Abstract
Two types of quantal spontaneous neurotransmitter release are present in the nervous system, namely action potential (AP)-dependent release and AP-independent release. Previous studies have identified and characterized AP-independent release during hypoxia and ischemia. However, the relative contribution of AP-dependent spontaneous release to the overall glutamate released during transient ischemia has not been quantified. Furthermore, the neuronal activity that mediates such release has not been identified. Using acute brain slices, we show that AP-dependent release constitutes approximately one-third of the overall glutamate-mediated excitatory postsynaptic potentials/currents (EPSPs/EPSCs) measured onto hippocampal CA1 pyramidal neurons. However, during transient (2 mins) in vitro hypoxia–hypoglycemia, large-amplitude, AP-dependent spontaneous release is significantly enhanced and contributes to 74% of the overall glutamatergic responses. This increased AP-dependent release is due to hyper-excitability in the presynaptic CA3 neurons, which is mediated by the activity of NMDA receptors. Spontaneous glutamate release during ischemia can lead to excitotoxicity and perturbation of neural network functions.
Keywords: action potential, CA1/CA3, hippocampus, ischemia, spontaneous transmitter release
Introduction
Extracellular accumulation of the excitatory neurotransmitter, glutamate, is believed to be an important mediator of hypoxic/ischemic injury in the CNS. Glutamate-induced cell damage involves depolarization of neurons and increases in intracellular Na+ and Ca2+. While Na+ triggers water shift and cell swelling, Ca2+ activates numerous intracellular cascades that trigger neuronal apoptosis (Kulik et al, 2000). Treatment with N-methyl--aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxalone (AMPA) antagonists significantly reduces neuronal death after ischemia in rodent models (Montero et al, 2007).
Many factors contribute to the release of glutamate and the rise of interstitial glutamate in hypoxia/ischemia. These factors include glutamate release from neurons at synaptic and extrasynaptic sites, as well as from glia (Back et al, 2007). Glutamate release could also be caused by reversed operation of neuronal glutamate transporters in hypoxia (Gebhardt et al, 2002). However, it is agreed that the initial increase is mainly due to spontaneously released glutamate from neuronal synapses (Krnjević, 2008), and this process is mediated by Ca2+-dependent exocytosis (Kulik et al, 2000; Rossi et al, 2000). In the hippocampus, intracellular calcium concentration rises during ischemia in the CA1 area (Allen et al, 2004), including in the Schaffer collateral presynaptic terminals (Tonkikh and Carlen, 2009). Electrophysiological evidence indicates that spontaneous glutamate release increases during the first several minutes of hypoxia (Katchman and Hershkowitz, 1993) and ischemia (Kulik et al, 2000).
What are the underlying mechanisms that mediate the enhanced spontaneous glutamate release during the early phase of hypoxia/ischemia? Previous studies have identified two distinct types of spontaneous release during early ischemia. The action potential (AP)-independent, spontaneous release (miniature release) reflects a quantal mechanism and is insensitive to the Na+ channel blocker, tetrodotoxin (TTX). An increase in the frequency of such release has been reported in CA1 hippocampal neurons (Katchman and Hershkowitz, 1993) and neocortical neurons (Fleidervish et al, 2001) during hypoxia, and in brainstem neurons during transient ischemia (Kulik et al, 2000). These studies concluded that AP-independent release during hypoxia/ischemia is mainly mediated by increased presynaptic calcium (as measured by Tonkikh and Carlen, 2009) from internal calcium stores that rely on ATP to maintain calcium homeostasis (Katchman and Hershkowitz, 1993). The AP-dependent, spontaneous release is mediated by a large phasic increase of presynaptic Ca2+, which is caused by an AP invading the presynaptic terminals (Goda and Stevens, 1994; Cummings et al, 1996). Compared with the spontaneous miniature release, these events are sensitive to TTX and are characterized by greater amplitude, as they reflect a multi-bouton glutamate release that is triggered by the presynaptic AP (Fleidervish et al, 2001). However, little is known about the relative contribution of this type of release to the overall glutamate released in hypoxia/ischemia, and the source of APs that trigger this release.
We chose to use the rodent hippocampus to study the AP-dependent spontaneous release during early transient ischemia, because of its well-defined tri-synaptic circuitry, and because this type of release has previously been documented in this structure. Here, we report that transient (2 mins) in vitro hypoxic–hypoglycemic (H/H) challenge leads to significant enhancement of the AP-dependent, spontaneous glutamatergic release in the CA1 area of the mouse hippocampus, which can account for 74% of the overall glutamatergic responses during this transient ischemic episode. The enhanced release is not due to altered postsynaptic efficiency. Rather, it is due to NMDA-receptor-mediated enhancement in the activity of presynaptic CA3 neurons.
Materials and methods
All tissue preparation was performed in accordance with the Canadian Animal Care Guidelines. Brain slices were obtained from 10- to 14-day-old mice (B3C6F1; Charles River, Wilmington, MA, USA). Mice were anesthetized with ketamine (10 mg/kg) administered intraperitoneally. Transcardial perfusion was performed with ice-cold oxygenated (95% O2+5% CO2) sucrose-based artificial cerebrospinal fluid (ACSF). The animals were then decapitated and the brain was quickly removed, hemisected, and placed into ice-cold high sucrose ACSF for ∼3 mins. The high-sucrose dissection solution contained the following (in mmol/L): 210 sucrose, 26 NaHCO3, 2.5 KCL, 1 CaCl2, 4 MgCl2, 1.25 NaH2PO4, and 10 -glucose (pH 7.4, 295 mOsm), and was saturated with 95%O2+5% CO2. Brain slices (400 μm) were obtained with a Vibratome (Series 1000; Technical Products Inc., St Louis, MO, USA) and incubated in normal ACSF at room temperature for a minimum of 1 h before recording. Normal ACSF contained the following (in mmol/L): 120 NaCl, 2.5 KCl, 2 CaCl2, 2 MgCl2, 25 NaHCO3, and 10 -glucose (pH 7.4), and was continuously bubbled with 95% O2+5% CO2. In some experiments, to isolate the effect of CA3 to the CA1 region, a surgical cut was made with a micro knife on the Schaffer collateral, to physically separate the CA3 and CA1 subfields.
