Abstract
Outcome from acute subdural hematoma is often worse than would be expected from the pure increase of intracranial volume by bleeding. The aim was to test whether volume-independent pathomechanisms aggravate damage by comparing the effects of blood infusion with those of an inert fluid, paraffin oil, on intracranial pressure (ICP), cerebral perfusion pressure (CPP), local cerebral blood flow (CBF), edema formation, glucose metabolism ([18F]-deoxyglucose, MicroPET ), and histological outcome. Rats were injured by subdural infusion of 300 μL venous blood or paraffin. ICP, CPP, and CBF changes, assessed during the first 30 mins after injury, were not different between the injury groups at most time points (n=8 per group). Already at 2 h after injury, blood caused a significantly more pronounced decrease in glucose metabolism in the injured cortex when compared with paraffin (P<0.001, n=5 per group). Ipsilateral brain edema did not differ between groups at 2 h, but was significantly more pronounced in the blood-treated groups at 24 and 48 h after injury (n=8 per group). These changes caused a 56.2% larger lesion after blood when compared with paraffin (48.1±23.0 versus 21.1±11.8 mm3; P<0.02). Blood constituent-triggered pathomechanisms aggravate the immediate effects due to ICP, CPP, and CBF during hemorrhage and lead to early reduction of glucose metabolism followed by more severe edema and histological damage.
Keywords: acute subdural hematoma, brain edema, FDG-PET, glucose metabolism, paraffin oil
Introduction
Acute subdural hematoma (ASDH) is a frequent complication of severe head injury. ASDH is associated with highest mortality and morbidity after severe head injury (e.g., Hlatky et al, 2004; Karabiyikoglu et al, 2005; Seelig et al, 1981). Improvements in diagnosis, surgical interventions, and medical care are not satisfactory. Even prompt surgical evacuation is often insufficient to alter the poor outcome of patients significantly, although it can reduce a high intracranial pressure (ICP) of >50 mm Hg drastically (Verweij et al, 2001). The volume and size of hematoma are often less marked than the secondary ischemic lesions found would suggest (Jenkins et al, 1990). Graham and Adams (1971) showed on autopsies of patients with fatal head injuries that this damage is secondary and occurred after trauma impact. The exact pathophysiological mechanisms remain unclear so far.
The first experimental ASDH model was developed by Miller et al (1990) with rats; further experiments used mice (Sasaki and Dunn, 2001) and pigs (for references see Durham and Duhaime, 2007; Timaru-Kast et al, 2008). The initially missing traumatic event had been introduced by Marmarou and co-workers (Sawauchi et al, 2002) by combining the original ASDH model with diffuse traumatic brain injury and could show significantly greater ischemia and brain damage.
These studies showed that there is a mismatch between increase of energy metabolism and decrease of cerebral blood flow (CBF) underneath the blood clot, which leads to elevated release of excitotoxic amino acids resulting in secondary brain damage (Bullock et al, 1991; Kuroda and Bullock, 1992). Besides involvement of ischemic blood flow and glutamate neurotoxicity due to increased intracranial volume, other factors are candidates to influence lesion development: blood constituents or mediators from plasma, erythrocytes, white cells, or monocytes entering the subdural space. Different hypotheses exist now about the role of blood in ASDH. Duhaime et al (1994) showed in an open-cranial model that blood alone in the absence of increased pressure or ischemia does not cause any brain damage. Although this could imply that blood itself has no detrimental effects on neuronal tissue, several studies suggest intravascular coagulation and effects of blood constituents as trigger mechanisms, which may worsen outcome after ASDH (Jenkins et al, 1990; Karabiyikoglu et al, 2005; Lee et al, 1997; Stein et al, 2002). For instance, injection of thrombin into the striatum is neurotoxic (Fujimoto et al, 2006), injections of blood or blood lysate affect blood–brain barrier integrity (Gao et al, 2007; Power et al, 2003), and contact of blood with brain tissue causes inflammation (Lee et al, 2006). Furthermore, superfusion of rat cortex with hemoglobin or products from hemolysis can induce cortical spreading depolarization and spreading ischemia with neuronal cell death (for definitions see secton Discussion and references Dreier et al, 1998, 2000).
An important first step in the process to elucidate the role of different blood-derived factors in lesion development would be substitution of blood by either an inflatable balloon or an inert fluid (paraffin oil, silicone). The same volume of blood, balloon, or silicone demands identical time courses of at least ICP to be able to separate blood-induced pathological consequences from those volume-dependent. Unfortunately, the time course of ICP changes is not comparable between blood and balloon inflation. At the end of infusion of the same volume, ICP remains at a much higher level using the balloon technique (Burger et al, 2002). This makes this technique unsuitable for our purpose. There were a few attempts to substitute blood by a silicone/mineral oil mixture with similar viscosity as blood. Although in some studies ICP was measured, silicone-induced ICP changes were monitored for a very short period (Jenkins et al, 1990), were not reported (Kuroda et al, 1992; Yilmazlar et al, 1997), induced high standard deviations (Uchida et al, 2001), or were not compared with blood volumes (Miller et al, 1987). Silicone induced less severe hypoglycolysis and a smaller lesion volume was reported following silicone when compared with blood (Jenkins et al, 1990; Kuroda et al, 1992; Yilmazlar et al, 1997). These differences were related to effects of blood constituents on pathophysiological processes, although this could be based simply on possible ICP differences between the two fluids for which these experiments were not controlled. Thus, before the trigger mechanisms of blood can be identified properly, a thorough investigation is indicated, which allows to differentiate the exact degree of damage due to subdural volume from that due to blood constituents. Therefore, the goal of this study was to separate the effects of blood and volume on acute pathophysiological parameters and to evaluate how early differences influence outcome parameters, by infusing the same subdural volume of autologous blood and paraffin oil. We monitored acute parameters such as ICP, cerebral perfusion pressure (CPP), CBF, and mean arterial blood pressure (MAP) in the same animals to observe the already early differences during and after infusion. Thereafter, we re-evaluated the changes in energy metabolism by [18F]-deoxyglucose positron-emission tomography (FDG-PET; see Kuroda et al, 1992) and tried to correlate the expected differences between blood and paraffin oil with early (ICP, CPP, CBF, MAP) and late pathological changes for up to 48 h after injury (edema formation, lesion volume) using an established ASDH model in rats.
