Abstract
We found that recombinant human erythropoietin (rhEPO) reduced significantly the development of brain edema in a rat model of diffuse traumatic brain injury (TBI) (impact-acceleration model). In this study, we investigated the molecular and intracellular changes potentially involved in these immediate effects. Brain tissue nitric oxide (NO) synthesis, phosphorylation level of two protein kinases (extracellular-regulated kinase (ERK)-1/-2 and Akt), and brain water content were measured 1 (H1) and 2 h (H2) after insult. Posttraumatic administration of rhEPO (5,000 IU/kg body weight, intravenously, 30 mins after injury) reduced TBI-induced upregulation of ERK phosphorylation, although it increased Akt phosphorylation at H1. These early molecular changes were associated with a reduction in brain NO synthesis at H1 and with an attenuation of brain edema at H2. Intraventricular administration of the ERK-1/-2 inhibitor, U0126, or the Akt inhibitor, LY294002, before injury showed that ERK was required for brain edema formation, and that rhEPO-induced reduction of edema could involve the ERK pathway. These results were obtained in the absence of any evidence of blood–brain barrier damage on contrast-enhanced magnetic resonance images. The findings of our study indicate that the anti edematous effect of rhEPO could be mediated through an early inhibition of ERK phosphorylation after diffuse TBI.
Keywords: brain trauma, cerebral edema, EPO (erythropoietin), inhibitors, MAPK (mitogen-activated protein kinase), MAPK activation after brain trauma
Introduction
Erythropoietin has triggered considerable interest because of several studies having indicated that it may confer neuroprotection and reduce neuronal death in in vivo and in vitro models of central nervous system injuries (Bernaudin et al, 1999; Brines et al, 2000; Cherian et al, 2007; Li et al, 2007a; Pacary et al, 2006; Sakanaka et al, 1998). In animal models of ischemia and of traumatic brain injury (TBI), erythropoietin has been found to very significantly affect neurogenesis, angiogenesis, and neuroinflammatory response (Lu et al, 2005; Siren et al, 2001; Yatsiv et al, 2005). In a rat model of diffuse TBI, magnetic resonance imaging (MRI) has shown that postinjury administration of recombinant human erythropoietin (rhEPO) reduces significantly the early development of diffuse brain edema and affects the apparent diffusion coefficient of brain water (Verdonck et al, 2007).
The immediate beneficial effect of rhEPO was conjectured to result from cellular processes. In an animal model of TBI, nitric oxide (NO) accumulation in the brain was observed within the first 5 to 30 mins after injury (Cherian et al, 2004). This accumulation was ascribed to the posttraumatic glutamate stimulation of N-methyl--aspartate receptors and calcium influx. In an animal model of brain ischemia, rhEPO reduced brain NO accumulation and brain edema, 6 h after the ischemic insult (Calapai et al, 2000). The neuroprotective potency of rhEPO observed 24 or 72 h after ischemia was ascribed to a reduction of neuronal NO synthase (NOS) expression (Kilic et al, 2005). Recombinant human erythropoietin was also shown in the same study to alter intracellular signaling mechanisms, in particular those involving extracellular-regulated kinase (ERK)-1/-2 and phosphatidylinositol-3 kinase-dependant factor Akt. Inhibition of ERK phosphorylation significantly reduced cortical lesion volume in a model of focal TBI (Mori et al, 2002b; Otani et al, 2007). Conversely, Akt phosphorylation (activation) was required to induce neuroprotection during heat acclimation (Shein et al, 2007). Therefore, we hypothesized that the early effects of rhEPO in our model of diffuse TBI could be associated with changes in the activation of erythropoietin signal-regulated protein kinases in the brain. To verify our hypothesis, we determined levels of phosphorylation (activation) of ERK-1/-2 and Akt as early as 1 h after trauma and we measured brain tissue NO synthesis and brain water content (BWC). Specific interactions between rhEPO, brain edema, and brain protein kinases were assessed using intracerebral pharmacologic inhibition of the ERK and Akt pathways before injury. Given the recent observation that rhEPO could restore the integrity of the blood–brain barrier (BBB) after disruption in cerebral ischemia (Li et al, 2007b), we further investigated the integrity of the BBB with contrast-enhanced MRI in this model of diffuse TBI.
Materials and methods
Male Wistar rats (weighting 300 to 450 g) were studied in three series of experiments. In the first series, we investigated the immediate biochemical effects of rhEPO on protein kinases, on NO synthesis, and on BWC at 1 (H1) and 2 h (H2) after TBI. Three groups of rats were studied for each of these two study periods. The first group of rats was subjected to TBI and saline sham treatment (TBI-saline, n=6 rats). The second group of rats was equally subjected to TBI and treated with rhEPO 30 mins after trauma (TBI-rhEPO, n=6 rats). Rats in the third group underwent surgical preparation of the TBI rats but eventually they were not subjected to the insult (sham-operated). They received either saline (n=4 rats) or rhEPO (n=4 rats). In the second series of experiments, the effects of ERK-1/-2 or Akt inhibition on BWC were determined at H2 after TBI. Six groups of five rats were pretreated with an intracerebroventricular administration of ERK-1/-2 inhibitor, Akt inhibitor, or vehicle before TBI, and were treated with rhEPO or saline. In the third series of experiments, we investigated the integrity of the BBB using contrast-enhanced MRI in two groups of rats: TBI-saline (n=4 rats) and sham-operated (n=4 rats).