For intracellular recordings, slices were transferred to a submerged recording chamber and continuously perfused at a speed of 4 to 6 mL/min, with bubbled ACSF at 35±0.5°C. The recording chamber was mounted on a Zeiss Axioskop FS upright microscope (Zeiss, Germany). We used infrared differential interference contrast microscopy to visualize individual neurons. Patch-clamp electrodes were positioned onto the cell membrane under visual guidance using a Newport crossed roller bearing an XYZ translation stage equipped with motorizers. Whole-cell recordings were performed using an Axoclamp 200B amplifier (Axon Instruments, Foster city, CA, USA). Digitization was performed by a 12-bit A/D board (Digidata 1200; Axon Instruments), and recorded using pCLAMP software (v 9.2). The Bessel filter of the Axopatch amplifier (200B) was set at 2 or 5 kHz. The components of the patch pipette (intracellular) solution were the following (in mmol/L): 150 potassium gluconate, 10 KCl, 2 HEPES, 0.1 EGTA (pH 7.25, adjusted with KOH, and 280 to 290 mOsm). Patch pipettes were pulled from borosilicate capillary tubing (World Precision Instruments, Sarasota, FL, USA) with a Narashige pipette puller (NG-811). Electrodes had tip resistances ranging from 4 to 6 MΩ when filled with internal solution. The resistance to ground of the whole-cell seal was 2 to 4 GΩ before breaking through the membrane and the series resistance was less than 20 MΩ. The resistance compensation of the patch amplifier was set near 80%. Pyramidal cells in the hippocampal CA1 were recorded with the whole-cell configuration in both the current-clamp mode and voltage-clamp mode. A cell was considered acceptable if it had a stable resting membrane potential of at least −50 mV.
After the cell was patched, constant 0.05-nA current steps, 900-ms in duration, were injected into the soma to obtain a current–voltage curve, to measure input resistance, AP amplitude, and AP threshold. The slope of the current–voltage plot represented the input resistance. AP threshold was calculated as the membrane voltage potential at which point the slope of the first (during positive-current injection) was greater than 10 V/s. AP amplitude was measured from the AP threshold to the AP peak. To record the excitatory spontaneous, glutamate responses, the cells were held at −70 mV (reversal potential for the GABAA-inhibitory postsynaptic potential) by injecting small positive or negative current, as needed. Spontaneous, excitatory postsynaptic potentials (sEPSPs) were recorded in the current-clamp mode, and spontaneous, excitatory postsynaptic currents (sEPSCs) were recorded in the voltage-clamp mode, from CA1 pyramidal neurons. Membrane potential and activity of CA3 pyramidal neurons were recorded in current-clamp mode (I=0). The H/H challenge was conducted by exposing brain slices to a glucose-free medium aerated with 95%N2–5% CO2 (Kulik et al, 2000) for 2 mins. The sucrose concentration of this solution was raised to 10 mmol/L to maintain appropriate osmolarity.
A template-matching technique (Clampfit 9; Axon Instruments, Foster city, CA, USA) was used to detect the spontaneous events. Briefly, a template was created by averaging multiple representative sEPSPs or sEPSCs. In some cases, more than one category of template was created to ensure reliable event detection. During event detection, experienced researchers visually accepted the matched sEPSPs/sEPSCs and rejected abnormal ones, which occurred on a few occasions, likely due to equipment noise. Using this method, over 95% of sEPSPs and sEPSCs were successfully detected, and their amplitudes and frequencies were further averaged and reported. sEPSPs smaller than 2 mV were not analyzed since their amplitude is comparable to the baseline noise level. Charge transfer from sEPSCs was calculated by integrating the area of a typical 20-sec recording to estimate the overall glutamate response due to these spontaneous events (Iremonger and Bains, 2007). All data were reported as mean±s.d. throughout the text and the figures. For statistical analysis, a Student's t-test was performed.
The blocker of AMPA/kainate glutamate receptors, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μmol/L), and the blocker of NMDA glutamate receptors, -(−)-2-amino-5-phosphonopentanoic acid (D-APV, 50 μmol/L), were used to block glutamate receptors. The sodium-channel blocker, TTX (1 μmol/L), was used to block the initiation and propagation of APs. All drugs were purchased from Sigma (St Louis, MO, USA).
Results
Spontaneous Glutamatergic Release in CA1 Pyramidal Cells Includes AP-Dependent and AP-Independent Components
To record spontaneous sEPSPs from CA1 pyramidal neurons, we recorded from the soma using whole-cell current clamp under visual guidance (Figure 1). To study the sEPSPs in isolation, neurons were held at −70 mV by small depolarizing or hyperpolarizing currents. Under current clamp, sEPSPs were visually recognized by upward deflections in the voltage recording traces. The amplitude of the apparent sEPSPs ranged from 0.5 mV to as large as 20 mV (n=8). Spontaneous release was also recorded in the voltage-clamp mode when neurons were clamped at −70 mV. The amplitude of the sEPSCs ranged from 4 to 40 pA (n=6). All sEPSPs and sEPSCs were completely eliminated by the ionotropic glutamate-receptor antagonists CNQX (10 μmol/L) and AP5 (50 μmpl/L), indicating that they were glutamate-mediated events (Figure 1A).
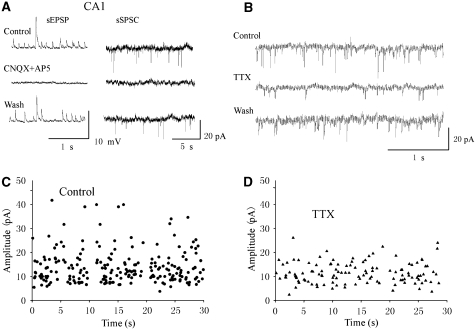
Figure 1.
Spontaneous neurotransmitter release recorded in CA1 pyramidal cells was glutamate-mediated and TTX-sensitive. (A) Spontaneous sEPSPs and currents (sEPSCs) recorded from a CA1 pyramidal neuron in current clamp (left) and from another neuron in voltage clamp (right), respectively. CNQX (10 μmol/L) and AP5 (50 μmol/L) completely and reversibly blocked sEPSPs/sEPSCs of all sizes in these neurons. The holding potentials for both neurons were −70 mV. (B) TTX (1 μmol/L) eliminated large-amplitude sEPSCs. (C) Plot of amplitude versus time of the sEPSCs before TTX application for a 30-min continuous recording. (D) Plot of amplitude versus time of the sEPSCs after TTX application for a 30-min continuous recording in the same cell as in panel C.