Materials and methods
Animals and Anesthesia
All experiments were performed with the approval of the governmental animal care and use committees. We used male Sprague–Dawley rats (Charles River Laboratories, Sulzfeld, Germany), which were divided in three groups (see below). All animals were housed at room temperature of 22±2°C and humidity >50%. The rats were intraperitoneally anesthetized with chloral hydrate (36 mg/mL) initially at 1 mL/100 g and afterwards at 1 mL/h. Premedication consisted of 1 mL atropine (1 mg subcutaneous) administration. Body temperature was maintained at 37°C with a heating pad and a rectal thermometer (Homeothermic Blanket Unit, Harvard, Kent, UK).
Study Design and Groups
This study was divided into three separate experiments. In the first experiment, acute pathophysiological parameters and histological outcome were assessed in a blood- and paraffin oil-treated group. In the second experiment, the time course of edema formation was investigated. In the third experiment, acute (120 mins after ASDH) changes of glucose metabolism were studied by PET. For each experiment animals were assigned randomly to a blood-treated group (subdural injection of 300 μL autologous venous blood), a paraffin-treated group (subdural injection of 300 μL paraffin oil; Sigma-Aldrich/Riedel-de Haën, no. 34920), or a sham-operated group (no injection, for studies 2 and 3 only). Wherever possible, the investigators were masked to the treatment applied.
Surgical Procedures and ASDH
An artery and the jugular vein were cannulated for measuring MAP and for tracer injection and withdrawal of autologous blood for subdural injection respectively. A craniotomy (∅3 mm) was made between the bregma and lambda suture on the left side as described previously (Alessandri et al, 2006). Briefly, the dura was opened and a bent, L-shaped, blunt cannula (23G) was inserted and secured with tissue glue (Histoacryl; B Braun, Melsungen, Germany) and dental cement. Depending on the experiment (see below) an ICP-sensor (Neurovent-P 3F, ∅1 mm; Raumedic, Helmbrechts, Germany) was inserted into the contralateral hemisphere and a laser Doppler (LD) probe was placed on the ipsilateral hemisphere frontal to the bregma (Vasamedics Laserflo BPM2, Vasamedics fiber-optic probe 8*200 mm; St Paul, MN, USA) (Figure 1). MAP, ICP, and CPP were recorded continuously every minute, while CBF could only be recorded just before and during subdural infusion, and at 30 mins after injury.
Figure 1.
Drawing of a rat skull showing the position of the sensors for local CBF and ICP measurement, and the position of craniotomy for subdural blood or paraffin oil infusion. Note that CBF was measured through the skull, which was thinned out at the indicated location. ICP was measured intraparenchymally.
For the ASDH, 300 μL autologous, unheparinized blood or paraffin oil was injected at a flow rate of 50 μL/min. After injection the catheter was removed and the scalp was sutured; the animal could recover and was returned to regular housing. The sham-operated groups received no infusion.
Experiment 1: Assessment of Acute Pathophysiological Changes and Histological Damage
Twenty-four rats were assigned to a sham-operated and a blood- and paraffin-treated group (n=8 per group) and were used to measure acute pathophysiological changes for 30 mins (MAP, ICP, CPP, CBF) and histological outcome at 48 h after subdural blood and paraffin oil infusion. Initially animals were surgically prepared for subdural infusion and ICP and CBF measurement. At the end of the experiment, they were perfusion-fixed using heparinized saline (10 IU/mL) followed by 4% paraformaldehyde (pH 7.4). Brains were removed carefully, post-fixed in the same solution, and embedded in paraffin wax. Coronal sections (3 μm thick) were cut throughout the visible lesion and stained with hematoxylin–eosin. The lesion areas were measured on sections using a computerized image-analysis system (Alessandri et al, 2006).
Experiment 2: Temporal Profile of Brain Edema
The temporal profile of edema formation was measured by the wet–dry weight method. Brains were removed quickly 2, 24, or 48 h after sham operation (n=8) or subdural infusion of blood (2 h, n=6; 24 h, n=6; 48 h, n=8) or paraffin oil (2 h: n=6; 24 h: n=6; 48 h: n=8), and separated into the ispi- and contralateral hemisphere. The hemispheres were then dried at 105°C in an oven for 24 h. Tissue weight before (wet weight) and after drying (dry weight) was measured and hemispheric water content was calculated for each animal (e.g., Timaru-Kast et al, 2008).
Experiment 3: Assessment of Glucose Metabolism by PET
Brain glucose metabolism was measured by a small-animal PET (MicroPET Focus 120; Concorde Microsystems Inc., Knoxville, TN, USA) using [18F]-deoxyglucose (FDG). Animals weighing 424±17 g were surgically prepared for subdural infusion as described above and randomly assigned to sham-operated, blood-, and paraffin-treated groups (n=5 per group). They received a single intravenous (jugular vein) injection of FDG (21.6±6.6 MBq) 90 mins after subdural injury induction. Data acquisition started 120 mins after injury since at this time point FDG uptake is essentially complete (see also reference Mori et al, 1990). Measurements lasted for 30 mins and were histogrammed into a single frame. The image data comprises 95 transaxial slices reconstructed iteratively with the OSEM3D/MAP algorithm in a 128 × 128 matrix, with a pixel size of 0.87 mm × 0.87 mm and a slice thickness of 0.8 mm (=voxel), resulting in an image resolution of 1.5 mm.
