Abstract
Obesity is an independent risk factor for stroke and is associated with poorer outcome after stroke. We investigated whether this poorer outcome is related to brain microvascular disruption. Focal cerebral ischaemia was induced in lean or obese (ob/ob) mice by transient middle cerebral artery occlusion. The incidence of haemorrhagic transformation and the volume of ischaemic brain damage were significantly greater in obese mice. Blood–brain barrier permeability and brain microvascular MMP-9 expression were also markedly increased in obese mice. These effects were independent of leptin or glycaemic status, suggesting that obesity potentiates brain microvascular disruption after experimental stroke.
Keywords: blood–brain barrier, haemorrhagic transformation, leptin, MMP-9, obesity, stroke
Introduction
Obesity is an independent risk factor for stroke, and is associated with atherosclerosis, diabetes, and hypertension, conditions that predispose to stroke (Winter et al, 2008). The increased risk of stroke in obese individuals may also be accompanied by poorer prognosis after the ischaemic insult (Razinia et al, 2007). In support of this, recent studies have demonstrated greater brain damage in obese rodents after experimental stroke (Mayanagi et al, 2008; Terao et al, 2008). However, the mechanisms responsible for these detrimental effects of obesity on cerebrovascular injury are poorly delineated.
A crucial site of convergence for pathophysiological mechanisms involved in obesity and stroke is the brain microvascular endothelium. Obesity is a state of chronic systemic inflammation and is associated with vascular oxidative stress (Dandona et al, 2004). Inflammation and oxidative stress are important factors that contribute to disruption of the blood–brain barrier (BBB). Mechanisms include disruption of inter-endothelial tight-junction complexes (Schreibelt et al, 2007) and induction of proteases, in particular matrix metalloproteinases (MMPs), that degrade constituents of the vascular basement membrane (Zhao et al, 2007). We have shown previously that systemic inflammation exacerbates ischaemic brain damage through MMP-9-dependent alterations in BBB integrity (McColl et al, 2008). Clinically, loss of BBB integrity after stroke is associated with serious complications such as brain oedema and haemorrhagic transformation (HT) that correlate with poor prognosis (Simard et al, 2007). Accordingly, it is feasible that obesity may predispose to poorer outcome after stroke through aggravation of brain microvascular disruption.
In the present study, we sought to determine if obesity exacerbates brain microvascular disruption after experimental stroke.
Materials and methods
Focal Cerebral Ischaemia
Experiments were performed on 8- to 12-week-old male obese ob/ob (C57BL/6OlaHsd-Lepob) or lean littermate mice (Harlan-Olac, Bicester, UK) according to the Animals (Scientific Procedures) Act (1986). Body weight and blood glucose concentration at the time of surgery were as follows: body weight, lean 29.0±0.5 g versus ob/ob 47.3±0.9 g (P<0.001) and glucose, lean 9.3±0.7 mmol/L versus ob/ob 10.1±1.4 mmol/L (P>0.05). Focal ischaemia was induced by transient (40 mins) middle cerebral artery occlusion (MCAo) as previously described (McColl et al, 2008). Briefly, under isoflurane anaesthesia, the carotid arteries were exposed and a 6-0 nylon monofilament (Dermalon) with a 2 mm tip (180 μm diameter) coated in thermo-melting glue (Jet Melt) was introduced into the external carotid artery and advanced 9 mm along the internal carotid artery until occluding the origin of the MCA. After 40 mins, the filament was withdrawn to establish reperfusion and the wound sutured.
In a separate experiment, lean and obese ob/ob mice were administered an intraperitoneal injection of vehicle (0.9% sterile saline) or leptin (4 mg/kg in saline; Peprotech, London, UK) at the onset of MCAo. This dose of leptin has been shown previously to reduce ischaemic injury (Zhang et al, 2007).
Tissue Processing
Twenty-four hours after MCAo mice were perfused transcardially with 0.9% saline followed by 4% paraformaldehyde and brains post-fixed, cryoprotected, and frozen. Coronal brain sections (20 μm) were prepared on a cryostat.
