Abstract
Neurovascular coupling provides the basis for many functional neuroimaging techniques. Nitric oxide (NO), adenosine, cyclooxygenase, CYP450 epoxygenase, and potassium are involved in dilating arterioles during neuronal activation. We combined inhibition of NO synthase, cyclooxygenase, adenosine receptors, CYP450 epoxygenase, and inward rectifier potassium (Kir) channels to test whether these pathways could explain the blood flow response to neuronal activation. Cerebral blood flow (CBF) and cerebral metabolic rate of oxygen (CMRO2) of the somatosensory cortex were measured during forepaw stimulation in 24 rats using a laser Doppler/spectroscopy probe through a cranial window. Combined inhibition reduced CBF responses by two-thirds, somatosensory evoked potentials and activation-induced CMRO2 increases remained unchanged, and deoxy-hemoglobin (deoxy-Hb) response was abrogated. This shows that in the rat somatosensory cortex, one-third of the physiological blood flow increase is sufficient to prevent microcirculatory increase of deoxy-Hb concentration during neuronal activity. The large physiological CBF response is not necessary to support small changes in CMRO2. We speculate that the CBF response safeguards substrate delivery during functional activation with a considerable ‘safety factor'. Reduction of the CBF response in pathological states may abolish the BOLD–fMRI signal, without affecting underlying neuronal activity.
Keywords: cerebral metabolic rate of oxygen, neurovascular coupling, somatosensory cortex
Introduction
During increases of neuronal activity, the brain increases local cerebral blood flow (CBF) within the area of activation (Roy and Sherrington, 1890). Since CBF increases are greater than activity-associated increases in oxygen metabolism, the result is a localized hyperoxygenation with a decrease in the deoxy-hemoglobin (deoxy-Hb) concentration (Fox and Raichle, 1986). These changes provide the basis for functional neuroimaging using blood oxygenation level-dependent functional magnetic resonance imaging (BOLD–fMRI; Ogawa et al, 1990). Neither the exact mechanisms nor the evolutionary advantage of such a CBF response are yet known. A straightforward interpretation holds that the stimulation-induced increase in blood flow serves to deliver oxygen to areas of increased oxidative metabolism. However, a large increase in CBF contrasts with a small increase in the cerebral metabolic rate of oxygen (CMRO2). There are three alternative explanations for this mismatch: first, a large increase in CBF could be necessary to support small increases in CMRO2 because of limitations of oxygen diffusion under physiological conditions (Gjedde, 2002; Buxton and Frank, 1997). In this case, inhibition of the blood flow response would result in oxygen supply insufficient for sustaining the CMRO2 increase observed under normal conditions. This relative hypoxia may impair neuronal activity. Second, the large CBF response may represent a safety mechanism as observed in many other physiological or biochemical systems providing protection against relative hypoxia and impairment of neuronal activity in pathological conditions of decreased CBF response. In this case, inhibition of the CBF response down to a certain critical level would not have any effect on neuronal function or CMRO2. Third, contrary to the classical view, the CBF responses to neuronal activity could serve a purpose other than oxygen delivery (e.g., temperature regulation, modification of neuronal activity). To test which of these alternatives best explains the CBF/CMRO2 response mismatch, we measured neuronal activity, CBF, hemoglobin oxygenation, and CMRO2 before and after inhibition of the blood flow response to neuronal activation in a rat model.
To date, complete pharmacological inhibition of the CBF response with intact neuronal activity has not been shown. Our strategy for maximal inhibition of the CBF response was a combination of known inhibitors of neurovascular coupling.
According to numerous studies in the literature, nitric oxide (NO), adenosine, cyclooxygenase (COX) products, cytochrome P450 (CYP450) products such as epoxyeicosatrienoic acids, and potassium play key roles in mediating neurovascular coupling. Adenosine, whose level increases in the areas of activation because of ATP breakdown, has been shown to participate in the CBF response as inhibition of adenosine receptors reduces this response by approximately 40% (Dirnagl et al, 1994). NO does not act as a mediator, but appears to be an important modulator (permissive factor), in neurovascular coupling. Inhibition of NO synthase (NOS) reduces the CBF responses by 50% to 60% (Lindauer et al, 1999; Peng et al, 2004). Larger CBF response reductions during NOS inhibitions have also been reported (Burke and Bührle, 2006; Stefanovic et al, 2007). However, in these studies neuronal activity was also reduced (which was attributed to NOS inhibition) and time control experiments are lacking. COX products have been shown to be involved in neurovascular coupling. While some groups claimed predominant or exclusive involvement of COX-2 (Niwa et al, 2000) without a significant contribution of COX-1 (Niwa et al, 2001; Bakalova et al, 2002; Stefanovic et al, 2006), others have suggested that COX-1 is also involved (Takano et al, 2006). The role of COX products may be modulatory, as CBF responses after COX-2 inhibition were partly recovered after PGE-2 superfusion, which is the main vasodilatory product of COX-2 (Stefanovic et al, 2006). More recently, involvement of astrocytic CYP450 epoxygenase in neurovascular coupling has been shown (Peng et al, 2002, 2004, Shi et al, 2008).
Potassium channels have been thought to play a key role in neurovascular coupling since the hypothesis of potassium siphoning was introduced (Paulson and Newman, 1987). Metea et al (2007) have refuted this mechanism. Their results, however, were obtained for the retina, and may not extend to the cerebral cortex. Inward rectifier potassium channels on vascular smooth-muscle cells have been shown to play a key role in potassium-dependent vasodilation in isolated rat cerebral arteries (Knot et al, 1996) and in neurovascular coupling in rat brain slice preparations (Filosa et al, 2006).
In view of the accumulating experimental evidence suggesting involvement of several molecules and enzymes in the process of neurovascular coupling, it seems reasonable to assume that different pathways, acting at least partly in parallel, link neuronal activity to CBF. Dirnagl et al (1994) have tested a combined inhibition of adenosine receptors and neuronal NOS (nNOS). No simple additive role of these interventions was found, but the CBF response tended to further decrease with combined inhibition. In contrast, Shi et al (2008) found no further reduction of CBF responses with combined inhibition of A2B receptors and nNOS as compared with individual effects. The same group found no additive effect on CBF responses for the combined inhibition of A2B receptors and metabotropic glutamate receptors (mGluR), or for the combined inhibition of A2B receptors, mGluR, and epoxyeicosatrienoic acids (Shi et al, 2008). Peng et al (2004) did not find an additive role for inhibition of epoxyeicosatrienoic acid synthesis and nNOS. So far, no study has been performed using combined inhibition of all the above-mentioned inhibitors of neurovascular coupling.
In our study, we tested the hypotheses that (1) combined inhibition of COX, nNOS, epoxyeicosatrienoic acid synthesis, and adenosine receptors abolishes the CBF response to neurovascular coupling; and, if the response cannot be fully blocked, whether (2) additional inhibition of inward rectifier potassium channels leads to further reduction or abolishment of the CBF response; (3) whether reduction of the CBF response can compromise neuronal activity or CMRO2, or unmask deoxygenation in the microcirculation, and if so, at what level of CBF response reduction this deoxygenation occurs. This is, with some caveats, equivalent to the determination of the safety factor of neurovascular coupling with respect to oxygen delivery to active brain regions.
