Abstract
MCT2 is the predominant neuronal monocarboxylate transporter allowing lactate use as an alternative energy substrate. It is suggested that MCT2 is upregulated to meet enhanced energy demands after modifications in synaptic transmission. Brain-derived neurotrophic factor (BDNF), a promoter of synaptic plasticity, significantly increased MCT2 protein expression in cultured cortical neurons (as shown by immunocytochemistry and western blot) through a translational regulation at the synaptic level. Brain-derived neurotrophic factor can cause translational activation through different signaling pathways. Western blot analyses showed that p44/p42 mitogen-activated protein kinase (MAPK), Akt, and S6 were strongly phosphorylated on BDNF treatment. To determine by which signal transduction pathway(s) BDNF mediates its upregulation of MCT2 protein expression, the effect of specific inhibitors for p38 MAPK, phosphoinositide 3-kinase (PI3K), mammalian target of rapamycin (mTOR), mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) kinase (MEK), p44/p42 MAPK (ERK), and Janus kinase 2 (JAK2) was evaluated. It could be observed that the BDNF-induced increase in MCT2 protein expression was almost completely blocked by all inhibitors, except for JAK2. These data indicate that BDNF induces an increase in neuronal MCT2 protein expression by a mechanism involving a concomitant stimulation of PI3K/Akt/mTOR/S6, p38 MAPK, and p44/p42 MAPK. Moreover, our observations suggest that changes in MCT2 expression could participate in the process of synaptic plasticity induced by BDNF.
Keywords: energy metabolism, lactate, MAPK, mTOR, PI3K, synaptic plasticity
Introduction
Brain-derived neurotrophic factor (BDNF) is a widely expressed neurotrophin in the central nervous system (Skup, 1994). Acting through specific tyrosine kinase receptors, BDNF affects neuronal survival, differentiation, and synaptic plasticity after the activation of multiple intracellular signal transduction mechanisms, such as phosphoinositide 3-kinase (PI3K)/Akt and mitogen-activated protein kinase (MAPK) signaling pathways (Bramham and Messaoudi, 2005). Presynaptically, BDNF potentiates depolarization-evoked Ca2+-dependent glutamate release while causing direct glutamate release through Ca2+ mobilization from Ins(1,4,5)P3-sensitive stores, whereas the postsynaptic actions of BDNF include changes in glutamate receptor phosphorylation, subcellular localization and synthesis, and local alterations in protein synthesis, as well as long-term changes in gene expression (Carvalho et al, 2008). These effects of BDNF contribute not only to synaptic plasticity but also to modifications in spine density and morphology (Carvalho et al, 2008). However, one aspect that has not been explored in the context of synaptic plasticity concerns putative changes in neuroenergetics. Indeed, it is likely that as a consequence of alterations in synaptic efficacy, the supply of energy substrates must be adapted to meet the energy needs imposed by new levels of synaptic response.
In recent years, the role of monocarboxylates such as lactate as additional energy substrates for neurons has attracted increasing attention (Pellerin, 2003), raising interest for the identification of specific transporters in the central nervous system. MCT2 was shown to be the predominant monocarboxylate transporter expressed by neurons (Pierre et al, 2002). It belongs to a family of proton-linked carriers involved in the transport of lactate, pyruvate, and ketone bodies (Garcia et al, 1994, 1995). MCT2 immunoreactivity was found to be abundant in the neuronal processes of various brain regions, including the cortex, hippocampus, and cerebellum (Bergersen et al, 2002; Pierre et al, 2002). At the subcellular level, MCT2 is expressed on axons and dendrites (Pierre et al, 2002, 2009). Moreover, MCT2 is present at glutamatergic synapses and exclusively on postsynaptic elements (Bergersen et al, 2002, 2005; Pierre et al, 2009). It is particularly enriched in the postsynaptic density as well as in an intracellular pool within the spines (Bergersen et al, 2005). Recent observations have shown that MCT2 expression can be regulated in cultured neurons. Thus, it was found that noradrenaline, insulin, and insulin-like growth factor-1 (IGF-1) increase MCT2 protein expression in neurons through a translational mechanism (Chenal and Pellerin, 2007; Chenal et al, 2008). Interestingly, each of these neuroactive substances is known to induce long-term changes in synaptic transmission (Kobayashi and Yasoshima, 2001; Trejo et al, 2007; van der Heide et al, 2006). Therefore, it became of interest to investigate whether BDNF could regulate MCT2 expression in cultured neurons and to characterize the signal transduction pathways involved in this effect. In addition, the nature of the mechanism (transcriptional or translational) by which MCT2 expression is regulated by BDNF has been investigated.
Material and methods
Neuronal Cultures and Pharmacological Treatments
Primary cultures of mouse cortical neurons were prepared from embryonic day 17 OF1 mice (Charles River, Lyon, France). As described previously (Debernardi et al, 2003), after decapitation and brain dissection, cortices were mechanically dissociated in phosphate-buffered saline (PBS) supplemented with glucose (NaCl, 150 mmol/L; KCl, 3 mmol/L; KH2PO4, 1.5 mmol/L; Na2HPO4, 7.9 mmol/L; glucose, 33 mmol/L; penicillin, 0.006 g/L; streptomycin, 0.1 g/L; pH 7.4). Cells were plated on poly -ornithine (15 mg/L)-precoated dishes and cultured in neurobasal-B27 medium (Brewer et al, 1993) supplemented with 0.5 mmol/L -glutamine. All experiments were carried out on day 7 in vitro. At this stage, cultures contained <5% of glial cells (Debernardi et al, 2003). Neuronal treatments with pharmacological agents were carried out without changing the medium before or during the incubation time. Brain-derived neurotrophic factor (CYT-207; Brunschwig, Basel, Switzerland) was added directly into the culture medium at various concentrations and cells were incubated for the indicated times. Rapamycin, 20 ng/mL (mammalian target of rapamycin (mTOR) inhibitor), SB202190 HCl, 10 μmol/L and SB203580 HCl, 10 μmol/L (p38 MAPK inhibitors), LY294002, 10 μmol/L (PI3K inhibitor), PD98059, 50 μmol/L (MAPK/ERK kinase (MEK inhibitor), UO126, 10 μmol/L (p44/p42 MAPK (ERK, extracellular signal-regulated kinase) inhibitor), and AG490 25 μmol/L (JAK2 (Janus kinase 2) inhibitor) were added directly to the medium 30 mins before BDNF. All these inhibitors were purchased from Alexis Biochemicals (Lausen, Switzerland), except LY294002 (L9908; Sigma, Buchs, Switzerland) and SB203580 (S8307; Sigma). Transcription and translation inhibitors (5 μmol/L actinomycin D (ActD) and 10 μmol/L cycloheximide, respectively) were added 30 mins before pharmacological agents. All other chemicals were purchased from Sigma. Data represent mean±s.e.m. of ‘n' determinations, which are independent measurements (from different culture plates) obtained from at least three separate neuronal cultures.
Immunocytochemistry and Related Quantification
After removal of the culture medium, cells were carefully rinsed in PBS at 37°C and directly postfixed in an ice-cold paraformaldehyde fixative (4% in PBS for 30 mins at 20°C). Fixed cells were treated with casein (0.5% in PBS) for 1 h at room temperature to block nonspecific sites. For immunostaining, cultures were incubated overnight at 4°C in 50 μL of freshly prepared MCT2 antibody solution (anti-MCT2 diluted 1:500 in PBS containing 0.25% bovine serum albumin) (Pierre et al, 2000). After carefully rinsing in PBS, cultures were incubated in a solution containing Cy3-conjugated anti-rabbit Igs (diluted 1:500 for 2 h at room temperature; Jackson Immunoresearch, Baltimore, MD, USA). After rinsing in PBS twice and a final rinsing in water, coverslips were mounted with Vectashield (Reactolab SA, Burlingame, CA, USA). Coverslips were examined and photographed with an Axioplan2 microscope (Zeiss, Hallbergmoos, Germany) using epifluorescence with an appropriate filter.
