimates of Kp,brain are very uncertain, it is difficult to discriminate between compounds that are truly effluxed (with a truly low Kp,uu,brain) and compounds not effluxed at the BBB level (with a Kp,uu,brain close to unity). This can have a major impact on drug discovery programs if there is a need to achieve good brain exposure to obtain the primary effect, and even more so if brain exposure should be avoided to limit potential CNS-related side effects.
Kp,uu,CSF, which is the ratio of the unbound drug concentration in the cerebrospinal fluid (CSF) to the unbound drug concentration in the plasma, can be used as a putative surrogate measure of Kp,uu,brain, especially as one avoids the problem of drug in the residual blood. However, the CSF is a different compartment from the ISF; hence, the CSF drug concentration is not necessarily equal to that in the brain ISF. The difference in concentration can occur because the systemically administered drug can reach the CSF either by diffusion from the ISF or by passage over the blood–CSF barrier at the choroid plexus (Shen et al, 2004).
The aim of this study was to improve the accuracy of brain exposure measurements by establishing a novel correction model for the drug present in brain vascular spaces. The key feature of the model is the separation of vascular spaces to accommodate the composition of brain residual blood and the different binding properties of drugs in those vascular spaces. The precision of brain exposure measurements was determined by a confidence interval of Kp,uu,brain for three model compounds, namely indomethacin, loperamide, and moxalactam, which have potential problems associated with vascular space correction.
Materials and methods
Chemicals
3H-inulin was purchased from American Radiolabeled Chemicals (St Louis, MO, USA), and 14C-dextran (molecular weight 70 kDa) was obtained from Moravek Biochemicals (Brea, CA, USA). Indomethacin and loperamide were purchased from Sigma (St Louis, MO, USA), and moxalactam was purchased from MP Biomedicals (Illkirsch, France).
Animals
The experiments were performed on male Sprague–Dawley rats (Harlan, Horst, The Netherlands) weighing 290 to 335 g. All animals were acclimatized for at least 7 days after arrival and were group housed at 18°C to 22°C under a 12-h light–dark cycle with free access to food and water at all times before the experiments. All studies were approved by the Animal Ethics Committee of Göteborg University (1692006, 2212008).
Standardized Procedures for Terminal Sampling of Cerebrospinal Fluid, Blood, and Brain Tissue
The following procedure was used throughout the study for terminal sampling of CSF, blood and brain tissues, or for sampling of only blood and brain tissues. Rats were anesthetized by inhalation of isoflurane (Abbot Scandinavia, Solna, Sweden). The CSF (50 μL) was collected by puncturing the cisterna magna using a fine needle connected to a cannula. The CSF sample was dispensed into a 96-well plate containing 5 μL blank plasma, followed by rinsing thrice with 50 μL methyl-tert-butyl-ether:hexane (1:1) to minimize adsorption of lipophilic drugs to the catheter. Immediately after the CSF sample was taken, a blood sample (2 mL) was collected in a heparinized tube from the abdominal aorta, followed by exsanguination by severing the heart. This method of killing the rat seemed, by visual inspection, to result in less blood in the brain sample than obtained with decapitation. The brain was removed and a coronal section (6 mm, bregma ±3 mm (Paxinos and Watson, 1989)) containing the cerebral cortex, striatum, etc. was cut and divided into smaller pieces. Surface vessels and the choroid plexus were not removed. The brain sample (350 to 375 mg) was transferred into an Eppendorf tube and homogenized in three volumes of deionized water using an ultrasonic probe (Branson, Sonifier 250, Danbury, CT, USA). The coronal section containing the striatum was used as a sample of ‘whole brain' so as to match the section used in the brain slice method (discussed below).
Determination of Vascular Spaces
Drug compounds can be present in various compartments of the blood, i.e., unbound in plasma water, bound to plasma proteins, or associated with erythrocytes. The volume of each of these compartments was determined in eight drug-naive rats. The brain residual plasma volumes were determined using two radiolabeled plasma space tracers of different sizes. The apparent vascular space of plasma water (Vwater) was measured using 3H-inulin (molecular weight 5 kDa). Vwater represents the physical volume of the plasma in brain vasculature. The apparent vascular space of plasma proteins (Vprotein) was measured using 14C-dextran (molecular weight 70 kDa). Vprotein is physically part of Vwater and is also dependent on the protein concentration in brain capillary plasma. It represents an apparent plasma volume with a protein concentration equal to arterial plasma. 3H-inulin and 14C-dextran are not bound to plasma proteins or to erythrocytes and do not measurably cross the BBB within the estimated time of the experiment (Reed and Woodbury, 1963; Smith et al, 1988; Todd et al, 1993). 3H-inulin and 14C-dextran were dissolved in saline and dialyzed overnight in Spectra/Pore 1,000 kDa molecular weight cutoff membrane tubing (Spectrum Laboratories, Rancho Dominguez, CA, USA), to remove radiolabeled impurities of low molecular weight. The dialyzed solution containing both 3H-inulin (80 μCi/kg) and 14C-dextran (80 μCi/kg) was administered as an infusion over 1 min in a volume of 0.75 mL through the tail vein. Blood and brain tissues were sampled 5 mins after the end of the infusion, allowing time to reach stable values for the vascular spaces of these tracers (Smith et al, 1988). Samples of blood (50 μL), plasma (50 μL), and brain homogenate (150 μL) were dissolved and bleached with 1 mL Solvable (Perkin-Elmer, Shelton, CT, USA) and hydrogen peroxide (30%, 160 μL), respectively. A volume of 15 mL OptiPhase HiSafe 3 liquid scintillation cocktail (Perkin-Elmer) was added, and radioactivity was measured in a Wallac Winspectral 1414 Liquid Scintillation Counter (Wallac, Turku, Finland). Vprotein and Vwater in the brain capillaries were calculated as distribution volumes (dimensions μL/g brain):
