Abstract
Hypothermia reduces neuronal damage after cerebral ischemia and traumatic brain injury, while hyperthermia exacerbates damage from these insults. Previously we have shown that temperature-dependent modulation of excitotoxic neuronal death is mediated in part by temperature-dependent changes in the synaptic release/translocation of Zn2+. In this study, we hypothesize that brain temperature also affects hypoglycemia-induced neuronal death by modulation of vesicular Zn2+ release from presynaptic terminals. To test our hypothesis, we used a rat model of insulin-induced hypoglycemia. Here we found that hypoglycemia-induced neuronal injury was significantly affected by brain temperature, that is, hypothermia inhibited while hyperthermia aggravated neuronal death. To investigate the mechanism of temperature-dependent neuronal death after hypoglycemia, we measured zinc release/translocation, reactive oxygen species (ROS) production, and microglia activation. Here we found that hypoglycemia-induced Zn2+ release/translocation, ROS production, and microglia activation were inhibited by hypothermia but aggravated by hyperthermia. Even when the insult was accompanied by hyperthermic conditions, zinc chelation inhibited ROS production and microglia activation. Zinc chelation during hyperthermia reduced neuronal death, superoxide production, and microglia activation, which was comparable to the protective effects of hypothermia. We conclude that neuronal death after hypoglycemia is temperature-dependent and is mediated by increased Zn2+ release, superoxide production, and microglia activation.
Keywords: hypoglycemia, hypothermia, hyperthermia, zinc, superoxide, microglia
Introduction
Hypoglycemia-induced neuronal death is a potential complication encountered during intensive control of type-1 diabetes. It may result from tight control of blood glucose levels with insulin or other hypoglycemic agents, such as sulfonylurea (Seltzer, 1989). The risk of hypoglycemia-induced brain injury is also increased in patients with frequent hypoglycemic episodes or in patients with long-standing, type-1 diabetes due to loss of early warning symptoms. Twenty-five years ago Auer et al (1984a) established that severe hypoglycemia results in neuronal cell death, which may lead to cognitive impairments (McCrimmon and Frier, 1994). Our laboratory has recently demonstrated that glucose reperfusion after a hypoglycemic episode can trigger the neuronal death cascade rather than rescuing neurons (Suh et al, 2007). Put simply, the neuronal cell death, which arises after hypoglycemia, is not simply a result of energy failure resulting from lack of glucose, but is instead the result of a cell death program that is initiated by the re-introduction of glucose after a period of hypoglycemia (herein referred to as glucose reperfusion; Auer et al, 1986; Suh et al, 2007). Unfortunately there is currently no available means to resuscitate a hypoglycemic individual other than glucose infusion. Thus, we have suggested several strategies to prevent the neuronal death cascade, including inhibition of PARP-1 activation, chelation of synaptically released zinc, prevention of superoxide production, or supplementation with pyruvate during the glucose reperfusion period (Suh et al, 2003; Suh et al, 2005; Suh et al, 2004; Suh et al, 2007; Suh et al, 2008). However, these approaches require experimental validation and refinement prior to clinical application. In this study we test the hypothesis that hypothermia may be neuroprotective in this setting.
Mild systemic hypothermia has been identified as one of the most protective approaches to prevent neuronal death after cerebral ischemia (Busto et al, 1987) and traumatic brain injuries (Clifton et al, 1991; Suh et al, 2006), although the clinical outcome of hypothermia on the above brain injuries is still controversial (Clifton et al, 2001). The proposed neuroprotective mechanisms of mild systemic hypothermia are based on decreases in cerebral metabolic requirement (Erecinska et al, 2003), intracranial pressure (Soukup et al, 2002), glutamate release from presynaptic vesicles (Arai et al, 1993), free-radical generation (Globus et al, 1995), and inflammatory reaction (Kumar and Evans, 1997).
Previously we have shown that hypoglycemia-induced neuronal death is mediated by presynaptic release and postsynaptic translocation of zinc (Suh et al, 2004). Subsequently, it has been shown that intracellular zinc accumulation promotes the production of reactive oxygen species (ROS) through NADPH oxidase activation after hypoglycemia (Suh et al, 2007). Hypoglycemia-induced ROS production and subsequent neuronal death were prevented by an extracellular zinc chelation. Therefore, it is clear that hypoglycemia-induced neuronal death is triggered by zinc translocation into postsynaptic neurons. In addition to this direct role of zinc on neuron death described in a variety of brain insults (Koh et al, 1996; Suh et al, 2000a; Suh et al, 2004), it has also been shown recently that synaptically released zinc triggers microglia activation in primary cell culture and in vivo after transient cerebral ischemia. This suggests that zinc release is not only directly neurotoxic, but also indirectly affects neurons through microglia activation (Kauppinen et al, 2008). In light of the finding that neuronal death after traumatic brain injury is mediated in part by temperature-dependent modulation of presynaptic release of zinc (Suh et al, 2000b) and postsynaptic zinc translocation (Suh et al, 2006), we here hypothesized that brain temperature also affects hypoglycemia-induced neuronal death by modulation of vesicular zinc release from presynaptic terminals, superoxide production, and microglia activation.
This study shows that hypoglycemia-induced neuronal death can be prevented by hypothermia and can be aggravated by hyperthermia. Thus, hypothermia represents a promising clinical intervention for treatment of hypoglycemia.