Materials and methods
Animals
We performed all animal experiments in strict accordance with national and international guidelines. All animal experiments described herein were approved by the local official committee (Landesamt für Gesundheit und Soziales, Berlin, Germany). Male Wistar rats (Charles River Laboratories, Sulzfeld, Germany) weighing 270 to 400 g were used for all experiments.
Surgery
Animals were anesthetized with isoflurane (initially 4% in 100% O2, continued with 2% in 70% N2O and 30% O2) and mechanically ventilated. Endexspiratory pCO2 and rectal temperature were continuously monitored and maintained within physiological limits. The femoral artery was cannulated to monitor mean arterial blood pressure and obtain arterial blood gases. The femoral vein was cannulated for infusion of anesthetics and fluid substitution.
A superfused cranial window (dura mater removed) was implanted over the right somatosensory cortex as described previously (Lindauer et al, 1999). The center of the cranial window was placed over the representational area of the left forepaw. Intracranial pressure was maintained within physiological limits by adjusting the outflow catheter. Electrocorticogram (ECoG) was recorded as described below. CBF and hemoglobin oxygenation were measured using a combined laser Doppler/reflectance spectroscopy probe. After termination of surgery, anesthesia was switched to infusion of α-chloralose and urethane (40/200 mg/kg as a bolus followed by 40/200 mg/kg/h). Superfusion of artificial cerebrospinal fluid (aCSF) was started a few minutes after implantation of the cranial window and continued at a rate of 1 mL/h. Superfusion rate was increased to 5 mL/h for 20 mins at the beginning of inhibitor superfusion, and then switched back to 1 mL/h.
Experimental Protocol
Stimulation electrodes were inserted into the left forepaw and stimulation was performed at intensities of 0.4, 0.8, 1.2, 1.6, and 2.0 mA with a frequency of 3 Hz for 16 secs. Six stimulations were performed for each intensity, ensuring minimal interstimulus interval of 75 secs. The same stimulation paradigm was performed 60 mins after the start of inhibitor superfusion. This interval has previously been shown to be sufficient to obtain maximum effect of the substances used in our study (Peng et al, 2002; Lindauer et al, 1999).
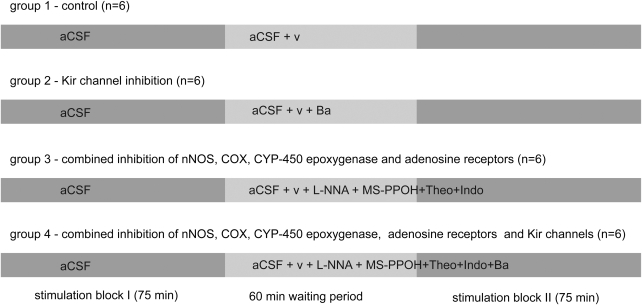
Experiments were performed on four groups (a total of 24 animals, six per group), schematically depicted in Figure 1. (1) In the control group (n=6) aCSF was superfused during the first block of stimulations followed by superfusion of aCSF plus vehicle (aCSF containing 0.5% ethanol in the second block for all groups), which was started immediately after termination of the first stimulation block. (2) In the Kir-channel inhibition group, aCSF containing 500 μmol/L barium chloride (BaCl2) was superfused during the second stimulation block. (3) In the third group, combined inhibition of adenosine receptors, nNOS, COX, and CYP450–epoxygenase was achieved by superfusion of aCSF containing L-NNA (1 mmol/L, nonselective NOS inhibition), indomethacin (500 μmol/L, nonselective COX inhibition), MS-PPOH (20 μmol/L, CYP450-epoxygenase inhibition), and theophylline (50 μmol/L, nonselective adenosine-receptor inhibition). (4) In the fourth group, additional inhibition of inward rectifier potassium channels was achieved by adding BaCl2 (500 μmol/L) to the inhibitory cocktail of the third group. The aCSF concentrations of the inhibitors were obtained from the literature (L-NNA, Lindauer et al, 1999; MS-PPOH, Peng et al, 2002; indomethacin, Takano et al, 2006; theophylline, Dirnagl et al, 1994). BaCl2 was used at a concentration (500 μmol/L) somewhat higher than that published for brain slice preparations (100 μmol/L; Filosa et al, 2006) or isolated artery experiments (10 to 300 μmol/L; Wellman and Bevan, 1995) to compensate for concentration gradients in deeper layers of the cortex that are likely present in in vivo experiments. As the concentration used in our study was rather high, effects on other potassium channels (e.g., ATP-sensitive potassium channels) or nonspecific effects cannot be excluded.
Figure 1.
The experimental paradigm. A stimulation block with randomly distributed stimulations at 0.4 to 2.0 mA was followed by a 60-min waiting period (wash in of vehicle/inhibitors), followed by a second stimulation block analogous to the first one. Each experimental group consisted of six animals. The first group served as the time control series. After the first block, superfusion was switched to aCSF+vehicle (v, ethanol 0.5%). In a second, Kir channel inhibition group, superfusion was switched to aCSF plus vehicle containing 500 μmol/L BaCl2. In the third group, combined inhibition of nNOS, COX, CYP450 epoxygenase, and adenosine receptors was achieved with superfusion of aCSF containing vehicle, L-NNA (1 mmol/L), indomethacin (500 μmol/L), MS-PPOH (20 μmol/L), and theophylline (50 μmol/L). In the fourth group, BaCl2 (500 μmol/L) was added on top of the inhibitory cocktail of group 3.
Combined Hemoglobin Oxygenation and Blood Flow Measurement
Combined measurement of regional hemoglobin oxygenation and regional CBF was performed as described previously (Royl et al, 2008). In brief, relative CBF changes were measured by Laser Doppler Flowmetry at 780 nm, with a distance of the optical fibers of 350 μm. CBF was expressed as relative changes from the baseline. It has been shown that relative CBF changes can be reliably measured by Laser Doppler Flowmetry, independently from baseline CBF (Fabricius and Lauritzen, 1996). In an x-shaped arrangement, two optical fibers for CBF measurement were combined with optical fibers for measurement of hemoglobin oxygenation (fiber distance 250 μm), resulting in a largely overlapping sample volume for flow and oxygenation measurements. Concentrations of deoxy-Hb and oxy-Hb, as well as cerebral blood volume (CBV, from oxy-Hb+deoxy-Hb), were determined from measured reflectance spectra in the wavelength range of 520 to 610 nm using an algorithm described previously (Kohl-Bareis et al, 2005; Royl et al, 2008). Average baseline oxygen saturation calculated by the algorithm was 43%±11% (mean±s.d.) in the visually located microcirculatory areas of the cortex. Similar values have been reported in the cortical capillaries of anesthetized rats using spectroscopic methods (Meyer et al, 2000). However, significantly higher values have been estimated from pO2 measurements (Vovenko, 1999). As our arterial pO2 and blood pressure were within the physiological range, hypoxia could be excluded in our animals.