Measurement of HT and Ischaemic Damage
To assess HT brain sections were stained with haematoxylin and eosin. Areas of HT were delineated at coronal levels (400 μm apart) and the total area of HT was calculated. For ischaemic damage, adjacent brain sections were stained with cresyl violet. Briefly, areas of damage were delineated at eight anatomically defined coronal levels and the total volume was calculated.
Immunohistochemistry
Primary antibodies were used as follows: goat anti-MMP-9 (1:400; R&D Systems, Abington, UK), and rabbit anti-laminin (1:25; Sigma, Poole, UK). Endogenous peroxidise activity (except for double immunofluorescence) and nonspecific binding sites were blocked before incubating (4°C) sections in primary antibody solution. For peroxidise-based staining, sections were then incubated in biotinylated secondary antibody (1:200; Vector Laboratories, Peterborough, UK), immersed in avidin–biotin–peroxidase complex (ABC; Vector Laboratories) and colour-developed using a 0.05% diaminobenzidine solution (in 0.01% H2O2). For assessment of BBB disruption, primary antibody was omitted and a biotinylated anti-mouse IgG secondary antibody was used. For double labelling immunofluorescence, after primary antibody application sections were incubated in donkey anti-goat Alexa 488 and donkey anti-rabbit 594 (Molecular Probes, Paisley, UK), mounted, and coverslipped with ProLong Gold mounted medium (Invitrogen, Paisley, UK).
Statistical Analysis
For all analyses, data are represented as mean±s.d. Parametric data were analysed using Student's t-test for single comparisons, or one-way analysis of variance followed by a Tukey's test for multiple comparisons. The incidence of HT was compared using χ2-test.
Results
Increased Incidence of HT and Ischaemic Brain Damage in Obese Mice
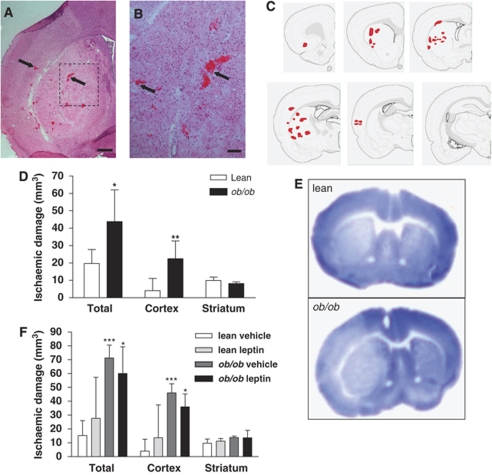
Haemorrhagic transformation was observed in the ipsilateral ischaemic hemisphere of ob/ob mice. Multiple foci of bleeds were evident throughout the striatum and cortex, and in some cases in the corpus callosum and hippocampus (Figure 1A–1C). HT was not detected in the contralateral hemisphere of ob/ob mice, suggesting that HT was a consequence of the ischaemic challenge and did not occur spontaneously in the absence of ischaemia in ob/ob mice. The incidence of HT was significantly increased in ob/ob mice as compared with that in the lean controls (lean, n=1/6 versus ob/ob, n=5/6; P<0.05, χ2-test of independence). The HT observed in the one lean mouse was restricted to a small striatal haemorrhage. The volume of ischaemic brain damage was significantly greater (120%) in ob/ob as compared with that in lean mice 24 h after MCAo (P<0.05; Figure 1D and 1E). There were two mortalities in the ob/ob group, and post mortem examination of their brains revealed extensive brain damage and HT.
Figure 1.
HT and ischaemic damage are increased in obese mice 24 h after MCAo. (A) Coronal brain sections stained with haematoxylin and eosin illustrate discrete foci of haemorrhages (arrows) in the ipsilateral striatum and cortex of ob/ob mice. (B) A higher magnification view of the area outlined by the dashed box in panel A. (C) The typical distribution of HT at six coronal brain sections. (D) An increase in the volume of ischaemic brain damage was observed in obese ob/ob mice 24 h after MCAo. (E) Representative cresyl violet-stained brain sections illustrate the exacerbation of brain damage in ob/ob mice. (F) Increased ischaemic damage in the brains of obese mice is independent of leptin. Leptin (4 mg/kg, intraperitoneal) or vehicle was administered at the onset of MCAo to lean and obese ob/ob mice and volume of ischaemic injury was assessed at 24 h. Data are mean±s.d. for n=4–6 mice per group. *P<0.05, **P<0.01 and ***P<0.001 versus lean mice. Scale bars, 200 μm (A) and 25 μm (B).