Materials and methods
Animal Surgery and Insulin-Induced Hypoglycemia
This study was approved by the San Francisco Veterans Affairs Medical Center animal studies committee. Hypoglycemia was induced by injection of regular human insulin as described by Auer et al (1984a), with minor modifications (Suh et al, 2003). Briefly, male Sprague–Dawley rats (Charles River Laboratories, Gilroy, CA, USA) weighing between 250 and 350 g were fasted overnight, but allowed free access to water. Hypoglycemia was induced by intraperitoneal injection of 10 IU/kg of insulin (Novolin-R, Novo Nordisk, Clayton, NC, USA). The rats were anesthetized with 2% isoflurane in a 75:25 mixture of nitrous oxide and oxygen. After tracheal intubation, controlled ventilation (Harvard Apparatus, South Natick, MA, USA) was initiated and anesthesia was maintained with 1% isoflurane. A 26-gauge polyvinyl catheter was introduced into the femoral artery for continuous arterial blood pressure monitoring and blood sampling, and another 26-gauge polyvinyl catheter was placed in the femoral vein for glucose infusion. Cranial trephinations were performed over bilateral parietal cortex for electroencephalogram (EEG) monitoring (BIOPAC system Inc., Santa Barbara, CA, USA). Two monopolar electrodes were placed beneath the dura and another reference electrode was inserted into neck muscle. Brain temperature was measured from temporalis muscle and maintained at 36.5°C to 37.5°C using warming pad until termination of isoelectric EEG (iso-EEG) period. Hypoglycemia was terminated after 30 mins of iso-EEG to generate a reproducible brain injury of moderate severity (Auer et al, 1984b). During the entire period of EEG isoelectricity, mean arterial blood pressure was maintained between 120 and 180 mm Hg by adjusting isoflurane. Bradycardia and respiratory tract secretions were prevented by administration of 1 mg/kg of atropine. Hypoglycemia was terminated by administration of 0.2 ml of 50% glucose through the femoral vein, followed by continuous intravenous infusion of 1:1 solution of 50% glucose and Krebs'–Henseleit buffer at a rate of 1.5 ml/h for 3 h. At the point of terminating hypoglycemia, subjects were allocated to one of three groups: hypothermia (33.0°C to 34.0°C, n=5), normothermia (36.5°C to 37.5°C, n=5), and hyperthermia (39.0°C to 40.0°C, n=6) during a glucose reperfusion period of 1 h. Body cooling was conducted by fanning after spraying 70% ethanol onto the rat's body in hypothermia group (Suh et al, 2006; Yanamoto et al, 2001). Body warming was performed with a heating pad and a heating lamp. Hypothermia or hyperthermia was achieved within 5 mins, and the designated brain temperature was maintained for 1 h. After 1-h glucose reperfusion, the hypothermia or hyperthermia groups were re-warmed or re-cooled to allow return to normothermia, respectively. Rats were recovered from anesthesia during two additional hours of intravenous glucose infusion under normothermic condition. The experimental protocol for brain temperature manipulations before and after hypoglycemia is illustrated in Figure 1A. For sham hypoglycemia, rats were fasted overnight and treated with the same dose of insulin, but maintained normoglycemia and normothermia followed by immediate administration of glucose.
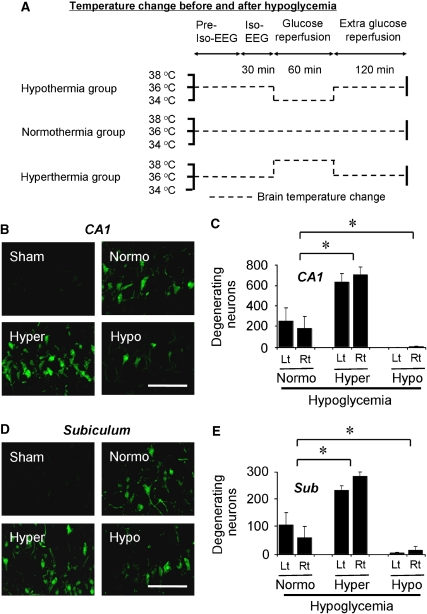
Figure 1.
Hypoglycemia-induced neuronal death is prevented by hypothermia. (A) Experimental protocol for brain temperature control before and after hypoglycemia. Brain temperature was maintained as in this schematic drawing. One hour after glucose reperfusion, brain temperature was raised or decreased, and then maintained at 36.5°C to 37.5°C until rats were fully recovered. (B–E) Hypoglycemia-induced neuronal death is aggravated by hyperthermia but is prevented by hypothermia. Confocal fluorescence images show neuronal death in hippocampal CA1 (B, C) and subiculum (Sub) (D, and E) at 7 days after hypoglycemia. Almost no FJB (+) neurons were seen in the section from sham-operated rat (Sham). FJB (+) neurons were apparent in normothermic-hypoglycemia (Normo) hippocampal section. The number of FJB (+) neurons was significantly reduced in hypothermic-hypoglycemia rats (Hypo). However, the number of positive neurons was substantially increased in hyperthermic-hypoglycemia rats (Hyper). The bar graph shows the quantitated neuronal degeneration in hippocampal CA1 (C) and in the Sub (E) from the normothermia, hyperthermia, and hypothermia-subjected group at 7 days after hypoglycemia. Lt, left hemisphere; Rt, right hemisphere. Scale bar=100 μm. Data are mean±s.e.m. (n=5 to 6); *P<0.05 compared with the normothermic glucose-reperfusion group.
Zinc Chelation
To deplete vesicular zinc in the synaptic space, this study used two different kinds of zinc chelators; the extracellular zinc chelator, CaEDTA (5 μl/100 mmol/L), was injected into the lateral ventricle (intracerebroventricularly) immediately before termination of iso-EEG. The cell-permeant zinc chelator, clioquinol (CQ), was injected into intraperitoneal space (intraperitoneally) immediately before termination of iso-EEG with a dose of 30 mg/kg after dilution with dimethylsulfoxide (final dimethylsulfoxide concentration was 1%).
Assessment of Neuronal Death
The rats were deeply anesthetized with isoflurane 7 days after hypoglycemia and killed by transcardial perfusion with 200 ml of 0.9% saline followed by 4% paraformaldehyde for 5 mins. The harvested brains were post-fixed for 24 h and immersed in 20% sucrose. Cryostat sections (25 μm thickness) were mounted on superfrosted coated slides (Fisher Scientific, Pittsburgh, PA, USA). For identifying degenerating neurons in the hippocampus, Fluoro-Jade B (FJB) staining was performed as described previously (Schmued and Hopkins, 2000). Briefly, sections were immersed in a basic alcohol solution for 5 min and 0.06% KMnO4 for 15 mins, and then the sections were incubated in 0.0004% FJB (Histo-Chem, Jefferson, AR, USA) for 20 mins. The slides were washed in distilled water and dried. To quantify neuronal death, sections were collected every third cut from 4.0 mm posterior to bregma and five coronal sections were analyzed from each animal. An observer masked to the temperature condition counted the number of FJB-positive (+) neurons in the hippocampal CA1, subiculum from both hemispheres. The mean numbers of FJB (+) neurons from each region were used for statistical analyses.
Zinc-Specific Fluorescence Staining
For fluorescence visualization of Zn2+, brain sections were stained with N-(6-methoxy-8-quinolyl)-para-toluenesulfonamide (TSQ) as described previously (Frederickson et al, 1987). To detect vesicular zinc release from hippocampal hilus, rats were killed 3 h after beginning of glucose reperfusion after hypoglycemia. To detect zinc translocation into hippocampal CA1 neurons, rats were killed 7 days after hypoglycemia. For TSQ staining, rats were anesthetized by 3% isoflurane and the brains were harvested without saline perfusion and frozen in powdered dry ice. Without paraformaldehyde fixation, coronal sections including hippocampus (20 μm thickness) were prepared using a cryostat, and then mounted on to gelatin-coated slides and dried by air. The sections were immersed in a solution of 4.5 μmol/L TSQ (Molecular Probes, Eugene, OR, USA) for 60 secs, and then rinsed with 0.9% saline for 60 secs. TSQ fluorescence was examined with a fluorescence microscope. For quantification of TSQ signal intensity, the mean fluorescence intensity within the designated regions of interest (mossy-fiber terminal area) was measured with Adobe Photoshop (6.0). Background correction was made by subtracting the mean intensity measured in 100 μm2 of lateral ventricle. An observer masked to the temperature condition measured TSQ intensity and counted TSQ-positive (+) neurons in hippocampi from both hemispheres.
