Abstract
Diabetes is an increased risk factor for stroke and results in increased brain damage in experimental animals and humans. The precise mechanisms are unclear, but our earlier studies in the db/db mice suggested that the cerebral inflammatory response initiating recovery was both delayed and diminished in the diabetic mice compared with the nondiabetic db/+ mice. In this study, we investigated the actions of the peroxisome proliferator-activated receptor (PPAR)-γ agonist darglitazone in treating diabetes and promoting recovery after a hypoxic-ischemic (H/I) insult in the diabetic ob/ob mouse. Male ob/+ and ob/ob mice received darglitazone (1 mg/kg) for 7 days before induction of H/I. Darglitazone restored euglycemia and normalized elevated corticosterone, triglycerides, and very-low-density lipoprotein levels. Darglitazone dramatically reduced the infarct size in the ob/ob mice at 24 h of recovery compared with the untreated group (30±13% to 3.3±1.6%, n=6 to 8) but did not show any significant effect in the ob/+ mice. Microglial and astrocytic activation monitored by cytokine expression (interleukin-1β and tumor necrosis factor-α) and in situ hybridization studies (bfl1 and glial fibrillary acidic protein) suggest a biphasic inflammatory response, with darglitazone restoring the compromised proinflammatory response(s) in the diabetic mouse at 4 h but suppressing subsequent inflammatory responses at 8 and 24 h in both control and diabetic mice.
Keywords: cytokines, microglia, stroke, thiazolidinediones, type II diabetes
Introduction
Stroke is the third leading cause of death and disability in the United States and diabetes is a major risk factor for stroke. Both type I and type II diabetic patients are two to six times more likely to experience a stroke than nondiabetic patients (Bonow and Gheorghiade, 2004); both morbidity and mortality after stroke are greatly enhanced, although the underlying mechanisms are still unknown. Initially, it was considered that preischemic hyperglycemia and consequent cerebral lactoacidosis were the primary cause (Folbergrova et al, 1992), however, we and others have indicated that additional factors have a significant role in the compromised recovery (Kumari et al, 2007; Nedergaard, 1987; Nedergaard and Diemer, 1987; Vannucci et al, 2001b; Zhang et al, 2004). Recently, we proposed that there is impairment in the acute inflammatory response in the diabetic db/db mouse after a unilateral cerebral hypoxic-ischemic (H/I) insult (Kumari et al, 2007; Zhang et al, 2004). This was manifested by a suppressed microglial and astrocytic activation coupled with a reduced and delayed expression of pro- and anti-inflammatory cytokines during the initial 12 h of recovery.
Thiazolidinediones (TZDs) such as rosiglitazone and pioglitazone are PPAR-γ agonists that are commonly prescribed for the treatment of type II diabetes, as they enhance insulin sensitivity and lower blood glucose levels and HbA1c (Stumvoll and Haring, 2002). In separate studies, beneficial effects of PPAR-γ agonists have been reported in rodent models of cerebral ischemic injury (Luo et al, 2006; Sundararajan et al, 2005). In these studies, rosiglitazone, troglitazone, and pioglitazone appeared to suppress the activation of microglia and infiltration of macrophages, and reduce the infarct size after cerebral ischemia by reducing levels of proinflammatory cytokines (Culman et al, 2007; Sundararajan et al, 2005). Only one such study was conducted in diabetic mice, showing a suppression of proinflammatory gene expression and reduction in infarct size (Tureyen et al, 2007). Darglitazone is a TZD that is 20 to 150 times more γ-receptor-selective than either rosiglitazone or pioglitazone, and is almost 10 times more orally potent in restoring euglycemia (Aleo et al, 2003; Oakes et al, 2001). Thus, the objectives of the current study were to investigate the effects of darglitazone treatment on H/I insult in the diabetic ob/ob mice with specific attention to the acute inflammatory response(s). We treated diabetic ob/ob mice with darglitazone (a generous gift from Pfizer, Groton, CT, USA) for 1 week before inducing a unilateral, cerebral H/I insult and then monitored the subsequent acute microglial and astrocytic inflammatory responses. We show that darglitazone normalized blood glucose and reduced circulating triglycerides (TG) and very-low-density lipoproteins (VLDL) in diabetic ob/ob mice without having any effect in the nondiabetic mice. Moreover, darglitazone treatment restored acute cerebral inflammatory responses that were absent in the diabetic mice and profoundly improved their recovery from H/I insult.
Materials and methods
Animal Procedures
Male diabetic ob/ob mice and their heterozygous control ob/+ mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA) at 6 weeks of age. After quarantine, mice were fed a powdered chow diet for a week. Food intake was monitored to calculate appropriate dosage of drug. Mice were then switched to powdered chow mixed with darglitazone (1 mg/kg) or vehicle. Earlier studies indicated that this level of darglitazone results in a 100% normalization of blood glucose in the ob/ob mouse (Hulin et al, 1996a, 1996b). Blood glucose was measured on alternate days during treatment. After 1 week of treatment, cerebral hypoxia–ischemia was induced as reported earlier (Kumari et al, 2007; Vannucci et al, 2001a). Briefly, the right carotid artery was exposed and permanently ligated. After a 3-h recovery, animals were exposed to 8% oxygen balanced with nitrogen for 24 mins at 35.5°C. In this model, reperfusion commences with return to normoxia. At 4, 8, and 24 h of recovery/reperfusion, mice were anesthetized with isoflurane and blood was collected by cardiac puncture for serum or plasma. The mice were decapitated and the brains were quickly frozen in cold isopentane (−40°C) and stored at −80°C until analysis.