Maximum FDG activity was calculated for each voxel and within five predefined cortical regions of interest (ROI; Figure 5). Values were shown using two different methods:
ROI analysis: The calculated values of each ipsilateral ROI were divided by the appropriate contralateral ROI and expressed as ipsi- to contralateral ratio.
Voxel-by-voxel analysis: The measured FDG activity of each voxel was corrected for body weight of the animal and injected FDG activity. The corrected voxels of each scan were sorted into 11 categories for each animal and normalized for the number of voxels per hemisphere.
Data Analysis
All values are given as means±s.d. Groups were compared by one-way analysis of variance (ANOVA) with a Student–Newman–Keuls post hoc test for individual group differences (SigmaStat 3.10; Systat Software Inc., Erkrath, Germany). In addition, hemispheric water content was compared within each group by paired t-test.
Results
Acute Changes of MAP, ICP, CPP, and CBF
MAP was stable during an acute monitoring period for the sham-operated, blood-, and paraffin-infused group (not significant, n.s.). MAP was 73.3±12.1 and 74.5±6.4 mm Hg before start of blood or paraffin oil infusion (sham: 72.5±3.4 mm Hg). MAP remained stable for the sham-operated group until the end of monitoring at 30 mins after injury (71.5±3.1 mm Hg). At the end of infusion (+6 mins) blood pressure reduced to 65.9±11.5 mm Hg (blood) and 56.5±7.5 mm Hg (paraffin; P=<0.006 versus baseline; P=<0.05 versus blood). Blood pressure then stabilized at around 64 mm Hg until the end of 30-min monitoring (blood: 64.1±9.7; paraffin: 63.2±7.7 mm Hg; between groups: n.s.).
As depicted in Figure 2A, ICP was similar for all groups before the start of blood or paraffin infusion (sham: 7.8±2.0 mm Hg; blood: 7.5±2.1 mm Hg; paraffin: 6.8±0.9 mm Hg). ICP increased during infusion of blood or paraffin oil above 30 mm Hg and recovered thereafter to around 10 mm Hg. There were significant but minor differences between blood and paraffin oil infusion only at the end of infusion at minute 6 to minute 8 after the start of infusion (P<0.03). At the end of monitoring, ICP was 9.0±2.3 mm Hg (sham), 11.8±2.5 mm Hg (blood), and 11.0±1.1 mm Hg (paraffin; n.s. versus blood).
Figure 2.
The time course of (A) ICP and (B) CPP before and after the start of infusion of 300 μL autologous blood or paraffin oil and sham operation (n=8 per group). The arrow indicates the start of subdural infusion at a rate of 50 μL/min. Note that there was a significant difference for ICP between injury groups only at three time points after the start of infusion (*P<0.05). For CPP, comparisons of injury groups were not significant at any time point. The calculated area under the curve for the post-ASDH monitoring period showed no significant difference between the injury groups for ICP (P=0.11) and CPP (P=0.89). The dotted horizontal line indicates the baseline (one-way ANOVA).
As depicted in Figure 2B, CPP was stable during baseline for all the groups and throughout the entire monitoring period for the sham-operated group (baseline: 64.7±3.6, end of monitoring: 62.5±1.8 mm Hg). CPP showed significant reduction from 65.8±12.2 mm Hg (blood) and 67.7±4.7 mm Hg, (paraffin) to a minimum of 26.1±9.0 mm Hg (blood) and 23.3±8.0 mm Hg (paraffin) (n.s. between groups; P=<0.001 versus baseline). At the end of 30-min monitoring after injury due to the injury-induced decrease of MAP, CPP only recovered to around 50 mm Hg (blood: 52.3±11.4 mm Hg; paraffin 52.2±7.8 mm Hg; P=0.880 between groups). There was no significant difference between the blood- and paraffin-treated groups at any time point of monitoring.
Baseline local CBF expressed in LD units (LDU) was 32.4±2.1 LDU for blood-, 34.0±2.3 LDU for paraffin oil-treated, and 37.2±4.1 LDU for sham-operated animals (sham versus blood P<0.05). CBF remained stable throughout the monitoring period for sham-operated animals (at 30 mins after injury: 37.6±3.8 LDU, 101.1±5% baseline). CBF reduced quickly in both injury groups and reached 5.0±2.0 LDU (15.5±8% baseline) and 9.7±4.9 LDU (28.6±15% baseline) at the end of subdural infusion (P=0.046). Both groups had increased CBF until the end of the monitoring period, which reached 15.5±3.7 LDU (47.9±13% baseline) and 18.8±4.3 LDU (55.3±10% baseline); this increase was not significantly different (P=0.15).
Injury Volume
As an outcome parameter, lesion volume was assessed 48 h after injury with 300 μL autologous blood or paraffin oil. Figure 3 shows that subdural blood produced a lesion volume of 48.1±23.0 mm3, whereas paraffin oil led to significantly smaller brain damage (21.1±11.8 mm3; P=<0.02). Both injury groups had significantly larger lesion than that in sham-operated animals (0.09±0.07 mm3; P<0.01). There was some degree of cell loss on the surface of the contralateral hemisphere in both the groups. This was partially due to the ICP probe. Hippocampal damage in the CA1 region was found mainly in blood-treated animals.
Figure 3.
(A) Lesion volume induced by sham operation or subdural infusion of either 300 μL autologous blood (B) or paraffin oil (C; n=8 per group). Brain damage was measured 48 h after injury on hematoxylin–eosin-stained coronal sections (A, *P<0.001 versus sham, one-way ANOVA). Images show an overview of the ipsilateral hemisphere from (B) blood- and (C) paraffin-treated animals. Arrows indicate the extension of the lesion (sections stained with cresyl violet).