To quantitatively assess the influence of different treatments on MCT2 protein expression, a quantitative analysis of images obtained by epifluorescence with a × 20 objective and acquired using a cooled CCD camera (Axiocam, Zeiss), together with the 4.6 Axiovision software (Zeiss) was carried out. Three fields were chosen randomly on each coverslip; they contained at least 20 MCT2-labeled neurons per field. All pictures were acquired and presented as different levels of gray with identical acquisition time for all. Pictures were then analyzed using NIH software (National Institutes of Health Image program, version 1.62, Rockville Pike, MD, USA). The fluorescence intensity of eight isolated cells taken randomly in each of the three captured areas was assessed. The average fluorescence intensity representing neuronal MCT2 expression was obtained by calculating the average of 24 measurements per coverslip. Measurements were obtained in a blinded manner with the investigator unaware of the culture treatments. Mean and s.e.m. for a particular condition were calculated from average fluorescence intensity values of distinct coverslips representing independent determinations (numbers indicated on each bar of the graph). Data were statistically analyzed with an ANOVA (analysis of variance), followed by Dunnett's or Bonferroni's test.
Western Blot and Related Quantification
Neurons in each culture dish were homogenized in 50 μL of buffer containing the following: Tris-HCl, pH 6.8, 20 mmol/L; sucrose, 0.27 mol/L; EGTA (ethylene glycol tetraacetic acid), 1 mmol/L; EDTA (ethylene diamine tetraacetic acid), 1 mmol/L; NaF, 50 mmol/L; Triton X-100, 1% β-glycerophosphate, 10 mmol/L; DTT (dithiothreitol), 10 mmol/L; 4-nitrophenylphosphate, 10 mmol/L; and a mixture of protease inhibitors (Complete, Roche Molecular Biochemicals, Mannheim, Germany). Each stimulated condition was examined in duplicate and the contents of the two Petri dishes were pooled. Protein samples were sonicated and heated at 95°C for 5 mins in half the final volume of SDS-PAGE sample buffer (Tris-HCl, 62.5 mmol/L; DTT, 50 mmol/L; SDS, 2% glycerol, 10% and bromophenol blue, 0.1%). Samples were loaded onto polyacrylamide gels composed of a 10% or 6% acrylamide-bisacrylamide running gel and a 4.5% acrylamide-bisacrylamide stacking gel. After electrophoresis, proteins were transferred onto nitrocellulose membranes (Trans-Blot Transfer Medium 162-0115; Bio-Rad, Reinach, Switzerland) using a Transblot semi-dry transfer cell (Bio-Rad). For protein detection, membranes were incubated in a blocking solution of Tris-buffered saline supplemented with Tween-20 (TBST; Tris-HCl, pH 7.5, 50 mmol/L; NaCl, 150 mmol/L; and Tween-20, 0.1%) containing 5% nonfat milk for 1 h at room temperature. Membranes were incubated overnight at 4°C with the antiphospho-serine/threonine protein kinase from AKT virus (Akt)-Ser473 (1:700), antiphospho-p44/p42 MAPK-Thr202/Tyr204 (both 1:1,000), antiphospho-mTOR-Ser1448 (1:1,000), anti-mTOR (1:1,000), antiphospho-S6-Ser235/236 ribosomal protein (1:1,000), and anti-β-actin (A5441; Sigma). All primary antibodies were purchased from Cell Signalling (BioConcept, Allschwil, Switzerland), except anti-β-actin (A5441; Sigma). After three washes in TBST, membranes were incubated with the secondary antibodies Alexa Fluor 680 goat anti-IgG (Juro, Lucerne, Switzerland) and IRDye 800 goat anti-mouse IgG (BioConcept), diluted at 1:5,000 in TBST containing 1% nonfat milk, for 2 h at room temperature, and protected from light. After three washes in TBST, membranes were scanned using the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA), which permits detection and quantification of proteins of interest. β-Actin, revealed in green, was used for normalization and the proteins of interest were revealed in red. As phospho-mTOR has a very high molecular weight (289 kDa), actin was not visible on the same gel. To normalize western blots for phospho-mTOR, samples were loaded in duplicate onto a 6% running gel and proteins were transferred onto a nitrocellulose membrane using a 10% methanol transfer buffer. The membrane was then cut into two identical pieces and probed either with the phospho-mTOR antibody (1:1,000) or with the mTOR antibody (1:1,000). Thus, quantifications were performed on samples resolved on the same gel and transferred onto the same membrane, and normalization was conducted against mTOR (instead of β-actin). The remaining of the procedure was unchanged.
Quantitative Real-Time Reverse Transcriptase-PCR
Quantitative determination of MCT2 mRNA expression levels was performed by quantitative reverse transcriptase-PCR according to Heid et al (1996) using an ABI Prism 7000 sequence detection system from Applied Biosystems (Rotkreuz, Switzerland). The following sets of oligonucleotides were used: 5′ → 3′ ActinFo, GCTTCTTTGCAGCTCCTTCGT; ActinRe, ATATCGTCATCCATGGCGAAC (Embl: X03672); MCT2Fo, CAGCAACAGCGTGATAGAGCTT; MCT2Re, TGGTTGCAGGTTGAATGCTAAT (Embl: NM_011391); NPYFo, ACCAGACAGAGATATGGCAAGAGA; NPYRe, GGCGTTTTCTGTGC (produced by Microsynth, Balgach, Switzerland).
Preparation and Stimulation of Synaptoneurosomes
Synaptoneurosomes were prepared from the forebrains of 8- to 14-day-old mice pups (10 to 15 pups per preparation) according to a previously published protocol (Rao and Steward (1991), as modified by Schratt et al (2004)). Briefly, the total forebrains were dissected in 20 mL of homogenization buffer (0.32 mol/L sucrose, 0.1 mmol/L EDTA, 0.25 mmol/L DTT, and 3 mmol/L HEPES, pH 7.4) and disrupted using a Teflon-coated Dounce-Potter homogenizer (B. Braun, Crissier, Switzerland) by eight up-and-down strokes. Nuclei and cell debris were pelleted by 2 mins of centrifugation at 2,000 g. The supernatant was collected and centrifuged for an additional 10 mins at 14,000 g to pellet a crude synaptoneurosome-containing fraction (P2). The pellet was then brought up to 8 mL total volume (with a solution of 0.32 mol/L sucrose and 1 mmol/L NaHCO3). This suspension (4 × 2 mL) was layered onto three different discontinuous sucrose gradients (0.85, 1, 1.2 mmol/L) that had been equilibrated at 4°C for 1 h. The gradient was centrifuged at 45,000 g for 45 mins in a Centrikon T-1075 ultracentrifuge using a SW 41.14Ti swinging bucket rotor. Synaptoneurosomes were collected from the 1/1.2 mol/L interface (500 μL for 2 mL of P2), washed twice in 1 × PBS (in a 2-mL tube), with a centrifugation at 7,000 g for 2 mins. Synaptoneurosomes were resuspended in 500 μL of synaptoneurosome incubation buffer (10 mmol/L Tris, pH 7.5, 2.2 mmol/L CaCl2, 0.5 mmol/L Na2HPO4, 0.4 mmol/L KH2PO4, 4 mmol/L NaHCO3, and 80 mmol/L NaCl). Synaptoneurosomes were centrifuged at 7,000 g for 2 mins, the supernatant was discarded, and synaptoneurosomes in the lower fraction were either used immediately or stored at −80°C. No significant differences were observed between results from freshly prepared or frozen synaptosomes such that results were pooled. Enrichment of the synaptosomal fraction was controlled by performing western blotting for the postsynaptic protein PSD-95 (data not shown).