The hemoglobin content in the brain homogenate was measured to obtain the volume occupied by erythrocytes in the brain residual blood (Ver). The spectrophotometric method used (Quantichrom Hemoglobin, Bioassay Systems, Hayward, CA, USA) converts hemoglobin to a colored end product that is measurable at 390 to 405 nm. Brain homogenate samples of 200 μL were transferred into Eppendorf tubes. A volume of 50 μL of blood diluted in deionized water was added to one of the tubes, corresponding to an addition of 3% blood in the brain. A volume of 50 μL of deionized water was added to the other tube, corresponding to an addition of 0% blood. In addition, 50 μL of deionized water was also added to a homogenate of 300-μm-thick brain slices that had been incubated for 5 h and were regarded as blood-free, on the basis of immunologic measurement of albumin content (Fridén et al, 2009). In all, 1 mL of Quantichrom reagent was added to each tube, and the tubes were vortexed and incubated at room temperature for 15 mins. The tubes were then centrifuged at 20,800 rcf for 60 mins to reduce turbidity in the samples. A volume of 200 μL of the supernatant was transferred from each tube into a 96-well plate, and absorbance was read at 405 nm using a Spectra Max 190 Plate Spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). The absorbance of blood-free brain slice homogenate, processed in the same manner, was subtracted from all samples to minimize the impact of the matrix on the absorbance. The absorbance was linear within additions of blood corresponding to 0% to 5% of whole blood added to the brain homogenate. The absorbance of brain samples containing unknown quantities of blood was plotted against the percentage of blood that had been added to the sample (3% and 0%). Linear regression analysis was performed to yield a y intercept representing the initial quantity of erythrocytes expressed as a percentage of whole arterial blood in the brain. Ver in the brain vascular space was calculated as the product of the arterial hematocrit (Hct) and the y intercept divided by the slope of the regression line:
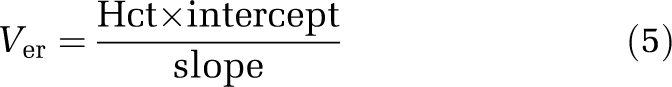 |
The hematocrit of arterial blood from the same animal was measured using a capillary centrifuge and a measuring scale. The hematocrit of brain capillary blood (Hctbrain) was calculated from Ver and Vwater according to
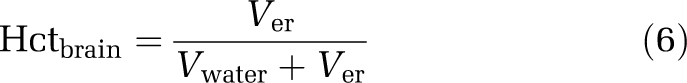 |
The degree of correlation between Ver and the plasma spaces Vwater and Vprotein were of particular interest. If a very strong correlation was found, the developed spectrophotometric method for Ver could be extended as a routine method for obtaining sample-specific estimates of residual blood without using radiolabeled chemicals.
Assessment of the Central Nervous System Exposure to Model Drug Compounds
Indomethacin, loperamide, and moxalactam were the model drug compounds for application of the correction model for Kp,uu,brain determinations. The drugs have different physiochemical properties and are expected to substantially differ in terms of binding to blood and brain tissues. Indomethacin, an acidic compound, was expected to bind more extensively to albumin in the plasma than to the membranes and proteins in the brain tissue. Loperamide, a moderately lipophilic basic compound, was expected to bind more strongly to the negatively charged membranes of the brain tissue than to plasma proteins. Moxalactam, which is more hydrophilic than either loperamide or indomethacin, was not expected to bind substantially in the brain and plasma. All three drugs were known a priori to have low Kp,brain values, and problems were therefore expected with the correction for drug in residual blood concentrations. The opioid loperamide was of particular interest as, because it is effluxed by P-glycoprotein, it has no clinical CNS effects.
The drugs were administered in a cassette to minimize the use of experimental animals. The femoral vein was surgically catheterized at least 24 h before the experiment. The drugs were administered to the rats as 4-h constant-rate intravenous infusions to approach steady state. The flow rate of 1 mL/kg per h corresponded to infusion rates of 2 μmol/kg per h for indomethacin and 4 μmol/kg per h for loperamide and moxalactam. The vehicle used was dimethylacetamide, tetraethyleneglycol, and water (1:1:1, w/w/w). Cerebrospinal fluid, blood, and brain tissues were sampled as described above. All samples were stored at −20°C until analysis by reversed-phase liquid chromatography and multiple reaction monitoring mass spectrometry (LC-MS/MS).
The delivery of unbound drug into the brain at steady state was assessed as Kp,uu,brain (Equations (1 and 2)). Abrain was estimated using the correction model for residual blood presented below (Equation (13)). Values for Vu,brain were obtained using the brain slice uptake technique exactly as described previously (Fridén et al, 2009). Vu,brain describes the uptake and binding of the drug in brain parenchymal cells and is the proportionality constant that relates Abrain to Cu,brainISF (Equations (1 and 2)). Cu,p was calculated as the product of plasma concentrations (Cp) and fu,p, determined by equilibrium dialysis (Wan and Rehngren, 2006), using plasma from control rats that had undergone the same surgical procedures and recovery period. The drug concentrations added to the plasma matched the concentrations observed in the in vivo experiment.
As a potential surrogate for Kp,uu,brain, Kp,uu,CSF was calculated using the unbound drug concentrations in the CSF (Cu,CSF), calculated as the product of CSF concentrations (CCSF) and the unbound fraction in the CSF (fu,CSF):
As large volumes of rat CSF for equilibrium dialysis were not available, fu,CSF was calculated from fu,p using a single-binding site model in which the drug–protein dissociation constant was considered as the same in the CSF and plasma (Equation (8)). Protein concentration in the CSF was assumed to be 0.3% of that in the plasma (Habgood et al, 1992).
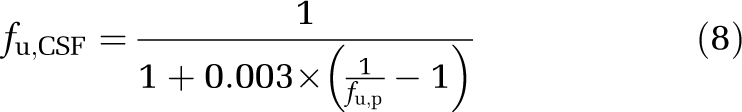 |
Drug Analysis by Liquid Chromatography and Multiple Reaction Monitoring Mass Spectrometry
Drug concentrations in blood, plasma, CSF, and brain tissue homogenate were quantified using LC-MS/MS with positive electrospray ionization. The LC-MS/MS system consisted of an LC-10AD pump (Schimadzu, Columbia, MA, USA), an Ascentis Express C18 column (3 cm × 2.1 mm, 2.7 μm, Supelco Analytical, Bellefonte, PA, USA), and a Micromass Ultima Platinum detector (Waters, Manchester, UK). The gradient elution from 5% to 95% mobile phase B was performed at a flow rate of 0.8 mL/min over 1.4 mins using mobile phases consisting of 0.2% formic acid in deionized water (A) and 0.2% formic acid in acetonitrile (B), respectively. Retention times and mass transitions were 0.50 min and m/z 521.0 → 198.9 for moxalactam, 0.77 min and m/z 477.2 → 266.0 for loperamide, and 1.00 min and m/z 357.9 → 138.9 for indomethacin, respectively.