Temporal Profile of Edema Formation
Figure 4 shows the ipsilateral brain water content 2, 24, and 48 h after infusion of 300 μL autologous blood, paraffin oil, or sham operation. The ipsilateral hemispheres of both groups had normal water content 2 h after injury, that is, no difference compared with the sham group (sham: 78.7±0.2% blood: 78.8±0.1% paraffin: 78.2±0.6%); 24 h later there was a significant difference (P<0.01) between the values for the blood group (80.3±1.2%) and that for both paraffin oil- (79.0±0.2%) and sham-treated groups (78.7±0.2%) (P-values <0.005). At 48 h after subdural infusion of blood or paraffin oil, ipsilateral water content was not further elevated in both injury groups (blood, n=8, 80.7±1.2% paraffin, n=8, 78.9±0.5%) and there was still a significant difference in the values from the blood-treated animals and sham-operated and paraffin-treated animals (P<0.001). Subdural blood as well as paraffin oil infusion increased ipsilateral water content over time, with a significant elevation already from 2 to 24 h (blood, P=0.016; paraffin, P=0.019), but not anymore from 24 to 48 h.
Figure 4.
Ipsilateral brain water content measured 2, 24, and 48 h after infusion of either 300 μL autologous venous blood or paraffin oil, or after sham operation. All values are presented as means±s.d. The asterisks (*) indicate significant difference between the values of the blood- and paraffin-treated group (one-way ANOVA; P<0.05).
Water content of the contralateral hemisphere showed no difference between groups at 2 and 24 h after injury, but blood increased water content in the contralateral hemisphere at 48 h after infusion when compared with that in sham- and paraffin-treated animals (sham, 78.7±0.2% blood, 79.2±0.3% paraffin, 78.8±0.3% P-vaues <0.05 versus the blood-treated group).
Glucose Metabolism (PET)
ROI analysis
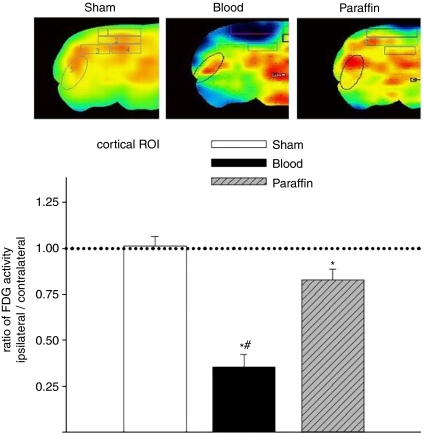
Analysis of FDG metabolism as the ratio between ipsi- and contralateral hemisphere showed significant reduction of cortical glucose metabolism in the cortical ROI underneath the subdural infusion site for both the blood- (0.36±0.07) and paraffin oil-treated group (0.83±0.07; sham, 1.03±0.05). This reduction of glucose metabolism was significantly more pronounced for the blood-treated group (P<0.001; blood versus paraffin oil) (Figure 5). The calculated ratios for the subcortical and lateral ROI did not differ significantly between the groups.
Figure 5.
Evidence of reduction in ipsilateral metabolism after ASDH with 300 μL autologous venous blood or paraffin oil. The micrographs show ipsilateral FDG activity maps of a sham, blood, and paraffin oil-treated animal. The drawn boxes and the circle indicate ROIs in which FDG activity was calculated for the ipsi- and contralateral hemisphere (upper graph). Metabolic reduction expressed as the ratio between ipsi- and contralateral FDG activity in the cortical ROI no. 1(n=5 per group, lower graph). Comparison between groups was significant for the cortical ROI (no. 1), but not for all other investigated ROIs (one-way ANOVA; *, versus sham; # , blood versus paraffin treatment).
Voxel-by-voxel analysis
In the voxel-by-voxel analysis (Figure 6), the normalized activities of all voxels were sorted into 11 categories, which represent low to high metabolism. Sorting the normalized activity of each voxel into these activity ranges showed that significantly more voxels of the blood infusion group were represented in the low-activity categories when compared with sham-operated and paraffin oil-treated animals. Almost 20% of all voxels were found in the six lower activity categories for the blood-treated group (18.5±3.1%), whereas paraffin-treated and sham-operated groups had only 2.2±0.8% and 0.1.0±0.9% of all voxels in the low-activity categories, respectively (P<0.01 versus blood; one-way ANOVA with Student–Newman–Keuls post hoc test).
Figure 6.
Voxel-by-voxel analysis of glucose metabolism (n=5 per group). FDG activity of each voxel was corrected for body weight, injected FDG activity, and number of voxels per hemisphere, and then normalized to values for the contralateral hemisphere. The resulting values were categorized from low to high activity; * and # indicate significant difference in values from blood-treated animals and sham-operated and paraffin oil-treated animals, respectively (P<0.05); § indicates significant difference between the values from the sham and paraffin treatment group (P<0.05; one-way ANOVA on ranks).
Discussion
ASDH is often associated with poor final outcome, and patients who survive are often disabled. Previous animal studies have shown that a pronounced ischemic infarction under the hematoma is commonly observed (e.g., Kuroda et al, 1992). Although the first hours after head injury are crucial for the development of acute brain damage, only few human studies have focused on this problem. (Verweij et al, 2001). ASDH can cause massive ischemic CBF underneath the hemorrhage and early surgical evacuation of the mass reduces mortality (Seelig et al, 1981), possibly by restoring CBF and energy supply. This would imply that the extravasated volume of blood is the main cause of ASDH-induced brain damage and mortality. Conversely, extravasated blood with all its components may induce additional pathophysiological mechanisms detrimental for brain tissue. Although there are a few studies, which addressed the issue of blood constituents after acute subdural hemorrhage, no thorough investigation is available to separate acute and chronic effects of volume-dependent and volume-independent factors. For instance, Kuroda et al (1992) studied glucose metabolism after subdural blood and silicone infusion, but they did not control for differences in ICP, CPP, or CBF . In a not well-controlled study Yilmazlar et al (1997) only described histological outcome at 24 h after subdural blood or silicone infusion. Both studies claim that differences in their results were due to pathological effects of blood constituents, without really proving it. On the basis of our data, this conclusion appears valid, but the degree of their silicone-induced findings might have been influenced by, for example, massive differences in ICP. Therefore, the goal of this study was to compare the effects of subdural blood or paraffin oil infusion on important acute pathophysiological parameters, to correlate them with early energy metabolism, brain edema formation, and late lesion development.