Measurement of Blood Glucose and Lipid Profile
Blood for glucose determination was obtained by tail prick and analyzed by glucose test strips (Nova Biomedical, Waltham, MA, USA) and BD Logic Monitors (Becton Dickson, Franklin Lakes, NJ, USA). To minimize daily variation in the results, the analysis was carried out between 0900 and 1000 hours. For measurement of cholesterol, TG, and VLDL, blood was collected by heart puncture in a Na-heparinized tube (BD Vacutainer, BD, Franklin Lakes, NJ, USA). The samples were centrifuged at 3,000g for 10 mins at 4°C and plasma was stored at −80°C until the analysis. Triglycerides and cholesterol were measured using VITROS Chemistry TRIG DT and CHOL DT Slides (Ortho-Clinical Diagnostics, Johnson & Johnson Company, Rochester, NY, USA) in a blood chemistry analyzer (DT60 II, Vitros Chemistry System, Ortho Clinical Diagnostics, Johnson & Johnson), according to the manufacturer's instructions. The VLDL value was calculated based on the corresponding TG measurement.
Corticosterone Measurement
Blood samples were collected by heart puncture at specific intervals of recovery times. Serum was obtained by centrifugation at 16,000g for 10 mins at room temperature and stored at −80°C until the analysis. Serum corticosterone (CORT) was measured by radioimmunoassay using ImmuChem Double Antibody Corticosterone 125I RIA Kit (MP Biomedicals, LLC Diagnostic Division, Orangeburg, NY), according to the manufacturer's instructions. Corticosterone values were calculated from a standard curve, which was generated using 0 to 1,000 ng/mL CORT standards.
Histologic Analysis
Coronal cryosections (16 μm) were cut at regular intervals from the striatum to the posterior hippocampus for histologic analysis and in situ hybridization. From the same brains, 4 × 60 μm coronal sections were collected and divided into contralateral and ipsilateral hemispheres, and stored separately for RNA isolation. To determine the infarct size at 24 h, cryosections were stained with hematoxylin and eosin. The area of infarction was measured using Scion Image analysis program (Scion Corp., Frederick, MD, USA) and calculated as a percentage of the ratio of the damaged area to the area of total hemisphere, with correction of hemisphere swelling due to edema as described earlier (Zhang et al, 2004).
In Situ Hybridization
The expression and localization of glial fibrillary acidic protein (GFAP) and bfl1 were analyzed by in situ hybridization. A 663-bp mouse bfl cDNA and 1,159 bp plasmid for GFAP were labeled with 35S for in situ hybridization, as previously described (Kumari et al, 2007). The 16 μm cryosections were hybridized with 35S-CTP- and 35S-UTP-labeled riboprobes in a humidified chamber at 59°C for 16 h and visualized using autoradiography (Vannucci et al, 1997).
Real-Time PCR
The samples for RNA isolation were obtained from ipsilateral and contralateral hemisphere cryosections (4 × 60 μm) of the brain at different time points of H/I recovery. The total RNA was isolated for each hemisphere using TRI reagent (Invitrogen Life Technology, Molecular Research Center, Cincinnati, OH, USA), according to the manufacturer's protocol. Single-stranded cDNA template was prepared using Omniscript RT Kit (Qiagen, Valencia, CA, USA). Different dilutions of cDNA were prepared, 1:20 for 18S internal control and 1:2 for cytokine primers. A volume of 2 μL of the 1:20 diluted cDNA template was treated with FAM-labeled 18S as an internal control and 2 μL of the 1:2 diluted cDNA were treated with mouse-specific cytokine primers (tumor necrosis factor-α (TNFα), interleukin-1β (IL-1β), and IL-6) (TaqMan Primers, Applied Biosystems, Foster City, CA, USA) to obtain the concentration of 250 nmol/L for probe and 900 nmol/L for primer. TaqMan Universal PCR Master Mix was used according to the manufacturer's protocol. Reactions of all samples, treated and nontreated ob/ob and ob/+ at a specific time point, were analyzed in 384-well plates to avoid plate-to-plate variations. The reaction plate was placed in Applied Biosystems 7900 HT PCR System and programmed for real-time PCR (RT-PCR). Data were analyzed using Sequence Detection System (SDS) 2.2.2 software (Applied Biosystems).
Statistical Analysis
Data were analyzed by one-way analysis of variance followed by Tukey's multiple comparisons using GraphPad Prism 2.01 (GraphPad Software Inc., San Diego, CA, USA). Relative Expression Software Tool (REST, Göteborg, Sweden) was used to analyze RT-PCR data. Significance was set at P<0.05.
Results
PPAR-γ Regulation of Blood Glucose, Lipids, and Corticosterone
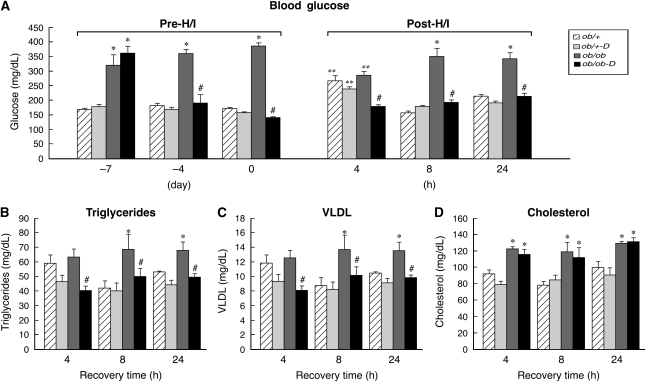
Blood glucose was measured in all diabetic ob/ob and control ob/+ mice immediately before darglitazone administration, during the ensuing week, before H/I, and at 4, 8, and 24 h of recovery. As illustrated in Figure 1A, blood glucose was significantly higher in the diabetic ob/ob (358±35 mg/dL) mice compared with their nondiabetic ob/+ littermates (166±9 mg/dL). Euglycemia in the ob/ob mice was restored and maintained by 48 h of darglitazone treatment, whereas glucose levels remained elevated in untreated ob/ob. Darglitazone had no effect on blood glucose level in the ob/+ mice. At 4 h after H/I, blood glucose levels were significantly elevated above the baseline (i.e., day 0 pre-H/I) in both treated and untreated ob/+ mice. In contrast, in the untreated ob/ob mice, blood glucose was significantly reduced relative to baseline, whereas the darglitazone-treated ob/ob mice remained normoglycemic throughout the entire experiment. At 8 and 24 h of recovery, euglycemia was restored in both groups of ob/+ mice.
Figure 1.
