Abstract
In a transient 90-min middle cerebral artery occlusion (MCAO) model of rats, a large ischemic lesion is formed where macrophage-like cells massively accumulate, many of which express a macrophage marker, Iba1, and an oligodendrocyte progenitor cell marker, NG2 chondroitin sulfate proteoglycan (NG2); therefore, the cells were termed BINCs (Brain Iba1+/NG2+ Cells). A bone marrow transplantation experiment using green-fluorescent protein-transgenic rats showed that BINCs were derived from bone marrow. 5-Fluorouracil (5FU) injection at 2 days post reperfusion (2 dpr) markedly reduced the number of BINCs at 7 dpr, causing enlargement of necrotic volumes and frequent death of the rats. When isolated BINCs were transplanted into 5FU-aggravated ischemic lesion, the volume of the lesion was much reduced. Quantitative real-time RT-PCR showed that BINCs expressed mRNAs encoding bFGF, BMP2, BMP4, BMP7, GDNF, HGF, IGF-1, PDGF-A, and VEGF. In particular, BINCs expressed IGF-1 mRNA at a very high level. Immunohistochemical staining showed that IGF-1-expressing BINCs were found not only in rat but also human ischemic brain lesions. These results suggest that bone marrow-derived BINCs play a beneficial role in ischemic brain lesions, at least in part, through secretion of neuroprotective factors.
Keywords: 5FU, Iba1, IGF-1, macrophage, MCAO, NG2
Introduction
Stroke is one of the most common causes of death and severe disability. Therefore, additional effective treatments must be developed to save lives and reduce neurological damage in patients with strokes. In fact, a number of studies have been conducted to find new therapeutic approaches to produce better outcomes. Most of these studies are designed to handle the acute phase of infarction and the ischemic penumbra, where still many neuronal cells are functional. Much less attention has been focused on the ischemic core than the penumbra, since the former has generally been considered unworthy of treatment as it presumably contains no living neurons (Donnan et al, 2008).
However, in the core are many living non-neural cells, most of which are macrophage-like cells expressing Iba1, a marker of macrophages or microglia (Matsumoto et al, 2008). Many of these macrophage-like cells express NG2 chondroitin sulfate proteoglycan (NG2), a marker of oligodendrocyte progenitor cells (Nishiyama et al, 1999). Oligodendrocytes are typical neuroectodermal cells, and NG2-expressing cells or oligodendrocyte progenitor cells with long ramified processes, often called NG2 cells or NG2 glia, are also considered neuroectodermal in origin. Conversely, microglia or macrophages expressing Iba1 are generally recognized as mesodermal cells (Kreutzberg, 1996; Streit, 2005). Thus, it is difficult to specify the origin of the vastly accumulating macrophage-like cells in the ischemic core, as these cells express both Iba1 and NG2. Therefore, Iba1+/NG2+ macrophage-like cells have simply been termed BINCs (Brain Iba1+/NG2+ Cells) to distinguish them from NG2 cells or microglia. In this study, we first aimed to determine whether BINCs are mesodermal or neuroectodermal, and then determined whether they are beneficial or detrimental in the ischemic brain. If they were found beneficial, a method or an agent that increases or activates BINCs may provide a new therapy for patients with brain infarctions. Conversely, if BINCs were found to be detrimental, a therapy that reduces their numbers or suppress their activity may be effective.
Materials and methods
Animals and Surgical Procedures
The experiments were conducted in accordance with the Guidelines for Animal Experimentation of Ehime University Graduate School of Medicine. Adult male Wistar rats (8 weeks old; body weight, 260 to 300 g) were subjected to middle cerebral artery occlusion (MCAO) for 90 mins, as described elsewhere (Matsumoto et al, 2007). In brief, the right MCA was occluded by an intraluminal filament technique; to obstruct the origin of the right MCA, a 4-0 nylon monofilament suture was inserted into the internal carotid artery through the right external carotid artery. After the intraluminal filament was placed in the correct position, the neck incision was sutured. The rats were re-anesthetized and their intraluminal suture was carefully removed 90 mins after MCAO. Some of the rats subjected to transient MCAO were intraperitoneally injected at 2 days post reperfusion (dpr) with 5-fluorouracil (5FU; Sigma, St Louis, MO, USA) at 100 mg/kg, dissolved in 0.4 M ammonia made in saline.