Traumatic Brain Injury Model
Animals were prepared in accordance with the guidelines of the French Government (decree nos 87 to 848 of 19 October 1987, licenses 006683 and A38071) and the European Guidelines for the Protection of the Vertebrate Animals (European Trading System (ETS) No. 123, Strasbourg, 18 March 1986). The experimental protocol was very similar to the one described previously (Verdonck et al, 2007). Briefly, each animal was positioned in a stereotactic frame under isoflurane anesthesia. A brass disc (10 mm in diameter and 3 mm thick) was glued to the skull vault between the coronal and lambdoid sutures. Catheters were inserted into the femoral artery and vein to allow the monitoring of mean arterial blood pressure and delivering drugs. Blood gases (PaO2 and PaCO2), arterial oxygen saturation of hemoglobin (SaO2), arterial pH (pHa), and hemoglobin content (tHb) were determined from arterial blood samples <0.1 mL (ABL 510, Radiometer, Copenhagen, Denmark). After tracheostomy, rats were mechanically ventilated with 60% air–40% oxygen using a rodent ventilator (SAR-830/P, CWE, Ardmore, PA, USA). After completion of the surgical procedures, isoflurane delivery was discontinued and anesthesia was maintained with intravenous administration of α-chloralose (80 mg/kg initially, followed by 26.7 mg/kg per h) and pancuronium bromide (0.5 mg/kg initially, followed by 0.2 mg/kg per h) in a saline solution at a rate of 2 mL/h throughout the study. Baseline physiological values under α-chloralose were measured after an isoflurane washout period of more than 30 mins. Ventilation was adjusted to maintain PaCO2 at 35 to 40 mm Hg. Rectal temperature was maintained at 36.5±0.5°C by placing a heating pad under the abdomen. Criteria for exclusion from the study were persistence, for 10 min at least during the experiment, of out of range values of the following physiological parameters: mean arterial blood pressure (<70 mm Hg), PaO2 (<100 mm Hg), PaCO2 (<30 mm Hg or >45 mm Hg), and tHb (<80 g/L).
We used the impact-acceleration model of TBI (Marmarou et al, 1994). Injury was induced by dropping a 500-g mass through a vertical cylinder from a height of 1.5 m onto a metallic disc glued to the skull. After the impact, the metallic disc was removed and the scalp sutured. The reference time (H0) corresponded to the moment of impact (TBI-rhEPO and TBI-saline) or to an equivalent time (sham-operated). At 30 mins after H0, animals were intravenously administered either 0.5 mL of an isotonic saline solution (TBI-saline) or 5,000 IU/kg of Epoetin alfa (Eprex, Janssen-Cilag Division Ortho Biotech, Issy-les-Moulineaux, France) diluted in 0.5 mL of saline solution (TBI-rhEPO). The sham-operated rats were treated similarly, but they were not submitted to TBI.
Biochemical Effects of Recombinant Human Erythropoietin
Brain water content, brain protein kinases, and NO synthesis were determined at each study period (H1 and H2) after killing the animals with an intravenous injection of a KCl solution. The brains were rapidly excised and placed in a rat brain matrix previously cooled in ice. Two, 2-mm thick, coronal slices were cut through the impact lesion. Considering the diffuse nature of the insult (Foda and Marmarou, 1994), samples of these whole slices were collected in the neocortex and caudoputamen for BWC measurements. Protein analysis, and nitrite and nitrate measurements were performed on the remaining brain tissue of the whole slices. Protein analysis was undertaken on the tissue from one hemisphere, whereas the tissue from the other hemisphere was frozen in −40°C isopentane, then immediately stored at −80°C, and eventually processed for measuring nitrite and nitrate.
Brain water content in the neocortex and caudoputamen was determined using a gravimetric technique as described previously (Verdonck et al, 2007). After calibration of a layered kerosene–bromobenzene linear column with drops of anhydrous K2SO4 solutions, brain samples (n=4 per hemisphere, 20 to 40 mg each) were deposited at the surface of the liquid column and their tissue-specific gravity (SG) was measured 2 mins later. The BWC was then derived according to the equation applicable to gray matter (Fatouros and Marmarou, 1999):
For the pharmacologic inhibitor studies, U0126 (1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene) and LY294002 (2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one) were used to specifically inhibit ERK-1/-2 and Akt phosphorylation, respectively. The compounds U0126 and LY294002 (Sigma-Aldrich, Saint-Quentin Fallavier, France) were dissolved in a saline solution containing 10% (v/v) DMSO (dimethylsulfoxide). U0126 (0.5 mg/mL), LY294002 (0.5 mg/mL), or vehicle (DMSO) was stereotactically administrated (2 μL) into each lateral ventricle 10 mins before trauma. At 30 mins after H0, rats were intravenously treated with 0.5 mL of saline solution or 5,000 IU/kg of rhEPO. The BWC was assessed at H2 after TBI.
For western blot analysis, brain samples were homogenized in a 2:1 volume-to-weight ratio of lysis buffer containing Tris (50 mmol/L), NaCl (150 mmol/L), 0.5% Triton X-100 (Sigma-Aldrich), and were supplemented with phosphatase and protease inhibitor cocktails (Sigma-Aldrich). Protein concentrations of the extracts were determined using the bicinchoninic acid protein assay kit (Perbio Science, Brebières, France). After separation by SDS-PAGE, proteins were transferred into polyvinylidene difluoride membranes (GE Healthcare, Uppsala, Sweden). Membranes were then blocked for 1 h in 5% (w/v) nonfat milk in TBS (Tris-buffered saline) containing 0.05% Tween 20 (T-TBS), and then incubated overnight a 4°C with the following primary antibodies: mouse anti-Phospho-ERK-1/-2, rabbit anti-total ERK-1/-2 antibodies (dilution rate: 1:200) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti-Phospho-Akt, rabbit anti-Akt antibodies (dilution rate: 1:1,000) (Upstate/Chemicon, Charlottesville, VA, USA). After washing in T-TBS, the membranes were incubated for 1 h at room temperature with peroxidase-labeled secondary antibodies. After washing, the immunoreactive bands were visualized by enhanced chemiluminescence (GE Healthcare). Blots were then incubated in stripping buffer (62 mmol/L Tris-HCl, pH 6.8; 2% SDS; and 100 mmol/L β-mercaptoethanol) for 30 mins at 50°C and reprobed with total ERK and Akt antibodies. Separated gels were also run and probed with anti-actin antibody (Sigma Aldrich) as additional loading controls. Band intensities of phosphorylated forms were normalized to their corresponding total forms and processed using the ImageJ software (W Rasband, National Institutes of Health, Bethesda, MD, USA).