Superoxide Detection
To evaluate the differences in superoxide production among these three groups, rats were administered with dihydroethidium (Molecular Probes, Invitrogen, Eugene, OR, USA) at a dose of 1 mg/kg (1 mg/ml solution in 1% dimethylsulfoxide) by the femoral vein at the onset of EEG isoelectricity (Murakami et al, 1998). Rats were killed 3 h after hypoglycemia and were transcardially perfused with 0.9% saline followed by 4% paraformaldehyde fixation. In 40-μm cryostat sections, ethidium (Et) signals were photographed with a confocal fluorescence microscope with an excitation of 510 to 550 nm and an emission greater than 580 nm. Signal intensity of Et was expressed as the ratio of the mean fluorescence in neuronal perikariya to that in the striatum radiatum of the hippocampal CA1 region.
Immunostaining for Evaluation of Microglia Activation
Immunostaining was performed on paraformaldehyde-fixed and coronally sectioned brain tissue at a thickness of 40 μm. After 1-mmol/L phosphate-buffered saline rinse, nonspecific protein binding of the brain tissue were blocked by a 1-h incubation in blocking buffer (10% goat serum and 0.1% Triton X-100 in 1 mmol/L phosphate-buffered saline) at room temperature. The sections were then immunostained with a mouse antibody to rat CD11b (Serotec, Raleigh, NC, USA) at 1:200 dilution. After washing, the sections were incubated with Alexa Fluor 488-conjugated goat anti-mouse IgG secondary antibody (Molecular Probes, Invitrogen) at a dilution of 1:500 for 2 h at room temperature. Negative control performed with secondary antibody alone showed no staining. Microglia activation was evaluated by an observer masked to the temperature condition. Three sections from each animal were evaluated for scoring. Microglia activation criteria were based on the number of CD11b-immunoreactive cells and their morphology (Kauppinen et al, 2008).
Statistical Analysis
Data are shown as means±s.e.m. Statistical significance was assessed by analysis of variance and post hoc testing was performed using Scheffe's test. P-value less than 0.05 was considered statistically significant. Microglia activation data were assessed by Kruskal–Wallis non-parametric one-way analysis of variance test followed by Dunn's test for multiple group comparison.
Results
Hypoglycemia-Induced Neuronal Cell Death is Temperature-Dependent
FJB staining at 7 days after hypoglycemia showed widespread hippocampal neuronal injury in the normothermia group, at a magnitude similar to that described in previous studies (Auer et al, 1984a; Suh et al, 2007). In sham-operated animals, no FJB (+) neurons were observed. Compared with the normothermia group, the hypothermia group showed significantly fewer FJB (+) neurons, while the hyperthermia group showed substantially increased number of FJB (+) neurons (Figures 1B–1E). FJB (+) neurons in the hippocampal CA1 (Figure 1C) and subiculum (Figure 1E) area, and dentate gyrus (DG) area (Supplementary Information) showed significant differences among those three groups (Table 1).
Table 1. Brain temperature changes in three different groups before, during, and after hypoglycemia (means±s.e.m.).
| Before Iso-EEG | During Iso-EEG | After Iso-EEG | |
|---|---|---|---|
| Normothermia (n=5) | 36.94±0.14 | 37.10±0.11 | 37.00±0.19 |
| Hypothermia (n=6) | 37.02±0.16 | 37.12±0.14 | 33.71±0.14 |
| Hyperthermia (n=5) | 36.96±0.15 | 37.10±0.19 | 39.26±0.11 |
EEG, electroencephalogram; Iso-EEG; isoelectric EEG.
Hypoglycemia-Induced Zinc Release is Strongly Influenced by Brain Temperature
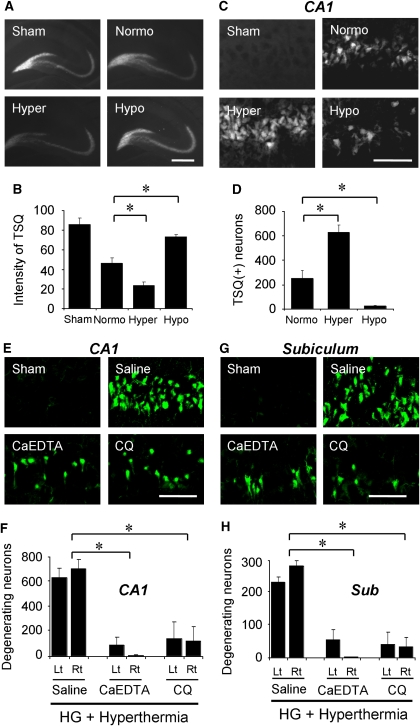
Previously, we showed that zinc release from presynaptic terminals and translocation into postsynaptic neurons contributed to hypoglycemic neuronal injury (Suh et al, 2004; Suh et al, 2008). Also, we showed that zinc release and translocation are strongly affected by brain temperature in traumatic brain injury (Suh et al, 2006). In this study, to investigate whether the vesicular zinc release and translocation is also affected by brain temperature after hypoglycemia, animals were subjected to hypoglycemia under different brain temperature conditions. Compared with sham-operated animals, hypoglycemia followed by normothermic glucose reperfusion showed decrease of TSQ intensity, which represents a release of vesicular zinc from presynaptic terminals (Suh et al, 2004). The magnitude of vesicular zinc release was inhibited by hypothermia, whereas it was increased by hyperthermia. Quantification of TSQ intensity from the hippocampal hilus showed significant differences among these three groups (Figures 2A and 2B).
Figure 2.
Temperature-dependent hypoglycemic neuronal death is mediated by zinc release and translocation. (A–D) Vesicular zinc release and translocation are aggravated by hyperthermia, but are prevented by hypothermia. (A) Representation of TSQ fluorescence images of hippocampus from sham-operated (Sham) and hypoglycemia (HG)-subjected rats. The hypothermia group (Hypo) almost completely prevented synaptic zinc release. Scale bar=500 μm. (B) The bar graph shows the quantitated TSQ fluorescence intensity from the hilar area. Data are mean±s.e.m. (n=7 to 12); *P<0.05 compared with the normothermic reperfusion group. (C) Photomicrographs of TSQ fluorescence staining shows zinc accumulation in the hippocampal CA1 neurons after hypoglycemia. Scale bar=100 μm. (D) The bar graph shows the quantitated TSQ (+) neurons in the CA1 area. Data are mean±s.e.m. (n=5 to 7); *P<0.05 compared with normothermic glucose reperfusion group. (E–H) Zinc chelator, CaEDTA or CQ, prevents hypoglycemia-induced neuronal death in the hyperthermia group. (E, G) FJB (+) neurons were reduced by CaEDTA or CQ injection even after hyperthermic reperfusion. Scale bar=100 μm. (F, H) The graphs represent quantitated neuronal death in the hippocampal CA1 and subiculum area after hypoglycemia. Data are the mean±s.e.m (n=5 to 7); *P<0.05 compared with saline-treated rats.