In our study we used equal volumes of blood and paraffin oil as well as identical infusion rates and we could not find prominent differences in ICP, CPP, and CBF between these groups. The almost identical ICP and CPP changes suggest that these are mainly volume-dependent changes during the acute ‘bleeding' period. The small standard deviations of ICP and CPP values (maximum: ±6 mm Hg) provides additional evidence that acute ICP and CPP changes are solely volume-dependent (Figures 2A and 2B). Local CBF, measured frontal to the craniotomy, changed in a time-dependent manner like ICP and CPP. In other words, experimental groups are very well comparable as far as acute changes are concerned. If the subdural volume would be the main factor influencing lesion development, we should not find huge differences in any measured parameters between the two groups at later time points. Nevertheless, already after 2 h glucose metabolism was significantly affected, indicating that the mediator mechanisms, which are volume-independent, had been initiated by blood infusion.
The above mentioned findings underline the fact that a mismatch between decreased CBF, mitochondrial dysfunction, and hypermetabolism appears to be an early occurring central point in the pathophysiology of ASDH. Kuroda et al (1992) very clearly showed uncoupling of CBF and glucose metabolism in a rat model of ASDH using glucose [14C]-2-deoxyglucose autoradiography. They could show a reduction of CBF and glucose metabolism in the core of infarction, whereas in the surrounding zone, the so-called peri-ischemic area, an increase of glucose use and global decrease of CBF could be seen. These findings could also be confirmed by others (Nedergaard et al, 1986; Tanaka et al, 1985). Comparably, decreased glucose metabolism was seen in FDG-PET already at 2 h after injury. We could not, however, detect clear hyperglycolysis, neither by ROI analysis nor by voxel-by-voxel analysis, which would challenge tissue with reduced CBF. The lack of such a finding might be due to the spatial resolution of the PET when compared with autoradiography. But decreased CPP and local CBF (frontal to infusion site) suggest that energy supply was certainly reduced in the penumbral region underneath the blood clot. Therefore, we may also have a mismatch between energy supply and demand resulting in energy crisis. Kuroda and Bullock (1992) proposed that the increase in glucose utilization is caused by excitatory amino-acid release (Kuroda et al, 1992) and other ion-channel activators, and by generation of free radicals (e.g., Kwon et al, 2003). The autoradiographic study performed by Kuroda et al (1992) using [14C]-deoxyglucose showed that substitution of blood by silicone induced no hyperglycolysis and formed a smaller hypoglycolytic zone underneath the infusion site. Our study of volume-dependent versus volume-independent effects of a subdural mass by means of FDG-PET is in agreement with these data. The blood group showed early metabolic changes already 2 h after injury, which correlated with development of significantly more pronounced lesion in the blood-treated group at 48 h after ASDH. It appears that early after ASDH, blood started to influence energy metabolism massively without having a similar effect on CPP or locally measured CBF, which both appeared to be influenced by volume only. This further suggests that the effects of sheer volume are potentiated by blood-derived factors, which influence cell death directly. Differences between volume- and blood-induced effects might be due to a more pronounced release of glutamate by blood treatment. However, we could not find higher glutamate concentrations in the extracellular space by means of microdialysis following subdural infusion of 5 mL paraffin oil or blood in a porcine model of ASDH (Timaru-Kast et al, 2008). It might be concluded that glutamate excitotoxicity is mainly dependent on subdural volume and a resulting underlying ischemia after ASDH, and that other factors are influencing energy metabolism.
Although it is unclear how blood constituents or breakdown products of blood cells can affect the tissue underneath the hemorrhage in such a localized manner, the involved mechanisms appear to interact with blood vessel perfusion. Mechanical obstruction with focal tissue destruction and swelling was thought to produce stasis in proximal vessels, with consecutive occlusion of the lumen. The reduction of endothelial swelling, ICP, and improvement of CBF by hypertonic–hyperoncotic treatment support this mode of action following subdural hematoma and focal venous occlusion (Heimann et al, 2003; Jussen et al, 2008). The almost identical time course of ICP for the blood- and paraffin-treated group indicates that similar mechanical pressure was applied to the brain and blood vessels with the used volume. Measurement of early edema formation 2 h after injury showed no difference between blood and paraffin treatment despite significantly more pronounced metabolic derangements after blood injection. Only at later time points (24 and 48 h) a more severe brain swelling developed with blood treatment when compared with that with paraffin oil. This ended up in a significantly larger lesion volume in the blood-treated group. It seems that early pathophysiological pathways are activated, which cause volume-independent, acute and delayed changes. Thus, blood constituents (e.g., thrombin from extravasated subdural blood) may be responsible for the observed differences between blood and paraffin oil treatment; which blood constituents are responsible for this has to be investigated in further studies.