The synaptoneurosome pellet was thawed on ice for 30 mins and diluted in a prewarmed (37°C) synaptoneurosome incubation buffer containing a mixture of antiproteases (Complete 11257000; Roche Molecular Biochemicals) to yield a protein concentration between 2 and 9 mg/mL. Synaptoneurosome samples (100 μL) were exposed to either ActD (5 μmol/L) or cycloheximide (10 μmol/L) for 30 mins before application of 100 ng/mL BDNF for 6 h. The reaction was stopped by adding 20 μL of SDS buffer (5 × ), and samples were boiled for 5 mins and served as starting material for western blotting.
Results
Effect of Brain-Derived Neurotrophic Factor on MCT2 Protein Expression in Cultured Neurons
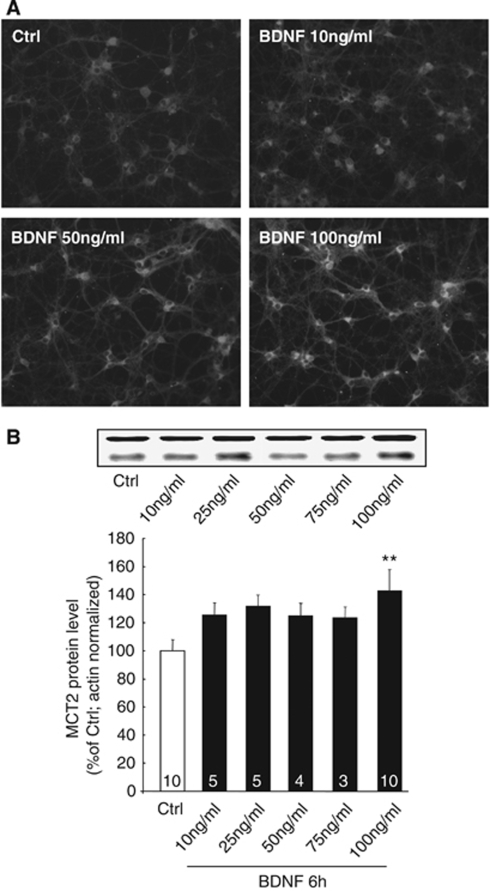
To assess the effect of BDNF on neuronal MCT2 protein expression, cultured cortical neurons were treated with BDNF for 6 h at various concentrations up to 100 ng/mL. Experiments conducted with BDNF concentrations of 10, 50, and 100 ng/mL followed by immunocytochemistry led to a striking enhancement of fluorescence intensity corresponding to higher levels of MCT2 immunoreactivity (IR) at 100 ng/mL (Figure 1A). Western blot analysis showed that the maximal increase of MCT2 expression was found with 100 ng/mL. At lower concentrations, MCT2 expression had a tendency to be increased, but the effect was not significant statistically (Figure 1B).
Figure 1.
Concentration-dependent effect of BDNF on MCT2 expression in primary cultures of mouse cortical neurons. (A) Immunocytochemical stainings for MCT2 in untreated cultures (Ctrl) and in cultures treated with BDNF for 6 h at different concentrations. (B) Western blot analysis of MCT2 expression in primary cultures of mouse cortical neurons treated with BDNF for 6 h at various concentrations up to 100 ng/mL. Western blots were quantified using Odyssey software (LI-COR Biosciences, Lincoln, NE, USA). Results were expressed as percentage of control after the values were normalized using β-actin signal as the reference. Statistical analysis was performed using ANOVA followed by Dunnett's test. ** indicates MCT2 protein levels significantly different from control with P<0.01. Numbers in the graph bars represent the number of independent experiments for each condition.
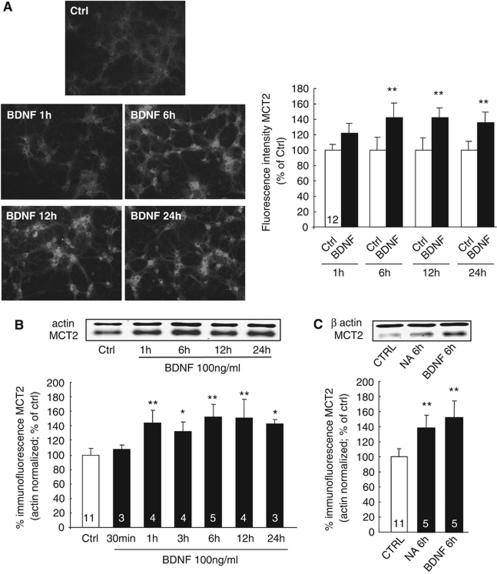
Changes in the levels of MCT2 IR induced by BDNF were also studied as a function of time (1, 6, 12, and 24 h). It was observed that 100 ng/mL BDNF caused a 40% increase in MCT2 IR 6 h after the beginning of the treatment that remained elevated at 12 and 24 h (Figure 2A). Western blot analysis showed that the increase of MCT2 expression was already found significant as early as after 1 h of BDNF stimulation (100 ng/mL) and that this significant increase was still present up to 24 h (Figure 2B). The effect of BDNF was found to be as robust as the previously described effect of noradrenaline (100 μmol/L) on MCT2 expression after 6 h of treatment (Figure 2C).
Figure 2.
Time course of the effect of BDNF on MCT2 protein expression in primary cultures of mouse cortical neurons. (A) MCT2 IR and related quantification of primary cultures of mouse cortical neurons treated with BDNF at a final concentration of 100 ng/mL for various periods of time up to 24 h (1, 6, 12, and 24 h). Left panels represent immunocytochemical stainings for MCT2 in untreated cultures (Ctrl) or cultures treated with BDNF for 6 h. The bar graph represents the quantitative determination of fluorescence intensity corresponding to MCT2 IR in cultured neurons treated with BDNF for various periods of time up to 24 h. Results are expressed as percentage of control fluorescence intensity and are represented as the mean±s.e.m. of independent determinations (numbers indicated on bars) from four distinct experiments. The value of fluorescence intensity for each determination represents the average level from 24 cells on the same coverslip. (B and C) Western blot analysis of MCT2 protein expression in cultures of mouse cortical neurons treated with BDNF 100 ng/mL or noradrenaline 100 μmol/L (NA 6 h) for the indicated times as compared with untreated cells (Ctrl). Western blots were quantified using Odyssey software (LI-COR Biosciences, Lincoln, NE, USA). Results are expressed as percentage of control (mean±s.e.m.) after the values had been normalized using β-actin signal as the reference. Statistical analysis was performed using ANOVA followed by Dunnett's test. *P<0.05, **P<0.01, ***P<0.001 versus control (Ctrl) for MCT2 protein levels. Numbers in the graph bars represent the number of independent experiments for each condition.