Calibration curves were established by serial dilution in 50% acetonitrile to cover all expected concentrations of the samples. A volume of 20 μL from each point of the serial dilution was mixed with 180 μL of blank matrices of blood, plasma, or brain homogenate. As sufficient amounts of blank CSF were not available, the calibration curve for CSF was established in 10% plasma in saline, to match the CSF samples, which were mixed with 5 μL blank plasma at the time of sampling. Four replicate samples of blood, plasma, brain homogenate, and CSF were protein precipitated by adding 200 μL of cold acetonitrile. The samples were vortexed for 2 mins and then centrifuged (Rotanta/TR; Hettich, Tuttlingen, Germany) at 4,000 r.p.m. at 4°C for 20 mins. A volume of 75 μL of 0.2% formic acid and 75 μL of supernatant were transferred into a new 96-well plate from which 5 μL samples were injected into the system.
Correction Model for Drug in Brain Vascular Spaces
The correction model for a drug present in the brain vascular spaces was established to increase the accuracy of the estimated amount of the drug in the brain tissue (Abrain). The concentrations of the drug in erythrocytes (Cer) and bound to plasma proteins (Cb,p) were calculated using Equations (9 and 10), respectively. Cblood is the drug concentration in whole arterial blood.
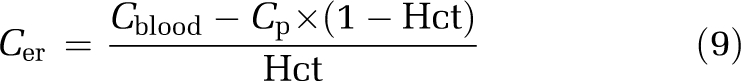 |
The effective plasma space in the brain for a given drug (Veff) depends on the protein-binding ability of the drug (fu,p) and has a value that is in between Vprotein and Vwater. Drugs that are essentially unbound have Veff close to Vwater, whereas highly bound drugs have Veff close to the smaller Vprotein (Equation (11)).
The amount of drug in the effective plasma space is given by:
Abrain was calculated from brain homogenate concentrations (Cbrain,h) corrected for dilutions and by subtracting the estimated amount of drug in the effective plasma space and the amount of drug associated with erythrocytes:
If the amount of drug associated with erythrocytes is negligible, a simplified approximate correction model is proposed that does not require measured drug concentrations in whole blood or arterial hematocrit (Equation (14)).
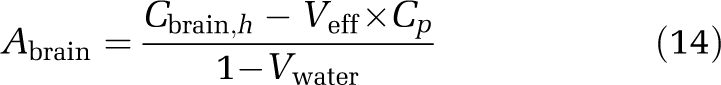 |
Statistical Analysis
The precision of Kp,brain and Kp,uu,brain in any one rat was assessed using the propagation of error method (Kendall et al, 1987). This statistical approach estimates the s.e. of a function Y=f(X, Z, …) with the following formula:
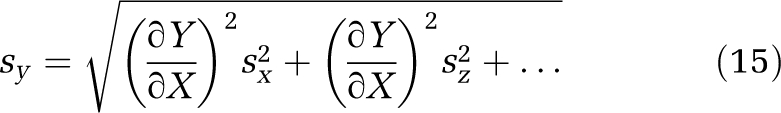 |
where sy, sx, and sz are the s.e. of function Y and measurements X and Z, assuming zero covariance between variables. ∂Y/∂X and ∂Y/∂Z are the partial derivatives of function Y with regard to X and Z, respectively. Kp,uu,brain was measured in this study as a function of nine different variables with replicated measurements (Equation (16)), from an amalgamation of Equations (12 and 13) after allowance for Cer in Equation (9):
The s.e. of Cbrain,h, Cp, and Cblood were calculated from the four replicates of the LC-MS/MS measurements. The s.e. of fu,p and Vu,brain were calculated from replicate equilibrium dialysis and brain slice experiments. This takes technical variability into account but assumes no interrat variability, on the basis of earlier findings that the interrat variability in Vu,brain is marginal compared with technical variability (Fridén et al, 2009). Instead of using the s.e. of Vwater, Vprotein, Ver, and Hct, a s.d. was calculated from the separate group of eight rats in which they had been determined. This also took into account the variability associated with sampling the brain. Values for Kp,uu,brain and Kp,brain are summarized across rats as averages±s.d.
Results
Determination of Brain Vascular Spaces
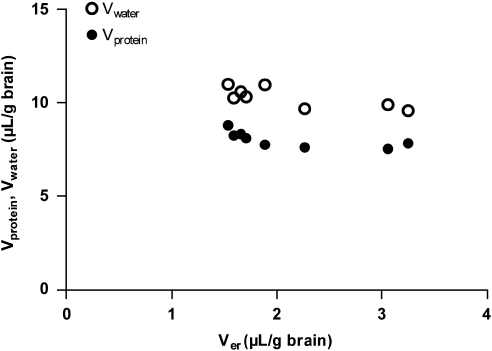
The apparent vascular space of plasma proteins measured by 14C-dextran (Vprotein, 7.99±0.43 μL/g brain) was smaller than the apparent plasma water space measured by 3H-inulin (Vwater, 10.3±0.54 μL/g brain). Protein concentration in the residual brain capillary plasma is therefore 20% lower than in the arterial plasma, indicating the need to incorporate protein binding of the drug into the correction for residual blood. The erythrocyte volume (Ver), measured spectrophotometrically, was 2.13±0.68 μL/g of the brain. The calculated hematocrit of brain capillary blood using Equation (6) (0.17±0.05) was smaller than the measured arterial hematocrit (0.42±0.016). As Vprotein represents a portion of Vwater, the total (physical) volume of residual blood was 12.4 μL/g brain, i.e., the sum of Vwater and Ver. Although the average value for Ver was in good agreement with previous literature data (Table 1), it was suspected that the matrix effect in the spectrophotometric method was variable, contributing to the large observed variability between rats for Ver (Figure 1). In contrast there was very little variability in the estimates for Vwater and Vprotein. Therefore, no correlation was observed between the brain plasma spaces of Vwater and Vprotein, and the erythrocyte space Ver (Figure 1). As it was consequently not possible to predict Vwater and Vprotein from values of Ver, it was decided not to make sample-specific measurements of Ver in subsequent experiments (below).
Figure 1.