The relative change of the regional CMRO2 was calculated from measurements of the relative changes (all data divided by the respective prestimulus baseline values) of CBF, deoxy-Hb, and CBV (Mayhew et al, 2000) using equation (1) as described by Royl et al, 2008 (following Dunn et al, 2005):
EEG and SEP
A continuous, epicortical electroencephalogram (EEG) was recorded using a silver ball electrode placed onto the cortical surface in close proximity to the representational area of the left forepaw. The reference electrode was inserted subcutaneously into the neck region. Intracranial pressure was controlled by adjusting the outflow catheter preventing brain herniation and movements of the recording electrode relative to the cortex. Somatosensory evoked potentials (SEPs) were recorded for the treatment and control groups to exclude potential confounding effects of the experimental setup and recording technique on SEP stability. Data were digitized at a rate of 5 kHz. A dynamic 50-Hz filter was used during data acquisition to suppress noise.
Data Analysis
Somatosensory evoked potentials were extracted from the epicortically recorded EEG and averaged for each stimulation intensity within each stimulation block. The relative change in the amplitude of the SEP (N1P1, first positive minus first negative peak) was calculated as a quantitative estimate of the neuronal activity induced by electrical forepaw stimulation. The relative change of CMRO2 during induced neuronal activity was assessed using the combined measurements of CBF and hemoglobin oxygenation using equation (1). As the vascular response occurs with a time lag of a few seconds, the amplitudes of all parameters (CBF, deoxy-Hb, CBV, CMRO2) were calculated from the average values 8 to 16 secs after stimulation onset divided by the baseline values immediately before stimulation.
The time courses of relative changes of oxy- and deoxy-Hb levels during stimulation were averaged for each stimulation intensity over all animals of the respective experimental group. To achieve maximal power in the detection of early deoxygenation (deoxy-Hb-increase, ‘initial dip'), averaging was also performed for the 12 animals of the inhibitory cocktail and inhibitory cocktail plus BaCl2 group together.
Statistical Analysis
Data were analyzed using custom-written software based on MATLAB (The Mathworks Inc., Natick, MA, USA). Statistical analysis was performed on CBF, CMRO2 responses, and SEPs exclusively for 2.0-mA stimulation intensity to avoid reduction of power because of multiple testing. CBF-response changes were calculated as percent changes of the stimulus related relative CBF increases during aCSF superfusion compared with inhibitor or vehicle superfusion (e.g., a CBF increase of 50% during aCSF superfusion, reduced to a 20% increase during inhibitor superfusion, corresponds to a 60% CBF response reduction). Changes in CMRO2 response were calculated as absolute changes of stimulus-induced relative CMRO2 increases, because stimulus-induced relative increases were close to zero for some animals. SEP changes were calculated as percent changes of the N1P1 amplitude of SEPs during aCSF superfusion compared with superfusion of inhibitors or vehicle. CBF, CMRO2, and SEP response changes were then compared for the four experimental groups (control, cocktail, cocktail+BaCl2, BaCl2, n=6 for each group) using Kruskal–Wallis one-way analysis of variance on ranks. If a significant difference was detected, Dunn's method was used as post hoc test. P<0.05/3 was considered significant, because three separate tests were performed for CBF, CMRO2, and SEP.
Results
Physiological Parameters
The physiological parameters are summarized in Table 1. Mean arterial blood pressure, arterial pO2, pCO2, and pH were maintained within physiological limits for all animals. Body temperature was kept constant at 37°C using a homeothermic heating blanket. Between the experimental groups, no significant difference was found for any physiological parameters. Concentrations of CO2 and pH were adjusted to physiological values in the aCSF (Supplementary Table S1).
Table 1. Physiological parameters.
| Experimental group | pH | paCO2 [mm Hg] | paO2 [mm Hg] | MABP [mm Hg] |
|---|---|---|---|---|
| Control block I | 7.41±0.02 | 34±2 | 124±8 | 116±11 |
| Control block II | 7.39±0.04 | 32±1 | 124±11 | 117±11 |
| Cocktail block I | 7.43±0.02 | 35±3 | 124±14 | 114±17 |
| Cocktail block II | 7.40±0.02 | 36±4 | 124±18 | 106±8 |
| Cocktail+Ba block I | 7.42±0.03 | 33±1 | 136±8 | 113±8 |
| Cocktail+Ba block II | 7.37±0.05 | 35±4 | 136±11 | 122±8 |
| Barium block I | 7.39±0.05 | 36±4 | 124±25 | 119±13 |
| Barium block II | 7.38±0.03 | 35±5 | 140±12 | 107±11 |
Abbreviation: MABP, mean arterial blood pressure.
Physiological parameters averaged across animals (mean±s.d.).
Resting Cerebral Blood Flow
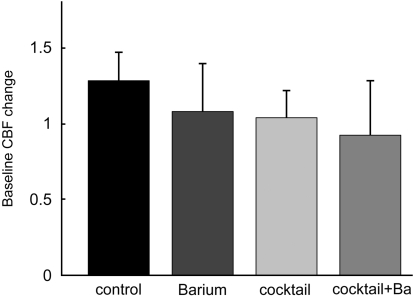
Resting CBF changes during superfusion of inhibitors were calculated as the ratio of mean CBF during the first block (superfusion of aCSF) to mean CBF during the second block (superfusion of vehicle and/or inhibitors). These changes are shown in Figure 2. A mild increase of resting CBF of 28%±19% (mean±s.d.) was detected for the control group. CBF remained largely stable in the three inhibition groups (+8%±32% for the BaCl2 group, +4%±18% for the cocktail group, and −8%±36% for the cocktail+BaCl2 group). In comparison with the control group (relative CBF change in the respective inhibitor group divided by relative CBF change in the control group) resting CBF was reduced by 16% after superfusion of BaCl2; by 19% after combined superfusion of MS-PPOH, L-NNA, indomethacin and theophylline; and by 28% after superfusion of this inhibitory cocktail plus BaCl2. Resting CBF during superfusion of cocktails showed rhythmic baseline oscillations, which are most likely attributable to superfusion of L-NNA as shown before (Lindauer et al, 1999).
Figure 2.
Baseline CBF changes. The relative changes in baseline CBF were calculated as the ratio of the mean of all CBF data of the first block (superfusion of aCSF in all groups) to the mean of all CBF data of the second block (superfusion of aCSF+vehicle in the control group, superfusion aCSF+inhibitors for the other groups). Baseline CBF increased by 28%±19% in the control group. In comparison with the control group, baseline CBF was reduced by approximately 20% in all other groups, with a nonsignificant trend towards stronger reduction in the cocktail plus BaCl2 group. Error bars denote 95% confidence intervals.
SEP Amplitudes
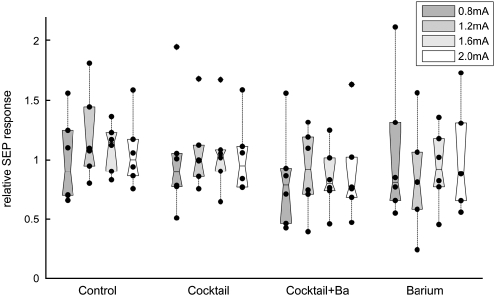
SEP amplitudes remained stable throughout the 16-s stimulation period (Supplementary Figure S1), without evidence of adaptation. To determine whether superfusion of inhibitory substances affected evoked neuronal activity, SEP amplitudes of the second stimulation block (after superfusion of vehicle/inhibitors) were divided by SEP amplitudes of the first block (baseline condition). The results are shown in Figure 3. SEP remained stable in the control group and in the inhibitory cocktail group without BaCl2. A trend towards reduction of the SEP amplitudes could be noted in the BaCl2 and inhibitory cocktail+BaCl2 group, but this was not statistically significant.