Putative Involvement of Distinct Signal Transduction Pathways in Brain-Derived Neurotrophic Factor-Induced MCT2 Expression
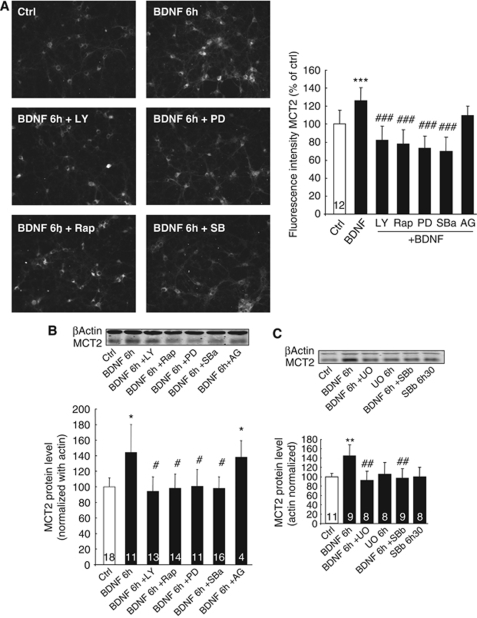
Involvement of specific signal transduction mechanisms involved in the effect of BDNF on MCT2 expression was investigated with a series of inhibitors. Cultured cortical neurons were pretreated with five different inhibitors (LY294002, a specific PI3K inhibitor; rapamycin, a specific mTOR inhibitor; PD98058, a specific MEK inhibitor; SB202190, a specific p38 MAPK inhibitor; and AG490, a specific JAK2 inhibitor) 30 mins before the addition of BDNF (100 ng/mL) for 6 h. The six panels on the left of Figure 3A show that a 30-min pretreatment of cultured mouse cortical neurons with LY294002 (10 μmol/L), rapamycin (20 ng/mL), PD98058 (50 μmol/L), or SB202190 (10 μmol/L) blocked the BDNF-induced increase in MCT2 IR after 6 h of treatment. In contrast, AG490 (25 μmol/L) had no effect (not shown). The quantification of MCT2 IR shows that all inhibitors (with the exception of AG490) prevented the induction of MCT2 protein expression by BDNF, reducing the overall expression by ∼40% (Figure 3A). Similar results were obtained by western blot, confirming the MCT2 IR data (Figure 3B).
Figure 3.
Effect of different signaling pathway inhibitors on BDNF-stimulated MCT2 expression in primary cultures of mouse cortical neurons. Primary cultures of mouse cortical neurons were pretreated for 30 mins with specific signaling pathway inhibitors (PD98095, 50 μmol/L (PD); SB202190, 10 μmol/L (SBa); LY294002, 10 μmol/L (LY); rapamycin, 20 ng/mL (Rap); AG490 25 μmol/L (AG); UO126, 10 μmol/L (UO); SB203580, 10 μmol/L (SBb)) before stimulation with BDNF 100 ng/mL for 6 h (BDNF 6 h). (A) Left panels represent immunocytochemical stainings for MCT2 in untreated cells (Ctrl), cultures treated with BDNF alone for 6 h (BDNF), or pretreated for 30 mins with each inhibitor (LY, Rap, PD, SBa) before the addition of BDNF. The bar graph represents the quantitative determination of fluorescence intensity corresponding to MCT2 IR in cultured neurons treated with the specific signaling pathway inhibitors followed by exposure to BDNF for 6 h. Results are expressed as percentage of control fluorescence intensity and represent the mean±s.e.m. of independent determinations (numbers indicated on bars) from four distinct experiments. The value of fluorescence intensity for each determination represents the average level from 24 cells on the same coverslip. (B and C) Western blot analysis of MCT2 expression in cultures of mouse cortical neurons treated with specific signaling pathway inhibitors (+LY, Rap, PD, SBa, AG, UO, SBb) before stimulation with BDNF 100 ng/mL for 6 h (BDNF 6 h). Western blots were quantified using Odyssey software (LI-COR Biosciences, Lincoln, NE, USA). Results are expressed as percentage of control (mean±s.e.m.) after the values had been normalized using β-actin signal as the reference. Statistical analysis was performed using ANOVA followed by Bonferroni's test. *P<0.05, **P<0.01 versus control (Ctrl). #P<0.05 versus 6 h BDNF treatment. Numbers in the graph bars represent the number of independent experiments for each condition.
The effect of all five inhibitors alone (without posttreatment with BDNF) was tested previously on cultured cortical neurons and no change in MCT2 IR was observed compared with the control condition (data not shown). To further validate our observations, two more inhibitors were tested for their effect on BDNF-induced MCT2 expression (UO126, a specific p44/p42 MAPK inhibitor, and SB203580, another specific p38 MAPK inhibitor). Cultured cortical neurons were pretreated with UO126 (10 μmol/L) and SB203580 (10 μmol/L) 30 mins before the addition of BDNF (100 ng/mL) for 6 h. Western blot analysis shows that UO126 and SB203580 significantly reduced MCT2 protein expression induced by BDNF (Figure 3C).
Activation by Brain-Derived Neurotrophic Factor of the Different Signal Transduction Pathways Implicated in MCT2 Upregulation
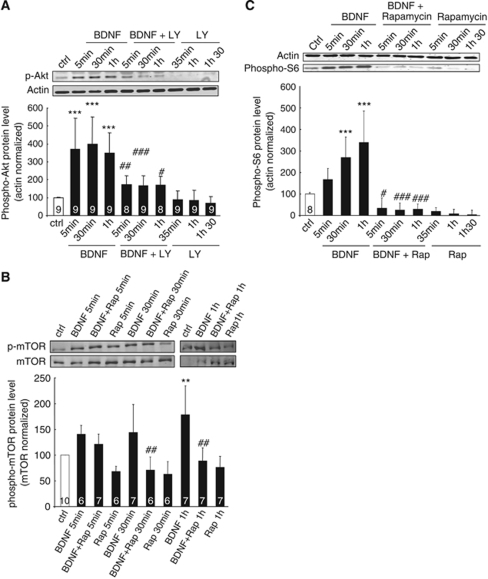
It became necessary to assess the effect of BDNF on each signal transduction pathway putatively implicated in MCT2 upregulation and to verify the efficacy of each inhibitor used. Cultured cortical neurons were treated with BDNF (100 ng/mL) for three time periods (5 mins, 30 mins, and 1 h). First, to evaluate the activation of the PI3K/Akt/mTOR/S6 pathway, the phosphorylation levels of Akt on Ser473, mTOR on Ser2448, and S6 ribosomal protein on Ser235/236 were determined by western blot. Brain-derived neurotrophic factor induced the phosphorylation of Akt after 5 mins of treatment (Figure 4A). The level of phosphorylation was increased by ∼300% above control (set at 100%) after 5 mins of treatment. Maximal activation was reached after 30 mins. Phosphorylation of Akt was sustained for 1 h of BDNF treatment. To obtain the confirmation that Akt signaling can be inhibited in a manner similar to MCT2 upregulation, cultured cortical neurons were pretreated with 10 μmol/L LY294002 for 30 mins before the addition of BDNF (100 ng/mL) for 5 mins, 30 mins, and 1 h. Indeed, LY294002 pretreatment prevented the phosphorylation of Akt induced by BDNF in cultured cortical neurons (Figure 4A).
Figure 4.