The volume of brain tissue samples occupied by plasma water (Vwater) and plasma proteins (Vprotein) plotted against the volume occupied by erythrocytes (Ver).
Assessment of Brain Exposure to Model Drug Compounds
The concentrations used in the correction model to calculate Abrain for indomethacin, loperamide, and moxalactam are presented in Table 2, together with values of Abrain and the corrections made for drug in brain capillaries. The parameter Vprotein × Cb,p contributed most to the total corrections made for any drug. For indomethacin, which is extensively bound to plasma proteins, Vprotein × Cb,p contributed 98% of the total correction. The amount of drug associated with erythrocytes was insignificant for indomethacin and moxalactam. For loperamide it contributed 26% of the total correction; however, the total correction itself was very small for loperamide (Table 2).
Table 2. Drug concentrations and the relative contribution of individual vascular spaces to the brain sample concentration and to total correction for drug in residual blooda.
| Cblood | Cp | Cbrain,h | Abrainb | Total correctionc | Contribution to total correction (%) | Contribution to Cbrain,h (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (μmol/L) | (μmol/L) | (μmol/L) | (μmol/kg brain) | (μmol/kg brain) | Ver × Cer | Vwater × Cu,p | Vprotein × Cb,p | Ver × Cer | Vwater × Cu,p | Vprotein × Cb,p | |
| Indomethacin | 37±7.0 | 24±5.3 | 0.61±0.19 | 0.309±0.14 | 0.31±0.061 | 4±3 | 1±0.02 | 96±3 | 2.0±2 | 0.35±0.05 | 49±7 |
| Loperamide | 0.91±0.09 | 1.03±0.1 | 0.15±0.03 | 0.137±0.03 | 0.01±0.0008 | 26±5 | 6±0.3 | 67±4 | 1.8±0.3 | 0.4±0.1 | 4.9±1 |
| Moxalactam | 12±1.4 | 7.50±1.1 | 0.15±0.02 | 0.042±0.015 | 0.11±0.012 | 2±4 | 37±1 | 61±2 | 1.5±3 | 27±3 | 45±4 |
Abrain, amount of drug in the brain after correction for residual blood; Cblood, drug concentration in whole blood; Cbrain,h, drug concentration in brain homogenate corrected for dilutions; Cp, drug concentration in the plasma; Ver × Cer, amount of drug associated with erythrocytes; Vwater × Cu,p, amount of unbound drug in plasma; Vprotein × Cb,p, amount of drug bound to plasma proteins.
Values are presented as means±s.d. for five rats.
Calculated with Equation (13).
Calculated as the sum of Vwater × Cu,p, Vprotein × Cb,p, and Ver × Cer, (Equations (12 and 13)).
The values of fu,p and Vu,brain (Table 3), obtained by equilibrium dialysis and the brain slice method, described the extent of binding to plasma proteins and the brain tissue, respectively. The value of Vu,brain for loperamide (376 mL/g brain) indicated extensive binding in brain cells, whereas the value of Vu,brain for moxalactam (0.46 mL/g brain) indicated predominant distribution outside brain cells and minimal binding to proteins or membranes. Indomethacin was extensively bound to plasma proteins, as indicated by the low value of fu,p (0.0054), whereas moxalactam bound moderately to plasma proteins (fu,p=0.32). The Vu,brain × fu,p product describing the ratio of unbound fractions in the brain to plasma differed 300-fold between loperamide and indomethacin and 150-fold between loperamide and moxalactam (Table 3).
Table 3. Binding of indomethacin, loperamide, and moxalactam in the plasma, brain tissue, and CSFa,b.
| fu,pa | Vu,brainb | fu,CSFc | fu,p × Vu,braind | |
|---|---|---|---|---|
| (mL/g brain) | ||||
| Indomethacin | 0.0054±0.001 | 13.8±0.7 | 0.50 | 0.074 |
| Loperamide | 0.059±0.013 | 370±9 | 0.86 | 21.9 |
| Moxalactam | 0.32±0.024 | 0.46±0.09 | 1.0 | 0.15 |
CSF, cerebrospinal fluid; fu,CSF, unbound fraction in cerebrospinal fluid; fu,p, unbound fraction in the plasma; Vu,brain, unbound brain volume of distribution.
Determined by ex vivo equilibrium dialysis using pooled plasma. Values are presented as means±s.d. for five dialysis cells.
Determined by brain slice method (Fridén et al, 2009).Values are presented as means±s.d. for six slices from an individual drug-naive rat.
Calculated from fu,p using Equation (8).
This number is understood as the ratio of unbound ‘fractions' in the brain and plasma, with higher or lower values representing greater or lesser binding, respectively, to brain tissue than to plasma proteins (Equation (2)).
Indomethacin, with a very low value for Kp,brain (0.0082), had the highest value for Kp,uu,brain (0.11) among the studied compounds. Loperamide, with the highest value for Kp,brain (0.15), had the lowest value for Kp,uu,brain (0.007). Moxalactam had the lowest value for Kp,brain (0.0035) and an intermediate value for Kp,uu,brain (0.024). The values of Kp,uu,brain were statistically different from 0 for all compounds, as illustrated by the 95% confidence interval of Kp,uu,brain (Table 4).
Table 4. Estimated values for Kp,brain, Kp,uu,brain, Kp,uu,CSF, and the relative contribution of experimental factors to imprecision in Kp,uu,braina.