Intravascular coagulation was a postulated mechanism of secondary ischemic damages in ASDH (Fujisawa et al, 1994; Stein et al, 2002), but Karabiyikoglu et al (2005) could disprove this in a rat model. Fibrin and thrombin immunoreactivity was increased in these lesions, but no intravascular fibrin deposits were found. None of the two coagulation inhibitors, argatroban and ginkolide-B, could prevent microvessel obstruction and reduce histological damage. Other mechanisms for secondary ischemic events are spreading depolarization (CSD) and spreading ischemia (Dreier et al, 1998, 2000), which have now also been shown in patients with subarachnoid hemorrhage (Dreier et al, 2009). Spreading depolarization describes a near-complete cortical depolarization associated with disruption of ionic homeostasis and vasodilatation in un-injured tissue. Under certain pathological conditions (e.g., critically reduced CBF and energy supply) CSD can cause vasoconstriction, that is, ‘spreading ischemia' (e.g., Shin et al, 2006). Petzold et al (2003) showed that spreading ischemia can be induced by blood-derived oxyhemoglobin in combination with increased endothelin-1 and reduced Na+/K+ ATPase activity. The fact that CBF is still reduced 30 mins after injury (baseline=32±2; +30 mins after injury=15.5±3.7 LDU) but cerebral perfusion pressure has recovered, may indicate that vasoconstriction occurred in the vicinity of the subdural blood clot. Normal CPP has also been found to co-occur with ischemic local CBF in brain-injured patients (Chieregato et al, 2003). Whether mechanisms such as spreading ischemia could be responsible for CBF effects described by Chieregato et al (2003) or for the acutely found differences between blood and paraffin oil in our model, has to be investigated thoroughly.
Independent of the effects of blood constituents on perfusion, direct contact of thrombin with brain cells is able to induce cell death (Fujimoto et al, 2006, 2007). This is in agreement with the fact that thrombin, produced by the coagulation cascade, is elevated in level in ischemic tissues and exerts cytotoxic effects in a dose-dependent manner (Lee et al, 1997). Furthermore, Ramos-Mandujano et al (2007) reported that thrombin enhances glutamate efflux from swelling astrocytes and consequently induces excitotoxicity. Likewise, this might trigger spreading ischemia. Recently Gao et al (2007) made an interesting observation that release of carbonic anhydrase-I from lysed red blood cells mediates an increase in the permeability of the blood–brain barrier and brain edema. However, we found a larger hypoglycolytic/ischemic area already at 2 h after subdural hematoma when compared with paraffin infusion. At such an early time point, blood cells are not degraded sufficiently that, for instance, oxyhemoglobin, iron, or carbonic anhydrase-I could play a major role for this tissue damage. Since acute measurements of ICP, CPP, and CBF did not provide decisive clues for the differences in lesion growth between the groups, it is more likely that compounds derived from the coagulation process induce early adverse effects after subdural hemorrhage. Recently, it could be shown that the known toxicity of thrombin is potentiated by plasminogen, which is readily available in blood as a precursor molecule for blood fibrinolysis (Fujimoto et al, 2008). Although thrombin seems not to have direct vasoactive properties (Lee et al, 1997), the effects on glutamate and endothelin release may contribute to spreading ischemia in the peri-ischemic area underneath the subdural hematoma, within the acute phase of injury development. Through these local mechanisms, blood contributes to a larger area of hypoglycolysis/ischemia despite similar acute ICP changes in the blood- and paraffin-treated groups. The thin membrane between the subdural space and subarachnoid space may allow rapid penetration of molecules toward the parenchyma, but prevents whole blood cells to massively invade brain tissue. The potential of such blood- and blood-derived factors for acute and late effects on lesion development has to be investigated in detail in future studies.
In conclusion, our data show that subdural blood itself aggravates brain edema and lesion development not only by increased ICP. Early changes in glucose metabolism and ICP/CPP-independent ischemia underneath the blood clot play an important role in lesion development. This study is the first in which different fluids in combination with glucose metabolisms and acute parameters were directly tested and compared with the extension of early and delayed brain damage by measuring brain edema and histological lesion volume. We could clearly show that acute changes in ICP, CPP, and local CBF are induced by sheer volume. Early thereafter, blood constituents started to affect energy metabolism and the degree of ischemia underneath the subdural hemorrhage, leading to more pronounced breakdown of the blood–brain barrier and brain damage when compared with volume alone. Thus, the subdural volume seems to be responsible for only 43.8% of the final histological damage following an experimental acute subdural hemorrhage. This indicates that further strategies to treat blood-dependent effects more efficiently are in view for patients with ASDH.
Acknowledgments
The data presented in the paper are part of the inaugural dissertations of Melika Behzad. We thank A Ehlert and M Malzahn for excellent technical assistance. The study was supported by a grant from ‘Deutsche Forschungsgemeinschaft' DFG (KE338/6-2; BA and OK).
Sources of support: Deutsche Forschungsgemeinschaft (DFG no. KE338/6-2) Schwerpunkt Neurowissenschaft of Johannes Gutenberg-University.
Footnotes
Disclosure/Conflict of interest
The authors declare no conflict of interest.