Figure 3.
SEP responses. The SEP amplitudes were calculated as amplitude between the first negative and positive peak (N1P1). Average amplitudes of the first stimulation block were divided by average amplitudes of the second block (after superfusion of vehicle/inhibitors) for each stimulation intensity. The figure shows the relative changes in SEP amplitude after superfusion of vehicle/inhibitors; black dots denote individual animals and boxes contain the second and third quartiles. There is no significant change in the SEP amplitudes, although a trend towards small reduction can be seen with the cocktail+BaCl2 and BaCl2 groups.
Time Courses of Cerebral Blood Flow, Cerebral Blood Volume, Cerebral Metabolic Rate of O2, Deoxy-Hemoglobin, and SEP
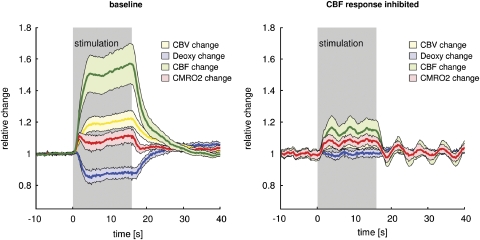
Figure 4 (left panel) shows the mean time courses of CBF, CMRO2, deoxy-Hb, and CBV during 2.0-mA forepaw stimulation averaged across the first stimulation block (superfusion of aCSF) of all 24 animals. The CBF response started within the first second, reaching a plateau at approximately 4 secs after the onset of stimulation, with a small further increase over the remaining stimulation period. The magnitude of the CBF response was within the expected range in previous experiments using this stimulation paradigm (Royl et al, 2006). There was no significant difference of the baseline CBF-response magnitude (block I) between the four experimental groups. At 2.0-mA stimulation intensity, CBV followed a similar temporal pattern, reaching a peak amplitude of approximately 20%. Using Grubbs relationship (Grubb et al, 1974), a steady-state CBF change (plateau of the CBF response) of 55% predicts a CBV change of 21%, which fits our measured CBV change. Deoxy-Hb level decreased by 15% during stimulation. CMRO2, calculated from CBF, CBV, and deoxy-Hb changes using equation (1), increased by about 10% (stimulation intensity 2.0 mA). The inhibitory cocktail reduced CBF responses by two-thirds, leaving CMRO2 unchanged (Figure 4, right panel). No change in deoxy-Hb level could be detected under these conditions (‘BOLD-blind' coupling).
Figure 4.
Major CBF-response reduction without CMRO2 impairment. Time courses of CBF, CBV, CMRO2, and deoxy-Hb during baseline conditions with superfusion of aCSF (no additional vehicle or inhibitor, n=24) and superfusion of inhibitory cocktails (CBF response inhibition, n=12) for a stimulation intensity of 2.0 mA. Shaded areas denote 95% confidence intervals. At baseline, CBF increases by ∼50%, CBV by ∼20% (in conformity with Grubbs relationship), CMRO2 by ∼10%, and deoxy-Hb level decreases by ∼15%. The inhibitory cocktail reduces CBF responses by two-thirds, leaving CMRO2 unchanged. No change in deoxy-Hb level can be detected under these conditions (‘BOLD-blind' coupling). Shaded areas denote 95% confidence intervals.
Cerebral Metabolic Rate of O2 Responses
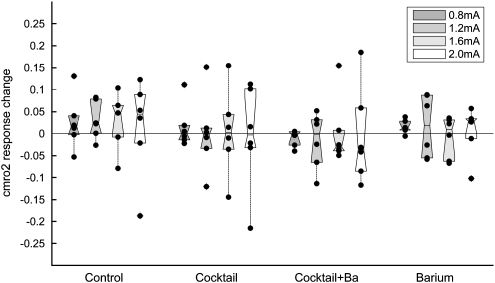
Changes in CMRO2 were calculated from combined measurements of CBF, CBV, and deoxy-Hb as described previously (Royl et al, 2008). Under control conditions (aCSF superfusion), a statistically significant increase in CMRO2 was noted for higher stimulation intensities. The CMRO2 increase, averaged across the baseline blocks of all 24 animals at a stimulation intensity of 2.0 mA, was approximately 10% as opposed to a 50% increase of CBF. As CMRO2 changes were small and close to zero for some animals, absolute rather than relative changes were evaluated (Figure 5). Superfusion of inhibitors did not significantly change activity-induced CMRO2 responses (P>0.05 for all groups).
Figure 5.
CMRO2 responses unaltered. Mean CMRO2 change before minus the mean CMRO2 change after superfusion of vehicle/inhibitors (ΔCMRO2 after−ΔCMRO2 before). Subtraction rather than division was used because CMRO2 changes were small and close to zero for some animals. Black dots denote individual animals and boxes contain the second and third quartiles. Superfusion of inhibitors did not lead to a significant change in CMRO2, despite a relevant reduction in CBF (see Figure 6).
Effects of Inhibitors on Cerebral Blood Flow Responses
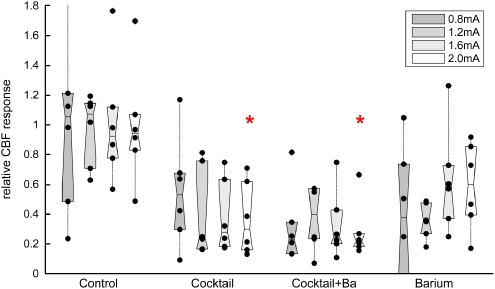
In the control group, SEP and CBF responses remained stable throughout the experiment (approximately four and a half hours after surgery and switching to α-chloralose/urethane anesthesia). Superfusion of vehicle (0.5% ethanol) in aCSF did not change SEP amplitudes or CBF responses. The effects of superfusion of vehicle or inhibitors are shown in Figure 6. Combined inhibition of COX, nNOS, adenosine receptors, and CYP450 epoxygenase lead to a statistically significant (P<0.01 at 2.0 mA) reduction of approximately two-thirds in CBF response. There was no significant difference in relative CBF response reduction for different stimulation intensities. Additional inhibition of inward rectifier potassium channels with BaCl2 led to a further, nonsignificant reduction of the CBF responses (reaching approximately 70%). Superfusion of BaCl2 alone led to a less pronounced, statistically not significant reduction of CBF responses.
Figure 6.
CBF responses pharmacologically reduced. The relative changes in activity-induced increase in CBF were calculated by individually dividing the average CBF increases of all animals of each group before by the average CBF increase after superfusion of vehicle/inhibitors. Black dots denote individual animals and boxes contain the second and third quartiles. CBF responses were largely reduced to 30% to 40% of the baseline response in the cocktail and cocktail+BaCl2 group. BaCl2 alone led to less pronounced reductions in CBF response. CBF response reduction was statistically tested for the 2.0-mA stimulation intensity. The red stars denote significant differences compared with the control group.