Previous studies have shown that spontaneous release in the CA1 area comprises AP-dependent and AP-independent components (Katchman and Hershkowitz, 1993; Raffaelli et al, 2004). To test whether the events we recorded were AP-dependent, we applied TTX (1 μmol/L) to the perfusate to block the sodium channels and APs. TTX administration completely eliminated the large-amplitude sEPSPs (n=4 cells, not shown). Since voltage clamp usually yields better recordings with stable baselines, we also used TTX to suppress large-amplitude sEPSCs during voltage-clamp recordings (Figure 1B, n=4). Figures 1C and 1D show a plot of the amplitude of the sEPSCs versus time before and after TTX application, respectively. The amplitude of sEPSCs range from 4 to 40 pA before TTX application. TTX administration eliminated sEPSCs greater than 25 pA. As consequence, the averaged amplitude and frequency of the overall spontaneous events were reduced by ∼40% and ∼20%, respectively. These results indicate that the AP-dependent spontaneous release in the CA1 area is likely associated with large-amplitude sEPSPs and sEPSCs, while the AP-independent release (i.e., miniature release) is associated with smaller amplitude sEPSPs and sEPSCs.
Both AP-Independent and AP-Dependent Spontaneous Release are Enhanced during Transient Ischemia
Previous studies have shown that transient ischemia can enhance spontaneous release (Fleidervish et al, 2001; Kulik et al, 2000). Knowing that spontaneous release contains AP-dependent and AP-independent components, and that AP-dependent release is associated with relatively larger sEPSPs and sEPSCs, we were able to study the effects of transient ischemia on spontaneous release specifically targeting each type of release.
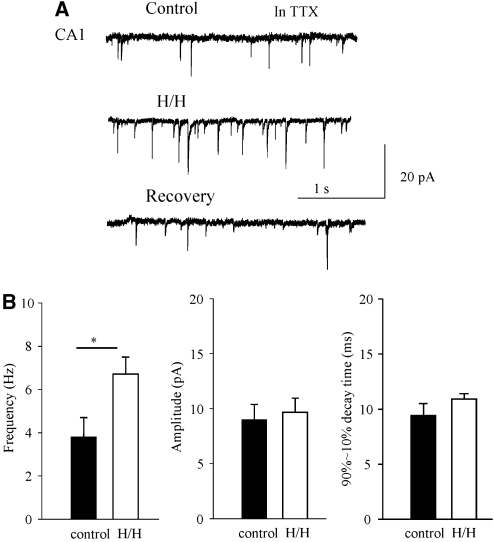
We first recorded and quantified the AP-independent release (n=6) during H/H in the presence of TTX (1.0 μmol/L). Figure 2A shows a typical miniature EPSC (mEPSC) recording before, during 2 mins of H/H, and 10 mins after recovery. Transient H/H induced an increase in the frequency of the mEPSCs (P<0.05), but the amplitude of the mEPSCs was unaltered (Figure 2B, P>0.05). In addition, the decay time (90% to 10%) of the mEPSCs was unaltered: 9.4±1.1 ms before and 10.9±0.5 ms during H/H. These results suggest that alteration of synaptic release during transient H/H is likely due to a presynaptic mechanism, and the properties of the postsynaptic glutamatergic receptors are not affected at this stage of H/H challenge (Tanaka et al, 2001).
Figure 2.
Effects of transient H/H on AP-independent, mEPSCs in CA1 neurons. Experiments were performed in the presence of TTX (1.0 μmol/L). (A) Voltage-clamp recording from a CA1 pyramidal cell before, during H/H, and 10 mins after recovery. Note that the transient inward currents, which represented mEPSCs, increased in frequency during H/H. (B) Statistical summarizing of the frequency, amplitude, and decay time before and during transient H/H (n=6); *P<0.05.
In the absence of TTX, the spontaneous release contains both AP-dependent and AP-independent components. We recorded the overall spontaneous release under a transient H/H challenge. A 2-min H/H episode caused a consistent increase in the overall spontaneous release, especially the large-amplitude, AP-dependent sEPSPs (Figure 3A). This increase in large-amplitude sEPSPs due to the H/H challenge was reversible after normal ACSF and oxygen supply were resumed for 10 mins (n=8 cells).
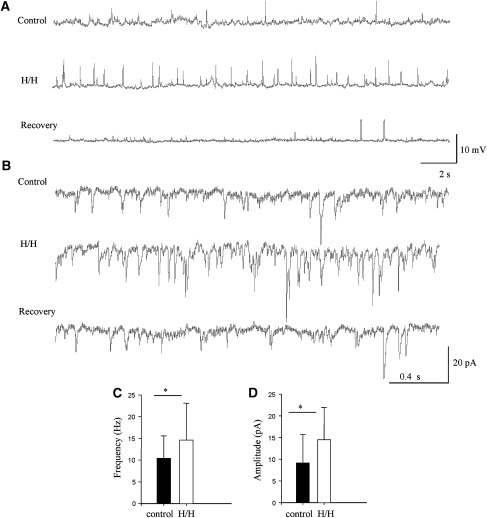
Figure 3.
Brief H/H challenge induced a rapid transient increase in the large-amplitude sEPSPs/sEPSCs in CA1 pyramidal cells. (A) Whole-cell current-clamp recording before, during, and 10 mins after a 2-min H/H challenge from a CA1 pyramidal neuron. H/H caused an obvious increase in large-amplitude sEPSPs. (B) Whole-cell voltage-clamp recording before, during, and 10 mins after H/H challenge from another CA1 pyramidal cell. The cell was held at −70 mV. H/H caused a significant increase in the large-amplitude sEPSCs. (C) Mean frequency of sEPSCs before and during H/H. (D) Mean amplitude of sEPSCs before and during H/H (n=8); *P<0.05.