Nitrite and nitrate levels were measured with the Griess reagent as described elsewhere (Calapai et al, 2000). Briefly, the brain hemisphere was homogenized (1:10; w/v) in buffer phosphate (0.1 mol/L; pH 7.4). Aliquots (250 μL) of the supernatant were diluted with ultra-pure water (500 μL) and incubated for 45 mins at room temperature with 250 μL substrate buffer (0.1 mol/L imidazole, 210 mmol/L NADPH, 3.8 mmol/L flavine adenine dinucleotide; pH 7.6) in the presence of nitrate-reductase (Aspergillus niger, 70 IU/L) (Sigma-Aldrich) so as to convert NO3 to NO2. Total NO synthesis (NO2+NO3) was then assessed using the Griess reagent (58 mmol/L sulfanilamide and 3.8 mmol/L naphthalene-ethylene diamine dihydrochloride in 0.5 mol/L H3PO4). Reacting samples were treated with 200 μL trichloroacetic acid (1.2 mol/L) and centrifuged for 5 mins at 8,000 × g. Supernatant absorbance was then measured at 540 nm. Amounts of NO2 were estimated from a standard enzymatic conversion curve of NaNO3 into NaNO2.
Evaluation of Blood–Brain Barrier Integrity
Integrity of the BBB was assessed with contrast-enhanced MRI using MnCl2 and gadolinium tetra-azacyclo-dodecane-tetra-aceticacid (Gd-DOTA). This approach was successfully applied to detect BBB damage after transient cerebral ischemia (Grillon et al, 2008). Magnetic resonance imaging was performed at 7 T, in a horizontal bore magnet (Magnex Scientific, Oxford, UK) equipped with a 200-mT/m gradient system (12 cm inner bore diameter) and a SMIS console (SMIS, Guildford, UK). Rats were placed prone, with the head secured with ear bars. A 79-mm internal diameter quadrature volume radiofrequency coil (Rapid Biomedical GmbH, Würzburg, Germany) was used for excitation and detection. A T1-weighted axial scout image was acquired to locate slices of interest underneath the metallic disc area.
Contrast-enhanced T1 images were acquired during a 1-h intravenous infusion of manganese (MnCl2, 0.1 mol/L, 9 μmol/kg per min) (Sigma-Aldrich), starting at H1. A bolus of Gd-DOTA(0.2 mmol/kg, Dotarem; Guerbet, Aulnay-sous-Bois, France) was injected intravenously 30 mins after the completion of the MnCl2 infusion. Tissue T1 was measured during a 3-h time span after TBI (or equivalent). Serial T1 maps from the brain and temporal muscle were acquired before the MnCl2 infusion (2 maps), during the MnCl2 infusion (14 maps), before the Gd-DOTA bolus (7 maps), and after the Gd-DOTA bolus (4 maps). The temporal muscle was used as a control for contrast agent leakage. The MR sequence used for T1-mapping was an inversion recovery Fast Low Angle Shot (FLASH) sequence (repetition time=10 secs; echo time=3.2 msecs; field of view=30 × 30 mm2; matrix=64 × 64; 4 averages). A total of 22 inversion times were applied ranging from 100 to 6,000 msecs. The inversion pulse was nonselective so as to minimize in-flow signal enhancement. Acquisition of each T1 map lasted ∼4 mins.
Statistical Analysis
Data are expressed as mean±s.d. Sham-operated animals having received saline or rhEPO in the first series of experiments were pooled into a single group as no statistically significant differences were found between these groups regarding treatment (data not shown). Comparisons between the groups of rats were subjected to factorial analysis of variance with a Protected Least Significant Difference (PLSD) Fisher's test as post hoc test (intergroup analysis) (StatView SE program, SAS Institute, Cary, NC, USA). Statistical significance of changes observed during the time course of the MRI experiment was assessed using analysis of variance for repeated measurements. The MRI values were compared with the corresponding values at the reference time (H1) (intragroup analysis). Statistical significance was declared when P<0.05.
Results
Recombinant Human Erythropoietin Reduces Traumatic Brain Injury-Induced Edema Formation
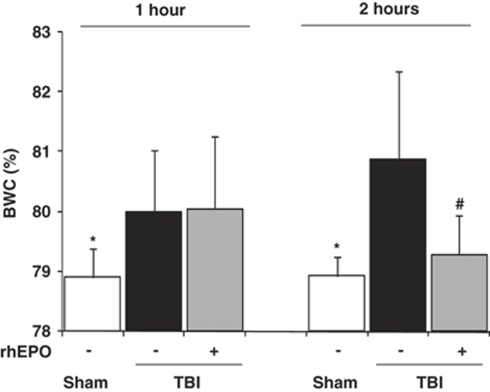
At H1, BWC in the neocortex and caudoputamen was significantly higher in rats from the two TBI groups than in sham-operated rats: 80.0±1.0% (TBI-saline) and 80.1±1.2% (TBI-rhEPO) versus 78.9±0.5% (sham-operated) (P<0.01) (first series of experiments) (Figure 1). No effect of rhEPO on BWC was found at this time point. At H2, rhEPO significantly reduced BWC in the TBI-treated group: 80.9±1.5% (TBI-saline) versus 79.3±0.7% (TBI-rhEPO) and 78.9±0.3% (sham-operated) (P<0.01). At this time point, BWC did not differ significantly between TBI-rhEPO and sham-operated groups. Physiological parameters were all within the normal ranges during experiments and were not affected by treatment with rhEPO (data not shown).
Figure 1.