Oxygenation Time Courses During Cerebral Blood Flow Response Inhibition
We averaged the time courses of CBF responses during forepaw stimulation at higher stimulation intensities (1.2 to 2.0 mA, averaged CBF response ∼50%) of all 24 animals during superfusion of aCSF (first block). These time courses were compared with those for the 12 animals in group-3 and group-4 after superfusion of inhibitors (cocktail or cocktail plus BaCl2). Both groups showed large reduction in CBF response as shown in Figure 6. No initial deoxygenation could be detected in the time courses of either oxy-Hb or deoxy-Hb, either before, or after reduction of CBF responses with local superfusion of inhibitory substances (Supplementary Figure S2). In contrast, during CBF response inhibition by approximately two-thirds the deoxy-Hb concentration remained unchanged during the entire stimulation period (Figure 4, right panel).
Discussion
The main findings of our study are as follows: in the somatosensory cortex of anesthetized rats (1) combined local inhibition of COX, nNOS, adenosine receptors, CYP450 epoxygenase, and Kir channels reduces the CBF response to functional activation by approximately two-thirds, independent of stimulation intensity; (2) during this major reduction of the CBF response, neuronal activity and CMRO2 remain largely unaffected. This argues for a CBF response that is not closely matched to oxygen demands, but rather overshoots with a considerable safety margin. The data are, thus, inconsistent with the diffusion limited model of oxygen transport; (3) selectively reducing the CBF response by two-thirds abolishes the CBF response-related decrease of deoxy-Hb concentration. Therefore, the safety factor of the CBF response with respect to relative hypoxia (as determined by microvascular deoxy-Hb concentration) is approximately three. This is well within the range of safety factors in many other physiological or biochemical systems; and (4) since deoxy-Hb responses disappear despite sustained neuronal activity with CBF responses, BOLD–fMRI is not reliable in pathological states.
Weaknesses of the Study
We performed experiments on anesthetized rats in a single brain region. It remains unclear whether our findings extend to the awake state, other brain regions, or other species. However, the phenomenon of neurovascular coupling has proved to be stable in evolution, keeping essential features such as the large mismatch between CBF and CMRO2 responses among brain regions and species.
The measurements for oxy-Hb, deoxy-Hb, and CBV were obtained at a wavelength range of 520 to 610 nm, while CBF recordings with Laser Doppler Flowmetry were obtained at 780 nm with probes in an x-shaped arrangement. Sample volumes, therefore, only partially overlapped. It has been shown that hemoglobin oxygenation time courses vary with cortical depth, although for a considerable range of 200 to 600 μmol/L these differences appear to be small (Hillman et al, 2007). Our measurement for the relationship between CBV and CBF fits well with the literature (Grubb et al, 1974), indicating similar compartments for CBF and CBV measurements. Furthermore, our CMRO2 measurements during hypothermia showed good agreement with the literature (Royl et al, 2008). However, incompletely overlapping sample volumes may introduce some uncertainty in our CMRO2 calculation. The CMRO2 calculation from equation (1) assumes measurement from a single venous compartment. While placing the probe over microcirculatory areas with large CBF and deoxy-Hb responses is likely to favor venously weighted sample volumes (in line with the low baseline saturation obtained), we are measuring from a mixed compartment including capillaries and arterioles. This could potentially affect accuracy of our CMRO2 calculation. We have simulated the CMRO2 calculation error resulting from mixed-compartment effects and found a relatively small error when assuming a venously weighted sample volume (10% arterioles, 30% capillaries, 60% venules), even if relative CBV changes are twice as large in the capillaries and arteries as compared with that in the venules (Hillman et al, 2007). Furthermore, evidence against significant errors resulting from mixed-compartment effects can be derived directly from our data. Comparison of CBF, CBV, and deoxy-Hb responses at a stimulation intensity of 0.8 mA during aCSF superfusion with responses at 1.6 mA during inhibitor superfusion (n=12) showed virtually identical CBF and CBV responses, with an abrogated deoxy-Hb response during inhibitor superfusion (Supplementary Figures S4 and S5). The sample volumes of CBF and CBV responses are identical, and, therefore, the lack of a deoxy-Hb level decrease during stimulation can only be explained by a higher level of CMRO2 during stimulations with 1.6 mA as compared with stimulations with 0.8 mA. This shows, excluding potential mixed-compartment effects, that the uninhibited, physiological CBF response can support larger CMRO2 responses than actually present during neuronal activation. Therefore, the physiological CBF response operates with a safety factor with respect to oxidative metabolism.
We used concentrations and superfusion times of the inhibitors as mentioned in the literature, if available for in vivo experiments. However, we did not assess the specificity or amount of enzyme, receptor or channel inhibition, and, therefore, cannot exclude the possibility that incomplete inhibition of enzymes, receptors or channels might account for incomplete inhibition of neurovascular coupling and no additional pathways are involved. However, we did not observe increasing reduction of CBF responses during a 1-h period of measurement beginning 1 h after the start of inhibitor superfusion. This suggests that diffusion of inhibitors into the relevant cortical layers had reached a steady-state during our waiting period.
Our examination of neuronal activity was limited to recording ECoG and SEP. In line with previous experiments by our own group and other groups during nNOS inhibition with L-NNA or 7-NI, we did not detect significant change in neuronal activity (Lindauer et al, 1996; Lindauer et al, 1999; Hoffmeyer et al, 2007). This contrasts with the results of Ngai et al (1995) who did observe decreases of SEPs during topical application of L-NNA, which depended on measurement technique (Ngai et al, 1998). As no change of SEP amplitudes was observed for our control group, effects of measurement technique on SEP amplitudes could be excluded in our current study. Decreases of SEP amplitudes have also been reported for intraperitoneal application of 7-NI (Stefanovic et al, 2007; Burke and Bührle, 2006). These effects on SEPs were temporarily and quantitatively different from the effects on CBF responses, indicating that experimental conditions influence differential effects of NOS inhibition on CBF and neuronal activity. With limited statistical power we did not detect large changes in SEP amplitudes. Nonetheless, using more complex neurophysiological assessment or a larger number of animals we might have revealed changes in neuronal activity not detectable with our approach. Therefore, we cannot rule out the possibility that other aspects of neuronal activity were changed as a result of decreased CBF responses or pharmacological agents used. However, in a model of severe hypotension, Masamoto et al (2008) detected unchanged local field potentials during somatosensory stimulation with no CBF response, further supporting the notion that the CBF response is not required to support CMRO2 or neuronal activity.