Effect of darglitazone on blood glucose, triglyceride, cholesterol, and very-low-density lipoprotein (VLDL) levels in ob/+ and ob/ob mice. (A) Blood glucose was measured by tail prick before treatment (−7 day), 3 days after the treatment onset (−4 day), before hypoxia/ischemia (H/I) (day 0), and at different intervals of H/I recovery (4, 8, and 24 h). (B to D) Darglitazone reduced triglycerides and VLDL but not cholesterol in ob/ob mice during recovery. Hypoxia/ischemia decreased triglyceride and VLDL values at all time points in both groups compared with their baseline values; ob/+: triglycerides (80±7 mg/dL) and VLDL (16±2 mg/dL) and ob/ob: triglycerides (94±5 mg/dL) and VLDL (19±1 mg/dL). Cholesterol did not change in either group compared with baseline: ob/+ (88±6 mg/dL) or ob/ob (128±6 mg/dL). Results are expressed as mean±s.e.m (n=8). *P<0.05 versus ob/+ (effect of gene), **P<0.05 versus 0 (effect of H/I), and #P<0.05 versus darglitazone (D) treatment (effect of drug).  , ob/+
, ob/+  , ob/+-D;
, ob/+-D;  , ob/ob; ▪, ob/ob-D.
, ob/ob; ▪, ob/ob-D.
Hypoxia–ischemia decreased TG and VLDL values at all time points in both groups compared with their baseline values (Figures 1B and 1C)—ob/+: TG (80±7 mg/dL), VLDL (16±2 mg/dL); ob/ob: TG (94±5 mg/dL) and VLDL (19±1 mg/dL). Cholesterol did not change in either group compared with baseline—ob/+: 88±6 or ob/ob: 128±6. Consistent with the observations of Li et al (2005), cholesterol levels were significantly higher in the ob/ob mice compared with the ob/+ mice, but were unaffected by H/I or darglitazone.
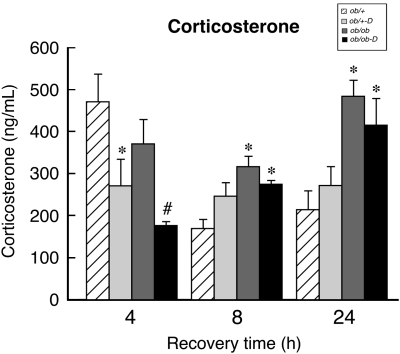
As indicated in the legend of Figure 2, the baseline levels of circulating CORT were elevated in the ob/ob mice relative to the ob/+ mice (395±40 versus 92±13 ng/mL). These data are consistent with previous observations in both db/db and ob/ob mice (Bernotiene et al, 2004; Liu et al, 2005). Figure 2 illustrates that H/I induced a profound, transient elevation of CORT in the ob/+ mice at 4 h of recovery, which was significantly reduced by darglitazone treatment (471±65 versus 273±63 ng/mL); the elevated levels rapidly declined at 8 h and remained the same at 24 h of recovery. In contrast, H/I had no effect on CORT levels in the untreated ob/ob mice at any time point of recovery. However, as with the ob/+ mice, darglitazone significantly reduced CORT levels in the ob/ob mice at 4 h of recovery, but they progressively returned to baseline at 8 and 24 h.
Figure 2.
Effects of darglitazone treatment on serum corticosterone (CORT) levels during stroke recovery. Corticosterone was measured by radioimmunoassay in serum before darglitazone treatment and at indicated time points during (H/I) recovery. Hypoxia–ischemia induced a significant increase in CORT levels in ob/+ but not in ob/ob group compared with the baseline values (92±13 and 395±40 ng/mL, respectively). Results are expressed as mean±s.e.m. (n=8). *P<0.05 versus ob/+ (effect of gene) and #P<0.05 versus darglitazone (D) treatment (effect of drug).  , ob/+
, ob/+  , ob/+-D;
, ob/+-D;  , ob/ob; ▪, ob/ob-D.
, ob/ob; ▪, ob/ob-D.
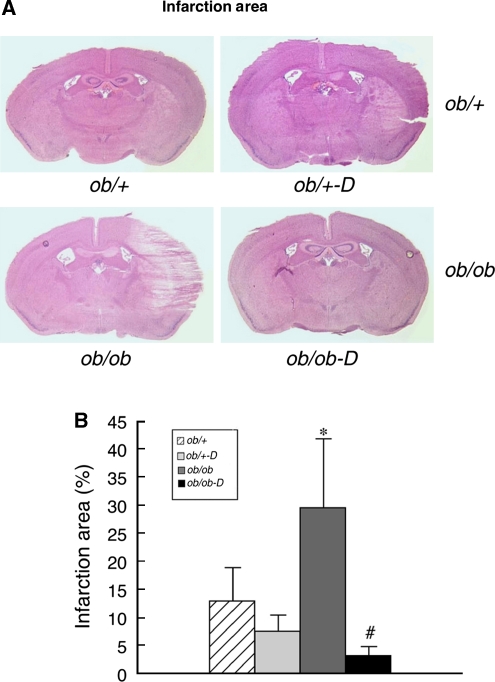
We have previously observed increased tissue damage after H/I in the diabetic, db/db, mouse relative to their normoglycemic control (Kumari et al, 2007; Vannucci et al, 2001a; Zhang et al, 2004). The same effect of diabetes was observed in the ob/ob mouse as illustrated in Figure 3A. Of the original eight diabetic mice subjected to H/I, two died during the initial 24-h interval and were excluded from the analysis and two of the remaining six did not show any damage, yielding the standard error depicted in Figure 3B. However, darglitazone dramatically reduced the area of infarction in the diabetic mice from 30% to 2%. Although darglitazone did appear to also protect the ob/+ mice, the damage in both nondiabetic populations was small and the effect did not reach statistical significance (Figures 3A and 3B).
Figure 3.