References
- Alessandri B, Nishioka T, Heimann A, Bullock RM, Kempski O. Caspase-dependent cell death involved in brain damage after acute subdural hematoma in rats. Brain Res. 2006;1111:196–202. doi: 10.1016/j.brainres.2006.06.105. [DOI] [PubMed] [Google Scholar]
- Bullock R, Butcher SP, Chen MH, Kendall L, McCulloch J. Correlation of the extracellular glutamate concentration with extent of blood flow reduction after subdural hematoma in the rat. J Neurosurg. 1991;74:794–802. doi: 10.3171/jns.1991.74.5.0794. [DOI] [PubMed] [Google Scholar]
- Burger R, Bendszus M, Vince GH, Roosen K, Marmarou A. A new reproducible model of an epidural mass lesion in rodents. Part I: characterization by neurophysiological monitoring, magnetic resonance imaging, and histopathological analysis. J Neurosurg. 2002;97:1410–1418. doi: 10.3171/jns.2002.97.6.1410. [DOI] [PubMed] [Google Scholar]
- Chieregato A, Fainardi E, Tanfani A, Sabia G, Martino C, Pascarella R, Servadei F, Targa L. Induced acute arterial hypertension and regional cerebral flow in intracontusional low density area. Acta Neurochir Suppl. 2003;86:361–365. doi: 10.1007/978-3-7091-0651-8_77. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Ebert N, Priller J, Megow D, Lindauer U, Klee R, Reuter U, Imai Y, Einhaupl KM, Victorov I, Dirnagl U. Products of hemolysis in the subarachnoid space inducing spreading ischemia in the cortex and focal necrosis in rats: a model for delayed ischemic neurological deficits after subarachnoid hemorrhage. J Neurosurg. 2000;93:658–666. doi: 10.3171/jns.2000.93.4.0658. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Korner K, Ebert N, Gorner A, Rubin I, Back T, Lindauer U, Wolf T, Villringer A, Einhaupl KM, Lauritzen M, Dirnagl U. Nitric oxide scavenging by hemoglobin or nitric oxide synthase inhibition by N-nitro--arginine induces cortical spreading ischemia when K+ is increased in the subarachnoid space. J Cereb Blood Flow Metab. 1998;18:978–990. doi: 10.1097/00004647-199809000-00007. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Major S, Manning A, Woitzik J, Drenckhahn C, Steinbrink J, Tolias C, Oliveira-Ferreira AI, Fabricius M, Hartings JA, Vajkoczy P, Lauritzen M, Dirnagl U, Bohner G, Strong AJ. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 2009;132:1866–1881. doi: 10.1093/brain/awp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhaime AC, Gennarelli LM, Yachnis A. Acute subdural hematoma: is the blood itself toxic. J Neurotrauma. 1994;11:669–678. doi: 10.1089/neu.1994.11.669. [DOI] [PubMed] [Google Scholar]
- Durham SR, Duhaime AC. Basic science; maturation-dependent response of the immature brain to experimental subdural hematoma. J Neurotrauma. 2007;24:5–14. doi: 10.1089/neu.2006.0054. [DOI] [PubMed] [Google Scholar]
- Fujimoto S, Katsuki H, Kume T, Akaike A. Thrombin-induced delayed injury involves multiple and distinct signaling pathways in the cerebral cortex and the striatum in organotypic slice cultures. Neurobiol Dis. 2006;22:130–142. doi: 10.1016/j.nbd.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Fujimoto S, Katsuki H, Ohnishi M, Takagi M, Kume T, Akaike A. Thrombin induces striatal neurotoxicity depending on mitogen-activated protein kinase pathways in vivo. Neuroscience. 2007;144:694–701. doi: 10.1016/j.neuroscience.2006.09.049. [DOI] [PubMed] [Google Scholar]
- Fujimoto S, Katsuki H, Ohnishi M, Takagi M, Kume T, Akaike A. Plasminogen potentiates thrombin cytotoxicity and contributes to pathology of intracerebral hemorrhage in rats. J Cereb Blood Flow Metab. 2008;28:506–515. doi: 10.1038/sj.jcbfm.9600547. [DOI] [PubMed] [Google Scholar]
- Fujisawa H, Maxwell WL, Graham DI, Teasdale GM, Bullock R. Focal microvascular occlusion after acute subdural haematoma in the rat: a mechanism for ischaemic damage and brain swelling. Acta Neurochir Suppl (Wien) 1994;60:193–196. doi: 10.1007/978-3-7091-9334-1_52. [DOI] [PubMed] [Google Scholar]
- Gao BB, Clermont A, Rook S, Fonda SJ, Srinivasan VJ, Wojtkowski M, Fujimoto JG, Avery RL, Arrigg PG, Bursell SE, Aiello LP, Feener EP. Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nat Med. 2007;13:181–188. doi: 10.1038/nm1534. [DOI] [PubMed] [Google Scholar]
- Graham DI, Adams JH. Ischaemic brain damage in fatal head injuries. Lancet. 1971;1:265–266. doi: 10.1016/s0140-6736(71)91003-8. [DOI] [PubMed] [Google Scholar]
- Heimann A, Takeshima T, Alessandri B, Noppens R, Kempski O. Effects of hypertonic/hyperoncotic treatment after rat cortical vein occlusion. Crit Care Med. 2003;31:2495–2501. doi: 10.1097/01.CCM.0000084893.44650.CB. [DOI] [PubMed] [Google Scholar]
- Hlatky R, Valadka AB, Goodman JC, Robertson CS.2004Evolution of brain tissue injury after evacuation of acute traumatic subdural hematomas Neurosurgery 551318–1323.discussion 24 [DOI] [PubMed] [Google Scholar]
- Jenkins A, Mendelow AD, Graham DI, Nath FP, Teasdale GM. Experimental intracerebral haematoma: the role of blood constituents in early ischaemia. Br J Neurosurg. 1990;4:45–51. doi: 10.3109/02688699009000681. [DOI] [PubMed] [Google Scholar]
- Jussen DS, Papaioannou C, Heimann A, Kempski O, Alessandri B. Effects of hypertonic/hyperoncotic treatment and surgical evacuation after acute subdural hematoma in rats. Crit Care Med. 2008;36:543–549. doi: 10.1097/01.CCM.0B013E3181620A0F. [DOI] [PubMed] [Google Scholar]