We also confirmed the above observation with the voltage-clamp recordings (n=6). Figure 3B illustrates a typical cell response to the H/H under this recording mode. Under control situation, units larger than 20 pA are rarely seen. Two minutes after initiation of H/H, we observed an increase in the spontaneous activity, especially the occurrence of large sEPSCs, with amplitudes between 20 to 40 pA. Ten minutes after recovery, the rate of spontaneous release returned to normal. As a consequence, the mean amplitude of the sEPSCs was increased from 10.4±5.2 (control) to 14.6±8.5 pA (H/H, P<0.05; Figure 3C). Increase in the incidence of large-amplitude sEPSCs during H/H also caused the mean frequency to increase from 9.1±6.6 to 14.5±7.4 Hz (P<0.05; Figure 3D).
Since both the frequency and amplitude of the spontaneous releases increased in the absence of TTX, we conclude that a portion of the large-amplitude EPSCs recorded during transient H/H are AP-dependent events, owning to a presynaptic mechanism.
Enhanced AP-Dependent Spontaneous Release during Transient Ischemia is not Due to Altered Intrinsic Properties of the Postsynaptic Neurons
Previous studies have shown that changes in intrinsic cell properties in the recorded neurons can alter the amplitude of spontaneous EPSPs/EPSCs (Raffaelli et al, 2004). Since we observed increase in the amplitude of AP-dependent spontaneous EPSPs/EPSCs during transient H/H, we asked whether the change is due to potential alterations in postsynaptic (CA1) cellular properties.
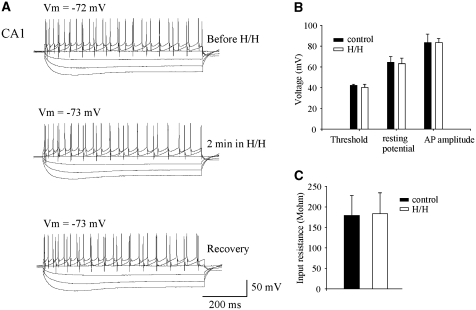
To measure the cell properties, we injected depolarizing and hyperpolarizing currents into the patched CA1 cells (Figure 4). We then created current–voltage responses before, 2 mins after initiation of H/H, and after 10 mins of recovery. We measured the resting membrane potentials, AP thresholds, and AP amplitudes of the patched cells (n=8). No significant changes were observed in these recordings (P>0.05). The resting potential was −64.3±5.6 mV in the control and −63.0±5.3 mV during H/H; AP threshold was −42.2±1.1 mV in the control and −40.3±3.0 mV in H/H; AP amplitude was 83.4±8.1 mV in the control and 83.4±4.1 mV in H/H. Although changes in input resistance could occur during longer (up to 6 mins) hypoxic/ischemic episodes (Zhang and Krnjevic, 1993; Fleidervish et al, 2001; Kulik et al, 2000), we did not observe any significant change in the input resistance (178.8±49.4 MΩ in the control and 183.1±51.1 MΩ in H/H, P>0.05; Figure 4C) during the short H/H challenge. Therefore, the enhanced glutamatergic response during transient ischemia was not due to intrinsic property changes in the patched postsynaptic neurons.
Figure 4.
Intrinsic properties of the postsynaptic CA1 neurons did not change during transient H/H. (A) Sample recording from a cell when constant current steps were applied through the patch electrode to quantify the spiking and membrane properties from the hyperpolarizing and depolarizing voltage response. The same current step protocol was used through all the experiments when current–voltage curves were obtained. (B) Two minutes of transient H/H did not cause significant changes in the transmembrane potential, threshold of firing AP, and the size of AP. (C) Two minutes of transient H/H did not cause significant changes in input resistance.
Contributions of AP-Dependent and AP-Independent Release to the Overall Glutamatergic Responses by the Spontaneous Mechanism
What are the individual contributions of the AP-dependent and AP-independent components to the overall glutamatergic responses under control condition and during transient ischemia? To estimate the glutamatergic responses due to these two different types of spontaneous activities on the control condition, we estimated the total charge transfer (Iremonger and Bains, 2007) by integrating the overall areas under sEPSCs (Figure 5A).
Figure 5.
Relative contribution of AP-independent and AP-dependent releases to the overall spontaneous release in control situation and during transient H/H. (A) The sEPSC events were detected and their areas were integrated to obtain the total area covered by the sEPSCs in a 10-sec trace. (B) The total area of the pies represents the total integrated area of EPSCs in control (left) and during transient H/H (right). The gray areas represent the glutamatergic responses mediated by the AP-dependent release, and the white areas represent the AP-independent release.
We first investigated the effects of TTX application on total charge transfer in the control situation (Figure 1B). TTX eliminated 34.5%±5% (n=4) of the total integrated area, suggesting that AP-dependent release can account for approximately one-third of the baseline glutamatergic response, while AP-independent mechanisms account for the remaining two-thirds of the glutamatergic response (Figure 5B, left).
Since H/H caused a significant increase in the AP-dependent, large-amplitude sEPSCs (Figure 3), we hypothesized that glutamate release through an AP-dependent mechanism would contribute largely to the overall integrated area during H/H. In transient H/H, the overall glutamatergic responses, including both AP-dependent and AP-independent, were increased by 417%±46% (n=6). However, AP-independent responses were only increased by 109%±32% during H/H (n=6; Figure 2). From such information, we estimated that the AP-dependent glutamatergic response is enhanced by approximately 11-fold, as measured by the integrated area of sEPSCs. As a consequence, AP-dependent release accounts for approximately 74.0% of the overall glutamatergic response during transient H/H, and AP-independent release accounts for 26.0% of the overall responses (Figure 5B, right).