In Vivo Immunohistochemistry
An indirect immunofluorescence was performed, as described elsewhere, using the primary antibodies listed in Table 1 (Matsumoto et al, 2007). Ischemic rat brains were sliced at the caudoputamen level into 4-μm-thick coronal sections using a cryostat. After the brain sections were incubated with the primary antibodies, and then with aminomethylcoumarin (AMCA)-, fluorescein isothiocyanate-, and/or Cy3-labeled secondary antibodies (Chemicon, Temecula, CA, USA). Hoechst 33258 (Sigma) was used for nuclear staining.
Table 1. Primary antibodies used in this study.
| Antigen | Antibody | Dilution | Source |
|---|---|---|---|
| BrdU | Mouse monoclonal | 1:500 | NeoMarkers (Fremont, CA, USA) |
| CD11b | Mouse monoclonal (MRC OX42) | 1:200 | Serotec (Oxford, UK) |
| GFAP | Mouse monoclonal | 1:500 | Chemicon (Temecula, CA, USA) |
| Iba1 | Rabbit polyclonal | 1:500 | Wako (Osaka, Japan) |
| IGF-1 | Goat polyclonal | 1:200 | Santa Cruz (Santa Cruz, CA, USA) |
| NG2 | Rabbit polyclonal | 1:500 | Chemicon |
| NG2 | Mouse monoclonal (132.39) | 1:500 | Chemicon |
Abbreviations: BrdU, bromodeoxyuridine; GFAP, glial fibrillary acidic protein; IGF-1, insulin-like growth factor-1; NG2, NG2 chondroitin sulfate proteoglycan.
Bone Marrow Transplantation
Four-week-old Sprague Dawley (SD) rats with a transgene of enhanced green-fluorescent protein (EGFP) under the control of the actin promoter (SLC, Hamamatsu, Japan) were intraperitoneally injected with 5FU (150 mg/kg body weight) (Tanaka et al, 2003). Two days later, bone marrow cells were harvested from the femoral and tibial cavities using a syringe filled with Dulbecco's modified Eagle's medium (Wako, Osaka, Japan). The harvested cells were filtered through a cell strainer of pore size of 70 μm (Becton Dickinson, Labware, NJ, USA); then the filtrate was centrifuged at 1500 r.p.m. for 5 mins at 4°C. The resulting pellet was resuspended in 2 mL of saline. Male Wistar recipient rats (4 weeks old) underwent whole-body irradiation (10 Gy) to destroy the bone marrow and were then injected, through the tail vein, with the 2 mL cell suspension. After the irradiation and transplantation, the rats were given water containing minocycline hydrochloride (100 μg/mL) for 1 week to prevent infection. The recipient rats were subjected to transient MCAO 2 months after the transplantation. The transplanted rats were fixed at 7 dpr. To confirm whether the bone marrow transplantation was successful, peripheral blood from the recipient rats was smeared on glass slides and stained with hematoxylin to identify white blood cells. All white blood cells were EGFP+, indicating that all bone marrow cells were completely replaced by those of the transgenic rats (data not shown).