Effects of darglitazone on the infarct area. (A) Representative hematoxylin and eosin (H&E) stained 16 μm cryosections from control and diabetic, treated and untreated, mice at 24 h of recovery from hypoxia–ischemia (H/I). (B) Sections from all animals were analyzed by Scion Image and the results calculated as the area of infarction as a percentage of the ipsilateral hemisphere relative to the contralateral hemisphere; mean±s.e.m. (n=6 to 8 per group). *P<0.05 versus ob/+ (effect of gene) and #P<0.05 versus darglitazone (D) treatment (effect of drug).  , ob/+
, ob/+  , ob/+-D;
, ob/+-D;  , ob/ob; ▪, ob/ob-D.
, ob/ob; ▪, ob/ob-D.
Activation of PPAR-γ and Response of Microglia and Astrocytes
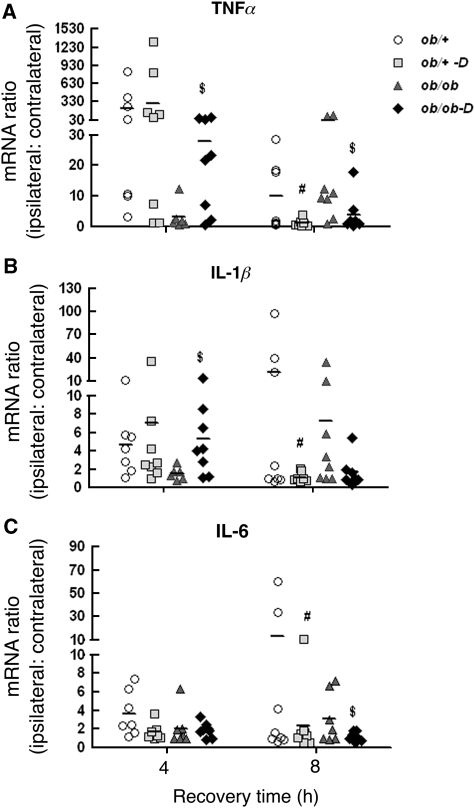
As we previously showed that the increased damage in the db/db mouse was associated with a reduced and delayed cerebral inflammatory response (Kumari et al, 2007), we next asked the question as to whether darglitazone treatment had any effect on this acute response in the ob/ob mouse. In this study, RNA was isolated from both contralateral and ipsilateral hemispheres and mRNA levels for the proinflammatory cytokines TNFα, IL-1β, and IL-6 were measured by RT-PCR. The results are expressed as a ratio of ipsilateral to contralateral hemisphere, as presented in Figure 4. Similar to previous results in the db/+ mice, H/I resulted in increased TNFα and IL-1β expression at 4 h in the ob/+ mice, with no effect of darglitazone (Figures 4A and 4B). However, by 8 h, the levels of both cytokines were significantly reduced in darglitazone-treated ob/+ mice, which then returned to nontreatment levels by 24 h (data not shown). Interleukin-6 expression (Figure 4C) was reduced at 8 h of recovery in the darglitazone group of ob/+ mice, and then returned to control levels at 24 h (data not shown).
Figure 4.
Effects of darglitazone on cytokine mRNA expression at early time points of hypoxic–ischemic (H/I) recovery: RT-PCR (real-time PCR) analysis. Proinflammatory cytokines tumor necrosis factor-α (TNFα), interleukin (IL)-1β, and IL-6 mRNA were measured by RT-PCR in both contralateral and ipsilateral hemispheres of untreated and darglitazone-treated ob/+ and ob/ob mice brain at 4 and 8 h of H/I recovery. The results are expressed as the ratio of ipsilateral to contralateral hemisphere (n=6 to 8). $P<0.05 versus ob/ob and #P<0.05 versus ob/+.  , ob/+
, ob/+  , ob/+-D;
, ob/+-D;  , ob/ob; ♦, ob/ob-D.
, ob/ob; ♦, ob/ob-D.
Furthermore, similar to previous reports in the db/db mice, the early cytokine responses were delayed in the ob/ob mouse, being completely absent at 4 h and elevated at 8 h. However, as seen in Figures 4A and 4B, darglitazone treatment normalized this early response in the diabetic ob/ob mice, with significantly increased TNFα and IL-1β expression at 4 h, whereas at 8 h, TNFα and IL-1β mRNA levels were suppressed by darglitazone in both ob/+ and ob/ob mice and this suppression persisted to 24 h (data not shown). IL-6 gene expression in the ob/ob mice was suppressed by darglitazone at 4 and 8 h of recovery compared with their nontreated controls, but returned to control levels at 24 h (data not shown) (Figure 4 C).
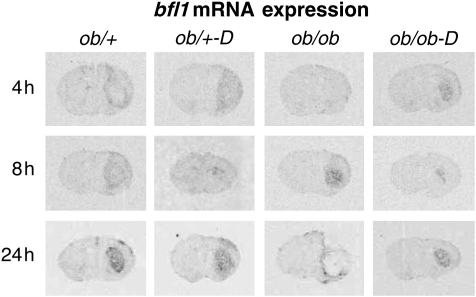
Activation of microglia and astrocytes and the release of numerous proinflammatory cytokines, such as TNFα, IL-1β, and IL-6, have been seen in many neurodegenerative diseases and are believed to be an integral component in the neuropathology of stroke (O'Connor et al, 2006). To determine the effect of darglitazone on microglia and astrocyte activation in the control and diabetic mice, we carried out in situ hybridization for GFAP mRNA as a marker of activated astrocytes and bfl1 mRNA, an antiapoptotic protein, as a marker for microglial activation (Zhang et al, 2004).
Our previous study showed that activation of microglia and astrocytes in nondiabetic db/+ mice could be detected as early as 4 h of recovery and progressively increased over 24 h. However, in the db/db mice, activation of either microglia or astrocytes was barely detectable at 12 h of recovery and never reached control levels. Moreover, the expression was predominantly surrounding the infarct (Zhang et al, 2004). In this study, we observed a similar response in the normoglycemic ob/+ mice, that is, clear bfl1 expression at 4 and 8 h with increases in the striatum at 24 h. However, there was no effect of darglitazone at 4 h with transient suppression at 8 h, consistent with the TNFα response. Furthermore, similar to db/db mice, microglial activation was absent at 4 h in the diabetic ob/ob mice compared with the ob/+ control. However, darglitazone treatment clearly normalized the bfl1 response in the diabetic ob/ob mice, such that the pattern of bfl1 expression was comparable with control mice. Darglitazone also appeared to suppress the bfl1 response in the ob/ob mice at 8 h of recovery (Figure 5).