| Contribution to imprecision in Kp,uu,brain(%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rat | Kp,brain | Kp,uu,brain | Cbl | Cp | Cbrain,h | Vwater | Vprotein | Ver | Hct | fu,p | Vu,brain | Kp,uu,CSF |
| Indomethacin | ||||||||||||
| 1 | 0.0125 (0.010–0.015) | 0.168 (0.13–0.21) | 1 | 5 | 36 | 0 | 8 | 0 | 0 | 48 | 3 | 0.241 |
| 2 | 0.0060 (0.004–0.008) | 0.081 (0.056–0.11) | 1 | 6 | 42 | 0 | 20 | 1 | 1 | 28 | 2 | 0.156 |
| 3 | 0.0071 (0.004–0.010) | 0.095 (0.056–0.14) | 1 | 12 | 61 | 0 | 8 | 1 | 0 | 16 | 1 | 0.148 |
| 4 | 0.0085 (0.005–0.012) | 0.114 (0.070–0.16) | 0 | 11 | 61 | 0 | 7 | 1 | 0 | 18 | 1 | 0.151 |
| 5 | 0.0067 (0.005–0.009) | 0.090 (0.062–0.12) | 1 | 6 | 47 | 0 | 17 | 0 | 1 | 28 | 1 | 0.161 |
| All | 0.0082±0.0026 | 0.110±0.035 | 1 | 8 | 49 | 0 | 12 | 1 | 0 | 27 | 1 | 0.172 |
| Loperamide | ||||||||||||
| 1 | 0.21 (0.19–0.23) | 0.0097 (0.008–0.012) | 0 | 2 | 18 | 0 | 0 | 0 | 0 | 79 | 1 | 0.056 |
| 2 | 0.12 (0.11–0.13) | 0.0056 (0.004–0.007) | 0 | 6 | 4 | 0 | 0 | 1 | 0 | 89 | 1 | 0.025 |
| 3 | 0.15 (0.13–0.17) | 0.0067 (0.005–0.008) | 0 | 1 | 30 | 0 | 0 | 0 | 0 | 68 | 1 | 0.033 |
| 4 | 0.17 (0.15–0.20) | 0.0079 (0.006–0.010) | 0 | 3 | 30 | 0 | 0 | 0 | 0 | 67 | 1 | 0.040 |
| 5 | 0.11 (0.10–0.11) | 0.0048 (0.004–0.006) | 0 | 2 | 7 | 0 | 0 | 0 | 0 | 89 | 1 | 0.034 |
| All | 0.15±0.042 | 0.0070±0.002 | 0 | 3 | 18 | 0 | 0 | 0 | 0 | 78 | 1 | 0.037 |
| Moxalactam | ||||||||||||
| 1 | 0.0049 (0.003–0.007) | 0.034 (0.018–0.050) | 2 | 7 | 66 | 2 | 6 | 0 | 0 | 4 | 12 | 0.028 |
| 2 | 0.0026 (0.001–0.004) | 0.018 (0.008–0.027) | 1 | 7 | 52 | 6 | 17 | 3 | 1 | 4 | 9 | 0.022 |
| 3 | 0.0033 (0.002–0.005) | 0.022 (0.013–0.032) | 5 | 3 | 48 | 6 | 17 | 1 | 1 | 6 | 14 | 0.013 |
| 4 | 0.0041 (0.002–0.006) | 0.028 (0.011–0.044) | 0 | 2 | 77 | 2 | 6 | 2 | 0 | 3 | 8 | 0.019 |
| 5 | 0.0024 (0.001–0.004) | 0.016 (0.006–0.027) | 0 | 14 | 54 | 5 | 14 | 1 | 1 | 3 | 7 | 0.016 |
| All | 0.0035±0.0011 | 0.024±0.007 | 2 | 7 | 59 | 4 | 12 | 2 | 1 | 4 | 10 | 0.020 |
Cbl, drug concentration in whole blood; Cbrain,h, drug concentration in brain homogenate corrected for dilutions; Cp, drug concentration in the plasma; fu,p, unbound fraction in plasma; Kp,uu,CSF, unbound CSF-to-plasma concentration ratio; Ver, brain vascular volume of erythrocytes; Vprotein, brain vascular volume of plasma proteins; Vwater, brain vascular volume of plasma water; Vu,brain, unbound brain volume of distribution.
Values for individual rats are presented as point estimates (95% confidence intervals). Values across rats are presented as means of point estimates±s.d.
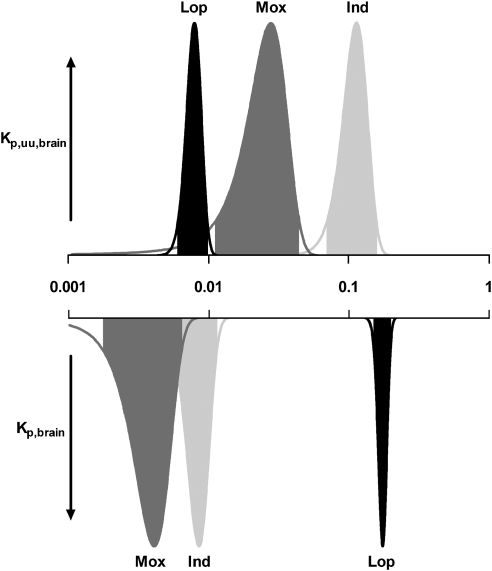
The normal distribution of the estimates of Kp,brain and Kp,uu,brain for the compounds is illustrated in Figure 2 for a representative rat. The shape of the distribution describes the uncertainty in the estimate of Kp,brain and Kp,uu,brain within a single rat. Loperamide, with the highest Kp,brain value, had the narrowest confidence interval for Kp,uu,brain, illustrating the high precision of the estimate. Kp,uu,brain for indomethacin and moxalactam, with lower values for Kp,brain than loperamide, were determined with somewhat lower precision, as illustrated by the broader shape of the distribution graph (Figure 2). The measurement variability in drug concentration in the brain homogenate and the unbound fraction in the plasma generally contributed most to the uncertainty of Kp,uu,brain. As there was very little variability in vascular spaces between individual rats, the contribution to the imprecision of Kp,uu,brain was small (Table 4).
Figure 2.
Probability density graph for Kp,uu,brain (upward) and Kp,brain (downward) for indomethacin (Ind), loperamide (Lop) and moxalactam (Mox) in a representative rat (Rat 4). The shaded areas define the 95% confidence intervals. The shift on the log scale for the peak of Kp,brain and Kp,uu,brain corresponds to the pattern of binding to plasma proteins and brain tissue (Vu,brain × fu,p) for each drug (Table 3).
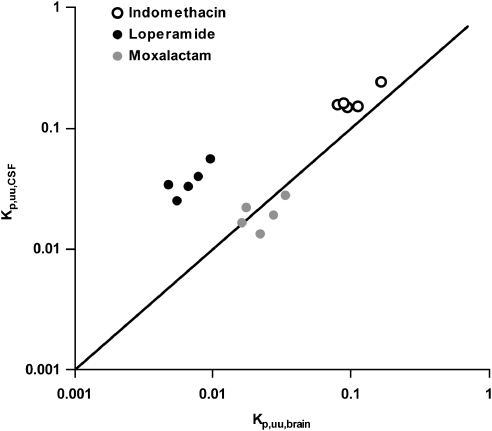
The agreement between Cu,CSF and Cu,brainISF was assessed by plotting Kp,uu,CSF against Kp,uu,brain for the model drugs (Figure 3). There was very close agreement between indomethacin and moxalactam, while concentrations of unbound loperamide in the CSF exceeded those in the brain ISF by approximately fivefold.