Labeling of Proliferating Cells with BrdU and their Immunohistochemical Detection
Bromodeoxyuridine (BrdU; Sigma) was dissolved at 10 mg/mL in saline, sterilized by filtration, and intraperitoneally injected into 8-week-old ischemic rats at 50 mg/kg three times at 2-h intervals at 2 dpr (Tatsumi et al, 2005). BrdU-administered rats were killed at 3 dpr and the brains were fixed and sectioned as described above. To detect BrdU, the sections were incubated in 2 N HCl for 30 mins at 37°C, neutralized with 0.1 M sodium borate for 10 mins, and washed with Tris-buffered saline. Anti-BrdU mouse monoclonal antibody was used with polyclonal antibodies to Iba1, or glial fibrillary acidic protein (GFAP).
Counting BrdU+ Cells in the Ischemic Brains
Brain sections from BrdU-administered rats fixed at 3 dpr were immunostained as described above (n=4). Micrographs were obtained with a CCD camera using a × 20 objective lens. Cells were counted in four or five × 20 fields (0.14 mm3 per field) of the ischemic core in the basal ganglia in the vicinity of the necrotic temporal cortex or in the temporal cortex. The total number of BrdU+ cells and the numbers of Iba1+/BrdU+ and GFAP+/BrdU+ cells in the fields were counted.
Isolation of Macrophage-Like Cells from the Ischemic Core
Macrophage-like cells were isolated from the ischemic hemisphere at 7 dpr and purified by allowing the cells to attach to suspension culture dishes, as described elsewhere (Matsumoto et al, 2008). For immunocytochemistry, isolated BINCs were scraped off with a rubber scraper and reseeded onto poly--lysine-coated glass coverslips placed in four-well plates. The cells were fixed 1 h after reseeding with 4% paraformaldehyde in phosphate-buffered saline. The fixed cells were rinsed with Tris-buffered saline, incubated in Tris-buffered saline containing 0.1% bovine serum albumin and 0.1% Tween-20 for 20 mins, and double immunostained with antibodies to NG2 and Iba1.
Transplantation of Macrophage-Like Cells into Ischemic Lesions
The recipient rats were subjected to transient MCAO and intraperitoneally injected with 5FU (100 mg/kg) at 2 dpr to eliminate the proliferating cells in the ischemic core. At 5 dpr, they were placed in a Kopf stereotaxic frame. Two holes were made through the skull, approximately 4 mm to the right of the midline and 0/4 mm posterior to the bregma. Macrophage-like cells, which were isolated from ischemic lesions as described above, were suspended in chilled saline at 1.25 × 107 cells/mL. The suspension was injected through the two holes using a 26-gauge needle (20 μL per hole, 2.5 × 105 cells per hole). The needle was inserted approximately 4 mm into the skull, as measured from the surface. At 9 days after the transplantation or at 14 dpr, the recipient rats were transcardially perfused with a fixative containing 4% paraformaldehyde. The fixed brains were sectioned and immunostained using antibodies listed in Table 1.
Primary Glial Cultures and Peritoneal Macrophages
Mixed glial cultures were prepared using forebrain cells of newborn Wistar rats (within 24 h after birth), and microglia and astrocytes were isolated from these mixed glial cultures as described previously (Tanaka et al, 1998, 1999). Resident peritoneal macrophages were isolated from the peritoneum of 8-week-old, male Wistar rats by lavage with 20 mL of chilled phosphate-buffered saline. The cells from the peritoneal macrophages suspended in the chilled phosphate-buffered saline were poured to suspension culture dishes. The macrophages attached shortly to the dishes, similar to BINCs, and they were purified with several washes to remove other types of cells, using Dulbecco's modified Eagle's medium containing 3% fetal calf serum. The purity was 91 to 94% as described elsewhere (Toku et al, 1999).