Figure 5.
Time course of bfl1 mRNA expression in darglitazone-treated ob/+ and ob/ob mice: In situ hybridization analysis. Brains were collected and rapidly frozen at indicated time points of recovery. Cryosections (16 μm) from control and darglitazone-treated ob/+ and ob/ob mice brains were analyzed by in situ hybridization using 35S-labeled riboprobes. Darglitazone activated the microglial response in ob/ob diabetic mice at 4 h of hypoxic–ischemic recovery, and initiated comparable responses with that observed in ob/+ mice throughout 24 h recovery.
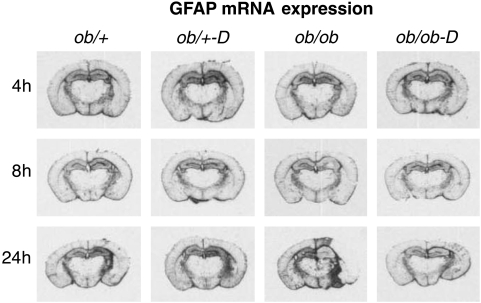
Figure 6 illustrates GFAP expression in the adjacent sections from the same animals as depicted in Figures 3A and 5. Glial fibrillary acidic protein mRNA was minimally altered in the ob/+ mice, with slightly increased expression in the ipsilateral hippocampus at 8 and 24 h. This is a pattern seen in the context of little to no damage after H/I (Zhang et al, 2004) and was unaffected by darglitazone. The pattern of GFAP expression in the ob/ob mice, that is, initial reduction in expression suggestive of early astrocytic death, followed by intense signal in the area surrounding the infarct, is also similar to what has been observed before in the diabetic brain in the context of extensive damage (Rosenson, 2007; Zhang et al, 2004). Darglitazone treatment completely normalized this response, apparently protecting the astrocytes from ischemic death (Figure 6).
Figure 6.
Time course of glial fibrillary acidic protein (GFAP) mRNA expression in control and darglitazone-treated ob/+ and ob/ob mice: In situ hybridization. Astrocytes response was measured by in situ hybridization using GFAP 35S-labeled riboprobe. Darglitazone did not significantly change the GFAP expression in ob/+ mice compared with untreated group. The pattern of GFAP expression in the darglitazone-treated ob/ob mice was comparable with both untreated and darglitazone-treated ob/+ mice and was consistent with minimal damage. The expression pattern seen in the control ob/ob mice was predominantly penumbral and reflected pronounced ischemic damage.
Discussion
This study confirms and extends our previous observations on the impact of stroke (H/I) on the diabetic brain. The results presented here in the ob/ob mice replicate what we have reported for the comparably diabetic db/db mouse. Both sets of diabetic mice show enhanced damage associated with a loss of the early microglial inflammatory response, followed by a delayed and diminished response. In addition, we have now measured CORT in these animals and observed that despite the somewhat elevated baseline CORT levels, the diabetic mice are not able to mount an appropriate CORT response to H/I and further, this response is delayed for 24 h after the insult. In addition, new to this study is the investigation of the role of the PPAR-γ agonist, darglitazone, which belongs to a class of TZD compounds commonly used to treat patients with type II diabetes. Overall, darglitazone treatment restored euglycemia and reduced circulating TG and VLDL in the ob/ob mice, but was without effect in the ob/+ mice. After H/I, darglitazone treatment of diabetic mice resulted in significant neuroprotection associated with a complete restoration of the initial microglial response observed in control mice, as evidenced by increased TNFα, IL-1β, and bfl1 expression in the diabetic brain at 4 h of recovery.
Thiazolidinedione compounds selectively bind and activate PPAR-γ receptors that are ligand-activated transcription factors of the nuclear hormone receptor superfamily, which modulate target genes involved in the regulation of glucose and lipid metabolism, cell growth and differentiation, and various inflammatory responses, both peripherally and in the central nervous system (Bernardo and Minghetti, 2008; Drew et al, 2006; Kapadia et al, 2008; Rosenson, 2007). Several studies have reported a TZD-mediated neuroprotection after middle cerebral artery occlusion (MCAO) in normal rats (Pereira et al, 2006; Shimazu et al, 2005; Sundararajan et al, 2005; Zhao et al, 2006) and mice (Luo et al, 2006; Tureyen et al, 2007). These reports suggest that PPAR-γ activation in brain suppresses microglial activation and macrophage accumulation, and reduces overexpression of neurodegenerative target genes such as inducible nitric oxide synthase and cyclooxygenase-2. It also promotes neuronal survival by decreasing the rates of neuronal apoptosis and reducing glutamate release, and subsequently N-methyl--aspartic acid agonist-mediated neuronal death (Culman et al, 2007; Sundararajan et al, 2005; Zhao et al, 2006). Various PPAR-γ agonists have been shown to suppress the proinflammatory response mediated by microglia/macrophage after cerebral ischemic–reperfusion injury (Luo et al, 2006; Woster and Combs, 2007).