Hypoglycemia-Induced Zinc Accumulation in CA1 Pyramidal Neurons is Strongly Influenced by Brain Temperature
Our previous study demonstrated that hypoglycemia induces zinc accumulation in hippocampal CA1 neurons (Suh et al, 2004; Suh et al, 2008). In this study, the number of TSQ (+) neurons in the hippocampal CA1 was significantly influenced by brain temperature. The hyperthermia group showed an apparent increase of TSQ (+) neurons, whereas the hypothermia group showed fewer TSQ (+) neurons (Figures 2C and 2D).
Hippocampal Neuronal Death is Prevented by Zinc Chelation Even Under Hyperthermic Glucose Reperfusion Condition Following Hypoglycemia
Zinc chelation has been shown to prevent hypoglycemia-induced zinc accumulation and neuronal death. (Suh et al, 2008). Here we tested whether zinc chelation prevents hypoglycemic neuronal death even under hyperthermic conditions. Both intracerebral zinc chelation by extracellular zinc chelator, CaEDTA, or systemic zinc chelation by CQ substantially decreased the number of FJB (+) neurons in the hippocampal CA1 and subiculum areas (Figures 2E–2H), and DG area (Supplementary Information) in the hyperthermia group. The degree of neuroprotection afforded by zinc chelation in the hyperthermic group was similar to the neuroprotective effects of hypothermia when compared with the normothermia group. Table 2 shows the brain temperature measured from the saline- or zinc chelator-treated groups.
Table 2. Brain temperature changes in the hyperthermia group with zinc chelators before, during, and after hypoglycemia (means±s.e.m.).
| Before Iso-EEG | During Iso-EEG | After Iso-EEG | |
|---|---|---|---|
| Saline (n=5) | 36.96±0.15 | 37.10±0.19 | 39.26±0.11 |
| CaEDTA (n=3) | 37.13±0.12 | 37.13±0.22 | 39.33±0.12 |
| Clioquinol (n=6) | 37.10±0.10 | 37.17±0.13 | 39.35±0.10 |
CaEDTA, calcium EDTA; EEG, electroencephalogram; Iso-EEG; isoelectric EEG.
Hypoglycemia-Induced Superoxide Production is Affected by Brain Temperature
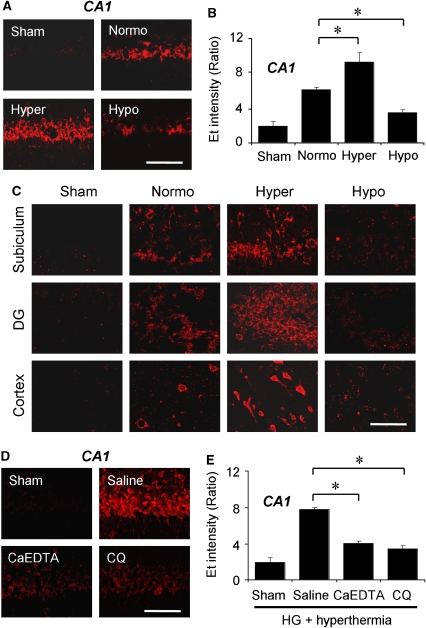
We examined the hypoglycemia-induced superoxide production in the hippocampal CA1, DG, subiculum, and cerebral cortex in the different brain temperature groups. Superoxide production was estimated by Et fluorescence intensity. Previously, we reported that hypoglycemic neuronal death is mediated by superoxide production through activation of neuronal NADPH oxidase (Suh et al, 2007). Therefore, we investigated whether superoxide production is influenced by brain temperature during the glucose reperfusion period. Here we found that Et fluorescence intensity was significantly reduced in hypothermia group as compared with that in the normothermia group, whereas in the hyperthermia group it was significantly increased (Figures 3A–3C).
Figure 3.
Hypoglycemia-induced ROS production is influenced by temperature and zinc translocation. (A) Neuronal superoxide production in the hippocampal CA1 region was detected by dihydroethidium fluorescence staining 3 h after hypoglycemia. Hyperthermia (Hyper) substantially increased Et intensity in CA1 pyramidal neurons an extent comparable to that in the normothermic (Normo) group. However, hypothermia (Hypo) reduced Et fluorescent intensity. Scale bar=100 μm. (B) Intensity of Et signal is expressed as the ratio of the mean fluorescence in neuronal perikaria to the background. Hyperthermia after hypoglycemia markedly increased the intensity of Et fluorescence, but hypothermia significantly reduced it. Data are the mean±s.e.m. (n=5 to 7); *P<0.05 compared with the normothermic glucose reperfusion group. (C) Neuronal superoxide production after hypoglycemia in the hippocampal subiculum, DG, and cortex was detected by dihydroethidium. Et fluorescence intensity is influenced by brain temperature after hypoglycemia. Scale bar=100 μm. (D, E) Zinc chelators prevent hypoglycemia-induced superoxide production in the hyperthermia group. (D) Et fluorescence intensity is reduced by CaEDTA or CQ injection. Scale bar=100 μm. (E) Quantitated Et intensity in the hippocampal CA1 area. Data are the mean±s.e.m. (n=5-7); *P<0.05 compared with saline-treated animals.
Hypoglycemia-Induced Superoxide Production is Inhibited by Zinc Chelation Even Under Hyperthermic Reperfusion Condition after Hypoglycemia
Both intracerebral zinc chelation by CaEDTA or CQ substantially decreased Et intensity in the hippocampal CA1 pyramidal area even under hyperthermic reperfusion conditions (Figures 3D and 3E). The reduction of Et fluorescence intensity by zinc chelation in hyperthermic group was similar to that seen when comparing hypothermia with normothermia.