RNA Isolation and RT-PCR Analysis
Isolated astrocytes, microglial cells, BINCs, and peritoneal macrophages were cultured on plastic dishes in 3% fetal calf serum-supplemented Dulbecco's modified Eagle's medium for 30 mins. Then, their total RNA was collected using ISOGEN (Nippon gene, Tokyo, Japan). cDNA was obtained from DNase-I-treated RNA by reverse transcription using an oligo-(dT) 15 primer as previously described (Takahashi et al, 2008). cDNA samples were prepared from four separate cultures of each cell type, astrocytes, microglia, BINCs, and peritoneal macrophages. Quantitative real-time reverse transcriptase PCR (RT-PCR) analysis was performed in triplicate using an ABI PRISM 7500 instrument with Power SYBR Green (Applied Biosystems, Foster City, CA, USA). PCR conditions were as follows: 50°C for 2 mins, 95°C for 10 mins followed by 40 cycles of 95°C for 15 secs, and 60°C for 1 mins. All gene-specific mRNA expression values were normalized against that of β-actin. The primer sequences for each gene, as well as the sizes of their products, are listed in Table 2. Statistical analyses were conducted using a one-way analysis of variance with Bonferroni's multiple-comparison post-test.
Table 2. Oligonucleotide primers for real-time RT-PCR.
| Gene | Sense/anti-sense |
|---|---|
| β-Actin | 5′-AGAAGAGCTATGAGCTGCCTGACG-3′ |
| 5′-TACTTGCGCTCAGGAGGAGCAATG-3′ | |
| bFGF | 5′-GACCCACACGTCAAACTACA-3′ |
| 5′-TTTCAGTGCCACATACCAAC-3′ | |
| BMP2 | 5′-CCAGGTTAGTGACTCAGAACAC-3′ |
| 5′-TCATCTTGGTGCAAAGACCTGC-3′ | |
| BMP4 | 5′-TGGACACTTCATCACACGACTA-3′ |
| 5′-GCGACGGCAGTTCTTATTCTTC-3′ | |
| BMP7 | 5′-AGACGCCAAAGAACCAAGAG-3′ |
| 5′-GCTGTCGTCGAAGTAGAGGA-3′ | |
| GDNF | 5′-CAGAGAATTCCAGAGGGAAA-3′ |
| 5′-TCACAGGAACCGCTACAATA-3′ | |
| HGF | 5′-TCTTGGTGTCATTGTTCCTG-3′ |
| 5′-CCATGGATGCTTCAAATACA-3′ | |
| IGF-1 | 5′-TTGCGGGGCTGAGCTGGTGG-3′ |
| 5′-GCGGTGACGTGGCATTTTCT-3′ | |
| PDGF-A | 5′-CCTGTGCCCATCCGCAGGAAGAGA-3′ |
| 5′-TTGGCCACCTTGACACTGCGGTG-3′ | |
| VEGF | 5′-TGGATGTCTACCAGCGAAGC-3′ |
| 5′-ACAAGGCTCACAGTGATTTT-3′ |
Abbreviations: bFGF, basic fibroblast growth factor; BMP, bone morphogenetic protein; GDNF, glial cell line-derived neurotrophic factor; HGF, hepatocyte growth factor; PDGF-A, platelet-derived growth factor-A; RT-PCR, reverse transcriptase PCR; VEGF, vascular endothelial growth factor.
Human Tissue
A tissue specimen was obtained from a patient with acute cerebellar infarction at the fifth day after onset. The patient (female, 76 years old) was admitted to Ehime University Hospital and underwent surgical decompression. Informed consent was obtained from the patient, with approval from the local ethics committee. Intracranial hemorrhages, arterial–venous malformations, and aneurysms were not found. The brain sections were immunostained with antibodies to Iba1, NG2, and insulin-like growth factor-1 (IGF-1). For secondary immunoreaction, Cy5-labeled anti-rabbit IgG donkey antibody was also used, in addition to fluorescein isothiocyanate-labeled anti-mouse IgG and Cy3-labeled anti-goat IgG donkey antibodies (Chemicon). The immunostained specimens were examined by conventional microscopy and laser-scanning microscopy (Nikon A1, Tokyo, Japan).