Consistent with the suppression of microglial activation, several studies have reported the reduction of proinflammatory cytokines by PPAR-γ agonists. Troglitazone treatment reduced IL-1β mRNA in rat brain after 24 h of reperfusion in the MCAO model (Sundararajan et al, 2005). Pioglitazone has been shown to reduce TNFα in the rat MCAO model after 24 h of recovery (Zhao et al, 2006). It is interesting that, despite their extensive use in the treatment of type II diabetes and several reviews indicating their efficacy in improving stroke outcome in diabetic patients (Culman et al, 2007; Kapadia et al, 2008), only one study examined the effects of PPAR-γ agonists on stroke recovery in an animal model of diabetes (Tureyen et al, 2007). In this study, long-term oral administration (21 days) of rosiglitazone reduced infarct volume equally in db/db and db/+ mice (47 and 50%, respectively), whereas acute administration, immediately before or after MCAO, reduced infarct volumes by only 21% in db/db, but by 54% in the db/+ mice (Tureyen et al, 2007). Surprisingly, chronic rosiglitazone treatment reduced only the blood glucose levels in the db/db mice by 32% (433 versus 303 mg/dL), and thus the animals would still be considered diabetic. The duration of the MCAO procedure had to be substantially reduced from the standard 2 h of occlusion to 45 mins, as the former condition resulted in 75% mortality in the db/db mice. Within the same study, metformin, another commonly prescribed diabetic medication, reduced blood glucose levels by the same percentage as rosiglitazone but was not neuroprotective. Tureyen et al (2007) also reported a reduced inflammatory gene expression such as IL-6 and IL-1β by rosiglitazone in db/+ and db/db mice after 45 mins of ischemia and 6 h of reperfusion, which represents the earliest time point investigated in any of the TZD studies.
As reported in our previous studies, there is a delay in the onset of proinflammatory cytokines TNFα and IL-1β after H/I. It is therefore somewhat intriguing to observe that darglitazone treatment in the ob/ob mice, rather than further suppressing the microglial activation, initiates an early upregulation of proinflammatory cytokine expression, especially TNFα, which is comparable with that observed in control mice. Tumor necrosis factor-α is unusual among stroke mediators, as it appears to be involved in every facet of stroke and has been considered an alarm hormone in response to stress, associated with both cell death and cell survival depending on the induction of inflammation or control and resolution of inflammation (Hallenbeck, 2002). Tumor necrosis factor-α knockout mice have shown a relatively normal cytokine response to lipopolysaccharide, but subsequently show a disorganized immune response that leads to cell death, whereas other studies have shown that TNFα is cytoprotective in acute brain ischemia (Bruce et al, 1996; Marino et al, 1997).
Interleukin-1β expression was also upregulated at 4 h of H/I recovery in darglitazone-treated diabetic mice. Earlier observations have indicated that IL-1β is necessary for the induction of growth factors such as ciliary neurotrophic factor (CNTF) and insulin growth factor-1 (IGF-1) (Herx et al, 2000; Mason et al, 2001). In our previous report, we also noted a delay in the CNTF expression but not in IGF-1 in db/db mice compared with db/+ after H/I (Kumari et al, 2007). Thus, the early activation of cytokines by darglitazone and the reduction in stroke size highlight a potential role of these proinflammatory cytokines to provide trophic support and protect the brain from ischemic cell death. It is important to note that darglitazone has no suppressive effect on the initial cytokine responses (4 h) in the control mice, but their subsequent expression in both control and diabetic mice is markedly reduced at 8 h, as is the microglial bfl1 response. These observations suggest a biphasic microglial response, with the early response essential to promote recovery and only the latter suppressed by the TZD, which is consistent with the studies cited above where the earliest recovery time point was 6 h.
At present, we do not know how PPAR-γ activation controls the microglial activity. Studies in cultured microglia and peripheral macrophages have suggested that PPAR-γ agonists, in addition to reducing TNFα and IL-1β expression, reduced Toll receptor (TLR2 and TLR4) expression and corresponding ligand-induced nuclear factor-κB activity (Dasu et al, 2009; Hounoki et al, 2008; Murakami et al, 2007; Woster and Combs, 2007). In all of the in vivo studies with the various PPAR-γ agonists, it is not clear whether there is a direct interaction with the microglia or whether they are mediated through an intermediate; however, they are consistent with that observed with in vitro and peripheral effects. In addition, in this study, the extent to which restoration of euglycemia and lipid balance with darglitazone treatment might influence the inflammatory response in the diabetic animals has yet to be determined. In the study by Tureyen et al (2007), the observation that rosiglitazone exerts a diminished acute neuroprotective response when given immediately before 2 h after occlusion suggests that both restoration of euglycemia and specific microglial interactions are important.
It has long been appreciated that the inhibition of leptin signaling that results from the mutations in the ob/ob mice and db/db mice leads to an elevation of CORT levels, which in turn have been linked to the suppression of inflammatory responses (Butcher and Lord, 2004; Fiuza and Suffredini, 2001). Therefore, it is important to note that accompanying the reduced blood glucose and lipid-lowering effect, darglitazone significantly decreased the CORT level in both control and diabetic ob/ob mice after 4 h of H/I recovery, but this effect was lost at later time points of H/I recovery. A similar reduction in CORT level was earlier reported in db/db mice after 2 weeks of treatment with rosiglitazone and muraglitazar (Harrity et al, 2006). Interestingly, the PPAR-γ agonist, rosiglitazone, markedly attenuated 11β hydroxysteroid dehydrogenase (11βHSD-1) gene expression in adipose tissue of db/db mice. 11βHSD-1 is responsible for the conversion of inactive glucocorticoids into active cortisol and CORT in the periphery, thus reducing the levels of this enzyme, which leads to reduced circulating levels CORT (Berger et al, 2001). If such a mechanism occurs in our ob/ob mice in response to darglitazone, it is tempting to speculate that restoration of near-normal CORT levels at 4 h of recovery promotes the proinflammatory cytokine secretion and enhanced microglial response that leads to improved recovery.
The review by Glezer and Rivest (2004) suggests that microglia are the primary sensors to changes in the microenvironment in the brain and their activation is believed to have a beneficial role in the host defense. They also proposed that ‘the identification of molecules having the ability to make the difference between beneficial and detrimental outcome will have a major impact for the treatment of neurodegenerative disease' (Glezer and Rivest, 2004). Darglitazone could be a potential candidate as it enhances the initial microglial response that initiates the inflammatory cascade in the diabetic mice to levels seen in control mice, but subsequently suppresses the inflammatory response as recovery progressed. Darglitazone is also clearly effective in controlling blood glucose and lipid metabolism, and in reducing CORT in diabetic ob/ob mice. The extent to which the actions of darglitazone are mediated by restoring metabolic homeostasis or by direct neural interaction remains to be determined.