Brain water content (BWC) in the neocortex 1 and 2 h after insult in three groups of rats: sham-operated (n=8), TBI with saline (n=6), TBI with rhEPO (n=6) (gravimetric experiments). Data are expressed as mean±s.d. *P<0.01 TBI-saline versus sham-operated; #P<0.01 TBI-saline versus TBI-rhEPO.
Recombinant Human Erythropoietin Modulates Extracellular-Regulated Kinase and Akt Phosphorylation
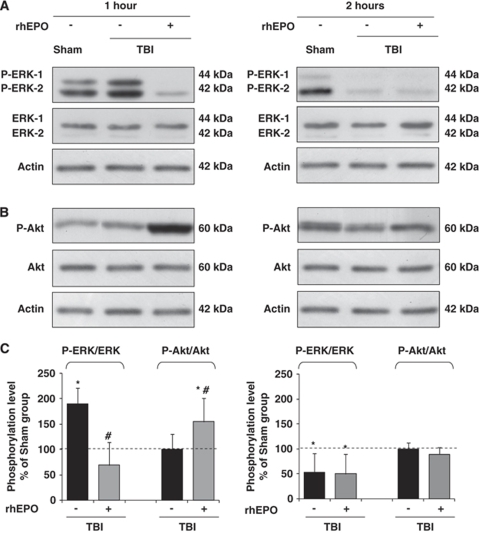
Phosphorylation levels of the protein kinases ERK-1/-2 and Akt were significantly altered according to the study period (H1 or H2) and group (Figure 2) (first series of experiments). At H1, an increase in phosphorylated ERK-1 and ERK-2 isoforms was detected in the TBI-saline group: 189±32% of sham-operated (P<0.01) (Figures 2A and 2C). By contrast, phospho-ERK was significantly reduced in the TBI-rhEPO group (69±45% of sham-operated; P<0.01 versus TBI-saline). Although the phosphorylation level of Akt was not altered at H1 in the TBI-saline group (100±30% of sham-operated), it was then significantly enhanced in the TBI-rhEPO group (154±47% of sham-operated; P<0.01 versus TBI-saline) (Figures 2B and 2C). These rhEPO-induced changes were not found at H2 for these two protein kinases (Figures 2A, 2B, and 2C), phospho-Akt being comparable in the three groups of animals at that time point. Phospho-ERK was similarly reduced in the two TBI groups: 54±37% of sham-operated value in the TBI-saline group and 50±40% in the TBI-rhEPO group (P<0.01).
Figure 2.
Western blot analysis of the phosphorylation of ERK-1/-2 (A) and Akt (B) after electrophoresis of brain extracted 1 h (left panel) and 2 h (right panel) after insult. Results are representative of three independent experiments. (C) Quantitative analysis of the p-ERK/ERK and p-Akt/Akt ratios after TBI, expressed in percentage of sham-operated phosphorylation level (dashed line). Data are expressed as mean±s.d. *P<0.01 sham-operated versus TBI-saline or TBI-rhEPO; #P<0.01 TBI-saline versus TBI-rhEPO.
Inhibition of Extracellular-Regulated Kinase-1/-2 is Required for Recombinant Human Erythropoietin-Induced Reduction of Brain Edema
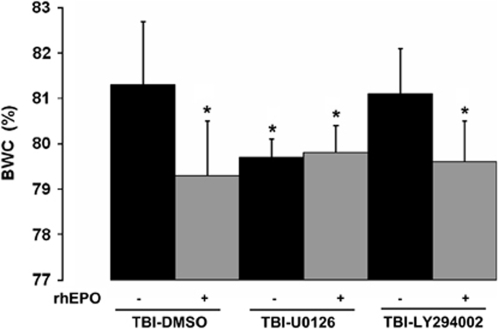
The pharmacologic inhibition of the two brain protein kinases, ERK-1/-2 and Akt, was effective in vivo because U0126 and LY294002 decreased the phosphorylation of ERK and Akt, respectively, using western blot analysis (data not shown) (second series of experiments). Inhibition of ERK-1/-2 before injury significantly reduced BWC at H2: 79.7±0.4% (TBI-U0126) versus 81.3±1.4% (TBI-vehicle) (P<0.01) (Figure 3). Decreased BWC was also found after postinjury treatment with rhEPO: 79.3±1.2% (TBI-vehicle-rhEPO). No further reduction in brain edema was found when ERK-1/-2 inhibition was combined with rhEPO treatment: 79.8±0.6% (TBI-U0126-rhEPO). Conversely, the inhibition of the Akt pathway before injury did not result in significant differences in BWC by comparison with TBI-vehicle: 81.1±1.0% (TBI-LY294002). The addition of postinjury treatment with rhEPO to Akt inhibition significantly decreased BWC: 79.6±0.9% (TBI-LY294002-rhEPO) (P<0.01 versus TBI-vehicle) (Figure 3). No difference in BWC was found between this group and the TBI-vehicle-rhEPO group.
Figure 3.
The effects of intraventricular administration of U0126 ERK-1/-2 inhibitor, LY294002 Akt inhibitor and DMSO vehicle before injury on brain water content (BWC) in the neocortex (n=5 animals per group). Rats were treated postinjury with an intravenous administration of rhEPO or saline. BWC was assessed 2 h after trauma. Data are expressed as mean±s.d. *P<0.01 versus TBI-vehicle. DMSO, dimethylsulfoxide.