Increased AP-Dependent Release is Correlated with the Enhanced Activity in the Presynaptic CA3 Pyramidal Cells During Transient Ischemia
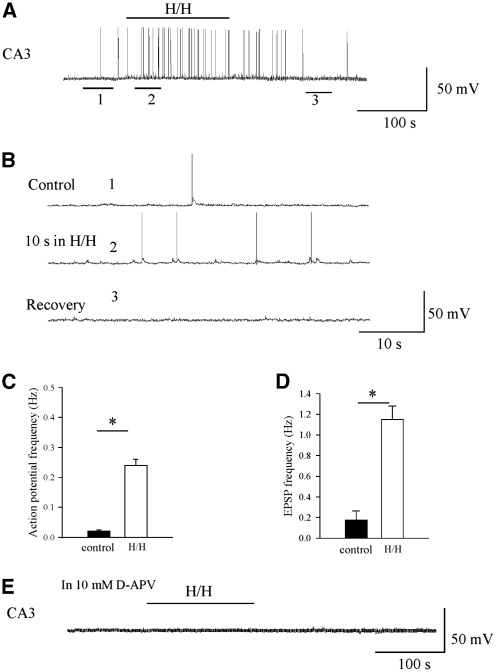
Is the enhanced AP-dependent release in the CA1 area during transient H/H due to enhanced activity in the presynaptic CA3 neurons? To answer this question, we investigated the firing frequency of the CA3 neurons. We found that under baseline conditions, the majority of CA3 neurons fire low-frequency, spontaneous APs (n=8), which is in agreement with previous observations (Raastad and Shepherd, 2003). Transient H/H increased the firing frequency in these neurons. Figure 6A shows the representative firing pattern of a CA3 cell before, during H/H, and 10 mins after recovery. The transient, 2-min H/H challenge caused greater excitatory synaptic input onto the CA3 neuron and more firing. The transmembrane potential of the cell was −66.7 mV before H/H. Approximately 10 secs after initiation of the transient H/H episode, the cell depolarized to −63.0 mV and fired at a significantly higher frequency (0.24±0.02 Hz, P<0.01) than the control (0.02±0.004 Hz; Figure 6B). In addition, we observed significant increase in the incidence of sEPSPs during H/H (0.18±0.09 Hz in control versus 1.15±0.13 Hz in H/H, P<0.01) in the recorded CA3 neurons. Application of 10 μmol/L APV in the bath solution prevented the sEPSPs, the minor depolarization, and enhanced firing of the recorded CA3 neuron during transient H/H (Figure 6E, n=4), suggesting the hyper-excitability observed in CA3 during transient H/H to be NMDA-receptor-mediated.
Figure 6.
CA3 activity was enhanced during transient H/H. (A) Voltage trace recorded from a CA3 neuron before, during, and after 2-min H/H challenge. No current (I=0) was injected into the cell. The black line represents the period of the H/H episode. Transient H/H caused minor depolarization and increase in the spontaneous firing frequency of the CA3 pyramidal cell. (B) Extended time-scale for the traces indicated by ‘1', ‘2', and ‘3' in panel A. (C) Statistical summary of the firing frequency observed before and during H/H. (D) Statistical summary of the incident of EPSPs observed before and during H/H in CA3 (n=8). (E) AP-V prevented the minor depolarization, high-frequency firing and the associated EPSPs in the CA3 neurons during transient H/H.
The similar time courses of the enhanced CA3 activity during H/H, and the increased AP-dependent spontaneous release in the CA1 area, suggest that the two may be correlated.
Lesioning the Schaffer Collaterals Prevented the Enhanced AP-Dependent Release
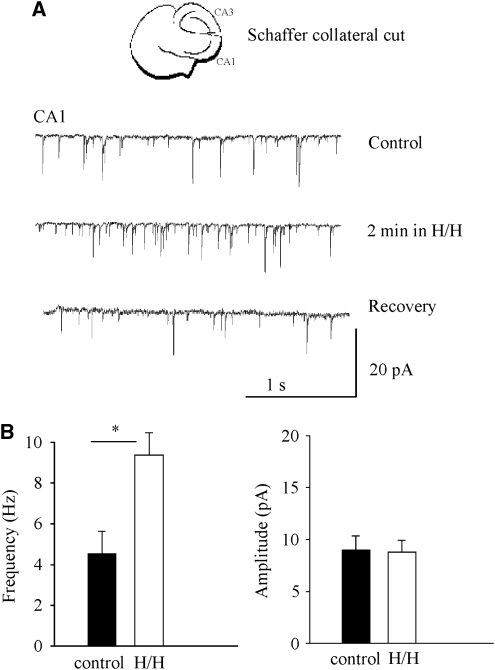
To confirm that the increased AP-dependent release was indeed due to the enhanced activities in the CA3 neurons during H/H, we sectioned the Schaffer collaterals and recorded the sEPSCs in CA1 during H/H (n=6). If the more activated CA3 neurons did contribute to the increased AP-dependent sEPSCs in CA1 during H/H, this lesion should prevent the large-amplitude sEPSCs during transient H/H. Indeed, this lesion resulted in the absence of large-amplitude EPSCs as normally seen in unlesioned slices (Figure 7A). However, the frequency of the smaller amplitude, spontaneous release was still increased by about two-fold during transient H/H (Figure 7B), as noted previously for the behavior of AP-independent miniatures in intact slices during transient H/H (Figure 2).
Figure 7.
Lesioning the Schaffer collaterals prevented the large amplitude AP-dependent release in CA1 area during H/H. (A) sEPSCs recorded from CA1 in a CA3/CA1 pathway lesioned brain slice before, during transient H/H, and after recovery. (B) Frequency of mEPSC increases significantly during H/H, but the amplitude of mEPSCs did not change during transient H/H (n=6).
In summary, increased activity in the presynaptic CA3 is sufficient and necessary for enhanced, AP-dependent spontaneous release in the CA1 region during transient H/H.
Discussion
Both AP-Dependent and AP-Independent Spontaneous Glutamate Release during Transient Ischemia are Enhanced in CA1
It has long been recognized that spontaneous glutamate release during hypoxia/ischemia is one of the major sources contributing to neuronal excitotoxicity. Spontaneous release includes both AP-dependent and AP-independent components (Figure 1), with AP-dependent components comprising larger amplitudes than the AP-independent miniatures. In this paper, we first show that H/H caused an increase in the frequency, but not the amplitude, of AP-independent release (Figure 2), as shown by others in CA1 pyramidal neurons (Katchman and Hershkowitz, 1993), neocortical neurons (Fleidervish et al, 2001), and in brainstem cells (Kulik et al, 2000). Although it is possible that there are large-amplitude, TTX-resistant miniatures that contain several presynaptic vesicles, due to the sum of several quanta (Llano et al, 2000), we did not observe large-amplitude sEPSCs in TTX experiments.