Acknowledgments
We thank Pfizer USA for providing darglitazone.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Aleo MD, Lundeen GR, Blackwell DK, Smith WM, Coleman GL, Stadnicki SW, Kluwe WM. Mechanism and implications of brown adipose tissue proliferation in rats and monkeys treated with the thiazolidinedione darglitazone, a potent peroxisome proliferator-activated receptor-gamma agonist. J Pharmacol Exp Ther. 2003;305:1173–1182. doi: 10.1124/jpet.102.042648. [DOI] [PubMed] [Google Scholar]
- Berger J, Michael T, Elbrecht A, Hermanowski-Vosatka A, Moller D, Wright S, Thieringer R. Peroxisome proliferators-activated receptor-y ligands inhibit adipocyte 11β-hydroxysteroid dehydrogenase type-1 expression and activity. J Biol Chem. 2001;276:12629–12635. doi: 10.1074/jbc.M003592200. [DOI] [PubMed] [Google Scholar]
- Bernardo A, Minghetti L. Regulation of glial cell functions by PPAR-gamma natural and synthetic agonists. PPAR Res. 2008;2008:864140. doi: 10.1155/2008/864140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernotiene E, Palmer G, Talabot-Ayer D, Szalay-Quinodoz I, Aubert ML, Gabay C. Delayed resolution of acute inflammation during zymosan-induced arthritis in leptin-deficient mice. Arthritis Res Ther. 2004;6:R256–R263. doi: 10.1186/ar1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonow RO, Gheorghiade M. The diabetes epidemic: a national and global crisis. Am J Med. 2004;116 (Suppl 5A:2S–10S. doi: 10.1016/j.amjmed.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Bruce A, Boling W, Kindy M, Peschon J, Kraemer P, Carpenter M, Holtsberg F, Mattson M. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. 1996;2:788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- Butcher SK, Lord JM. Stress responses and innate immunity: aging as a contributory factor. Aging Cell. 2004;3:151–160. doi: 10.1111/j.1474-9728.2004.00103.x. [DOI] [PubMed] [Google Scholar]
- Culman J, Zhao Y, Gohlke P, Herdegen T. PPAR-gamma: therapeutic target for ischemic stroke. Trends Pharmacol Sci. 2007;28:244–249. doi: 10.1016/j.tips.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Dasu MR, Park S, Devaraj S, Jialal I. Pioglitazone inhibits Toll-like receptor expression and activity in human monocytes and db/db mice. Endocrinology. 2009;150:3457–3464. doi: 10.1210/en.2008-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew PD, Xu J, Storer PD, Chavis JA, Racke MK. Peroxisome proliferator-activated receptor agonist regulation of glial activation: relevance to CNS inflammatory disorders. Neurochem Int. 2006;49:183–189. doi: 10.1016/j.neuint.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Fiuza C, Suffredini AF. Human models of innate immunity: local and systemic inflammatory responses. J Endotoxin Res. 2001;7:385–388. [PubMed] [Google Scholar]
- Folbergrova J, Memezawa H, Smith ML, Siesjo BK. Focal and perifocal changes in tissue energy state during middle cerebral artery occlusion in normo- and hyperglycemic rats. J Cereb Blood Flow Metab. 1992;12:25–33. doi: 10.1038/jcbfm.1992.4. [DOI] [PubMed] [Google Scholar]
- Glezer I, Rivest S. Glucocorticoids: protectors of the brain during innate immune responses. Neuroscientist. 2004;10:538–552. doi: 10.1177/1073858404263494. [DOI] [PubMed] [Google Scholar]
- Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nat Med. 2002;8:1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- Harrity T, Farrelly D, Tieman A, Chu C, Kunselman L, Gu L, Ponticiello R, Cap M, Qu F, Shao C, Wang W, Zhang H, Fenderson W, Chen S, Devasthale P, Jeon Y, Seethala R, Yang WP, Ren J, Zhou M, Ryono D, Biller S, Mookhtiar KA, Wetterau J, Gregg R, Cheng PT, Hariharan N. Muraglitazar, a novel dual (alpha/gamma) peroxisome proliferator-activated receptor activator, improves diabetes and other metabolic abnormalities and preserves beta-cell function in db/db mice. Diabetes. 2006;55:240–248. [PubMed] [Google Scholar]
- Herx LM, Rivest S, Yong VW. Central nervous system-initiated inflammation and neurotrophism in trauma: IL-1 beta is required for the production of ciliary neurotrophic factor. J Immunol. 2000;165:2232–2239. doi: 10.4049/jimmunol.165.4.2232. [DOI] [PubMed] [Google Scholar]
- Hounoki H, Sugiyama E, Mohamed SG, Shinoda K, Taki H, Abdel-Aziz HO, Maruyama M, Kobayashi M, Miyahara T. Activation of peroxisome proliferator-activated receptor gamma inhibits TNF-alpha-mediated osteoclast differentiation in human peripheral monocytes in part via suppression of monocyte chemoattractant protein-1 expression. Bone. 2008;42:765–774. doi: 10.1016/j.bone.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Hulin B, McCarthy PA, Gibbs EM. The glitazone family of antidiabetic agents. Current Pharmaceutical Design. 1996a;2:85–102. [Google Scholar]
- Hulin B, Newton LS, Lewis DM, Genereux PE, Gibbs EM, Clark DA. Hypoglycemic activity of a series of alpha-alkylthio and alpha-alkoxy carboxylic acids related to ciglitazone. J Med Chem. 1996b;39:3897–3907. doi: 10.1021/jm960230h. [DOI] [PubMed] [Google Scholar]
- Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci. 2008;13:1813–1826. doi: 10.2741/2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari R, Willing LB, Krady JK, Vannucci SJ, Simpson IA. Impaired wound healing after cerebral hypoxia–ischemia in the diabetic mouse. J Cereb Blood Flow Metab. 2007;27:710–718. doi: 10.1038/sj.jcbfm.9600382. [DOI] [PubMed] [Google Scholar]