Hypothermia Inhibits Hypoglycemia-Induced Microglia Activation
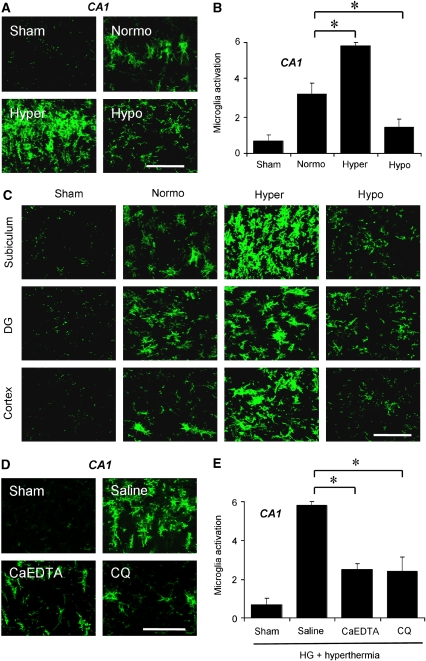
During severe hypoglycemia, glucose reperfusion and its neurotoxic cascade may not only damage neurons directly, but may also promote neuronal injury indirectly through microglia activation. Microglia activation is a gradual process including change of morphology from highly ramified into an amoeboid shape, proliferation, migration to injury site, increased expression of surface molecules, increased secretion of cytokines, chemokines, free radicals and proteases, and assumption of phagocytotic activity (Kreutzberg, 1996). In an effort to elucidate another neuroprotective effect of hypothermia, we investigated a degree of microglia activation after hypoglycemia. Compared with sham-operated rats, microglia were highly active after hypoglycemic insult in the normothermic group. In parallel with vesicular zinc translocation and superoxide formation, microglia activation was also affected by brain temperature. Microglia activation was significantly reduced by hypothermia, but aggravated by hyperthermia in the hippocampal CA1, subiculum, and hilus, and in the cerebral cortex (Figures 4A–4C).
Figure 4.
Hypoglycemia-induced microglia activation is influenced by temperature and zinc translocation. (A) Morphological change and intensity of immunostaining of microglia after hypoglycemia is affected by brain temperature. Hyperthermia (Hyper) substantially increased microglia activation in the hippocampal CA1 region to an extent comparable to that in normothermic (Normo) animals. However, hypothermia (Hypo) apparently reduced microglia activation in the above areas. Scale bar=100 μm. (B) Quantification of microglia activation was performed in the hippocampal CA1 area. As shown in the images, microglia activation is strongly affected by brain temperature. Data are mean±s.e.m. (n=3 to 6); *P<0.05 compared with the normothermic glucose reperfusion group. (C) Hyperthermia substantially increased microglia activation in the hippocampal subiculum and DG, and the cerebral cortex to an extent comparable to that in the normothermia group. However, hypothermia apparently reduced microglia activation in the above areas. Scale bar=100 μm. (D, E) Zinc chelator, CaEDTA or CQ, prevented hypoglycemia-induced microglia activation even in the hyperthermia group. (D) Microglia activation is reduced by CaEDTA or CQ injection. Scale bar=100 μm. (E) Quantitated microglia activation in the hippocampal CA1 area after hypoglycemia. Data are the mean±s.e.m. (n=3 to 6); *P<0.05 compared with saline-treated animals.
Hypoglycemia-Induced Microglia Activation is Inhibited by Zinc Chelation Even Under Hyperthermic Reperfusion Condition
Here we tested whether zinc chelation prevents microglia activation even under hyperthermic conditions. Both CaEDTA and CQ substantially decreased hypoglycemia-induced microglia activation in the hippocampal CA1 pyramidal area even under hyperthermic reperfusion condition (Figures 4D and 4E). The degree of microglia activation by zinc chelation in hyperthermic group was similar with hypothermia effects when compared to the normothermia group.
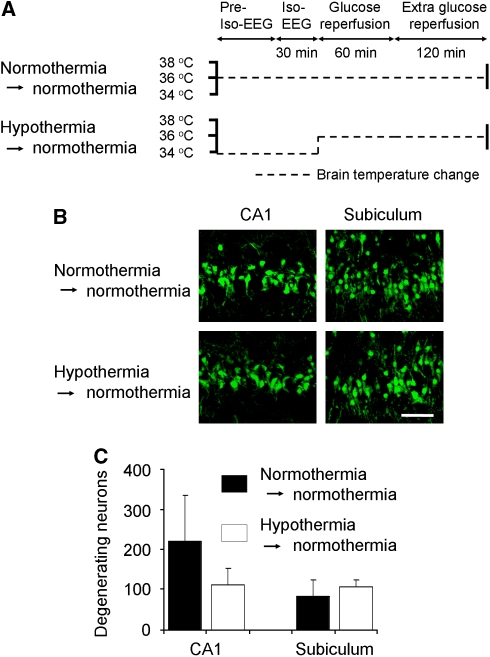
Hypothermia Before Glucose Reperfusion (Before and During Iso-EEG) Shows no Neuroprotection after Hypoglycemia
Since Agardh et al (1992) previously showed that application of hypothermia before and during the iso-EEG period afforded no neuroprotection in the hippocampal brain, we performed the same procedure to confirm their results in the context of this study. Brain temperature was maintained from 33.0°C to 34.0°C before and during the iso-EEG period, and then brain temperature was increased to 36.5°C to 37.5°C during glucose reperfusion period. One week later, brains were harvested and examined for neuronal death after FJB staining. Figure 5 shows that the number of FJB (+) neurons in the hippocampal CA1 area was slightly less in the hypothermia → normothermia group as compared with that in the normothermia → normothermia group rats. However, the difference of FJB (+) neurons in the CA1 and subiculum between these two groups was not statistically significant (Supplementary Information). This result confirms that the previous results of Agardh et al (1992 are consistent with our experimental setting and further strengthen our hypothesis that brain temperature during the glucose reperfusion period is critical to hypoglycemia-induced neuronal death.
Figure 5.
Predisposed hypothermia before glucose reperfusion shows no neuroprotection after hypoglycemia. (A) The experimental protocol for brain temperature control before and after hypoglycemia. Brain temperature change is depicted as a schematic representation. Normothermia → normothermia group: Brain temperature was maintained at 36.5°C to 37.5°C for the entire experimental period. Hypothermia → normothermia group: Brain temperature was maintained at 33.0°C to 34.0°C before and during the iso-EEG period. After the iso-EEG period, brain temperature was raised and maintained at 36.5°C to 37.5°C until rats fully recovered. (B) Confocal fluorescence images show neuronal death in the hippocampal CA1 and subiculum area at 7 days after hypoglycemia. The number of FJB (+) neurons in the hippocampal CA1 area was slightly less in the hypothermia → normothermia group as compared with that in the normothermia → normothermia group. However, the difference of FJB (+) neurons in the CA1 and subiculum between these two groups is not statistically significant. Scale bar=100 μm. (C) Bar graph shows the quantitated neuronal degeneration in hippocampal CA1 and subiculum from normothermia → normothermia and hypothermia → normothermia-subjected group at 7 days after hypoglycemia. Data are mean±s.e.m. (n=3 to 7).
Discussion
This study shows that mild hypothermia reduces hypoglycemia-induced neuronal death in the hippocampus, whereas hyperthermia aggravates those brain injuries. Here we report that hypothermia (lowering brain temperature) prevents hypoglycemia-induced neuronal death by reduction of vesicular zinc release, superoxide production, and microglia activation, where temperature-dependent vesicular zinc release was a key event upstream of hypoglycemia-induced superoxide production and microglia activation.