Results
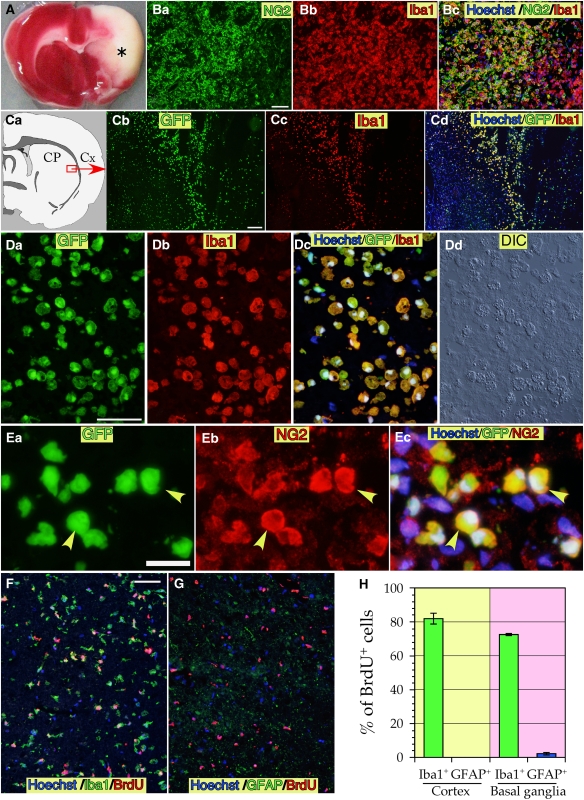
Transient MCAO for 90 mins produced large ischemic lesions in the basal ganglia and adjacent cerebral cortex, visible at 7 dpr as shown by 2,3,5-triphenyltetrazolium chloride (TTC) staining (Figure 1A). At this time point, marked accumulation of macrophage-like cells in the lesion was observed. About two-thirds of the macrophage-like cells expressed both Iba1 and NG2; they were, therefore, considered to be BINCs (Figure 1B; Matsumoto et al, 2008). To determine the origin of the BINCs, a bone marrow transplantation experiment was performed. After lethal irradiation, rats were intravenously transplanted with cells obtained from the bone marrow of transgenic rats that ubiquitously expressed EGFP. Two months after the transplantation (3 months of age), they were subjected to transient MCAO for 90 mins. At 7 dpr, many Iba1+ macrophage-like cells accumulated in the ischemic core in the basal ganglia and the temporal cortex, and all the Iba1+ cells bore EGFP fluorescence (Figures 1C and 1D). These EGFP+/Iba1+ macrophage-like cells had large vesicles in their cytoplasm, as identified by examination with differential interference microscopy (Figure 1D-d). These large granules may have been phagosomes, as described elsewhere (Matsumoto et al, 2008). The bone marrow-derived Iba1+ cells also expressed NG2 (yellow arrowheads in Figure 1E). Thus, BINCs were bone marrow-derived.
Figure 1.
Ischemic lesion identified by TTC staining (A), BINCs accumulated in the ischemic core (B), and the results of bone marrow transplantation experiments with EGFP-transgenic rats (C–E). BINCs were highly proliferative as revealed by a BrdU-labeling experiment (F–H). (A) An ischemic brain whose right MCA was transiently occluded for 90 mins and then coronally dissected at the caudoputamen level (∼0.5 mm anterior to the bregma) and stained with TTC at 7 dpr. The asterisk denotes an ischemic core region. (B) There are many amoeboid cells in the ischemic core expressing NG2 and Iba1, which are termed BINCs. (C) After lethal irradiation of the bone marrow, normal rats underwent transplantation of bone marrow cells obtained from rats that ubiquitously expressed EGFP. The transplanted rats were subjected to transient MCAO 2 months after the transplantation. Almost all Iba1+ cells accumulated in the basal ganglia in the ischemic core (boxed region in Ca) of the transplanted rats at 7 dpr were EGFP+. CP, caudoputamen; Cx, cerebral cortex. (D) High-magnification view of the EGFP+/Iba1+ cells in the ischemic basal ganglia. (E) EGFP+ macrophage-like cells also express NG2. (F) Most BrdU+ cells in the temporal cortex were Iba1+, implying that macrophage-like cells including BINCs were highly proliferative during the acute phase of the ischemic event. (G) No astrocytes were found that incorporated BrdU at 3 dpr in the basal ganglia. Virtually no viable astrocytes were present. (H) Most BrdU+ cells in the temporal cortex and basal ganglia located in the ischemic core at 3 dpr were Iba1+ cells, as revealed by counting the numbers of Iba1+ or GFAP+ cells among total BrdU+ cells. There were no GFAP+/BrdU+ cells in the cortex and only 2.2% of BrdU+ cells were GFAP+ in the basal ganglia. Data obtained from eight rats are expressed as mean±s.e. Scale bars=50 μm.