Effect of BDNF on the phosphorylation levels of Akt, mTOR, and S6 in cultured mouse cortical neurons. Western blot analysis of phospho-Akt (A), phospho-mTOR (B), and phospho-S6 (C) levels in cultures of mouse cortical neurons treated with BDNF 100 ng/mL for the indicated times as compared with untreated cells (ctrl). (Panel A) LY294002, a specific PI3K inhibitor, was added to the culture medium at the concentration of 10 μmol/L, 30 mins before incubation with BDNF 100 ng/mL during 5 mins, 30 mins, and 1 h. Western blots were quantified using Odyssey software (LI-COR Biosciences, Lincoln, NE, USA). Results are expressed as percentage of control after the values had been normalized using β-actin signal as the reference. (Panels B and C) Rapamycin, a specific mTOR inhibitor, was added to the culture medium at a concentration of 20 ng/mL, 30 mins before incubation with BDNF 100 ng/mL. Results are expressed as percentage of control after the values had been normalized using either β-actin signal (panel C) or mTOR signal (panel B) as the reference. Statistical analysis was performed using ANOVA followed by Bonferroni's test. **, *** indicates phospho-Akt, phospho-mTOR, or phospho-S6 protein levels significantly different from control with P<0.01, P<0.001 respectively. #, ##, ### indicates phospho-Akt, phospho-mTOR, or phospho-S6 protein levels significantly different from the BDNF-treated condition with P<0.05, P<0.01, and P<0.001, respectively. Numbers in the graph bars represent the number of independent experiments for each condition.
Activation of mTOR by BDNF treatment was investigated by monitoring its level of phosphorylation. Brain-derived neurotrophic factor induced the phosphorylation of mTOR within 5 mins after the beginning of the treatment (Figure 4B). Phosphorylation of mTOR was further increased by more than 50% above the control level after 1 h of BDNF treatment. To determine whether mTOR activation can be prevented in the same manner as MCT2 upregulation, cultured cortical neurons were pretreated with 20 ng/mL rapamycin for 30 mins before adding BDNF (100 ng/mL) for 5 mins, 30 mins, and 1 h. Rapamycin pretreatment completely prevented the phosphorylation of mTOR induced by BDNF in cultured cortical neurons at 30 mins (Figure 4B).
S6 phosphorylation represents one of the late steps in the activation of the PI3K/Akt/mTOR/S6 pathway. Phosphorylation levels of S6 were assessed after BDNF treatment. Brain-derived neurotrophic factor induced the phosphorylation of S6 within 5 mins after the beginning of the treatment (80% above control; Figure 4C). Maximal activation was reached after 1 h with an increase of 350% above control. As the S6 protein is known to be a downstream effector of the Akt/mTOR signaling pathway, cultured cortical neurons were pretreated with 20 ng/mL rapamycin (inhibitor of mTOR) for 30 mins before the addition of BDNF for 5 mins, 30 mins, and 1 h. It was observed that rapamycin pretreatment completely prevented the phosphorylation of S6 ribosomal protein induced by BDNF in cultured cortical neurons (Figure 4C).
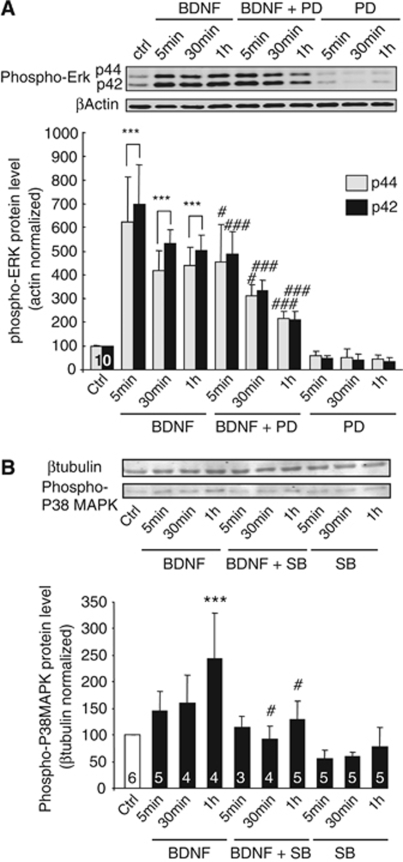
Mitogen-activated protein kinase activation is purported to be involved in the effect of BDNF on MCT2 expression. Phosphorylation levels of p44 and p42 MAPK (phospho-ERK) on Thr202/Tyr204 were determined by western blot after treatment of cultured cortical neurons with BDNF (100 ng/mL) for three different time periods (5 mins, 30 mins, and 1 h). Brain-derived neurotrophic factor induced the phosphorylation of p44 and p42 MAPK after 5 mins of treatment (Figure 5A). The level of phosphorylation was increased by more than 500% (for both p44 and p42) above control (set at 100%) at that time. Phosphorylation of p44 and p42 MAPK was sustained for 1 h of BDNF treatment. To ascertain whether MAPK activation can be prevented similar to MCT2 upregulation, cultured cortical neurons were pretreated with 50 μmol/L PD98059 for 30 mins before the addition of BDNF (100 ng/mL) for 5 mins, 30 mins, and 1 h. As expected, PD98059 pretreatment attenuated the phosphorylation of p44 and p42 MAPK induced by BDNF in cultured cortical neurons (Figure 5A).
Figure 5.
Effect of BDNF on the phosphorylation levels of ERK and p38 MAPK in cultured mouse cortical neurons. Western blot analysis of phospho-ERK (A) and phospho-p38 MAPK (B) levels in cultures of mouse cortical neurons treated with BDNF 100 ng/mL for the indicated times as compared with untreated cells (ctrl). (Panel A) PD98058, a specific MEK inhibitor, was added to the culture medium at a concentration of 50 μmol/L, 30 mins before incubation with BDNF 100 ng/mL during 5 mins, 30 mins, and 1 h. (Panel B) SB202190, a specific p38 MAPK inhibitor, was added to the culture medium at a concentration of 10 μmol/L, 30 mins before incubation with BDNF 100 ng/mL. Western blots were quantified using Odyssey software (LI-COR Biosciences, Lincoln, NE, USA). Results are expressed as percentage of control after the values had been normalized using β-actin signal as reference. Statistical analysis was performed using ANOVA followed by Bonferroni's test. *** indicates phospho-ERK or phospho-p38 MAPK levels significantly different from control with P<0.001. ##, ### indicates phospho-ERK or phospho-p38 MAPK levels significantly different from BDNF-treated condition with P<0.01 and P<0.001, respectively. Numbers in the graph bars represent the number of independent experiments for each condition.
Activation of p38 MAPK is another possible signaling mechanism involved in BDNF-induced MCT2 upregulation. Phosphorylation levels of p38 MAPK on Thr180/Tyr182 were determined by western blot. Brain-derived neurotrophic factor induced the phosphorylation of p38 MAPK that reached a maximum after 1 h with an increase of ∼150% above control (set at 100% Figure 5B). To investigate whether p38 MAPK activation can be prevented using the inhibitor previously used, cultured cortical neurons were pretreated with 10 μmol/L SB202190 for 30 mins before the addition of BDNF (100 ng/mL) for 5 mins, 30 mins, and 1 h. SB202190 pretreatment was found to prevent phosphorylation of p38 MAPK induced by BDNF in cultured cortical neurons (Figure 5B).