Figure 3.
Relationship between the concentration of unbound drug in the CSF and that in brain ISF normalized to unbound plasma concentrations (Kp,uu,CSF and Kp,uu,brain). Markers represent estimates for individual rats and compounds. The solid line illustrates the line of identity.
Discussion
Among a number of essential steps for accurate in vivo measurement of drug exposure in the brain, correction for the drug that is present in the residual blood of the brain tissue sample is critical. The hematocrit and concentration of plasma proteins are lower in brain capillary blood than in arterial blood, yet an equal composition has been inherently assumed by previous approaches using the measured drug concentration in a concomitant arterial blood sample. This paper presents a novel correction model that corrects for the drug present in various compartments of the brain residual blood (unbound drug in the plasma, drug bound to plasma proteins, and drug associated with erythrocytes). The correction model was applied for the determination of the unbound brain-to-plasma concentration ratio, Kp,uu,brain, for indomethacin, loperamide, and moxalactam.
Estimates of the brain vascular spaces vary substantially in the literature and seem to depend on the method used to kill the experimental animals and the vascular tracer used. Decapitation or, as in this study, severing the heart before removing the brain, results in lower estimates for vascular spaces than observed with in vivo measurements or in situ fixation (Table 1). This can be explained by the fact that the intravascular pressure decreases to zero when animals are killed by decapitation or by severing the heart, resulting in blood drain from the tissue (Todd et al, 1992, 1993). The composition of brain residual blood is also different from that of arterial blood in that the hematocrit is lower. This was explained by experimental data supporting a model in which essentially all brain capillaries are continuously perfused with plasma, whereas the flow of erythrocytes is intermittently restricted by precapillary sphincters (Tajima et al, 1992). In addition, erythrocytes move through capillaries faster than the plasma, as the plasma is exposed to shear stresses near the vessel wall, which result in its retention (Preston and Haas, 1986; Smith et al, 1988; Todd et al, 1992). Furthermore, the estimated volume of the vascular plasma space seems to be dependent on the size of the vascular tracer used. Reports show that vascular tracers with a molecular size of ≤20 kDa occupy a 60% to 90% greater volume of brain capillaries in rats than tracers >40 kDa (Reed and Woodbury, 1963; Sarna et al, 1977; Smith et al, 1988). This suggests that plasma water has a larger volume of distribution within the brain capillaries than do plasma proteins (α1-acid glycoprotein 42 kDa, albumin 67 kDa, lipoproteins 200 to 2,400 kDa (Rowland and Tozer, 1995)). The reasons for the difference are not clarified; however, they may relate to restricted entry of large dextrans into the gel-like endothelial glycocalyx layer (Vink and Duling, 2000) or selective draining of larger solutes during exsanguination or decapitation. It is unlikely that passage across the BBB influences the estimates of vascular spaces as these remained at fairly constant levels after the rapid increase during the first few minutes (Smith et al, 1988). The values for Vprotein, Vwater, and Ver determined in this study (Table 2) agree well with published values of experiments wherein animals have been killed in a similar manner (Table 1).
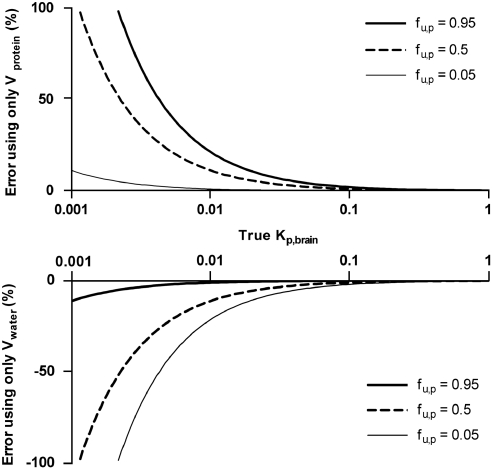
Correction for the drug in all the vascular compartments by using the full correction model (Equation (13)) requires, in addition to brain homogenate and plasma concentrations, analysis of the drug concentration in whole blood, which significantly adds to the experimental workload. As Ver was the smallest of the vascular volumes in the brain and the amount of associated drug (Ver × Cer) contributed least to the total correction, a simplified model is proposed which ignores erythrocytes (Equation (14)). The error associated with ignoring erythrocytes is expected to be small even for drugs such as loperamide that significantly distribute into phospholipid-rich erythrocytes, because such compounds also tend to bind to brain cells, thereby reducing the importance of correction for residual blood by its inherently higher Kp,brain (Table 2). The main sources of variability for Kp,uu,brain were the measured drug concentrations in the brain homogenate and the unbound fraction in the plasma. This is because of their leverage for Kp,uu,brain (Equation (16)) and the proportionally lower precision, versus other variables, in their determination. To further improve the precision of Kp,uu,brain, it would be reasonable to focus on the plasma protein binding assay and the LC-MS/MS analysis of brain homogenate. The variability between rats in the estimated vascular plasma volumes (Figure 1) was so small that there was very little contribution to the imprecision of Kp,uu,brain. Therefore, we propose that the values for Vwater (10.3 μL/g brain) and Vprotein (7.99 μL/g brain) determined in this study can be used henceforth in any laboratory with the simplified version of the correction model (Equation (14)) for measurement of brain exposure with sufficient accuracy, provided similar methods for euthanasia and brain tissue sampling. Conservative experimenters may, however, choose to reestablish the vascular volumes for particular experimental conditions or procedures using vascular markers. For less conservative experimenters, a reasonable approximation is to correct only for Vprotein, particularly for highly protein-bound drugs. An error analysis of correcting for either only Vprotein or only Vwater is shown in Figure 4.
Figure 4.
Expected error in Kp,brain and Kp,uu,brain associated with the correction for residual blood using a single vascular marker for various unbound fractions in the plasma. Errors associated with markers of Vprotein, i.e., dextran, are shown in the upper panel. Errors associated with markers of Vwater, i.e., inulin, are shown in the lower panel.