BINCs have been reported as highly proliferative (Matsumoto et al, 2008). When BrdU was intraperitoneally injected at 2 dpr and the rats were killed at 3 dpr, ∼80% of BrdU+ cells were Iba1+ (Figures 1F and 1H). The density of macrophage-like cells in the ischemic core was not as high at 7 dpr. In contrast, at 3 dpr almost all the GFAP+ cells in the ischemic core were degenerated (Figure 1G), as described elsewhere (Nakagomi et al, 2009), and very few GFAP+/BrdU+ cells were found only in the basal ganglia. This finding is in accordance with previous reports dealing with traumatic brain lesions (Tatsumi et al, 2005).
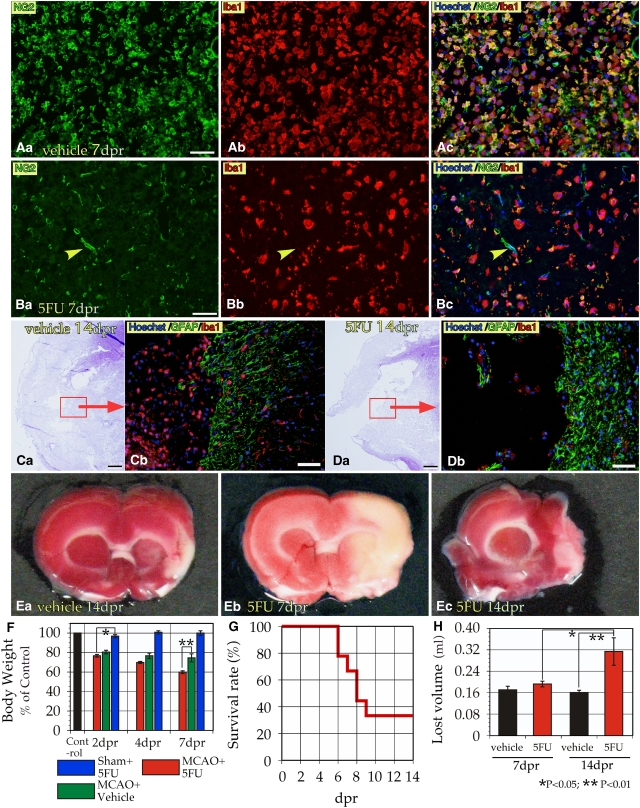
The BrdU experiment indicates that administration of 5FU, which induces apoptosis in proliferating cells, to ischemic rats at 2 dpr can selectively eliminate BINCs. In fact, much fewer macrophage-like cells were found in the ischemic brain lesions of the rats injected with 5FU at 2 dpr than in the rats injected with vehicle alone (Figures 2A and 2B). At 14 dpr, there were still many Iba1+ cells in the ischemic lesions in the vehicle-injected rats (Figure 2C). Yet, at the same time point, Iba1+ cells in the ischemic lesions of 5FU-injected rats were quite few in and around the degenerating tissue (Figure 2D). In most vehicle-injected cases, the ischemic core lesions were limited to the temporal cortex and the lateral portion of the basal ganglia (Figure 2Ea). Conversely, in the 5FU-injected cases, the lesions were markedly larger in the direction of the midline (Figures 2Eb and 2Ec). The 5FU-injection at 2 dpr caused no apparent differences in the body weights of sham-operated rats (Figure 2F). Although body weights of ischemic rats with vehicle injection apparently decreased, the decrease was greater in ischemic rats that underwent 5FU injections, reaching a nearly 40% loss. 5FU injection of ischemic rats was associated with higher mortality; six out of nine 5FU-injected ischemic rats died by 14 dpr (Figure 2G). In contrast, neither vehicle-injected ischemic rats nor 5FU-injected, sham-operated rats died before the end of the experiment. At 14 dpr, the necrosis-induced volume loss of ischemic brains with 5FU injection was twice as large as that of ischemic brains with vehicle injection (Figure 2H).