The mechanism of hypoglycemia-induced neuronal injury is more complex than the simple deprivation of glucose, the primary energy source for neurons (Suh et al, 2007). Rather, several contributing factors act downstream of glucose deprivation/glucose reperfusion to influence hypoglycemia-induced neuronal death, including sustained activation of glutamate receptor (Wieloch, 1985), increased mitochondrial membrane permeability (Friberg et al, 1998), poly(ADP-ribose) polymerase activation (Suh et al, 2003), and zinc translocation (Suh et al, 2004). Although several studies have reported findings in which neuronal injury was ameliorated, there are still no clinically applicable intervention strategies available for treatment of this devastating brain injury. Therefore, this study has sought a simple, safe, and applicable treatment for preventing severe hypoglycemia-induced neuronal death using body (brain) temperature modulation.
Mild hypothermia has been known as the most effective approach to prevent neuronal death after cerebral ischemia (Busto et al, 1987), traumatic brain injury (Clifton et al, 1991; Suh et al, 2006), and prolonged seizure (Liu et al, 1993). Here we found that mild hypothermia also can prevent hypoglycemia-induced neuronal death. Neuronal death evaluated in hippocampal area shows that hypothermia significantly reduced neuronal death while hyperthermia applied after hypoglycemic events aggravated the neuronal death. The neuroprotective effects of hypothermia after hypoglycemia reported in this study, however, differ from those reported in previous studies (Agardh et al, 1992). Agardh et al reported that mild hypothermia applied before and during of hypoglycemia (before and during the entire iso-EEG period) produced a similar degree of neuronal death compared to normothermic animals. No neuroprotective effect of hypothermia was seen in hypoglycemic animals. The differences between this study and that by Agardh et al may be explained by the onset of hypothermia application. Agardh et al applied hypothermia before and during the iso-EEG period. However, in this study hypothermia applied after the iso-EEG period was terminated, that is, brain temperature was decreased during the glucose reperfusion period after hypoglycemia. Since we have previously shown that hypoglycemia-induced neuronal death is not initiated during the period of glucose deprivation, but instead during the glucose reperfusion period, it may be that the hypothermic application before and during the isoelectric period was not sufficient to prevent neuronal death after hypoglycemic events. In our experimental setting we also found that hypothermia application before and during the iso-EEG period had no statistically significant neuroprotective effects as seen in the previous study (Agardh et al, 1992), strengthening our hypothesis that brain temperature is a critical factor during glucose reperfusion period after hypoglycemia.
The suggested neuroprotective mechanisms of mild hypothermia on several brain injuries are based on decreases in cerebral metabolic requirement (Erecinska et al, 2003), intracranial pressure (Soukup et al, 2002), glutamate release from presynaptic vesicles (Arai et al, 1993), free radical generation (Globus et al, 1995), and inflammatory reaction (Kumar and Evans, 1997). Previously, we have shown that hypothermia reduced vesicular zinc release and subsequent neuronal death after traumatic brain injury (Suh et al, 2006). We also have shown that hypoglycemia-induced neuronal death is mediated by vesicular zinc release and translocation (Suh et al, 2004; Suh et al, 2008). Therefore, in this study we hypothesized that mild hypothermia exerts neuroprotective effects by reducing vesicular zinc release after hypoglycemia. Although zinc is released from presynaptic terminals as a component of normal physiological signaling at zinc-modulated synapses, a large amount of vesicular zinc released together with glutamate may enter postsynaptic neurons through glutamate receptors (Weiss et al, 2000) or voltage-sensitive calcium channels (Sensi et al, 1999). Zinc translocation into postsynaptic neurons after hypoglycemia has been demonstrated by our laboratory (Suh et al, 2004; Suh et al, 2008). Many brain areas with high vesicular zinc level exhibit high vulnerability to hypoglycemia, but this correlation is not always true. Some brain areas with high vesicular zinc concentration are not correspondingly sensitive to hypoglycemia, and conversely some brain areas that are highly sensitive to hypoglycemia are not rich in vesicular zinc (Frederickson et al, 1992). Thus, vesicular zinc is not the sole determinant of neuronal vulnerability to hypoglycemia, but may be a contributory factor in areas where vesicular concentrations are high. The zinc chelator CaEDTA was used to evaluate a causal role for extracellular zinc elevations in subsequent postsynaptic neuronal zinc accumulation and death after hypoglycemia. The utility of CaEDTA as a zinc chelator has been established in ischemia and brain trauma studies (Koh et al, 1996; Suh et al, 2000a). Interestingly, Aizenmann et al. suggested that the large fraction of zinc existing in the form of thiol-zinc metalloproteins can be released from oxidation of intracellular zinc-binding proteins (e.g., metallothionein) by oxidative stress. Zinc liberated in such a manner may then become cytotoxic (Aizenman et al, 2000). This study shows that application of mild hypothermia significantly reduced hypoglycemia-induced neuronal death by reducing presynaptic zinc release and translocation into postsynaptic neurons. Hyperthermia applied after hypoglycemia aggravates this zinc release and translocation comparable to that in normothermia-subjected animals. From these results, we conclude that the neuroprotective effects of mild hypothermia after hypoglycemia can be achieved by reduction of synaptic zinc release and subsequent zinc translocation. However, our present study also found that zinc-dependent DG neuron degeneration was prevented by the cell-permeant zinc chelator, CQ. We therefore cannot exclude the possibility that intracellularly originated free zinc also contributes to hippocampal neuronal cell death after hypoglycemia as previously suggested (Aizenman et al, 2000). Anatomical and physiological studies have shown that DG neurons contain high concentration of vesicular zinc in their synaptic terminals, which is released with neuronal activity. Intraneuronal accumulation of zinc may arise from cytoplasmic organelles or proteins rather than from presynaptic terminals of stratum moleculare. However, the source of intraneuronal accumulation of zinc in DG neurons still requires further study. An additional unsolved question arises regarding how the extracellular zinc chelator, CaEDTA also prevented DG neuron death if intraneuronal zinc accumulation originates from cytoplasmic sources. Further studies investigating the neuroprotective effects of CaEDTA are warranted.
Recently, our laboratory presented an interesting finding that hypoglycemia-induced superoxide production is triggered during glucose reperfusion period and not during the iso-EEG period. We showed that glucose reperfusion after hypoglycemia caused NADPH oxidase activation-induced superoxide production, which causes delayed neuronal death after hypoglycemia (Suh et al, 2007). Thus, we investigated the role of brain temperature on superoxide production after hypoglycemia. For these reasons, we applied three different brain temperatures (normothermic, hyperthermic, or hypothermic) not during hypoglycemia but during the glucose reperfusion period after hypoglycemia. Here we found that hypothermia significantly reduced while hyperthermia increased superoxide production in the hippocampal pyramidal neurons. Also, when two different kinds of zinc chelators, CaEDTA and CQ, were applied after hypoglycemia even after hyperthermic reperfusion, the superoxide production is significantly decreased. These results suggest that hypoglycemia-induced superoxide production is also modulated by brain temperature through vesicular zinc release and translocation.