Recombinant Human Erythropoietin Reduces Nitrite and Nitrate Production
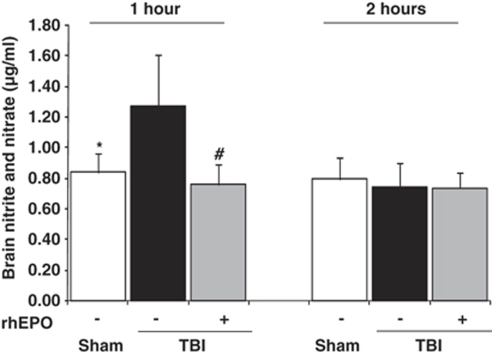
Brain nitrite and nitrate levels (index of NO synthesis) were significantly higher in TBI-saline animals than in the sham-operated group at H1: 1.28±0.33 versus 0.84±0.12 μg/mL, respectively (P<0.01) (first series of experiments) (Figure 4). However, the rhEPO-treated TBI group had significantly lower NO levels at H1 (0.75±0.13 μg/mL) than the TBI-saline group (P<0.01), whereas its NO levels were comparable with those from the sham-operated group. No significant differences between the three groups of rats were further found in NO synthesis at H2 (Figure 4).
Figure 4.
Brain nitrite and nitrate levels performed on brain tissue samples taken 1 and 2 h after insult in three groups of rats: sham-operated (n=8), TBI with saline (n=6), TBI with rhEPO (n=6). Data are expressed as mean±s.d. *P<0.01 TBI-saline versus sham-operated; #P<0.01 TBI-saline versus TBI-rhEPO.
Traumatic Brain Injury does not Induce Blood–Brain Barrier Damage
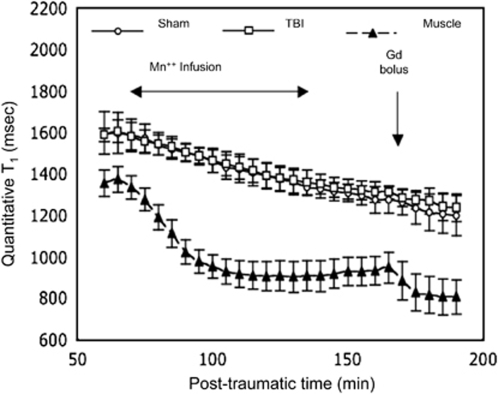
In the third series of experiments, brain T1 evolved very similarly in TBI and sham-operated groups during the sequential administration of the two contrast agents (MnCl2 and Gd-DOTA) (Figure 5). There was no significant interaction between groups of animals and time (F-test=0.44; P=0.99). In both groups of rats, the decrease in T1 became significant at the fifth acquisition, i.e., at 10 mins after the start of MnCl2 infusion and it remained so until the end of the experiment (Gd-DOTA bolus).
Figure 5.
Time course of mean T1 values (±s.d.) in the brain tissue and in temporal muscle. Manganese (MnCl2) was infused during 1 h and Gd-DOTA was injected as a bolus in TBI-saline and sham-operated groups (n=4 for each group). T1 values in brain tissue were significantly lower than baseline (P<0.05) from the fifth data point onwards.
Discussion
Using a model of diffuse TBI in rats, we found that postinjury administration of rhEPO rapidly and transiently alters the phosphorylation level of two protein kinases, ERK-1/-2 and Akt. Specifically, western blot analysis showed that rhEPO decreases phosphorylated ERK and increases phosphorylated Akt 1 h after trauma. These molecular changes are associated with a reduction in brain NO synthesis at H1 and with an attenuation of postTBI brain edema as shown by BWC measurements at H2. The pretreatment with inhibitors of these two protein kinases showed that the ERK pathway was required for the development of posttraumatic brain edema. To our knowledge, this is the first study suggesting that rhEPO-mediated reduction of brain edema after diffuse TBI in rats could involve changes in phosphorylation levels of brain protein kinases, particularly the ERK-1/-2 signaling pathway, and in brain NO synthesis at an early stage.
Changes in ERK-1/-2 and Akt were assessed 1 h after trauma and 30 mins after rhEPO administration. In fact, rhEPO can rapidly cross the BBB as shown by a significant increase in the concentration of erythropoietin in cerebrospinal fluid 30 mins after intraperitoneal administration of rhEPO (Brines et al, 2000). The exact mechanisms by which rhEPO mediates its cellular effects are still uncertain. As rhEPO can also restore the integrity of the BBB after ischemia-induced BBB disruption (Li et al, 2007b), we tested BBB integrity with serial T1 mapping study. It has been shown that, after transient cerebral ischemia in rat, BBB disruption results in a marked decrease in tissue T1 due to the extravasation of two contrast agents (Gd-DOTA, 590 Da; MnCl2, 55 Da) (Grillon et al, 2008). Using the same methodology, we found no evidence of BBB damage, a result that is in line with an earlier study using contrast-enhanced MRI in this model of diffuse TBI (Beaumont et al, 2006). Moreover, an early reduction in brain apparent diffusion coefficient was found in this model of TBI, which is ascribed to mainly cellular (cytotoxic) edema (Ito et al, 1996; Verdonck et al, 2007). These findings confirm that this model of diffuse TBI induces brain edema of cellular origin. The effect of rhEPO administration is thus likely related to mechanisms involving intracellular targets.