Brain-Derived Neurotrophic Factor Enhances MCT2 Expression by a Translational Mechanism
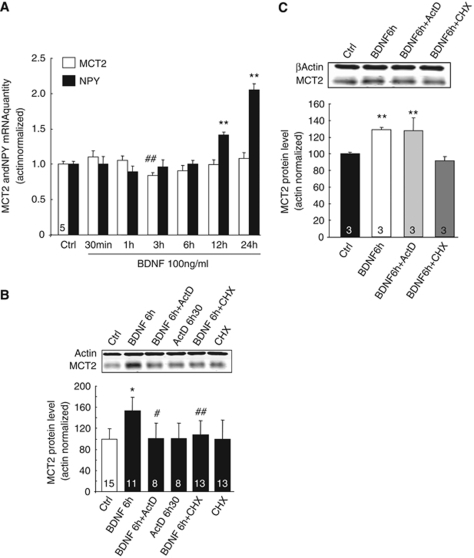
The effect of BDNF on MCT2 mRNA expression was investigated by quantitative reverse transcriptase-PCR on total RNA from mouse cortical neurons. Neurons were treated with BDNF (100 ng/mL) for various time points up to 24 h. Figure 6A shows that BDNF exerted no enhancing effect on MCT2 mRNA levels at any time point, whereas NPY mRNA levels (a peptide known to be transcriptionally regulated) showed a significant increase after 12 and 24 h. A small but significant decrease in MCT2 mRNA levels was observed at 3 h. To ascertain whether the effect of BDNF on MCT2 expression requires the activation of translation but not of transcription, cultured neurons were treated with inhibitors for these two processes. Application of cycloheximide (10 μmol/L), a protein synthesis inhibitor, 30 mins before BDNF stimulation (100 ng/mL), prevented the enhancement of MCT2 protein expression (Figure 6B). Quite unexpectedly, the mRNA synthesis inhibitor ActD (5 μmol/L) also blocked the effect of BDNF on MCT2 protein expression. To determine whether the enhancement in MCT2 protein synthesis occurs at the synaptic level and requires transcriptional activation, an experiment was conducted on synaptoneurosomes (Figure 6C). Brain-derived neurotrophic factor (100 ng/mL) induced an increase in MCT2 protein expression after 6 h in this preparation. The effect of BDNF was prevented by cycloheximide (10 μmol/L) but not by ActD (5 μmol/L).
Figure 6.
Synaptic regulation of MCT2 protein synthesis by BDNF. (A) Primary cultures of mouse cortical neurons were treated with BDNF 100 ng/mL for various periods of time. Neuronal cultures were harvested, and total RNA was extracted and analyzed for MCT2 and NPY mRNA expression by quantitative real-time RT-PCR. Results are expressed as percentages of control (mean±s.d.) after the values had been normalized using β-actin gene as the internal reference and were expressed as a relative mRNA quantity compared with control. Five independent experiments were pooled after performing quantitative RT-PCR. (B) Western blot analysis of MCT2 expression in primary cultures of mouse cortical neurons exposed to either actinomycin D (ActD) or cycloheximide (CHX) for 30 mins before the application of 100 ng/mL BDNF for 6 h. (C) Western blot analysis of MCT2 expression in synaptoneurosomes treated with BDNF 100 ng/mL in the presence or absence of ActD or CHX. Western blots were quantified using the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA). Results are expressed as percentage of control after the values had been normalized using β-actin signal as the reference. Statistical analysis was performed using ANOVA followed by Bonferonni's test. *,** indicate MCT2 protein levels significantly different from control with P<0.05 and P<0.01. #, ## indicate MCT2 protein levels significantly different from BDNF-treated condition with P<0.05 and P<0.01, respectively. Numbers in the graph bars represent the number of independent experiments for each condition.
Discussion
In recent years, BDNF has emerged as a major regulator of synaptic transmission and plasticity at adult synapses in many regions of the central nervous system (Bramham and Messaoudi, 2005). Among the mechanisms subserving synaptic plasticity, enhancement of localized protein synthesis constitutes a critical process for long-term adaptation of synaptic efficacy. Brain-derived neurotrophic factor is one of the major activity-dependent modulators of dendritic protein synthesis and it is known to activate specific components of the translational machinery in neurons (Bramham and Messaoudi, 2005; Steward and Schuman, 2003). Previously, it has been shown that the rapid induction of protein synthesis by BDNF is mediated both through the PI3K pathway, as it involves the activation of PI3K and mTOR (Bramham and Messaoudi, 2005; Schratt et al, 2004; Yoshii and Constantine-Paton, 2007), as well as through the MAPK signaling pathway (Kelleher et al, 2004). Interestingly, BDNF was shown in this study to enhance MCT2 protein expression in cultured cortical neurons through a stimulation of translation. This is supported by the observations that no changes in MCT2 mRNA levels were detected, whereas MCT2 protein expression was enhanced by BDNF in synaptoneurosomes, a preparation that can sustain translation but not transcriptional activation. The observation that the mRNA synthesis inhibitor ActD can prevent BDNF-induced enhancement of MCT2 protein expression in intact cells but not in synaptoneurosomes is intriguing. It cannot be excluded that as a general transcription inhibitor, ActD indirectly interferes with the translation process in intact cells by reducing the expression of some essential components. The effect of BDNF on MCT2 protein expression was shown to involve the activation of three distinct signaling pathways, all classically implicated in the regulation of translation initiation (Hay and Sonenberg, 2004). Thus, a concomitant activation of PI3K/Akt/mTOR, p38 MAPK as well as MEK/ERK kinases was found to be necessary for the enhancement of MCT2 protein expression by BDNF (Figure 7). Such an observation suggests a putative cross talk between the different signaling pathways activated by BDNF to regulate translational activation, a possibility previously proposed by others (Almeida et al, 2005; Bramham and Messaoudi, 2005). It is noted that noradrenaline (a neurotransmitter), insulin (a hormone), and IGF-1 (a neurotrophic factor) were also previously shown to regulate MCT2 protein expression at the translational level, notably by activating the PI3K/Akt/mTOR/S6 kinase pathway in cultured cortical neurons (Chenal and Pellerin, 2007; Chenal et al, 2008). As each of these neuroactive substances, similar to BDNF, has been implicated in different forms of synaptic plasticity, it suggests that MCT2 represents a common target for such signals, pointing to a putatively important role of MCT2 in relation to synaptic modifications.
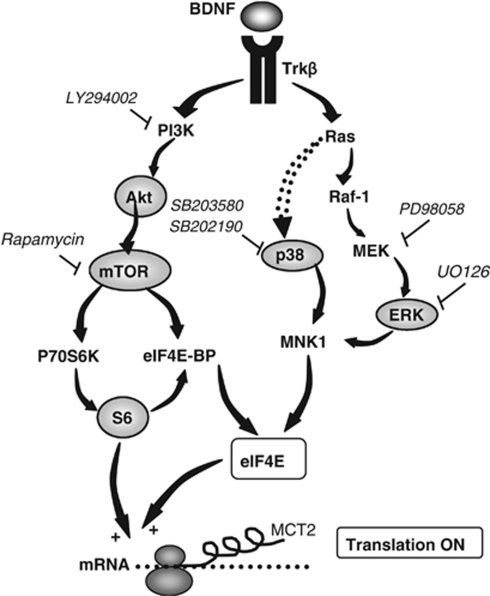
Figure 7.
Putative signaling pathways leading to translational activation and enhanced MCT2 protein synthesis after BDNF treatment in cultured cortical neurons. BDNF and TrkB can activate distinct signal transduction pathways involving specific kinases leading to translation initiation. Two pathways investigated in this study are illustrated herein (PI3K/Akt/mTOR/S6 and MAPK signaling pathways). The surrounding proteins were directly investigated for their level of phosphorylation. First, phosphorylation of PI3K can cause the phosphorylation and activation of Akt, which then directly phosphorylates mTOR, which in turn phosphorylates p70S6K. The target of p70S6K, the ribosomal S6 protein, once phosphorylated, participates in the translational machinery as part of the 40S complex. Second, the MAPK cascade is also activated by BDNF requiring the activation of MEK. MEK phosphorylates the p44 and p42 MAPK, which can activate, among others, MNK1. When activated, MNK1 phosphorylates eIF4E on Ser209 that correlates with enhanced rates of translation of capped mRNA. Specific inhibitors for some kinases have been used to distinguish the implication of each pathway in the effect of BDNF: LY294002, PI3K inhibitor; rapamycin, mTOR inhibitor; PD98058, MEK inhibitor; SB202190 and SB203580, p38 MAPK inhibitors.