Figure 2.
Intraperitoneal injection of 5FU at 2 dpr reduced the number of BINCs accumulated in ischemic lesions and exacerbated the ischemic damage. (A) Dense accumulation of BINCs at 7 dpr in the ischemic core in the basal ganglia of an ischemic rat injected with vehicle at 2 dpr. (B) When injected with 5FU at 2 dpr, the ischemic basal ganglia contained much fewer BINCs. Note that many of the Iba1+ macrophage-like cells did not express NG2. Yellow arrowheads denote the NG2+ blood vessels. Thus, the single 5FU injection was associated with a reduced number of BINCs. (C) Density of BINCs in the ischemic lesion at 14 dpr in the cortex and basal ganglion of a rat with a vehicle injection at 2 dpr. A brain section stained with hematoxylin (C, (a)) and immunostained with antibodies to GFAP and Iba1 (C, (b)). Many Iba1+ cells were present in the ischemic core. (D) Density of BINCs reduced at 14 dpr when 5FU was injected at 2 dpr; note the difference from the vehicle-injected rat brain (C). (E) Ischemic lesions at ∼1.5 mm anterior to the bregma identified with TTC staining. When vehicle was injected, the necrotic lesion was limited to the temporal cortex and the lateral portion of the basal ganglia. The core lesion was lost at 14 dpr (E, (a)). The 5FU injection markedly enlarged the core lesion close to the midline at 7 dpr (E, (b)). Such 5FU-indued enlarged lesions were lost by 14 dpr (E, (c)). (F) Sham operation plus 5FU injection was not associated with a loss of body weight. When subjected to 5FU injection, ischemic rats had significantly reduced body weights beginning at 2 dpr. At 7 dpr, the decrease reached 40%, which was significantly more severe than in the ischemic rats that did not receive a 5FU injection. (G) 5FU injection at 2 dpr was associated with the death of rats beginning at 6 dpr. Six out of nine ischemic rats with 5FU injection died by 14 dpr. (H) The lost volume of the ischemic brain tissue at 7 and 14 dpr. In spite of the 5FU injection, the loss of ischemic tissue was not evident at 7 dpr (n=5). At 14 dpr, the 5FU-induced tissue loss became twice as large as the loss in vehicle-injected controls (n=3 each). Note, however, the data in panel H were only obtained from rats that survived to 7 or 14 dpr. Scale bars=50 μm.