The use of the correction model for indomethacin and moxalactam was valuable, as realistic values for Kp,uu,brain could be determined in spite of extremely low values of Kp,brain. In contrast, had a correction model based on the in situ blood volume (∼3%), a blood-to-plasma ratio of unity, and arterial blood composition been used, large negative Kp,uu,brain values would have resulted for both indomethacin (−0.19) and moxalactam (−0.13). Intricate patterns of brain exposure are illustrated by the results for the model drugs. Indomethacin, with a very low Kp,brain, had the highest Kp,uu,brain among the studied compounds and was thus the least efficiently effluxed drug. The most efficiently effluxed drug, loperamide, with the lowest Kp,uu,brain, had a remarkably high Kp,brain. This, relatively speaking, high Kp,brain is due to extensive binding to brain tissue, with proportionally less binding in the plasma. The extremely low Kp,brain of indomethacin despite less efficient efflux is a result of extensive binding to plasma proteins and proportionally much less binding in the brain. In addition to significant efflux, the very low value of Kp,brain for moxalactam is caused by its distribution predominantly outside the brain cells (very low Vu,brain) in combination with some plasma protein binding. The results highlight the fact that, to identify and quantify efflux at the BBB, it is not sufficient to determine the value of Kp,brain. The value of Kp,brain must be used in combination with Vu,brain and fu,p to give an estimate of Kp,uu,brain, which describes the extent of transport across the BBB. In drug discovery programs, it is necessary to have a good resolution to rank compounds correctly with regard to Kp,uu,brain to build structure activity relationships for chemical design and select the most appropriate compound(s) for further development. The precision of Kp,uu,brain, as illustrated by the shapes of the Kp,uu,brain distribution graphs (Figure 2), was partly related to Kp,brain.
When Kp,brain values are so low or so variable that they cannot be quantitatively relied on, it is important to ask the following question: what is the maximum value for Kp,uu,brain for the particular drug that could have resulted in such a low Kp,brain value? The answer can be approached by assigning a reasonable but arbitrary maximal value for Kp,brain, such as 0.005, and transposing this to the maximal value for Kp,uu,brain using values for Vu,brain and fu,p (Equations (1 and 2)). As a result, it can be seen that, for the development of centrally acting drugs, low Kp,brain values should not mean discarding the drug before the Kp,uu,brain is estimated.
Although it was possible in this study to determine Kp,uu,brain with a reasonable precision for the model drugs, surrogate CSF measurements (Kp,uu,CSF) can potentially be used in the event of unacceptably imprecise estimates of Kp,uu,brain. There was close agreement between Kp,uu,CSF and Kp,uu,brain for indomethacin and moxalactam; however, for loperamide, Kp,uu,CSF was approximately fivefold higher than Kp,uu,brain (Figure 3). This may have been the result of more efficient efflux at the BBB than at the blood–CSF barrier, which has been reported for other extensively effluxed compounds (Shen et al, 2004; Takasawa et al, 1997). This suggests that Kp,uu,CSF may not be a reliable surrogate measure for Kp,uu,brain. It is noteworthy that loperamide is a P-glycoprotein substrate (Schinkel et al, 1996), and that P-glycoprotein is reported to be oriented at the blood–CSF barrier to transport its substrates into the CSF (Kusuhara and Sugiyama, 2001).
This study has shown that the composition of the brain residual blood is different from that of arterial blood; separate values were obtained for the brain vascular spaces of plasma proteins, erythrocytes, and plasma water. These values (Vprotein 7.99 μL/g brain, Ver, 2.13 μL/g brain, and Vwater 10.3 μL/g brain) can be used with a novel, drug-specific correction model for residual blood to quantify drug exposure in the brain. It was found that the erythrocyte binding had minimal influence on the correction. By using protein binding information together with the brain vascular volumes of plasma water and plasma protein, a correction for the drug present in the brain vasculature can be calculated for most drugs through the effective plasma space in brain, Veff (Equation 11). The described methodology for measurement of the pharmacologically relevant unbound brain-to-plasma concentration ratio, Kp,uu,brain, offers a method of consistently providing high-quality in vivo measurement of brain exposure, as input for drug design with the aim to maximize the therapeutic window.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Bickel U, Schumacher OP, Kang YS, Voigt K. Poor permeability of morphine 3-glucuronide and morphine 6-glucuronide through the blood-brain barrier in the rat. J Pharmacol Exp Ther. 1996;278:107–113. [PubMed] [Google Scholar]
- Cremer JE, Seville MP. Regional brain blood flow, blood volume, and haematocrit values in the adult rat. J Cereb Blood Flow Metab. 1983;3:254–256. doi: 10.1038/jcbfm.1983.35. [DOI] [PubMed] [Google Scholar]
- Everett NB, Simmons B, Lasher EP. Distribution of blood (Fe 59) and plasma (I 131) volumes of rats determined by liquid nitrogen freezing. Circ Res. 1956;4:419–424. doi: 10.1161/01.res.4.4.419. [DOI] [PubMed] [Google Scholar]
- Fridén M, Ducrozet F, Antonsson M, Middleton B, Bredberg U, Hammarlund-Udenaes M. Development of a high-throughput brain slice method for studying drug distribution in the CNS. Drug Metab Dispos. 2009;37:1226–1233. doi: 10.1124/dmd.108.026377. [DOI] [PubMed] [Google Scholar]
- Fridén M, Gupta A, Antonsson M, Bredberg U, Hammarlund-Udenaes M. In vitro methods for estimating unbound drug concentrations in the brain interstitial and intracellular fluids. Drug Metab Dispos. 2007;35:1711–1719. doi: 10.1124/dmd.107.015222. [DOI] [PubMed] [Google Scholar]