Increased HT and Ischaemic Brain Damage in Obese Mice are Independent of Leptin Deficiency
Neuroprotective actions of leptin have been demonstrated previously (Zhang et al, 2007); therefore, in a separate experiment, we assessed the effect of acute leptin replacement on ischaemic brain damage in lean and obese mice. Leptin administration did not affect the incidence of HT, as haemorrhages were present in the striatum and cortex of all ob/ob mice (ob/ob vehicle, n=4/4 versus ob/ob leptin, n=4/4) 24 h after MCAo. The total area of HT in the ischaemic tissue of ob/ob mice was also unaffected by leptin treatment (ob/ob vehicle, 0.35±0.15 mm2 versus ob/ob leptin, 0.56±0.09 mm2; P>0.05). There was no HT observed in the brains of lean mice treated with either vehicle or leptin (lean vehicle, n=0/6 versus lean leptin, n=0/5). Leptin administration did not affect the exacerbation of ischaemic damage observed in obese mice (Figure 1F). One mortality after MCAo was noted in both obese groups (ob/ob vehicle and ob/ob leptin) and a post-mortem examination of the brains of these mice revealed the presence of HT and extensive ischaemic damage.
To verify that the absence of an effect of leptin administration on brain pathology was not due to lack of biological activity, we assessed the effect of leptin on food intake and body weight. Leptin (4 mg/kg, intraperitoneal.) significantly reduced food intake (vehicle, 4.0±0.2 g versus leptin, 3.2±0.3 g, P<0.05; n=5–6) and caused body weight loss (Δ body weight; vehicle, 0.2±0.1 g versus leptin, −0.4±0.1 g, P<0.01; n=5–6) 24 h after injection in lean mice.
BBB Permeability and Brain Microvascular MMP-9 Expression are Increased after Focal Cerebral Ischaemia in Obese Mice
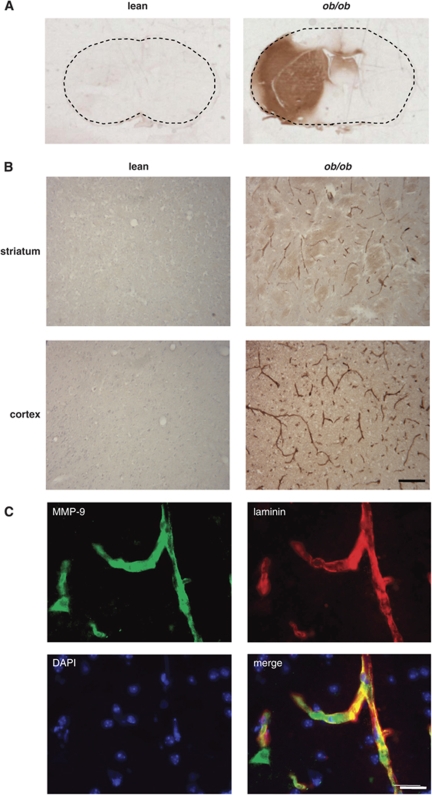
The marked increase in susceptibility to ischaemia-induced HT observed in ob/ob mice suggests severe loss of BBB integrity and an increase in cerebrovascular permeability. BBB permeability was determined by assessing the accumulation of endogenous IgG (which is excluded by an intact BBB) in the brain by immunohistochemistry. There was minimal IgG immunoreactivity in the brain tissue of lean mice after MCAo (Figure 2A). Marked increases in distribution and intensity of IgG immunoreactivity were observed in the ipsilateral hemisphere of brain sections from ob/ob mice 24 h after MCAo. Intense IgG staining was present in both the striatum and cortex, corresponding to areas of ischaemic damage.
Figure 2.