We then show, using both current and voltage recordings, that the overall spontaneous glutamatergic response is enhanced during transient H/H in hippocampal CA1 neurons, in the absence of TTX. This enhancement is characterized as the increment in both amplitude and frequency of the sEPSPs and sEPSCs (Figure 3). Therefore, the enhanced large units seen in this experiment are likely AP-dependent events. Enhancement of AP-dependent release has also been reported in other systems during hypoxia/ischemia. They were observed 30 secs after onset of hypoxia, and persisted for 2 to 3 mins in mouse somatosensory cortex (Fleidervish et al, 2001). In that study, ∼20% of the hypoxia-triggered spontaneous EPSCs were >30 pA. The authors concluded that those events likely reflected a multi-bouton glutamate release synchronized by presynaptic APs, since they could be completely abolished in the presence of TTX and in Ca2+-free bath solution. In addition, large-amplitude, AP-dependent release has been observed immediately after the onset of in vitro ischemia (Rossi et al, 2000) in hippocampal CA1 neurons. Our observations, along with that of others, suggest that AP-dependent release is contributing to the overall enhanced glutamate release during H/H.
CA3 Hyper-excitability is Responsible for the Enhanced AP-Dependent Responses in CA1 during Transient H/H
The AP-dependent postsynaptic potentials are generated by APs that propagate to the presynaptic terminals through the afferent axons. After the arrival of a presynaptic impulse at an excitatory synapse, spontaneous release increases through persistent residual increase in the intraterminal Ca2+ concentration (Goda and Stevens, 1994). Several lines of evidence suggest that the increase in the spontaneous, AP-dependent activity in the CA1 neurons during transient H/H is due to excessive activation of the presynaptic CA3 neurons.
First, TTX abolished the large-amplitude sEPSCs during transient ischemia (Figure 2), suggesting that sodium channel-mediated AP is needed for such enhanced responses.
Second, enhanced spontaneous release is likely due to a presynaptic mechanism. We observed an increase in the frequency of mEPSC in TTX but not in the amplitude or decay time (Figure 2B), indicating that the H/H-mediated change in synaptic transmission is presynaptic and is not likely mediated by postsynaptic mechanisms. We further showed that the intrinsic properties of the postsynaptic neurons were not altered in transient ischemia (Figure 4). This result is consistent with that of Fleidervish et al (2001), where they observed large-amplitude EPSPs during early transient hypoxia, but unaltered input resistance in the first 3 mins of hypoxia (Figure 3 in reference Fleidervish et al, 2001). It should be noted that if the ischemic episode is prolonged, it causes depressed sEPSPs (Raffaelli et al, 2004), since ATP-dependent K+ channels or calcium-dependent K+ channels (Haddad and Jiang, 1993) could be opened by prolonged challenge.
Third, activity in the CA3 neurons was enhanced, as shown by the increased rate of spiking during transient H/H (Figures 6A and 6B). Depolarizations are seen in several cell types during hypoxia/ischemia, including those in the neocortex (Rosen and Morris, 1991) and dentate gyrus (Krnjević and Ben-Ari, 1989), and respiratory neurons in the brainstem (Haddad and Jiang, 1993). Since the time course and degree of increase in the firing frequency of CA3 neurons (Figure 6) were comparable to the enhancement of AP-dependent EPSPs/EPSCs observed in CA1 (Figure 3), the two events are likely related.
Finally, lesioning the Schaffer collaterals, the major afferent fiber pathway from CA3 neurons to CA1 neurons, greatly reduced the AP-dependent release, and prevented its increase during H/H (Figure 7). This result supports the previous in vivo observation that lesioning excitatory afferents to the CA1 area could prevent ischemia-induced cell death. Destruction of CA3 neurons prevented ischemic damage to CA1 cells, by reducing the calcium influx through glutamate-operated channels (Benveniste et al, 1989). Removal of excitatory inputs (dentate granule cells) attenuated hippocampal injury in global ischemia (Johansen et al, 1986). Lesioning of a small region of the deep prepiriform cortex attenuates neuronal cell death in the hippocampus after global ischemia, and this lesion decreases ischemia-induced elevation of glutamate in the extracellular space around the target neurons (Kawaguchi et al, 1997). Lesioning afferent excitatory connections has been proposed as a neuroprotective strategy (Mulholland and Prendergast, 2003).
Possible Mechanisms that Mediate Enhanced CA3 Activity
Many factors can potentially enhance the activity of CA3 neurons in ischemia. Intrinsically, blockade of the Na–K pump (Somjen, 2001) could have a significant depolarizing effect. Similarly, activation of a persistent, voltage-dependent Na+ current can depolarize the cell (Crill, 1996). Enhanced Ca2+ influx due to ischemia-induced acidosis (MacDonald et al, 2006) could cause depolarization. An increase in input resistance could also lead to increased space constant, thereby allowing distant depolarization (synaptic noise and dendrite calcium spikes) to influence the axon hillock of the cell. Unfortunately, our data do not provide support for this argument, since the input resistance in CA3 neurons did not change during 2-min H/H challenge (2%±13.3%, P>0.05). It is possible, however, that this change might appear during longer H/H periods, and might play a significant role in the pathology of ischemia (Zhang and Krnjevic, 1993; Fleidervish et al, 2001).
Extrinsically, suppression of GABA-mediated inhibition can render neurons susceptible to depolarization in hypoxia (Fujiwara et al, 1987). Depolarization can be further enhanced by adenosine acting on excitatory A2 receptors, which induces an inward Ca2+ current, enhancing glutamate release and NMDA-receptor actions (Marcoli et al, 2004). It is also possible that when CA3 neurons receive excitatory drive from the local associative network, the membrane potential approaches the AP threshold, thus allowing the unitary mossy-fiber EPSPs to trigger APs (Mori et al, 2004).
We observed more EPSPs in the CA3 neurons during transient ischemia. When the NMDA antagonist D-AP5 was applied, it prevented enhanced AP-dependent release and eliminated the excitatory drive to the CA3 neurons during transient H/H (Figure 7), suggesting that the enhanced CA3 activity depended on the activation of the NMDA receptors. One potential mechanism is through blocking presynaptic NMDA receptors and preventing calcium influx and synaptic release.
Pathological Implications of Enhanced AP-Dependent Release in Transient H/H
The spontaneous release mediated by enhanced presynaptic neural activity, as shown by this study, constitutes one of the many mechanisms mediating the ischemic glutamate accumulation and disturbance of normal network functions.