Cerebral Blood Flow Response Inhibition
Combined local inhibition of adenosine receptors, NOS, CYP450 epoxygenase, and COX reduced the CBF responses by approximately two-thirds. Of note, the amount of CBF response inhibition was independent of stimulation intensity over a wide range of SEP and CBF changes (Figure 6). This finding indicates incomplete additive effects of the investigated inhibitors when compared with that in the literature. CBF-response reductions of 40% to 60% have been shown for adenosine-receptor inhibition (Dirnagl et al, 1994; Shi et al, 2008), for unspecific as well as for nNOS inhibition (Lindauer et al, 1999; Niwa et al, 1993; Peng et al, 2004), for COX inhibition (Niwa et al, 2000; Stefanovic et al, 2006), and for inhibition of CYP450 epoxygenase (Peng et al, 2002, 2004; Shi et al, 2008). Larger CBF-response reductions have been reported after NOS inhibition by intraperitoneal application of 7-NI (Stefanovic et al, 2007; Burke and Bührle, 2006); however, in these studies, neuronal activity (SEPs) did not remain stable. In our study, inhibition of Kir channels with BaCl2 lead to reduction in CBF response by approximately 40%, with a trend towards stronger reduction with lower stimulation intensities. This indicates involvement of inward rectifier potassium channels in neurovascular coupling, in accordance with data obtained in brain slice preparations (Filosa et al, 2006). As we used relatively high concentrations to achieve maximal effects (Wellman and Bevan, 1995) and compensate for concentration gradients likely present in in vivo experiments, effects on other potassium channels cannot be excluded. Adding BaCl2 to the above mentioned inhibitory cocktail did not significantly further reduce the CBF responses. However, a trend towards further reduction was noted. CBF response dropped to 30% of baseline responses in this group. Assuming complete inhibition of the target enzymes or receptors, as suggested in former studies (Lindauer et al, 1999; Dirnagl et al, 1994; Peng et al, 2002; Filosa et al, 2006), our results indicate that additional, yet unknown pathways are involved in neurovascular coupling. However, incomplete inhibition of target enzymes and receptors has to be considered as an alternative explanation for incomplete CBF-response reduction.
Cerebral Blood Flow/Cerebral Metabolic Rate of O2 Mismatch
Relative CBF responses exceeded CMRO2 responses by a factor of approximately five to six in our study. This is well in line with the early and subsequent results of Fox and Raichle (1986) and recent data showing ‘coupling ratios' between two and 10 using positron emission tomography or MRI for visual stimulation for humans (e.g., Lin et al, 2009). However, recent MRI studies of rats have found values at the low end of the large interval shown for visual cortex for humans (e.g., ∼3.5, Sicard and Duong, 2005, ∼3, Mandeville et al, 1999). It has been shown that coupling ratios determined with MRI might vary largely depending on the calculation algorithm used (Lin et al, 2009). In addition, the CMRO2 calculation employed in the current study might somewhat underestimate CMRO2 changes and could, therefore, overestimate the coupling ratio.
Since the surprising finding by Fox and Raichle, explanations have been discussed for the mismatch between a large CBF response and a small CMRO2 response to neuronal activation. As oxidative metabolism is the most efficient way to generate energy for neuronal activity and blood flow primarily serves to deliver substrates for neuronal energy metabolism, it is natural to assume that increases of CBF serve the delivery of oxygen to activated brain regions. Explanations that elegantly resolve the surprising mismatch between CBF and CMRO2 increases have been proposed. A theoretical model predicted much larger CBF than CMRO2 responses under the assumption that all oxygen leaving brain capillaries is metabolized and all capillaries are perfused at rest (Buxton and Frank, 1997). A second, similar explanation holds that increased oxygen consumption critically depends on a large increase of blood flow because of a negligible oxygen tension in brain mitochondria (Vafaee and Gjedde, 2000; Gjedde, 2002). However, the experimental evidence supporting this hypothesis is controversial (Mintun et al, 2001). The data of our current study clearly contradict this hypothesis. Largely reduced CBF responses left accompanying CMRO2 increases and neuronal activity unchanged (Figure 4). Identical CBF and CBV responses supported larger CMRO2 responses during CBF response inhibition (Supplementary Figure S4 and S5). This is in line with experiments showing intact neuronal activity during severe hypotension with absent CBF responses, and providing evidence for intact oxidative metabolism (Masamoto et al, 2008, 2009).
We propose an alternative explanation for the CBF/CMRO2 response mismatch. As neurovascular coupling with this feature has been shown for many species (humans, monkeys, cats, rats) with quite similar quantitative characteristics, it is likely that neurovascular coupling, which comes at the ‘cost' of increased blood supply, must provide some evolutionary benefit. Mismatches between capacity and demand (safety factors) are key features of many physiological and biochemical systems. Such safety factors generally range from 1.2 to 10 (Diamond, 2002). Their values are determined, among other factors, by cost of failure, cost of construction, maintenance and operation, variation of capacity or load, and deterioration of capacity with time. In case of oxygen supply to active brain regions, cost of failure (impaired neuronal activity) would be high, whereas cost of operation (increasing the CBF to a small brain area with constant whole-brain CBF) is low, favoring a large safety factor. The variations of capacity (variation of the amount of oxygen delivered at a certain level of CBF increase) and of load (variation of oxygen demand for a certain level of neuronal activity) are expected to be small. However, they may increase substantially under pathological conditions.
From our experiments one may deduce a safety factor of the CBF response for an activity-induced increase in the deoxy-Hb concentration of around three (in good agreement with Masamoto et al, 2009), compatible with data on safety factors of many other systems (Diamond, 2002). However, other explanations for a large CBF/CMRO2 response mismatch have to be considered. Temperature regulation has been proposed as an important function of the CBF response (Katz-Brull et al, 2005). However, neurovascular coupling was preserved during severe hypothermia (Royl et al, 2008). Moore and Cao (2008) have suggested that in contrast to the classical metabolic view the CBF response could primarily have a neuro-modulatory function. We did not detect altered neuronal activity during inhibition of neurovascular coupling, but SEPs are a coarse correlate of neuronal activity and our study was not powered to detect small changes. A more complex assessment of neuronal activity during impaired neurovascular coupling may reveal changes not detectable with our simple approach.
Initial Oxygenation Time Courses
In agreement with our previous findings (Lindauer et al, 2001), using spectroscopic measurements of oxy- and deoxy-Hb we did not detect an initial increase of deoxy-Hb (‘initial dip') or decrease of oxy-Hb level in the first seconds of somatosensory stimulation in the rat. It has been argued that an initial deoxygenation could be masked by rapid and overshooting CBF response. Initial deoxygenation might be spatially more confined to the area of neuronal activity than blood flow response-related deoxy-Hb concentration changes, a few seconds later. Unmasking initial deoxygenation, might, therefore be an approach to improve spatial resolution of functional imaging. However, in our current study, after reduction of the CBF response by two-thirds, no deoxygenation was unmasked in the first seconds after stimulation (Supplementary Figure S2).
Implications for Functional Imaging
Our data demonstrate that a reduction of the CBF response by two-thirds abolishes the stimulation-induced decrease in deoxy-Hb level in the rat somatosensory cortex (Figure 4 and Supplementary Figures S4 and S5). This decrease, however, provides the basis for functional imaging using BOLD–fMRI. Under such conditions, neuronal activity (unaffected by the CBF-response reduction) would no longer be detectable by BOLD–fMRI. For one animal, CBF-response reduction reached 85% and reproducible increases of deoxy-Hb during neuronal activity with inhibited CBF response were detected (Supplementary Figure S3). From these data it can be speculated that under the conditions of a complete inhibition of the CBF response, a deoxygenation will be unmasked. However, apart from anecdotal evidence from one animal we have no proof that the deoxy-Hb concentration does indeed increase if CBF responses are blocked beyond two-thirds. Deoxygenation during complete inhibition of the CBF responses has been demonstrated during conditions of severe hypotension (Masamoto et al, 2008, 2009), where cerebral vessels are maximally dilated. This deoxygenation could again be detected by BOLD–fMRI (with inverse signal characteristics) and might be more closely confined to the area of neuronal activation. However, to date a simple pharmacological approach for complete CBF-response inhibition is not available, leaving this approach only a theoretical option.