In view of the current results, it appears that MCT2 belongs to a group of neuronal proteins specifically regulated at the translational level. Until now, more than 100 different mRNAs coding for proteins involved in neurotransmission and in the modulation of synaptic activity have been identified in dendrites. Local protein synthesis from these mRNAs is postulated to provide the basic mechanism of long-term changes in the strength of neuronal connections (Skup, 2008). Postsynaptic proteins that undergo enhancement of their synthesis locally include calmodulin kinase II (Ouyang et al, 1999; Wu and Cline, 1998), MAP2 (Blichenberg et al, 1999), Arc/Arg 3.1 (Steward and Schuman, 2001), and GluR1, as well as GluR2 AMPA receptor subunits (Ju et al, 2004). Although the presence of MCT2 mRNA has not been described yet in dendrites, it seems likely that the translational regulation of MCT2 expression would occur locally. Our results obtained with synaptoneurosomes support this conclusion. The MCT2 protein is not only present in dendrites but it is also specifically associated with spines, where it was found to be expressed in the postsynaptic density as well as on vesicle-like structures forming an intracellular pool (Bergersen et al, 2005). MCT2 was recently found to interact with a specific subset of postsynaptic proteins (Pierre et al, 2009). This is particularly the case with GluR2, a subunit of the glutamatergic AMPA receptor subtype. It was shown that MCT2 seems not only to determine the subcellular localization of GluR2 in neural cells but also to regulate its expression levels (Maekawa et al, 2009). In addition, it was observed that MCT2 together with GluR2 undergoes a trafficking process between the plasma membrane and an intracellular pool under conditions inducing synaptic plasticity (Pierre et al, 2009). Translocation of GluR2 to and from the plasma membrane has been implicated in synaptic plasticity mechanisms, such as long-term potentiation and long-term depression (Kessels and Malinow, 2009; Malenka, 2003; Sheng and Kim, 2002). Thus, MCT2 localization and interaction with specific synaptic proteins involved in plasticity mechanisms strengthen the view that its expression may be regulated in a manner similar to its partners. In this regard, increased MCT2 protein expression by BDNF may be part of a coordinated mechanism of local synthesis for various postsynaptic proteins involved in the long-term regulation of glutamatergic transmission.
Although a major effort has been devoted to decipher the molecular events involved in synaptic plasticity, including those induced by BDNF, few studies have explored the concomitant changes in neuroenergetics that could take place with alterations in synaptic transmission. Recently, the concept that energy metabolism might be coupled to synaptic plasticity has been proposed together with the implication of BDNF in such interactions (Vaynman et al, 2006). Indeed, BDNF was shown to enhance mitochondrial activity (El Idrissi and Trenkner, 1999). Regarding the energy substrates that might be concerned, lactate has attracted much attention recently. Lactate has been shown to be a preferential oxidative substrate for neurons both in vitro (McKenna et al, 1993, 1994) and in vivo (Hyder et al, 2006; Serres et al, 2005). Lactate is not only able to sustain synaptic vesicle turnover and synaptic transmission (Morgenthaler et al, 2006; Rouach et al, 2008; Schurr et al, 1988) but it is also shown to allow, at least in part, the establishment of long-term potentiation (Izumi et al, 1997; Yang et al, 2003). In addition, glutamatergic activity increases the production and release of lactate by astrocytes (Pellerin and Magistretti, 1994), and it has been purported that such an effect participates in a mechanism to provide lactate as an additional energy substrate to neurons to sustain their activity (Pellerin et al, 2007). As the primary role of MCT2 is to supply neurons with nonglucose energy substrates, e.g., lactate, changes in MCT2 expression might facilitate the utilization of alternative substrates. Indeed, overexpression of MCT2 in neurons was shown to enhance lactate consumption by neurons stimulated with kainate (Bliss et al, 2004). Our observation that MCT2 can be upregulated by BDNF is consistent with the possibility of a coupling between lactate utilization and synaptic plasticity. It is hypothesized that to meet higher energy demands caused by enhanced synaptic transmission after synaptic plasticity, MCT2 expression is increased to facilitate lactate supply at potentiated synapses.
In conclusion, we have shown that BDNF enhances the expression of the monocarboxylate transporter MCT2 in cultured cortical neurons through a translational regulation. A possible role for such an effect could be to enlarge the local lactate transporter pool to allow potentiated synapses to meet higher energy demands on activation.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Graos MM, Carvalho RF, Carvalho AP, Duarte CB. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12:1329–1343. doi: 10.1038/sj.cdd.4401662. [DOI] [PubMed] [Google Scholar]
- Bergersen L, Rafiki A, Ottersen OP. Immunogold cytochemistry identifies specialized membrane domains for monocarboxylate transport in the central nervous system. Neurochem Res. 2002;27:89–96. doi: 10.1023/a:1014806723147. [DOI] [PubMed] [Google Scholar]
- Bergersen LH, Magistretti PJ, Pellerin L. Selective postsynaptic co-localization of MCT2 with AMPA receptor GluR2/3 subunits at excitatory synapses exhibiting AMPA receptor trafficking. Cereb Cortex. 2005;15:361–370. doi: 10.1093/cercor/bhh138. [DOI] [PubMed] [Google Scholar]
- Blichenberg A, Schwanke B, Rehbein M, Garner CC, Richter D, Kindler S. Identification of a cis-acting dendritic targeting element in MAP2 mRNAs. J Neurosci. 1999;19:8818–8829. doi: 10.1523/JNEUROSCI.19-20-08818.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TM, Ip M, Cheng E, Minami M, Pellerin L, Magistretti P, Sapolsky RM. Dual-gene, dual-cell type therapy against an excitotoxic insult by bolstering neuroenergetics. J Neurosci. 2004;24:6202–6208. doi: 10.1523/JNEUROSCI.0805-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Carvalho AL, Caldeira MV, Santos SD, Duarte CB. Role of the brain-derived neurotrophic factor at glutamatergic synapses. Br J Pharmacol. 2008;153 (Suppl 1:S310–S324. doi: 10.1038/sj.bjp.0707509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenal J, Pellerin L. Noradrenaline enhances the expression of the neuronal monocarboxylate transporter MCT2 by translational activation via stimulation of PI3K/Akt and the mTOR/S6K pathway. J Neurochem. 2007;102:389–397. doi: 10.1111/j.1471-4159.2007.04495.x. [DOI] [PubMed] [Google Scholar]
- Chenal J, Pierre K, Pellerin L. Insulin and IGF-1 enhance the expression of the neuronal monocarboxylate transporter MCT2 by translational activation via stimulation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin pathway. Eur J Neurosci. 2008;27:53–65. doi: 10.1111/j.1460-9568.2007.05981.x. [DOI] [PubMed] [Google Scholar]
- Debernardi R, Pierre K, Lengacher S, Magistretti PJ, Pellerin L. Cell-specific expression pattern of monocarboxylate transporters in astrocytes and neurons observed in different mouse brain cortical cell cultures. J Neurosci Res. 2003;73:141–155. doi: 10.1002/jnr.10660. [DOI] [PubMed] [Google Scholar]