We examined total and phosphorylated Akt and ERK-1/-2, the latter being one of the mitogen-activated protein kinases. These protein kinases are known to be involved in neuronal death/survival pathways (Subramaniam and Unsicker, 2006) and were then proposed to have a key role in erythropoietin-mediated neuroprotection (Digicaylioglu and Lipton, 2001; Ghezzi and Brines, 2004). Although there are conflicting results regarding the neuroprotective role of ERK-1/-2 activation after focal brain ischemia (Kilic et al, 2005; Namura et al, 2001), there are consistent results about the harmful effect on phosphorylation of ERK in brain trauma models. An increased phosphorylation of ERK was found as early as 2 mins after controlled cortical impact in mouse (Mori et al, 2002a). These immediate changes in the phosphorylation of ERK were transient and returned to baseline within 1 to 3 h (Mori et al, 2002b). The use of an inhibitor of ERK phosphorylation (PD98059) significantly attenuated posttraumatic brain edema, whereas no protective effect was shown with the use of an inhibitor of other mitogen-activated protein kinases (p38 and c-jun N terminal) (Mori et al, 2002b). Similarly, ERK activation in the nucleus of damaged CA3 neurons occurred 30 mins after a focal TBI, and pretreatment with the U0126 ERK inhibitor reduced contusional lesion volume 7 days after injury (Otani et al, 2007). These findings indicate a causal relationship between activation of the ERK pathway and development of posttraumatic brain edema and cortical lesions. This study led to similar observations in describing biochemical events occurring very early after TBI that were affected by post-TBI administration of rhEPO: A transient increase in phosphorylated ERK was found 1 h after TBI and postinjury administration of rhEPO both reduced phosphorylated ERK at H1 and brain edema at H2. Evidence of the involvement of the ERK-1/-2 pathway in the development of brain edema has been subsequently provided with the use of ERK inhibitor before injury (TBI-U0126 group). The absence of further reduction in brain edema when ERK-1/-2 inhibition was combined with rhEPO (TBI-U0126-rhEPO group) strongly suggests a causal link between rhEPO and the ERK-1/-2 pathway. Treatment with rhEPO was associated with a decreased tissue expression of ERK in rats subjected to a spinal cord trauma (Huang et al, 2009). It should be noted that the use of intracerebroventricular route to deliver ERK-1/-2 and Akt inhibitors before applying the trauma has never been reported in vivo with the diffuse TBI model. The consistency of the results regarding BWC measured in the TBI-saline group (first series of experiments) and in the TBI-vehicle group (second series) suggests that this preinjury intervention did not alter the subsequent development of brain edema. Overall, these results support the fact that ERK-1/-2 could be one of the molecular targets of rhEPO, and that inhibition of ERK phosphorylation with rhEPO contributes to preventing the development of posttraumatic brain edema.
The serine–threonine kinase, or Akt, has a critical role in neuronal survival because phospho-Akt promotes cell survival and prevents apoptosis by inactivating several targets, including caspase-9 (Cardone et al, 1998). Transient changes in Akt phosphorylation in the hippocampal region were found in saline-treated mice subjected to focal TBI, with a decrease 1 h after the insult, followed by an increase 3 h later (Noshita et al, 2002). We did not find similar changes in phospho-Akt in the TBI-saline group at H1 and H2. Instead, we observed a transient increase in phospho-Akt in the TBI-rhEPO group at H1, in line with the results from other studies showing neuroprotective effects related to activation of the phosphatidylinositol-3 kinase/Akt pathway (Kilic et al, 2005; Liot et al, 2004; Valable et al, 2003). In an isolated preparation of rabbit hearts, erythropoietin induced phosphorylation of Akt during the early phase of ischemia only (Kobayashi et al, 2008). However, inhibition of the Akt pathway by LY294002 failed to aggravate the development of brain edema in this study (TBI-LY294002 group). Moreover, rhEPO was still efficient in reducing brain edema in the presence of Akt inhibition (TBI-LY294002-rhEPO group), indicating that Akt activation after treatment with rhEPO is not drug specific. A causal link between the effects of erythropoietin on posttraumatic edema and Akt activation cannot be thus established.
In addition to changes in phosphorylation of ERK and Akt, we found a reduction in brain NO synthesis 1 h after trauma in the TBI-rhEPO group. We evaluated brain tissue nitrite and nitrate production to assess the activity of the brain NO pathway (Salter et al, 1996). There is convincing evidence that NO has a role in the pathogenesis of brain trauma and in the formation of cellular brain edema (Cherian et al, 2004; Gahm et al, 2005; Nagafuji et al, 1992; Thippeswamy et al, 2006). Excess production of NO after TBI is mediated mainly through upregulation of NOS. It may result in oxidative brain damage as NO is metabolized to peroxynitrite. In animal models of brain ischemia, posttreatment with rhEPO was shown to significantly inhibit the inducible form of NOS and to reduce NO toxicity to neurons (Noguchi et al, 2001; Sakanaka et al, 1998). However, induction of the inducible form of NOS is a slow process and, according to the kinetics of NO production described in this study, a constitutive form of NOS, i.e., neuronal or endothelial, is likely to be involved in this process. Those constitutive NOSs contributed to the neuronal injury in a model of diffuse TBI (Gahm et al, 2005). In addition, the rhEPO-induced inhibition of NO production was shown to reduce the formation of brain edema induced by ischemia (Calapai et al, 2000). Our findings are in line with the results of these studies. They suggest that the reduction in brain NO synthesis could be one of the key mechanisms explaining the early effects of rhEPO on posttraumatic brain edema formation. No causal relationship between rhEPO-induced changes in protein kinases and in NO synthesis can presently be inferred from our results. However, there is evidence that NO overproduction might depend on the phosphorylation level of ERK-1/-2 (Zhang et al, 2007). The swelling in cultured astrocytes after mechanical trauma was accompanied with increased mitogen-activated protein kinase phosphorylation; it was prevented by the use of NOS inhibitors (Jayakumar et al, 2008). Overall, these findings suggest that the development of postinjury brain edema might be closely related to the activation of the ERK pathway and NO production, in a direct crosstalk between both systems. Our concomitant and transient changes in the phosphorylation level of ERK-1/-2 and in NO production at H1 and at H2 support this hypothesis. Inhibiting the ERK pathway and NO production with rhEPO would prevent the development of brain edema. As erythropoietin was recently shown to reduce the activation of water channel aquaporin 4, then to decrease the susceptibility to brain edema (Gunnarson et al, 2009), it would be of value to see whether erythropoietin regulates aquaporin 4 through the inhibition of ERK-1/-2.