Several studies have shown that the inflammatory process involved in the progression of neuronal death after brain ischemia (Inamasu et al, 2000) and brain trauma (Thompson et al, 2005). Activated microglia have been shown to be neurotoxic. During activation, microglia produce both ROS and nitrogen species, which are toxic to neurons. Hypothermia has been shown to attenuate microglia activation after ischemia (Han et al, 2002). Therefore, in this study we investigated whether microglia activation is modulated by brain temperature after hypoglycemia. Microglia constituting about 5 to 12% of total glial population are the first non-neuronal immunocompetent phagocytic cells responding to the inflammatory (Vaughan and Peters, 1974), ischemic, and traumatic brain injury (Inamasu et al, 2000; Thompson et al, 2005). Activation of microglia is characterized by a change of morphology into an amoeboid shape, increased proliferation, and increased phagocytosis (Kreutzberg, 1996). Activated microglia releases ROS, including superoxide and nitric oxide, glutamate, extracellular protease, and cytokines (Chao et al, 1992). Here, we found that hypothermia significantly reduced microglia activation after hypoglycemia, while hyperthermia increased activation. Although models of how hypothermia mediates attenuation of microglia activation after brain insults has been suggested, the precise mechanism is not clear (Han et al, 2002; Inamasu et al, 2000). Recently, our laboratory reported that zinc release is an event upstream of microglia activation through poly(ADP-ribose) polymerase-1 and NADPH oxidase activation both in in vitro and in vivo ischemia models (Kauppinen et al, 2008). Thus we also tested the effects of zinc chelation on hypoglycemia-induced microglia activation. The zinc chelation by CaEDTA or CQ significantly attenuated hypoglycemia-induced microglia activation even after hyperthermic glucose reperfusion. Therefore we speculate that vesicular zinc release is an upstream event of hypoglycemia-induced microglia activation, and attenuation of microglia activation by hypothermia is related to inhibition of vesicular zinc release. We believe that vesicular zinc is an important mediator in this cell death process; however, we cannot exclude the possibility that calcium, copper, or iron also have neurotoxic effects, either directly on neurons or indirectly through microglia activation.
Taken together, this study shows that post-hypoglycemic (glucose reperfusion period) brain temperature can modulate the outcome of brain injury, that is, hypothermia significantly reduces, while hyperthermia aggravates, neuronal death after hypoglycemia through inhibition of vesicular zinc release, reduction of ROS production, and prevention of microglia activation. Therefore, cautious brain temperature monitoring and maintaining lower brain temperature during glucose reperfusion period may predict better clinical outcome after severe hypoglycemic episode.
Acknowledgments
We greatly appreciate Dr Midori Yenari's critical comments on this study, and technical assistance provided by Aaron Hamby. This work was supported by the Juvenile Diabetes Research Foundation (2-2006-113, SWS.) and KOSEF2009-0078399 (SWS).
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Conflict of interest
The authors declare no conflict of interest.
Supplementary Material
References
- Agardh CD, Smith ML, Siesjo BK. The influence of hypothermia on hypoglycemia-induced brain damage in the rat. Acta Neuropathol. 1992;83:379–385. doi: 10.1007/BF00713529. [DOI] [PubMed] [Google Scholar]
- Aizenman E, Stout AK, Hartnett KA, Dineley KE, McLaughlin B, Reynolds IJ. Induction of neuronal apoptosis by thiol oxidation: putative role of intracellular zinc release. J Neurochem. 2000;75:1878–1888. doi: 10.1046/j.1471-4159.2000.0751878.x. [DOI] [PubMed] [Google Scholar]
- Arai H, Uto A, Ogawa Y, Sato K. Effect of low temperature on glutamate-induced intracellular calcium accumulation and cell death in cultured hippocampal neurons. Neurosci Lett. 1993;163:132–134. doi: 10.1016/0304-3940(93)90363-p. [DOI] [PubMed] [Google Scholar]
- Auer RN, Hall P, Ingvar M, Siesjo BK. Hypotension as a complication of hypoglycemia leads to enhanced energy failure but no increase in neuronal necrosis. Stroke. 1986;17:442–449. doi: 10.1161/01.str.17.3.442. [DOI] [PubMed] [Google Scholar]
- Auer RN, Olsson Y, Siesjo BK. Hypoglycemic brain injury in the rat. Correlation of density of brain damage with the EEG isoelectric time: a quantitative study. Diabetes. 1984a;33:1090–1098. doi: 10.2337/diab.33.11.1090. [DOI] [PubMed] [Google Scholar]
- Auer RN, Wieloch T, Olsson Y, Siesjo BK. The distribution of hypoglycemic brain damage. Acta Neuropathol. 1984b;64:177–191. doi: 10.1007/BF00688108. [DOI] [PubMed] [Google Scholar]
- Busto R, Dietrich WD, Globus MY, Valdes I, Scheinberg P, Ginsberg MD. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab. 1987;7:729–738. doi: 10.1038/jcbfm.1987.127. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol. 1992;149:2736–2741. [PubMed] [Google Scholar]
- Clifton GL, Jiang JY, Lyeth BG, Jenkins LW, Hamm RJ, Hayes RL. Marked protection by moderate hypothermia after experimental traumatic brain injury. J Cereb Blood Flow Metab. 1991;11:114–121. doi: 10.1038/jcbfm.1991.13. [DOI] [PubMed] [Google Scholar]
- Clifton GL, Miller ER, Choi SC, Levin HS, McCauley S, Smith KR., Jr, Muizelaar JP, Wagner FC., Jr, Marion DW, Luerssen TG, Chesnut RM, Schwartz M. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344:556–563. doi: 10.1056/NEJM200102223440803. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in mammalian central nervous system. J Cereb Blood Flow Metab. 2003;23:513–530. doi: 10.1097/01.WCB.0000066287.21705.21. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Kasarskis EJ, Ringo D, Frederickson RE. A quinoline fluorescence method for visualizing and assaying the histochemically reactive zinc (bouton zinc) in the brain. J Neurosci Methods. 1987;20:91–103. doi: 10.1016/0165-0270(87)90042-2. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Rampy BA, Reamy-Rampy S, Howell GA. Distribution of histochemically reactive zinc in the forebrain of the rat. J Chem Neuroanat. 1992;5:521–530. doi: 10.1016/0891-0618(92)90007-d. [DOI] [PubMed] [Google Scholar]