- Gupta A, Chatelain P, Massingham R, Jonsson EN, Hammarlund-Udenaes M. Brain distribution of cetirizine enantiomers: comparison of three different tissue-to-plasma partition coefficients: K(p), K(p,u), and K(p,uu) Drug Metab Dispos. 2006;34:318–323. doi: 10.1124/dmd.105.007211. [DOI] [PubMed] [Google Scholar]
- Gupta A, Gillard M, Christophe B, Chatelain P, Massingham R, Hammarlund-Udenaes M. Peripheral and central H1 histamine receptor occupancy by levocetirizine, a non-antihistamine; a time course study in the guinea pig. Br J Pharmacol. 2007;151:1129–1136. doi: 10.1038/sj.bjp.0707318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habgood MD, Sedgwick JE, Dziegielewska KM, Saunders NR. A developmentally regulated blood-cerebrospinal fluid transfer mechanism for albumin in immature rats. J Physiol. 1992;456:181–192. doi: 10.1113/jphysiol.1992.sp019332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund-Udenaes M, Bredberg U, Friden M. Methodologies to assess brain drug delivery in lead optimization. Curr Top Med Chem. 2009;9:148–162. doi: 10.2174/156802609787521607. [DOI] [PubMed] [Google Scholar]
- Heykants J, Michiels M, Knaeps A, Brugmans J. Loperamide (R 18 553), a novel type of antidiarrheal agent. Part 5: the pharmacokinetics of loperamide in rats and man. Arzneimittel-Forschung. 1974;24:1649–1653. [PubMed] [Google Scholar]
- Kendall M, Stuart A, Ord K. Advanced Theory of Statistics. London: Hodder Arnold; 1987. [Google Scholar]
- Khor SP, Bozigian H, Mayersohn M. Potential error in the measurement of tissue to blood distribution coefficients in physiological pharmacokinetic modeling.Residual tissue blood. II. Distribution of phencyclidine in the rat. Drug Metab Dispos. 1991;19:486–490. [PubMed] [Google Scholar]
- Kusuhara H, Sugiyama Y. Efflux transport systems for drugs at the blood-brain barrier and blood-cerebrospinal fluid barrier (Part 1) Drug Discov Today. 2001;6:150–156. doi: 10.1016/s1359-6446(00)01632-9. [DOI] [PubMed] [Google Scholar]
- Lin SZ, Nakata H, Tajima A, Gruber K, Acuff V, Patlak C, Fenstermacher J. Quantitative autoradiographic assessment of 55Fe-RBC distribution in rat brain. J Appl Physiol. 1990;69:1637–1643. doi: 10.1152/jappl.1990.69.5.1637. [DOI] [PubMed] [Google Scholar]
- Mandula H, Parepally JM, Feng R, Smith QR. Role of site-specific binding to plasma albumin in drug availability to brain. J Pharmacol Exp Ther. 2006;317:667–675. doi: 10.1124/jpet.105.097402. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1989. [Google Scholar]
- Preston E, Haas N. Defining the lower limits of blood-brain barrier permeability: factors affecting the magnitude and interpretation of permeability-area products. J Neurosci Res. 1986;16:709–719. doi: 10.1002/jnr.490160411. [DOI] [PubMed] [Google Scholar]
- Reed DJ, Woodbury DM. Kinetics of movement of iodide, sucrose, inulin and radio-iodinated serum albumin in the central nervous system and cerebrospinal fluid of the rat. J Physiol. 1963;169:816–850. doi: 10.1113/jphysiol.1963.sp007298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland M, Tozer T. Clinical Pharmacokinetics Concepts and Applications. Media: Williams & Wilkins; 1995. [Google Scholar]
- Sandor P, Cox-van Put J, de Jong W, de Wied D. Continuous measurement of cerebral blood volume in rats with the photoelectric technique: effect of morphine and naloxone. Life Sci. 1986;39:1657–1665. doi: 10.1016/0024-3205(86)90163-3. [DOI] [PubMed] [Google Scholar]
- Sarna GS, Bradbury MW, Cavanagh J. Permeability of the blood-brain barrier after portocaval anastomosis in the rat. Brain Res. 1977;138:550–554. doi: 10.1016/0006-8993(77)90692-8. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Wagenaar E, Mol CA, van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97:2517–2524. doi: 10.1172/JCI118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen DD, Artru AA, Adkison KK. Principles and applicability of CSF sampling for the assessment of CNS drug delivery and pharmacodynamics. Adv Drug Deliv Rev. 2004;56:1825–1857. doi: 10.1016/j.addr.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Shockley RP, LaManna JC. Determination of rat cerebral cortical blood volume changes by capillary mean transit time analysis during hypoxia, hypercapnia and hyperventilation. Brain Res. 1988;454:170–178. doi: 10.1016/0006-8993(88)90816-5. [DOI] [PubMed] [Google Scholar]
- Smith QR, Ziylan YZ, Robinson PJ, Rapoport SI. Kinetics and distribution volumes for tracers of different sizes in the brain plasma space. Brain Res. 1988;462:1. doi: 10.1016/0006-8993(88)90577-x. [DOI] [PubMed] [Google Scholar]
- Tajima A, Nakata H, Lin SZ, Acuff V, Fenstermacher J. Differences and similarities in albumin and red blood cell flows through cerebral microvessels. [erratum appears in Am J Physiol 1992 Sep;263(3 Pt 2):section H followi] Am J Physiol. 1992;262:H1515–H1524. doi: 10.1152/ajpheart.1992.262.5.H1515. [DOI] [PubMed] [Google Scholar]
- Takasawa K, Terasaki T, Suzuki H, Ooie T, Sugiyama Y. Distributed model analysis of 3′-azido-3′-deoxythymidine and 2′,3′-dideoxyinosine distribution in brain tissue and cerebrospinal fluid. J Pharmacol Exp Ther. 1997;282:1509–1517. [PubMed] [Google Scholar]
- Todd MM, Weeks JB, Warner DS. Cerebral blood flow, blood volume, and brain tissue hematocrit during isovolemic hemodilution with hetastarch in rats. Am J Physiol. 1992;263:H75–H82. doi: 10.1152/ajpheart.1992.263.1.H75. [DOI] [PubMed] [Google Scholar]
- Todd MM, Weeks JB, Warner DS. Microwave fixation for the determination of cerebral blood volume in rats. J Cereb Blood Flow Metab. 1993;13:328–336. doi: 10.1038/jcbfm.1993.41. [DOI] [PubMed] [Google Scholar]
- Wan H, Rehngren M. High-throughput screening of protein binding by equilibrium dialysis combined with liquid chromatography and mass spectrometry. J Chromatogr A. 2006;1102:125–134. doi: 10.1016/j.chroma.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Vink H, Duling BR. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am J Physiol—Heart Circ Physiol. 2000;278:H285–H289. doi: 10.1152/ajpheart.2000.278.1.H285. [DOI] [PubMed] [Google Scholar]