In conclusion, this study reinforces previous findings that rhEPO reduces the early development of brain edema in a rat model of diffuse TBI (Verdonck et al, 2007). It provides true insight into possible cellular mechanisms underlying the versatile properties of rhEPO in experimental brain TBI. The immediate changes in phosphorylation levels of two protein kinases and in NO production induced by postinjury administration of rhEPO, in association with a significant reduction in brain edema, strongly suggest that ERK-1/-2 and NO pathways are possible mediators in the antiedematous effect of rhEPO.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Beaumont A, Fatouros P, Gennarelli T, Corwin F, Marmarou A. Bolus tracer delivery measured by MRI confirms edema without blood-brain barrier permeability in diffuse traumatic brain injury. Acta Neurochir Suppl. 2006;96:171–174. doi: 10.1007/3-211-30714-1_38. [DOI] [PubMed] [Google Scholar]
- Bernaudin M, Marti HH, Roussel S, Divoux D, Nouvelot A, MacKenzie ET, Petit E. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calapai G, Marciano MC, Corica F, Allegra A, Parisi A, Frisina N, Caputi AP, Buemi M. Erythropoietin protects against brain ischemic injury by inhibition of nitric oxide formation. Eur J Pharmacol. 2000;401:349–356. doi: 10.1016/s0014-2999(00)00466-0. [DOI] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Cherian L, Goodman JC, Robertson C. Neuroprotection with erythropoietin administration following controlled cortical impact injury in rats. J Pharmacol Exp Ther. 2007;322:789–794. doi: 10.1124/jpet.107.119628. [DOI] [PubMed] [Google Scholar]
- Cherian L, Hlatky R, Robertson CS. Nitric oxide in traumatic brain injury. Brain Pathol. 2004;14:195–201. doi: 10.1111/j.1750-3639.2004.tb00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- Fatouros PP, Marmarou A. Use of magnetic resonance imaging for in vivo measurements of water content in human brain: method and normal values. J Neurosurg. 1999;90:109–115. doi: 10.3171/jns.1999.90.1.0109. [DOI] [PubMed] [Google Scholar]
- Foda MA, Marmarou A. A new model of diffuse brain injury in rats. Part II: morphological characterization. J Neurosurg. 1994;80:301–313. doi: 10.3171/jns.1994.80.2.0301. [DOI] [PubMed] [Google Scholar]
- Gahm C, Danilov A, Holmin S, Wiklund PN, Brundin L, Mathiesen T. Reduced neuronal injury after treatment with NG-nitro-L-arginine methyl ester (L-NAME) or 2-sulfo-phenyl-N-tert-butyl nitrone (S-PBN) following experimental brain contusion. Neurosurgery. 2005;57:1272–1281. doi: 10.1227/01.neu.0000187317.32529.06. [DOI] [PubMed] [Google Scholar]
- Ghezzi P, Brines M. Erythropoietin as an antiapoptotic, tissue-protective cytokine. Cell Death Differ. 2004;11 (Suppl 1:S37–S44. doi: 10.1038/sj.cdd.4401450. [DOI] [PubMed] [Google Scholar]
- Grillon E, Provent P, Montigon O, Segebarth C, Remy C, Barbier EL. Blood-brain barrier permeability to manganese and to Gd-DOTA in a rat model of transient cerebral ischaemia. NMR Biomed. 2008;21:427–436. doi: 10.1002/nbm.1206. [DOI] [PubMed] [Google Scholar]
- Gunnarson E, Song Y, Kowalewski JM, Brismar H, Brines M, Cerami A, Andersson U, Zelenina M, Aperia A. Erythropoietin modulation of astrocyte water permeability as a component of neuroprotection. Proc Natl Acad Sci USA. 2009;106:1602–1607. doi: 10.1073/pnas.0812708106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Fan S, Ji X, Zhang Y, Bao F, Zhang G. Recombinant human erythropoietin protects against experimental spinal cord trauma injury by regulating expression of the proteins MKP-1 and p-ERK. J Int Med Res. 2009;37:511–519. doi: 10.1177/147323000903700227. [DOI] [PubMed] [Google Scholar]
- Ito J, Marmarou A, Barzo P, Fatouros P, Corwin F. Characterization of edema by diffusion-weighted imaging in experimental traumatic brain injury. J Neurosurg. 1996;84:97–103. doi: 10.3171/jns.1996.84.1.0097. [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, Rao KV, Panickar KS, Moriyama M, Reddy PV, Norenberg MD. Trauma-induced cell swelling in cultured astrocytes. J Neuropathol Exp Neurol. 2008;67:417–427. doi: 10.1097/NEN.0b013e31816fc9d4. [DOI] [PubMed] [Google Scholar]
- Kilic E, Kilic U, Soliz J, Bassetti CL, Gassmann M, Hermann DM. Brain-derived erythropoietin protects from focal cerebral ischemia by dual activation of ERK-1/-2 and Akt pathways. FASEB J. 2005;19:2026–2028. doi: 10.1096/fj.05-3941fje. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Miura T, Ishida H, Miki T, Tanno M, Yano T, Sato T, Hotta H, Shimamoto K. Limitation of infarct size by erythropoietin is associated with translocation of Akt to the mitochondria after reperfusion. Clin Exp Pharmacol Physiol. 2008;35:812–819. doi: 10.1111/j.1440-1681.2008.04925.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Lu Z, Keogh CL, Yu SP, Wei L. Erythropoietin-induced neurovascular protection, angiogenesis, and cerebral blood flow restoration after focal ischemia in mice. J Cereb Blood Flow Metab. 2007a;27:1043–1054. doi: 10.1038/sj.jcbfm.9600417. [DOI] [PubMed] [Google Scholar]
- Li Y, Lu ZY, Ogle M, Wei L. Erythropoietin prevents blood brain barrier damage induced by focal cerebral ischemia in mice. Neurochem Res. 2007b;32:2132–2141. doi: 10.1007/s11064-007-9387-9. [DOI] [PubMed] [Google Scholar]