- Karabiyikoglu M, Keep R, Hua Y, Xi G.2005Acute subdural hematoma: new model delineation and effects of coagulation inhibitors Neurosurgery 57565–572.discussion 72 [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Bullock R.1992Failure of cerebral blood flow-metabolism coupling after acute subdural hematoma in the rat Neurosurgery 311062–1071.discussion 71 [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Inglis FM, Miller JD, McCulloch J, Graham DI, Bullock R. Transient glucose hypermetabolism after acute subdural hematoma in the rat. J Neurosurg. 1992;76:471–477. doi: 10.3171/jns.1992.76.3.0471. [DOI] [PubMed] [Google Scholar]
- Kwon TH, Chao DL, Malloy K, Sun D, Alessandri B, Bullock MR. Tempol, a novel stable nitroxide, reduces brain damage and free radical production, after acute subdural hematoma in the rat. J Neurotrauma. 2003;20:337–345. doi: 10.1089/089771503765172291. [DOI] [PubMed] [Google Scholar]
- Lee KR, Kawai N, Kim S, Sagher O, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage: effects of thrombin on cerebral blood flow, blood-brain barrier permeability, and cell survival in a rat model. J Neurosurg. 1997;86:272–278. doi: 10.3171/jns.1997.86.2.0272. [DOI] [PubMed] [Google Scholar]
- Lee ST, Chu K, Sinn DI, Jung KH, Kim EH, Kim SJ, Kim JM, Ko SY, Kim M, Roh JK. Erythropoietin reduces perihematomal inflammation and cell death with eNOS and STAT3 activations in experimental intracerebral hemorrhage. J Neurochem. 2006;96:1728–1739. doi: 10.1111/j.1471-4159.2006.03697.x. [DOI] [PubMed] [Google Scholar]
- Miller JD, Bullock R, Graham DI, Chen MH, Teasdale GM. Ischemic brain damage in a model of acute subdural hematoma. Neurosurgery. 1990;27:433–439. doi: 10.1097/00006123-199009000-00016. [DOI] [PubMed] [Google Scholar]
- Miller JD, Peeler DF, Pattisapu J, Parent AD. Supratentorial pressures. Part I: differential intracranial pressures. Neurol Res. 1987;9:193–197. doi: 10.1080/01616412.1987.11739794. [DOI] [PubMed] [Google Scholar]
- Mori K, Schmidt K, Jay T, Palombo E, Nelson T, Lucignani G, Pettigrew K, Kennedy C, Sokoloff L. Optimal duration of experimental period in measurement of local cerebral glucose utilization with the deoxyglucose method. J Neurochem. 1990;54:307–319. doi: 10.1111/j.1471-4159.1990.tb13316.x. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Gjedde A, Diemer NH. Focal ischemia of the rat brain: autoradiographic determination of cerebral glucose utilization, glucose content, and blood flow. J Cereb Blood Flow Metab. 1986;6:414–424. doi: 10.1038/jcbfm.1986.74. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Einhaupl KM, Dirnagl U, Dreier JP. Ischemia triggered by spreading neuronal activation is induced by endothelin-1 and hemoglobin in the subarachnoid space. Ann Neurol. 2003;54:591–598. doi: 10.1002/ana.10723. [DOI] [PubMed] [Google Scholar]
- Power C, Henry S, Del Bigio MR, Larsen PH, Corbett D, Imai Y, Yong VW, Peeling J. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann Neurol. 2003;53:731–742. doi: 10.1002/ana.10553. [DOI] [PubMed] [Google Scholar]
- Ramos-Mandujano G, Vazquez-Juarez E, Hernandez-Benitez R, Pasantes-Morales H. Thrombin potently enhances swelling-sensitive glutamate efflux from cultured astrocytes. Glia. 2007;55:917–925. doi: 10.1002/glia.20513. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Dunn L. A model of acute subdural hematoma in the mouse. J Neurotrauma. 2001;18:1241–1246. doi: 10.1089/089771501317095278. [DOI] [PubMed] [Google Scholar]
- Sawauchi S, Beaumont A, Signoretti S, Tomita Y, Marmarou C, Marmarou A. Diffuse brain injury complicated by acute subdural hematoma in the rodents: the effect of early or delayed surgical evacuation. Acta Neurochir Suppl. 2002;81:243–244. doi: 10.1007/978-3-7091-6738-0_63. [DOI] [PubMed] [Google Scholar]
- Seelig JM, Becker DP, Miller JD, Greenberg RP, Ward JD, Choi SC. Traumatic acute subdural hematoma: major mortality reduction in comatose patients treated within four hours. N Engl J Med. 1981;304:1511–1518. doi: 10.1056/NEJM198106183042503. [DOI] [PubMed] [Google Scholar]
- Shin HK, Dunn AK, Jones PB, Boas DA, Moskowitz MA, Ayata C. Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J Cereb Blood Flow Metab. 2006;26:1018–1030. doi: 10.1038/sj.jcbfm.9600252. [DOI] [PubMed] [Google Scholar]
- Stein SC, Chen XH, Sinson GP, Smith DH. Intravascular coagulation: a major secondary insult in nonfatal traumatic brain injury. J Neurosurg. 2002;97:1373–1377. doi: 10.3171/jns.2002.97.6.1373. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Welsh FA, Greenberg JH, O'Flynn R, Harris VA, Reivich M. Regional alterations in glucose consumption and metabolite levels during postischemic recovery in cat brain. J Cereb Blood Flow Metab. 1985;5:502–511. doi: 10.1038/jcbfm.1985.76. [DOI] [PubMed] [Google Scholar]
- Timaru-Kast R, Meissner A, Heimann A, Holper B, Kempski O, Alessandri B. Acute subdural hematoma in pigs: role of volume on multiparametric neuromonitoring and histology. J Neurotrauma. 2008;25:1107–1119. doi: 10.1089/neu.2008.0517. [DOI] [PubMed] [Google Scholar]
- Uchida K, Nakakimura K, Kuroda Y, Haranishi Y, Matsumoto M, Sakabe T. Dizocilpine but not ketamine reduces the volume of ischaemic damage after acute subdural haematoma in the rat. Eur J Anaesthesiol. 2001;18:295–302. doi: 10.1046/j.0265-0215.2000.00825.x. [DOI] [PubMed] [Google Scholar]
- Verweij BH, Muizelaar JP, Vinas FC. Hyperacute measurement of intracranial pressure, cerebral perfusion pressure, jugular venous oxygen saturation, and laser Doppler flowmetry, before and during removal of traumatic acute subdural hematoma. J Neurosurg. 2001;95:569–572. doi: 10.3171/jns.2001.95.4.0569. [DOI] [PubMed] [Google Scholar]
- Yilmazlar S, Hanci M, Oz B, Kuday C. Blood degradation products play a role in cerebral ischemia caused by acute subdural hematoma. J Neurosurg Sci. 1997;41:379–385. [PubMed] [Google Scholar]