Conclusion
Combined local inhibition of nNOS, COX, adenosine receptors, CYP450-epoxygenase, and inward rectifier potassium channels reduces the blood flow responses to neuronal activation by approximately two-thirds in the rat somatosensory cortex. Neuronal activity and oxidative metabolism remain largely unaffected. This provides evidence that the observed mismatch between a large increase of CBF and a small increase of the cerebral metabolic rate of oxygen might be the result of an evolutionary development that has favored a generous safety margin for oxygen supply to the brain during increases of neuronal activity. BOLD–fMRI might not detect neuronal activity in pathological states when this safety margin is partially depleted.
Acknowledgments
We express special thanks to Dr Falck for providing the MS-PPOH. This work was supported in part by the Deutsche Forschungsgemeinschaft (UL, UD, GR) and the Hermann and Lilly Schilling Stiftung (UD).
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Disclosure/conflict of interest
The authors declare no conflicts of interest.
Supplementary Material
References
- Bakalova R, Matsuura T, Kanno I. The cyclooxygenase inhibitors indomethacin and rofecoxib reduce regional cerebral blood flow evoked by somatosensory stimulation in rats. Exp Med Biol (Maywood) 2002;227:465–473. doi: 10.1177/153537020222700710. [DOI] [PubMed] [Google Scholar]
- Burke M, Bührle C.2006BOLD response during uncoupling of neuronal activity and CBF Neuroimage 321–8.e-pub 2006 May 3 [DOI] [PubMed] [Google Scholar]
- Buxton RB, Frank LR. A model for the coupling between cerebral blood flow and oxygen metabolism during neural activation. J Cereb Blood Flow Metab. 1997;17:64–72. doi: 10.1097/00004647-199701000-00009. [DOI] [PubMed] [Google Scholar]
- Diamond J. Quantitative evolutionary design. J Physiol. 2002;542 (Pt 2:337–345. doi: 10.1113/jphysiol.2002.018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Niwa K, Lindauer U, Villringer A. Coupling of cerebral blood flow to neuronal activation: role of adenosine and nitric oxide. Am J Physiol. 1994;267 (1 Pt 2:H296–H301. doi: 10.1152/ajpheart.1994.267.1.H296. [DOI] [PubMed] [Google Scholar]
- Dunn AK, Devor A, Dale AM, Boas DA. Spatial extent of oxygen metabolism and hemodynamic changes during functional activation of the rat somatosensory cortex. NeuroImage. 2005;27:279–290. doi: 10.1016/j.neuroimage.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Fabricius M, Lauritzen M. Laser–Doppler evaluation of rat brain microcirculation: comparison with the [14C]-iodoantipyrine method suggests discordance during cerebral blood flow increases. J Cereb Blood Flow Metab. 1996;16:156–161. doi: 10.1097/00004647-199601000-00018. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci USA. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb RL, Jr, Raichle ME, Eichling JO, Ter-Pogossian MM. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke. 1974;5:630–639. doi: 10.1161/01.str.5.5.630. [DOI] [PubMed] [Google Scholar]
- Gjedde A. Cerebral blood flow change in arterial hypoxemia is consistent with negligible oxygen tension in brain mitochondria. Neuroimage. 2002;17:1876–1881. doi: 10.1006/nimg.2002.1272. [DOI] [PubMed] [Google Scholar]
- Hillman EM, Devor A, Bouchard MB, Dunn AK, Krauss GW, Skoch J, Bacskai BJ, Dale AM, Boas DA.2007Depth-resolved optical imaging and microscopy of vascular compartment dynamics during somatosensory stimulation Neuroimage 3589–104.e-pub 2007 Jan 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeyer HW, Enager P, Thomsen KJ, Lauritzen MJ.2007Nonlinear neurovascular coupling in rat sensory cortex by activation of transcallosal fibers J Cereb Blood Flow Metab 27575–587.e-pub 2006 Aug 9 [DOI] [PubMed] [Google Scholar]
- Katz-Brull R, Alsop DC, Marquis RP, Lenkinski RE. Limits on activation induced temperature and metabolic changes in the human primary visual cortex. Magn Reson Med. 2005;56:348–355. doi: 10.1002/mrm.20972. [DOI] [PubMed] [Google Scholar]
- Knot HJ, Zimmermann PA, Nelson MT. Extracellular K(+)-induced hyperpolarizations and dilatations of rat coronary and cerebral arterioles involve inward rectifier K(+) channels. J Physiol. 1996;492 (Pt 2:419–430. doi: 10.1113/jphysiol.1996.sp021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl-Bareis M, Guertler R, Lindauer U, Leithner C, Sellien H, Royl G, Dirnagl U.2005System for the measurement of blood flow and oxygenation in tissue applied to neurovascular coupling in brain Photon migration and diffuse-light imaging II(Licha K, Cubeddu R eds). Vol. 5859 of Proc. SPIE, Optical Society of America, paper WA3
- Lin AL, Fox PT, Yang Y, Lu H, Tan LH, Gao JH.2009Time-dependent correlation of cerebral blood flow with oxygen metabolism in activated human visual cortex as measured by fMRI Neuroimage 4416–22.e-pub 2008 Sept 4 [DOI] [PubMed] [Google Scholar]
- Lindauer U, Megow D, Schultze J, Weber JR, Dirnagl U. Nitric oxide synthase inhibition does not affect somatosensory evoked potentials in the rat. Neurosci Lett. 1996;216:207–10. doi: 10.1016/0304-3940(96)13044-5. [DOI] [PubMed] [Google Scholar]
- Lindauer U, Megow D, Matsuda H, Dirnagl U. Nitric oxide: a modulator, but not a mediator, of neurovascular coupling in rat somatosensory cortex. Am J Physiol. 1999;277 (2 Pt 2:H799–H811. doi: 10.1152/ajpheart.1999.277.2.H799. [DOI] [PubMed] [Google Scholar]
- Lindauer U, Royl G, Leithner C, Kühl M, Gold L, Gethmann J, Kohl-Bareis M, Villringer A, Dirnagl U. No evidence for early decrease in blood oxygenation in rat whisker cortex in response to functional activation. Neuroimage. 2001;13 (6 Pt 1:988–1001. doi: 10.1006/nimg.2000.0709. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJ, Ayata C, Moskowitz MA, Weisskoff RM, Rosen BR. MRI measurement of the temporal evolution of relative CMRO(2) during rat forepaw stimulation. Magn Reson Med. 1999;42:944–951. doi: 10.1002/(sici)1522-2594(199911)42:5<944::aid-mrm15>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Masamoto K, Vazquez A, Wang P, Kim SG.2008Trial-by-trial relationship between neuronal activity, oxygen consumption, and blood flow responses Neuroimage 40442–450.e-pub 2008 Jan 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamoto K, Vazquez A, Wang P, Kim SG. Brain tissue oxygen consumption and supply induced by neural activation: determined under suppressed hemodynamic response conditions in the anesthetized rat cerebral cortex. Adv Exp Med Biol. 2009;645:287–292. doi: 10.1007/978-0-387-85998-9_43. [DOI] [PubMed] [Google Scholar]