- Li J, Thorne LN, Punjabi NM, Sun CK, Schwartz AR, Smith PL, Marino RL, Rodriguez A, Hubbard WC, O'Donnell CP, Polotsky VY. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res. 2005;97:698–706. doi: 10.1161/01.RES.0000183879.60089.a9. [DOI] [PubMed] [Google Scholar]
- Liu Y, Nakagawa Y, Wang Y, Sakurai R, Tripathi PV, Lutfy K, Friedman TC. Increased glucocorticoid receptor and 11{beta}-hydroxysteroid dehydrogenase type 1 expression in hepatocytes may contribute to the phenotype of type 2 diabetes in db/db mice. Diabetes. 2005;54:32–40. doi: 10.2337/diabetes.54.1.32. [DOI] [PubMed] [Google Scholar]
- Luo Y, Yin W, Signore A, Zhang F, Hong Z, Wang S, Graham S, Chen J. Neuroprotection against focal ischemic brain injury by the peroxisome proliferated-activated receptor-y agonist rosiglitazone. J Neurochem. 2006;97:435–448. doi: 10.1111/j.1471-4159.2006.03758.x. [DOI] [PubMed] [Google Scholar]
- Marino MW, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, Jungbluth A, Wada H, Moore M, Williamson B, Basu S, Old LJ. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JL, Suzuki K, Chaplin DD, Matsushima GK. Interleukin-1beta promotes repair of the CNS. J Neurosci. 2001;21:7046–7052. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K, Bujo H, Unoki H, Saito Y. Effect of PPARalpha activation of macrophages on the secretion of inflammatory cytokines in cultured adipocytes. Eur J Pharmacol. 2007;561:206–213. doi: 10.1016/j.ejphar.2006.12.037. [DOI] [PubMed] [Google Scholar]
- Nedergaard M. Transient focal ischemia in hyperglycemic rats is associated with increased cerebral infarction. Brain Res. 1987;408:79–85. doi: 10.1016/0006-8993(87)90360-x. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Diemer NH. Focal ischemia of the rat brain, with special reference to the influence of plasma glucose concentration. Acta Neuropathol. 1987;73:131–137. doi: 10.1007/BF00693778. [DOI] [PubMed] [Google Scholar]
- O'Connor JC, Johnson DR, Freund GG. Psychoneuroimmune implications of type 2 diabetes. Neurol Clin. 2006;24:539–559. doi: 10.1016/j.ncl.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Oakes ND, Thalen PG, Jacinto SM, Ljung B. Thiazolidinediones increase plasma-adipose tissue FFA exchange capacity and enhance insulin-mediated control of systemic FFA availability. Diabetes. 2001;50:1158–1165. doi: 10.2337/diabetes.50.5.1158. [DOI] [PubMed] [Google Scholar]
- Pereira MP, Hurtado O, Cardenas A, Bosca L, Castillo J, Davalos A, Vivancos J, Serena J, Lorenzo P, Lizasoain I, Moro MA. Rosiglitazone and 15-deoxy-delta12,14-prostaglandin J2 cause potent neuroprotection after experimental stroke through noncompletely overlapping mechanisms. J Cereb Blood Flow Metab. 2006;26:218–229. doi: 10.1038/sj.jcbfm.9600182. [DOI] [PubMed] [Google Scholar]
- Rosenson RS. Effects of peroxisome proliferator-activated receptors on lipoprotein metabolism and glucose control in type 2 diabetes mellitus. Am J Cardiol. 2007;99:96B–104B. doi: 10.1016/j.amjcard.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Shimazu T, Inoue I, Araki N, Asano Y, Sawada M, Furuya D, Nagoya H, Greenberg JH. A peroxisome proliferator-activated receptor-gamma agonist reduces infarct size in transient but not in permanent ischemia. Stroke. 2005;36:353–359. doi: 10.1161/01.STR.0000152271.21943.a2. [DOI] [PubMed] [Google Scholar]
- Stumvoll M, Haring HU. Glitazones: clinical effects and molecular mechanisms. Ann Med. 2002;34:217–224. [PubMed] [Google Scholar]
- Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator-activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005;130:685–696. doi: 10.1016/j.neuroscience.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Tureyen K, Kapadia R, Bowen KK, Satriotomo I, Liang J, Feinstein DL, Vemuganti R. Peroxisome proliferator-activated receptor-gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J Neurochem. 2007;101:41–56. doi: 10.1111/j.1471-4159.2006.04376.x. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Maher F, Simpson IA. Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia. 1997;21:2–21. doi: 10.1002/(sici)1098-1136(199709)21:1<2::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Willing LB, Goto S, Alkayed NJ, Brucklacher RM, Wood TL, Towfighi J, Hurn PD, Simpson IA. Experimental stroke in the female diabetic, db/db, mouse. J Cereb Blood Flow Metab. 2001a;21:52–60. doi: 10.1097/00004647-200101000-00007. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Willing LB, Goto S, Alkayed NJ, Brucklacher RM, Wood TL, Towfighi J, Hurn PD, Simpson IA. Experimental stroke in the female diabetic, db/db, mouse. J Cereb Blood Flow Metab. 2001b;21:52–60. doi: 10.1097/00004647-200101000-00007. [DOI] [PubMed] [Google Scholar]
- Woster AP, Combs CK. Differential ability of a thiazolidinedione PPARgamma agonist to attenuate cytokine secretion in primary microglia and macrophage-like cells. J Neurochem. 2007;103:67–76. doi: 10.1111/j.1471-4159.2007.04706.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Nair A, Krady K, Corpe C, Bonneau RH, Simpson IA, Vannucci SJ. Estrogen stimulates microglia and brain recovery from hypoxia–ischemia in normoglycemic but not diabetic female mice. J Clin Invest. 2004;113:85–95. doi: 10.1172/JCI200418336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Patzer A, Herdegen T, Gohlke P, Culman J. Activation of cerebral peroxisome proliferator-activated receptors gamma promotes neuroprotection by attenuation of neuronal cyclooxygenase-2 overexpression after focal cerebral ischemia in rats. FASEB J. 2006;20:1162–1175. doi: 10.1096/fj.05-5007com. [DOI] [PubMed] [Google Scholar]