BBB permeability and MMP-9 expression are increased in obese mice after MCAo. BBB permeability was assessed by immunostaining for IgG. (A) Representative sections from lean and obese ob/ob mice 24 h after MCAo illustrate marked increased in the intensity and distribution of IgG immunoreactivity in ob/ob mice in the cortex and striatum indicating greater BBB disruption in obese mice. (B) Representative photomicrographs of MMP-9 expression in the brain of lean and obese ob/ob mice 24 h after MCAo. Increase in MMP-9 immunoreactivity was observed in the striatum and cortex of ob/ob mice. (C) Multi-labelling immunofluorescence shows colocalisation of laminin-positive blood vessels (red) and MMP-9 expression (green) in ob/ob mice indicating that the majority of MMP-9 is localised to cerebral blood vessels. Scale bars, 100 μm (B) and 25 μm (C).
Experimental and clinical evidence implicates proteolytic disruption of BBB substrates by proteases such as MMP-9 in cerebrovascular disruption. We, therefore, assessed the expression of MMP-9 in the brain of obese and lean mice after MCAo. Extensive MMP-9 immunoreactivity was detected in the ipsilateral ischaemic striatum and cortex of ob/ob mice (Figure 2B), but not in the contralateral hemisphere. No MMP-9-positive cells were seen in the ipsilateral striatum or cortex of lean mice. The majority of MMP-9 in ob/ob mice was localised to blood vessels, which was confirmed by strong colocalisation between MMP-9 and laminin, a major basement membrane component of blood vessels (Figure 2C).
Discussion
In the present study, we show elevated microvascular MMP-9 expression and increased incidence of HT in obese, leptin-deficient ob/ob mice after experimental stroke. BBB permeability and infarct volume were also exacerbated in obese mice. Our data suggest that these effects are not due to hyperglycaemia or leptin deficiency, and, therefore, implicate obesity as an independent factor that promotes severe brain microvascular disruption after ischaemic brain injury.
In addition to clinical data that have indicated increased risk of stroke in obese individuals (Winter et al, 2008), recent studies have shown that the severity of brain damage is increased after experimental stroke in obese rodents (Mayanagi et al, 2008; Terao et al, 2008). Our data expand on these previous findings to show that obese mice are highly susceptible to HT, which is associated with elevated microvascular MMP-9 immunoreactivity. HT is a serious complication in ischaemic stroke patients that correlates with poor prognosis (Paciaroni et al, 2008). Conventional risk factors for HT include hyperglycaemia and hypertension (Paciaroni et al, 2008), both of which are common in obese subjects. However, these factors are unlikely to account for the increased frequency of HT in the present study, because pre-ischaemic blood glucose levels were similar in lean and obese mice, and previous studies have not found significant differences in blood pressure (Vachharajani et al, 2005). Thrombolytic treatment also predisposes to HT (Zhao et al, 2007). Our data suggest that obese patients may be at further risk of HT if treated with thrombolytic agents.
The extravasation of all blood constituents, including erythrocytes, that occurs during HT is indicative of a catastrophic failure of microvascular integrity. This failure also underlies the increased permeability of the BBB and brain oedema that accompanies HT. In the present study, we found marked increase in BBB permeability to IgG in obese mice, which is consistent with their increased susceptibility to HT and confirms that obesity promotes severe disruption to the BBB. We also observed localisation of extensive MMP-9 immunoreactivity to the microvasculature in obese mice, suggesting that MMP-9 may be an important mediator underlying increased BBB disruption and HT in obese mice. Quantitative assays of MMP-9 activity will be required to verify changes on a functional level. Previous studies have shown that MMP-9 can disrupt multiple components of the BBB, including the tight-junction proteins claudin-5 and occludin, and the basement membrane protein collagen-IV (Candelario-Jalil et al, 2009). Furthermore, inhibition of MMPs attenuates BBB disruption and reduces thrombolysis-induced HT after experimental stroke (Candelario-Jalil et al, 2009).
A chronically elevated inflammatory profile is a feature of obesity and inflammation is implicated in microvascular dysfunction associated with obesity (Singer and Granger, 2007). Increased leukocyte–endothelium interactions and intracellular adhesion molecule-1 (ICAM-1) expression after experimental stroke in obese mice have recently been reported (Mayanagi et al, 2008; Terao et al, 2008). Leukocytes, in particular neutrophils, contain abundant proteases, including MMP-9; therefore, increased leukocyte adhesion could be a mechanism underlying the increased microvascular MMP-9 expression in the present study. In support of this, brain neutrophil accumulation was significantly greater after MCAo in obese mice (data not shown). More generally, growing evidence suggests that systemic inflammatory conditions, such as obesity, are important modifiers of stroke outcome (McColl et al, 2009).