- Friberg H, Ferrand-Drake M, Bengtsson F, Halestrap AP, Wieloch T. Cyclosporin A, but not FK 506, protects mitochondria and neurons against hypoglycemic damage and implicates the mitochondrial permeability transition in cell death. J Neurosci. 1998;18:5151–5159. doi: 10.1523/JNEUROSCI.18-14-05151.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Globus MY, Busto R, Lin B, Schnippering H, Ginsberg MD. Detection of free radical activity during transient global ischemia and recirculation: effects of intraischemic brain temperature modulation. J Neurochem. 1995;65:1250–1256. doi: 10.1046/j.1471-4159.1995.65031250.x. [DOI] [PubMed] [Google Scholar]
- Han HS, Qiao Y, Karabiyikoglu M, Giffard RG, Yenari MA. Influence of mild hypothermia on inducible nitric oxide synthase expression and reactive nitrogen production in experimental stroke and inflammation. J Neurosci. 2002;22:3921–3928. doi: 10.1523/JNEUROSCI.22-10-03921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamasu J, Suga S, Sato S, Horiguchi T, Akaji K, Mayanagi K, Kawase T. Post-ischemic hypothermia delayed neutrophil accumulation and microglial activation following transient focal ischemia in rats. J Neuroimmunol. 2000;109:66–74. doi: 10.1016/s0165-5728(00)00211-3. [DOI] [PubMed] [Google Scholar]
- Kauppinen TM, Higashi Y, Suh SW, Escartin C, Nagasawa K, Swanson RA. Zinc triggers microglial activation. J Neurosci. 2008;28:5827–5835. doi: 10.1523/JNEUROSCI.1236-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kumar K, Evans AT. Effect of hypothermia on microglial reaction in ischemic brain. Neuroreport. 1997;8:947–950. doi: 10.1097/00001756-199703030-00026. [DOI] [PubMed] [Google Scholar]
- Liu Z, Gatt A, Mikati M, Holmes GL. Effect of temperature on kainic acid-induced seizures. Brain Res. 1993;631:51–58. doi: 10.1016/0006-8993(93)91185-u. [DOI] [PubMed] [Google Scholar]
- McCrimmon RJ, Frier BM. Hypoglycaemia, the most feared complication of insulin therapy. Diabete Metab. 1994;20:503–512. [PubMed] [Google Scholar]
- Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, Chan PH. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998;18:205–213. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade: novel fluorochromes for detecting toxicant-induced neuronal degeneration. Toxicol Pathol. 2000;28:91–99. doi: 10.1177/019262330002800111. [DOI] [PubMed] [Google Scholar]
- Seltzer HS. Drug-induced hypoglycemia. A review of 1418 cases. Endocrinol Metab Clin North Am. 1989;18:163–183. [PubMed] [Google Scholar]
- Sensi SL, Yin HZ, Carriedo SG, Rao SS, Weiss JH. Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc Natl Acad Sci USA. 1999;96:2414–2419. doi: 10.1073/pnas.96.5.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukup J, Zauner A, Doppenberg EM, Menzel M, Gilman C, Young HF, Bullock R. The importance of brain temperature in patients after severe head injury: relationship to intracranial pressure, cerebral perfusion pressure, cerebral blood flow, and outcome. J Neurotrauma. 2002;19:559–571. doi: 10.1089/089771502753754046. [DOI] [PubMed] [Google Scholar]
- Suh SW, Aoyama K, Chen Y, Garnier P, Matsumori Y, Gum E, Liu J, Swanson RA. Hypoglycemic neuronal death and cognitive impairment are prevented by poly(ADP-ribose) polymerase inhibitors administered after hypoglycemia. J Neurosci. 2003;23:10681–10690. doi: 10.1523/JNEUROSCI.23-33-10681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh SW, Aoyama K, Matsumori Y, Liu J, Swanson RA. Pyruvate administered after severe hypoglycemia reduces neuronal death and cognitive impairment. Diabetes. 2005;54:1452–1458. doi: 10.2337/diabetes.54.5.1452. [DOI] [PubMed] [Google Scholar]
- Suh SW, Chen JW, Motamedi M, Bell B, Listiak K, Pons NF, Danscher G, Frederickson CJ. Evidence that synaptically-released zinc contributes to neuronal injury after traumatic brain injury. Brain Res. 2000a;852:268–273. doi: 10.1016/s0006-8993(99)02095-8. [DOI] [PubMed] [Google Scholar]
- Suh SW, Danscher G, Jensen MS, Thompson R, Motamedi M, Frederickson CJ. Release of synaptic zinc is substantially depressed by conventional brain slice preparations. Brain Res. 2000b;879:7–12. doi: 10.1016/s0006-8993(00)02675-5. [DOI] [PubMed] [Google Scholar]
- Suh SW, Frederickson CJ, Danscher G. Neurotoxic zinc translocation into hippocampal neurons is inhibited by hypothermia and is aggravated by hyperthermia after traumatic brain injury in rats. J Cereb Blood Flow Metab. 2006;26:161–169. doi: 10.1038/sj.jcbfm.9600176. [DOI] [PubMed] [Google Scholar]
- Suh SW, Garnier P, Aoyama K, Chen Y, Swanson RA. Zinc release contributes to hypoglycemia-induced neuronal death. Neurobiol Dis. 2004;16:538–545. doi: 10.1016/j.nbd.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest. 2007;117:910–918. doi: 10.1172/JCI30077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh SW, Hamby AM, Gum ET, Shin BS, Won SJ, Sheline CT, Chan PH, Swanson RA. Sequential release of nitric oxide, zinc, and superoxide in hypoglycemic neuronal death. J Cereb Blood Flow Metab. 2008;28:1697–1706. doi: 10.1038/jcbfm.2008.61. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, Hoover RC, Tkacs NC, Saatman KE, McIntosh TK. Development of posttraumatic hyperthermia after traumatic brain injury in rats is associated with increased periventricular inflammation. J Cereb Blood Flow Metab. 2005;25:163–176. doi: 10.1038/sj.jcbfm.9600008. [DOI] [PubMed] [Google Scholar]
- Vaughan DW, Peters A. Neuroglial cells in the cerebral cortex of rats from young adulthood to old age: an electron microscope study. J Neurocytol. 1974;3:405–429. doi: 10.1007/BF01098730. [DOI] [PubMed] [Google Scholar]
- Weiss JH, Sensi SL, Koh JY. Zn(2+): a novel ionic mediator of neural injury in brain disease. Trends Pharmacol Sci. 2000;21:395–401. doi: 10.1016/s0165-6147(00)01541-8. [DOI] [PubMed] [Google Scholar]
- Wieloch T. Hypoglycemia-induced neuronal damage prevented by an N-methyl--aspartate antagonist. Science. 1985;230:681–683. doi: 10.1126/science.2996146. [DOI] [PubMed] [Google Scholar]
- Yanamoto H, Nagata I, Niitsu Y, Zhang Z, Xue JH, Sakai N, Kikuchi H. Prolonged mild hypothermia therapy protects the brain against permanent focal ischemia. Stroke. 2001;32:232–239. doi: 10.1161/01.str.32.1.232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.