AP-dependent release could contribute greatly to the spontaneous glutamate release in early ischemia. The AP-dependent EPSPs can be as large as 10 mV in current-clamp recordings (Figure 3A) and 40 pA in voltage clamp recordings (Figure 3B) during H/H. This enhanced release constitutes 74% of the overall glutamatergic responses measured in CA1 during transient ischemia (Figure 5). These results support the early speculation that AP-dependent release could contribute to a large portion of the overall spontaneous release in early ischemia (Fleidervish et al, 2001). These changes are expected to be more pronounced in vivo, when neuronal activity is not already compromised as in vitro, where dissection damages, at least in part, neurons, glia, and axonal connections. Oxygen consumption in the relatively quiescent brain slice is lower than in the in vivo situation (Raichle, 1998). The distance of the oxygen diffusion in our experimental chamber is larger than that between neurons and capillaries (Reina-De La Torre et al, 1998). Therefore, the increase in AP-dependent spontaneous releases that we observed during in vitro H/H could occur almost immediately in a behaving animal after a critical decrease in oxygenated blood supply. Eventually, this early outpouring of transmitter could play a significant role in later irreversible neuronal depolarization (Krnjević, 2008) and excitotoxicity (Lee et al, 2000).
AP-dependent release may have significant functional implications for normal neural networks during transient ischemia. First, normal information processing could be disrupted by such release. Normal information processing by the neocortical circuit must entail precise timing of individual spikes and their postsynaptic consequences (Victor and Purpura, 1996). The increased AP-dependent spontaneous activity could be expected to introduce incoherent noise (Stacey and Durand, 2001) to the circuit, and to disturb normal neuronal integration of inputs. Second, the increased spontaneous transmitter release may deplete the readily releasable vesicles for normal synaptic transmission. The AP-dependent spontaneous release, combined with AP-independent miniature release, could interfere with the release that is synchronized by presynaptic APs, because the synaptic vesicles that are available for these processes are drawn from the same readily limited releasable pool (Rosenmund and Stevens, 1996). Third, the excitatory/inhibitory balance in the neuronal circuit could be disrupted by the changes in the AP-dependent, spontaneous release. Although we only focused on glutamate release, evidence has been shown that early ischemia/hypoxia is associated with increase in both AP-dependent (Allen et al, 2004) and AP-independent, miniature GABA release (Katchman et al, 1994). Functionally, ischemia-induced increase in the GABAergic interneurons could inhibit excitatory transmission.
Acknowledgments
This work was supported by CIHR, Heart and Stroke Foundation of Canada, Alzheimer Society of Canada, Pfizer Canada, and a Canadian Heart and Stroke Foundation postdoctoral fellowship to Hui Ye. Chiping Wu provided technical support for this work, and Tariq Zahid proofread the paper.
Footnotes
Disclosure/conflict of interest
We have no actual or potential conflicts of interest including financial, personal or other relationships with other people or organizations while conducting the study presented in this manuscript.
References
- Allen NJ, Rossi DJ, Attwell D. Sequential release of GABA by exocytosis and reversed uptake leads to neuronal swelling in simulated ischemia of hippocampal slices. J Neurosci. 2004;24:3837–3849. doi: 10.1523/JNEUROSCI.5539-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Craig A, Kayton RJ, Luo NL, Meshul CK, Allcock N, Fern R. Hypoxia-ischemia preferentially triggers glutamate depletion from oligodendroglia and axons in perinatal cerebral white matter. J Cereb Blood Flow Metab. 2007;27:334–347. doi: 10.1038/sj.jcbfm.9600344. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Jorgensen MB, Sandberg M, Christensen T, Hagberg H, Diemer N. Ischemic damage in hippocampal CA1 is dependent on glutamate release and intact innervation from CA3. J Cereb Blood Flow Metab. 1989;9:626–639. doi: 10.1038/jcbfm.1989.90. [DOI] [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol. 1996;58:349–362. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- Cummings DD, Wilcox KS, Dichter MA. Calcium-dependent paired-pulse facilitation of the miniature EPSC frequency accompanies depression of EPSC at hippocampal synapses in culture. J Neurosci. 1996;16:5312–5323. doi: 10.1523/JNEUROSCI.16-17-05312.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleidervish IA, Gebhardt C, Astman N, Gutnick MJ, Heinemann U. Enhanced spontaneous transmitter release is the earliest consequence of neocortical hypoxia that can explain the disruption of normal circuit function. J Neurosci. 2001;21:4600–4608. doi: 10.1523/JNEUROSCI.21-13-04600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara N, Higashi H, Shimoji K, Yoshimura M. Effects of hypoxia on rat hippocampal neurones in vitro. J Physiol. 1987;384:131–151. doi: 10.1113/jphysiol.1987.sp016447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt C, Körner R, Heinemann U. Delayed anoxic depolarizations in hippocampal neurons of mice lacking the excitatory amino acid carrier 1. J Cereb Blood Flow Metab. 2002;22:569–575. doi: 10.1097/00004647-200205000-00008. [DOI] [PubMed] [Google Scholar]
- Goda Y, Stevens CF. Two components of transmitter release at a central synapse. Proc Natl Acad Sci USA. 1994;91:12942–12946. doi: 10.1073/pnas.91.26.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad GG, Jiang C. Mechanisms of anoxia-induced depolarization in brainstem neurons: in vitro current and voltage clamp studies in the adult rat. Brain Res. 1993;625:261–268. doi: 10.1016/0006-8993(93)91067-3. [DOI] [PubMed] [Google Scholar]