- Liot G, Gabriel C, Cacquevel M, Ali C, MacKenzie ET, Buisson A, Vivien D. Neurotrophin-3-induced PI-3 kinase/Akt signaling rescues cortical neurons from apoptosis. Exp Neurol. 2004;187:38–46. doi: 10.1016/j.expneurol.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Lu D, Mahmood A, Qu C, Goussev A, Schallert T, Chopp M. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J Neurotrauma. 2005;22:1011–1017. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: pathophysiology and biomechanics. J Neurosurg. 1994;80:291–300. doi: 10.3171/jns.1994.80.2.0291. [DOI] [PubMed] [Google Scholar]
- Mori T, Wang X, Aoki T, Lo EH. Downregulation of matrix metalloproteinase-9 and attenuation of edema via inhibition of ERK mitogen activated protein kinase in traumatic brain injury. J Neurotrauma. 2002a;19:1411–1419. doi: 10.1089/089771502320914642. [DOI] [PubMed] [Google Scholar]
- Mori T, Wang X, Jung JC, Sumii T, Singhal AB, Fini ME, Dixon CE, Alessandrini A, Lo EH. Mitogen-activated protein kinase inhibition in traumatic brain injury: in vitro and in vivo effects. J Cereb Blood Flow Metab. 2002b;22:444–452. doi: 10.1097/00004647-200204000-00008. [DOI] [PubMed] [Google Scholar]
- Nagafuji T, Matsui T, Koide T, Asano T. Blockade of nitric oxide formation by N omega-nitro-L-arginine mitigates ischemic brain edema and subsequent cerebral infarction in rats. Neurosci Lett. 1992;147:159–162. doi: 10.1016/0304-3940(92)90584-t. [DOI] [PubMed] [Google Scholar]
- Namura S, Iihara K, Takami S, Nagata I, Kikuchi H, Matsushita K, Moskowitz MA, Bonventre JV, Alessandrini A. Intravenous administration of MEK inhibitor U0126 affords brain protection against forebrain ischemia and focal cerebral ischemia. Proc Natl Acad Sci USA. 2001;98:11569–11574. doi: 10.1073/pnas.181213498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, Yamashiro S, Matsuzaki T, Sakanashi M, Nakasone J, Miyagi K. Effect of 1-week treatment with erythropoietin on the vascular endothelial function in anaesthetized rabbits. Br J Pharmacol. 2001;133:395–405. doi: 10.1038/sj.bjp.0704083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noshita N, Lewen A, Sugawara T, Chan PH. Akt phosphorylation and neuronal survival after traumatic brain injury in mice. Neurobiol Dis. 2002;9:294–304. doi: 10.1006/nbdi.2002.0482. [DOI] [PubMed] [Google Scholar]
- Otani N, Nawashiro H, Fukui S, Ooigawa H, Ohsumi A, Toyooka T, Shima K. Role of the activated extracellular signal-regulated kinase pathway on histological and behavioral outcome after traumatic brain injury in rats. J Clin Neurosci. 2007;14:42–48. doi: 10.1016/j.jocn.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Pacary E, Petit E, Bernaudin M. Erythropoietin, a cytoprotective and regenerative cytokine, and the hypoxic brain. Neurodegener Dis. 2006;3:87–93. doi: 10.1159/000092098. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci USA. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter M, Duffy C, Garthwaite J, Strijbos PJ. Ex vivo measurement of brain tissue nitrite and nitrate accurately reflects nitric oxide synthase activity in vivo. J Neurochem. 1996;66:1683–1690. doi: 10.1046/j.1471-4159.1996.66041683.x. [DOI] [PubMed] [Google Scholar]
- Shein NA, Tsenter J, Alexandrovich AG, Horowitz M, Shohami E. Akt phosphorylation is required for heat acclimation-induced neuroprotection. J Neurochem. 2007;103:1523–1529. doi: 10.1111/j.1471-4159.2007.04862.x. [DOI] [PubMed] [Google Scholar]
- Siren AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, Cerami A, Ehrenreich H, Ghezzi P. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S, Unsicker K. Extracellular signal-regulated kinase as an inducer of non-apoptotic neuronal death. Neuroscience. 2006;138:1055–1065. doi: 10.1016/j.neuroscience.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Thippeswamy T, McKay JS, Quinn JP, Morris R. Nitric oxide, a biological double-faced janus–is this good or bad. Histol Histopathol. 2006;21:445–458. doi: 10.14670/HH-21.445. [DOI] [PubMed] [Google Scholar]
- Valable S, Bellail A, Lesne S, Liot G, Mackenzie ET, Vivien D, Bernaudin M, Petit E. Angiopoietin-1-induced PI3-kinase activation prevents neuronal apoptosis. FASEB J. 2003;17:443–445. doi: 10.1096/fj.02-0372fje. [DOI] [PubMed] [Google Scholar]
- Verdonck O, Lahrech H, Francony G, Carle O, Farion R, Van de Looij Y, Remy C, Segebarth C, Payen JF. Erythropoietin protects from post-traumatic edema in the rat brain. J Cereb Blood Flow Metab. 2007;27:1369–1376. doi: 10.1038/sj.jcbfm.9600443. [DOI] [PubMed] [Google Scholar]
- Yatsiv I, Grigoriadis N, Simeonidou C, Stahel PF, Schmidt OI, Alexandrovich AG, Tsenter J, Shohami E. Erythropoietin is neuroprotective, improves functional recovery, and reduces neuronal apoptosis and inflammation in a rodent model of experimental closed head injury. FASEB J. 2005;19:1701–1703. doi: 10.1096/fj.05-3907fje. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brovkovych V, Brovkovych S, Tan F, Lee BS, Sharma T, Skidgel RA. Dynamic receptor-dependent activation of inducible nitric-oxide synthase by ERK-mediated phosphorylation of Ser745. J Biol Chem. 2007;282:32453–32461. doi: 10.1074/jbc.M706242200. [DOI] [PubMed] [Google Scholar]