- Mayhew J, Johnston D, Berwick J, Jones M, Coffey P, Zheng Y. Spectroscopic analysis of neuronal activity in brain: increased oxygen consumption following activation of barrel cortex. Neuroimage. 2000;12:664–675. doi: 10.1006/nimg.2000.0656. [DOI] [PubMed] [Google Scholar]
- Metea MR, Kofuji P, Newman EA. Neurovascular coupling is not mediated by potassium siphoning from glial cells. J Neurosci. 2007;27:2468–2471. doi: 10.1523/JNEUROSCI.3204-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B, Schultheiss R, Schramm J. Capillary oxygen saturation and tissue oxygen pressure in the rat cortex at different stages of hypoxic hypoxia. Neurol Res. 2000;22:721–726. doi: 10.1080/01616412.2000.11740746. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Lundstrom BN, Snyder AZ, Vlassenko AG, Shulman GL, Raichle ME. Blood flow and oxygen delivery to human brain during functional activity: theoretical modeling and experimental data. Proc Natl Acad Sci USA. 2001;98:6859–6864. doi: 10.1073/pnas.111164398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CI, Cao R.2008The hemo-neural hypothesis: on the role of blood flow in information processing J Neurophysiol 992035–2047.e-pub 2007 Oct 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai AC, Meno JR, Winn HR. L-NNA suppresses cerebrovascular response and evoked potentials during somatosensory stimulation in rats. Am J Physiol. 1995;269 (5 Pt 2:H1803–H1810. doi: 10.1152/ajpheart.1995.269.5.H1803. [DOI] [PubMed] [Google Scholar]
- Ngai AC, Meno JR, Jolley MA, Winn HR. Suppression of somatosensory evoked potentials by nitric oxide synthase inhibition in rats: methodological differences. Neurosci Lett. 1998;245:171–174. doi: 10.1016/s0304-3940(98)00213-4. [DOI] [PubMed] [Google Scholar]
- Niwa K, Araki E, Morham SG, Ross ME, Iadecola C. Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J Neurosci. 2000;20:763–770. doi: 10.1523/JNEUROSCI.20-02-00763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa K, Haensel C, Ross ME, Iadecola C. Cyclooxygenase-1 participates in selected vasodilator responses of the cerebral circulation. Circ Res. 2001;88:600–608. doi: 10.1161/01.res.88.6.600. [DOI] [PubMed] [Google Scholar]
- Niwa K, Lindauer U, Villringer A, Dirnagl U. Blockade of nitric oxide synthesis in rats strongly attenuates the CBF response to extracellular acidosis. J Cereb Blood Flow Metab. 1993;13:535–539. doi: 10.1038/jcbfm.1993.70. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson OB, Newman EA. Does the release of potassium from astrocyte endfeet regulate cerebral blood flow . Science. 1987;237:896–898. doi: 10.1126/science.3616619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Zhang C, Alkayed NJ, Harder DR, Koehler RC. Dependency of cortical functional hyperemia to forepaw stimulation on epoxygenase and nitric oxide synthase activities in rats. J Cereb Blood Flow Metab. 2004;24:509–517. doi: 10.1097/00004647-200405000-00004. [DOI] [PubMed] [Google Scholar]
- Peng X, Carhuapoma JR, Bhardwaj A, Alkayed NJ, Falck JR, Harder DR, Traystman RJ, Koehler RC. Suppression of cortical functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am J Physiol Heart Circ Physiol. 2002;283:H2029–H2037. doi: 10.1152/ajpheart.01130.2000. [DOI] [PubMed] [Google Scholar]
- Roy CS, Sherrington CS. On the regulation of the blood-supply of the brain. J Physiol. 1890;11:85–158. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royl G, Füchtemeier M, Leithner C, Offenhauser N, Megow D, Steinbrink J, Kohl-Bareis M, Dirnagl U, Lindauer U. Hypothermia effects on neurovascular coupling and cerebral metabolic rate of oxygen. Neuroimage. 2008;40:1523–1532. doi: 10.1016/j.neuroimage.2008.01.041. [DOI] [PubMed] [Google Scholar]
- Royl G, Leithner C, Sellien H, Müller JP, Megow D, Offenhauser N, Steinbrink J, Kohl-Bareis M, Dirnagl U, Lindauer U. Functional imaging with laser speckle contrast analysis: vascular compartment analysis and correlation with laser Doppler flowmetry and somatosensory evoked potentials. Brain Res. 2006;1121:95–103. doi: 10.1016/j.brainres.2006.08.125. [DOI] [PubMed] [Google Scholar]
- Shi Y, Liu X, Gebremedhin D, Falck JR, Harder DR, Koehler RC.2008Interaction of mechanisms involving epoxyeicosatrienoic acids, adenosine receptors, and metabotropic glutamate receptors in neurovascular coupling in rat whisker barrel cortex J Cereb Blood Flow Metab 28111–125.e-pub 2007 May 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard KM, Duong TQ. Effects of hypoxia, hyperoxia, and hypercapnia on baseline and stimulus-evoked BOLD, CBF, and CMRO2 in spontaneously breathing animals. Neuroimage. 2005;25:850–858. doi: 10.1016/j.neuroimage.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic B, Bosetti F, Silva AC.2006Modulatory role of cyclooxygenase-2 in cerebrovascular coupling Neuroimage 3223–32.e-pub 2006 Apr 19 [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Schwindt W, Hoehn M, Silva AC.2007Functional uncoupling of hemodynamic from neuronal response by inhibition of neuronal nitric oxide synthase J Cereb Blood Flow Metab 27741–754.e-pub 2006 Aug 2 [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M.2006Astrocyte-mediated control of cerebral blood flow Nat Neurosci 9260–267.e-pub 2005 Dec 25 [DOI] [PubMed] [Google Scholar]
- Vafaee MS, Gjedde A. Model of blood-brain transfer of oxygen explains nonlinear flow-metabolism coupling during stimulation of visual cortex. J Cereb Blood Flow Metab. 2000;20:747–754. doi: 10.1097/00004647-200004000-00012. [DOI] [PubMed] [Google Scholar]
- Vovenko E. Distribution of oxygen tension on the surface of arterioles, capillaries and venules of brain cortex and in tissue in normoxia: an experimental study on rats. Pflugers Arch. 1999;437:617–623. doi: 10.1007/s004240050825. [DOI] [PubMed] [Google Scholar]
- Wellman GC, Bevan JA. Barium inhibits the endothelium-dependent component of flow but not acetylcholine-induced relaxation in isolated rabbit cerebral arteries. J Pharmacol Exp Ther. 1995;274:47–53. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.