- Iremonger KJ, Bains JS. Integration of asynchronously released quanta prolongs the postsynaptic spike window. J Neurosci. 2007;27:6684–6691. doi: 10.1523/JNEUROSCI.0934-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen FF, Jorgensen MB, Diemer NH. Ischemic CA-1 pyramidal call loss is prevented by preischemic colchicine destruction of dentate gyrus granule cells. Brain Res. 1986;377:344–347. doi: 10.1016/0006-8993(86)90878-4. [DOI] [PubMed] [Google Scholar]
- Katchman AN, Hershkowitz N. Early anoxia-induced vesicular glutamate release results from mobilization of calcium from intracellular stores. J Neurophysiol. 1993;70:1–7. doi: 10.1152/jn.1993.70.1.1. [DOI] [PubMed] [Google Scholar]
- Katchman AN, Vicini S, Hershkowitz N. Mechanism of early anoxia-induced suppression of GABAA-mediated inhibitory postsynaptic current. J Neurophysiol. 1994;71:1128–1138. doi: 10.1152/jn.1994.71.3.1128. [DOI] [PubMed] [Google Scholar]
- Kawaguchi K, Huerbin M, Simon RP. Lesioning of deep prepiriform cortex protects against ischemic neuronal necrosis by attenuating extracellular glutamate concentrations. J Neurochem. 1997;69:412–417. doi: 10.1046/j.1471-4159.1997.69010412.x. [DOI] [PubMed] [Google Scholar]
- Krnjević K. Electrophysiology of cerebral ischemia. Neuropharmacology. 2008;55:319–333. doi: 10.1016/j.neuropharm.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Krnjević K, Ben-Ari Y. Anoxic changes in dentate granule cells. Neurosci Lett. 1989;107:89–93. doi: 10.1016/0304-3940(89)90796-9. [DOI] [PubMed] [Google Scholar]
- Kulik A, Trapp S, Ballanyi K. Ischemia but not anoxia evokes vesicular and Ca2+ independent glutamate release in the dorsal vagal complex in vitro. J Neurophysiol. 2000;83:2905–2915. doi: 10.1152/jn.2000.83.5.2905. [DOI] [PubMed] [Google Scholar]
- Lee JM, Grabb MC, Zipfel GJ, Choi DW. Brain tissue responses to ischemia. J Clin Invest. 2000;106:723–731. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I, Gonzalez J, Caputo C, Lai FA, Blayney LM, Tan YP, Marty A. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Xiong ZG, Jackson MF. Paradox of Ca2+ signaling, cell death and stroke. Trends Neurosci. 2006;29:75–81. doi: 10.1016/j.tins.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Marcoli M, Bonfanti A, Roccatagliata P, Chiaramonte G, Ongini E, Raiteri M, Maura G. Glutamate efflux from human cerebrocortical slices during ischemia: vesicular-like mode of glutamate release and sensitivity to A(2A) adenosine receptor blockade. Neuropharmacology. 2004;47:884–891. doi: 10.1016/j.neuropharm.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Montero M, Nielsen M, Rønn LC, Møller A, Noraberg J, Zimmer J. Neuroprotective effects of the AMPA antagonist PNQX in oxygen–glucose deprivation in mouse hippocampal slice cultures and global cerebral ischemia in gerbils. Brain Res. 2007;1177:124–135. doi: 10.1016/j.brainres.2007.08.038. [DOI] [PubMed] [Google Scholar]
- Mori M, Abegg MH, Gahwiler BH, Gerber U. A frequency-dependent switch from inhibition to excitation in a hippocampal unitary circuit. Nature. 2004;431:453–456. doi: 10.1038/nature02854. [DOI] [PubMed] [Google Scholar]
- Mulholland PJ, Prendergast MA. Transection of intrinsic polysynaptic pathways reduces N-methyl--aspartate neurotoxicity in hippocampal slice cultures. Neurosci Res. 2003;46:369–376. doi: 10.1016/s0168-0102(03)00102-0. [DOI] [PubMed] [Google Scholar]
- Raastad M, Shepherd GMG. Single-axon action potentials in the rat hippocampal cortex. J Physiol. 2003;548:745–752. doi: 10.1113/jphysiol.2002.032706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaelli G, Saviane C, Mohajerani MH, Pedarzani P, Cherubini EJ. BK potassium channels control transmitter release at CA3–CA3 synapses in the rat hippocampus. J Physiol. 2004;557 (Pt 1:147–157. doi: 10.1113/jphysiol.2004.062661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. Behind the scenes of functional brain imaging: a historical and physiological perspective. Proc Natl Acad Sci USA. 1998;95:765–772. doi: 10.1073/pnas.95.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reina-De La Torre F, Rodriguez-Baeza A, Sahuquillo-Barris J. Morphological characteristics and distribution pattern of the arterial vessels in human cerebral cortex: a scanning electron microscope study. Anat Rec. 1998;251:87–96. doi: 10.1002/(SICI)1097-0185(199805)251:1<87::AID-AR14>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Rosen AS, Morris ME. Depolarizing effects of anoxia on pyramidal cells of rat neocortex. Neurosci Lett. 1991;124:169–173. doi: 10.1016/0304-3940(91)90086-9. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stevens CF. Definition of readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- Rossi D, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev. 2001;81:1065–1096. doi: 10.1152/physrev.2001.81.3.1065. [DOI] [PubMed] [Google Scholar]
- Stacey WC, Durand DM. Synaptic noise improves detection of subthreshold signals in hippocampal CA1 neurons. J Neurophysiol. 2001;86:1104–1112. doi: 10.1152/jn.2001.86.3.1104. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Yasumoto S, Hattori G, Niiyama S, Matsuyama S, Higashi H. Mechanisms underlying the depression of evoked fast EPSCs following in vitro ischemia in rat hippocampus CA1 neurons. J Neurophysiol. 2001;86:1095–1103. doi: 10.1152/jn.2001.86.3.1095. [DOI] [PubMed] [Google Scholar]
- Tonkikh AA, Carlen PL. Impaired presynaptic cytosolic and mitochondrial calcium dynamics in aged compared to young adult hippocampal CA1 synapses ameliorated by calcium chelation. Neuroscience. 2009;159:1300–1308. doi: 10.1016/j.neuroscience.2008.12.057. [DOI] [PubMed] [Google Scholar]
- Victor JD, Purpura KP. Nature and precision of temporal coding in visual cortex: a metric-space analysis. J Neurophysiol. 1996;76:1310–1326. doi: 10.1152/jn.1996.76.2.1310. [DOI] [PubMed] [Google Scholar]
- Zhang L, Krnjevic K. Whole-cell recording of anoxic effects on hippocampal neurons in slices. J Neurophysiol. 1993;69:118–127. doi: 10.1152/jn.1993.69.1.118. [DOI] [PubMed] [Google Scholar]