One important caveat of the present study is that we cannot exclude that the effects of obesity are mediated through alterations in cerebral perfusion (e.g., through collateral vessels) and, indeed, given the pro-coagulatory state that is associated with obesity, it will be important to consider this potential mechanism in future studies. In summary, we have shown that obese ob/ob mice are highly susceptible to HT after experimental stroke, an effect that is associated with increased microvascular MMP-9 expression and BBB opening. Further work is ongoing to explore the mechanisms in more detail.
Acknowledgments
We acknowledge financial support by the Research Councils UK and the Medical Research Council.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Mayanagi K, Katakam PV, Gaspar T, Domoki F, Busija DW. Acute treatment with rosuvastatin protects insulin resistant (C57BL/6J ob/ob) mice against transient cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:1927–1935. doi: 10.1038/jcbfm.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl BW, Allan SM, Rothwell NJ. Systemic infection, inflammation and acute ischemic stroke. Neuroscience. 2009;158:1049–1061. doi: 10.1016/j.neuroscience.2008.08.019. [DOI] [PubMed] [Google Scholar]
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci. 2008;28:9451–9462. doi: 10.1523/JNEUROSCI.2674-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciaroni M, Agnelli G, Corea F, Ageno W, Alberti A, Lanari A, Caso V, Micheli S, Bertolani L, Venti M, Palmerini F, Biagini S, Comi G, Previdi P, Silvestrelli G. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke. 2008;39:2249–2256. doi: 10.1161/STROKEAHA.107.510321. [DOI] [PubMed] [Google Scholar]
- Razinia T, Saver JL, Liebeskind DS, Ali LK, Buck B, Ovbiagele B. Body mass index and hospital discharge outcomes after ischemic stroke. Arch Neurol. 2007;64:388–391. doi: 10.1001/archneur.64.3.388. [DOI] [PubMed] [Google Scholar]
- Schreibelt G, Kooij G, Reijerkerk A, van Doorn R, Gringhuis SI, van der Pol S, Weksler BB, Romero IA, Couraud PO, Piontek J, Blasig IE, Dijkstra CD, Ronken E, de Vries HE. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 2007;21:3666–3676. doi: 10.1096/fj.07-8329com. [DOI] [PubMed] [Google Scholar]
- Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer G, Granger DN. Inflammatory responses underlying the microvascular dysfunction associated with obesity and insulin resistance. Microcirculation. 2007;14:375–387. doi: 10.1080/10739680701283158. [DOI] [PubMed] [Google Scholar]
- Terao S, Yilmaz G, Stokes KY, Ishikawa M, Kawase T, Granger DN. Inflammatory and injury responses to ischemic stroke in obese mice. Stroke. 2008;39:943–950. doi: 10.1161/STROKEAHA.107.494542. [DOI] [PubMed] [Google Scholar]
- Vachharajani V, Russell JM, Scott KL, Conrad S, Stokes KY, Tallam L, Hall J, Granger DN. Obesity exacerbates sepsis-induced inflammation and microvascular dysfunction in mouse brain. Microcirculation. 2005;12:183–194. doi: 10.1080/10739680590904982. [DOI] [PubMed] [Google Scholar]
- Winter Y, Rohrmann S, Linseisen J, Lanczik O, Ringleb PA, Hebebrand J, Back T. Contribution of obesity and abdominal fat mass to risk of stroke and transient ischemic attacks. Stroke. 2008;39:3145–3151. doi: 10.1161/STROKEAHA.108.523001. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang S, Signore AP, Chen J. Neuroprotective effects of leptin against ischemic injury induced by oxygen-glucose deprivation and transient cerebral ischemia. Stroke. 2007;38:2329–2336. doi: 10.1161/STROKEAHA.107.482786. [DOI] [PubMed] [Google Scholar]
- Zhao BQ, Tejima E, Lo EH. Neurovascular proteases in brain injury, hemorrhage and remodeling after stroke. Stroke. 2007;38:748–752. doi: 10.1161/01.STR.0000253500.32979.d1. [DOI] [PubMed] [Google Scholar]