- El Idrissi A, Trenkner E. Growth factors and taurine protect against excitotoxicity by stabilizing calcium homeostasis and energy metabolism. J Neurosci. 1999;19:9459–9468. doi: 10.1523/JNEUROSCI.19-21-09459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia CK, Goldstein JL, Pathak RK, Anderson RG, Brown MS. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. 1994;76:865–873. doi: 10.1016/0092-8674(94)90361-1. [DOI] [PubMed] [Google Scholar]
- Garcia CK, Brown MS, Pathak RK, Goldstein JL. cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. J Biol Chem. 1995;270:1843–1849. doi: 10.1074/jbc.270.4.1843. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Katsuki H, Zorumski CF. Monocarboxylates (pyruvate and lactate) as alternative energy substrates for the induction of long-term potentiation in rat hippocampal slices. Neurosci Lett. 1997;232:17–20. doi: 10.1016/s0304-3940(97)00567-3. [DOI] [PubMed] [Google Scholar]
- Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci. 2004;7:244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, III, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Yasoshima Y. The central noradrenaline system and memory consolidation. Neuroscientist. 2001;7:371–376. doi: 10.1177/107385840100700506. [DOI] [PubMed] [Google Scholar]
- Maekawa F, Tsuboi T, Fukuda M, Pellerin L. Regulation of the intracellular distribution, cell surface expression, and protein levels of AMPA receptor GluR2 subunits by the monocarboxylate transporter MCT2 in neuronal cells. J Neurochem. 2009;109:1767–78. doi: 10.1111/j.1471-4159.2009.06100.x. [DOI] [PubMed] [Google Scholar]
- Malenka RC. Synaptic plasticity and AMPA receptor trafficking. Ann N Y Acad Sci. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Tildon JT, Stevenson JH, Boatright R, Huang S. Regulation of energy metabolism in synaptic terminals and cultured rat brain astrocytes: differences revealed using aminooxyacetate. Dev Neurosci. 1993;15:320–329. doi: 10.1159/000111351. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Tildon JT, Stevenson JH, Hopkins IB. Energy metabolism in cortical synaptic terminals from weanling and mature rat brain: evidence for multiple compartments of tricarboxylic acid cycle activity. Dev Neurosci. 1994;16:291–300. doi: 10.1159/000112122. [DOI] [PubMed] [Google Scholar]
- Morgenthaler FD, Kraftsik R, Catsicas S, Magistretti PJ, Chatton JY. Glucose and lactate are equally effective in energizing activity-dependent synaptic vesicle turnover in purified cortical neurons. Neuroscience. 2006;141:157–165. doi: 10.1016/j.neuroscience.2006.03.065. [DOI] [PubMed] [Google Scholar]
- Ouyang Y, Rosenstein A, Kreiman G, Schuman EM, Kennedy MB. Tetanic stimulation leads to increased accumulation of Ca(2+)/calmodulin-dependent protein kinase II via dendritic protein synthesis in hippocampal neurons. J Neurosci. 1999;19:7823–7833. doi: 10.1523/JNEUROSCI.19-18-07823.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L. Lactate as a pivotal element in neuron-glia metabolic cooperation. Neurochem Int. 2003;43:331–338. doi: 10.1016/s0197-0186(03)00020-2. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre K, Chatton JY, Parent A, Repond C, Gardoni F, Di Luca M, Pellerin L. Linking supply to demand: the neuronal monocarboxylate transporter MCT2 and the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid receptor GluR2/3 subunit are associated in a common trafficking process. Eur J Neurosci. 2009;29:1951–1963. doi: 10.1111/j.1460-9568.2009.06756.x. [DOI] [PubMed] [Google Scholar]
- Pierre K, Magistretti PJ, Pellerin L. MCT2 is a major neuronal monocarboxylate transporter in the adult mouse brain. J Cereb Blood Flow Metab. 2002;22:586–595. doi: 10.1097/00004647-200205000-00010. [DOI] [PubMed] [Google Scholar]
- Pierre K, Pellerin L, Debernardi R, Riederer BM, Magistretti PJ. Cell-specific localization of monocarboxylate transporters, MCT1 and MCT2, in the adult mouse brain revealed by double immunohistochemical labeling and confocal microscopy. Neuroscience. 2000;100:617–627. doi: 10.1016/s0306-4522(00)00294-3. [DOI] [PubMed] [Google Scholar]
- Rao A, Steward O. Evidence that protein constituents of postsynaptic membrane specializations are locally synthesized: analysis of proteins synthesized within synaptoneurosomes. J Neurosci. 1991;11:2881–2895. doi: 10.1523/JNEUROSCI.11-09-02881.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME. BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci. 2004;24:7366–7377. doi: 10.1523/JNEUROSCI.1739-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurr A, West CA, Rigor BM. Lactate-supported synaptic function in the rat hippocampal slice preparation. Science. 1988;240:1326–1328. doi: 10.1126/science.3375817. [DOI] [PubMed] [Google Scholar]
- Serres S, Bezancon E, Franconi JM, Merle M. Ex vivo NMR study of lactate metabolism in rat brain under various depressed states. J Neurosci Res. 2005;79:19–25. doi: 10.1002/jnr.20277. [DOI] [PubMed] [Google Scholar]
- Sheng M, Kim MJ. Postsynaptic signaling and plasticity mechanisms. Science. 2002;298:776–780. doi: 10.1126/science.1075333. [DOI] [PubMed] [Google Scholar]
- Skup M. BDNF and NT-3 widen the scope of neurotrophin activity: pharmacological implications. Acta Neurobiol Exp (Wars) 1994;54:81–94. [PubMed] [Google Scholar]
- Skup M. Dendrites as separate compartment–local protein synthesis. Acta Neurobiol Exp (Wars) 2008;68:305–321. doi: 10.55782/ane-2008-1697. [DOI] [PubMed] [Google Scholar]
- Steward O, Schuman EM. Protein synthesis at synaptic sites on dendrites. Annu Rev Neurosci. 2001;24:299–325. doi: 10.1146/annurev.neuro.24.1.299. [DOI] [PubMed] [Google Scholar]
- Steward O, Schuman EM. Compartmentalized synthesis and degradation of proteins in neurons. Neuron. 2003;40:347–359. doi: 10.1016/s0896-6273(03)00635-4. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Piriz J, Llorens-Martin MV, Fernandez AM, Bolos M, LeRoith D, Nunez A, Torres-Aleman I. Central actions of liver-derived insulin-like growth factor 1 underlying its pro-cognitive effects. Mol Psychiatry. 2007;12:1118–1128. doi: 10.1038/sj.mp.4002076. [DOI] [PubMed] [Google Scholar]
- van der Heide LP, Ramakers GM, Smidt MP. Insulin signaling in the central nervous system: learning to survive. Prog Neurobiol. 2006;79:205–221. doi: 10.1016/j.pneurobio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Wu A, Gomez-Pinilla F. Coupling energy metabolism with a mechanism to support brain-derived neurotrophic factor-mediated synaptic plasticity. Neuroscience. 2006;139:1221–1234. doi: 10.1016/j.neuroscience.2006.01.062. [DOI] [PubMed] [Google Scholar]
- Wu GY, Cline HT. Stabilization of dendritic arbor structure in vivo by CaMKII. Science. 1998;279:222–226. doi: 10.1126/science.279.5348.222. [DOI] [PubMed] [Google Scholar]
- Yang B, Sakurai T, Takata T, Yokono K. Effects of lactate/pyruvate on synaptic plasticity in the hippocampal dentate gyrus. Neurosci Res. 2003;46:333–337. doi: 10.1016/s0168-0102(03)00096-8. [DOI] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat Neurosci. 2007;10:702–711. doi: 10.1038/nn1903. [DOI] [PubMed] [Google Scholar]