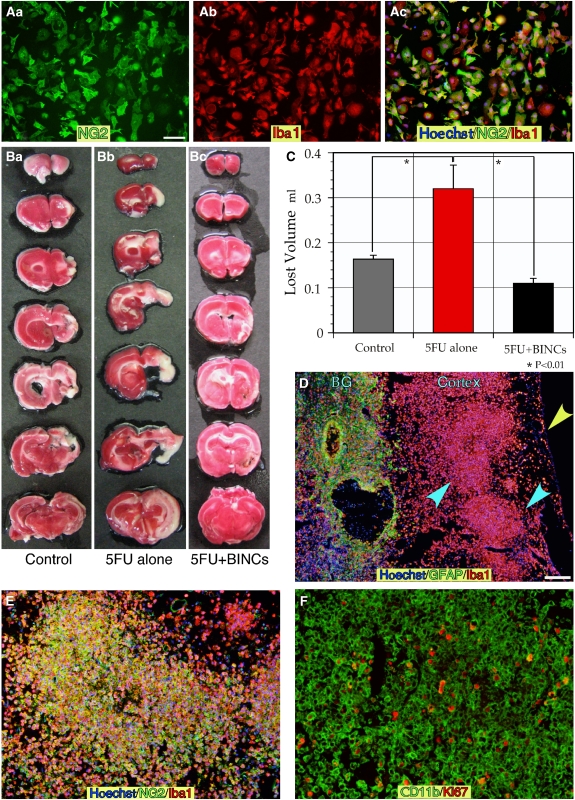
To gain more insight into the role of BINCs in ischemic brains, we transplanted these cells into the ischemic lesions of rats that had been injected with 5FU. By treating the minced ischemic hemisphere at 7 dpr with EDTA, many floating cells were obtained, which were then seeded onto polystyrene dishes for suspension culture. In this way, BINC cultures were prepared (Figure 3A) and the purity was better than the 95% described elsewhere (Matsumoto et al, 2008). The major cell type contaminated in BINC culture was Iba1+/NG2− cells. Iba1− cells were very few. The isolated BINCs were suspended in saline at 500 000 cells/40 μL and injected to the core lesions of other rats through two holes opened over the parietal cortex at 5 dpr when the ischemic lesions were spread over wide regions of the right hemisphere due to 5FU injection (Figures 3B and 3C). In the control ischemic rats with vehicle injection, the lesions were limited to the cerebral cortex and the lateral portion of the basal ganglia at 14 dpr (Figure 3Ba) and the mean lost volume was about 0.16 mL (Figure 3C). The 5FU injection at 2 dpr was associated with larger ischemic lesions at 14 dpr. These enlarged lesions spread over the whole right hemisphere, included the thalamus, and the mean lost volume was about 0.32 mL. However, the lesions identified with TTC staining became much smaller when BINCs were transplanted at 5 dpr to 5FU-aggravated ischemic lesions. The mean lost volume was 0.11 mL, which was significantly smaller than that of the 5FU-injected ischemic rats without BINC transplantation (P<0.01). Note that no 5FU-injected ischemic rats with the transplantation died by 14 dpr. Many BINCs formed large aggregates in the cerebral cortex (blue arrowheads in Figure 3D). Thus, the results of TTC staining shown in Figures 3Bc and 3C were not due to regeneration of neural tissue. Iba1+ cells and GFAP+ cells were colocalized in basal ganglia (denoted ‘BG' in Figure 3D) where gliosis was presumably progressing. These Iba1+ cells forming large aggregates were also NG2+ (Figure 3E). The aggregates of BINCs may be formed through their active proliferation in the ischemic lesions because many cells expressing another macrophage marker CD11b+ had Ki67+ nuclei (Figure 3F).
Figure 3.
Isolated BINCs were transplanted at 5 dpr to ischemic lesions in rats injected with 5FU at 2 dpr. (A) Isolated BINCs from ischemic rat brains at 7 dpr, which were seeded on glass coverslips, fixed and immunostained with antibodies to Iba1 and NG2. As shown here, cells isolated in this way were almost all BINCs. (B) Brains of ischemic rats injected either with vehicle (B, (a)), or 5FU (B, (b) and (c)) at 2 dpr. ‘5FU+BINCs' groups were transplanted with 5 × 105 BINCs at 5 dpr (B, (c)). Photographs show the representative results in each group. Although 5FU caused marked loss of brain tissue (B, (b)), the BINCs transplantation was associated with less loss (B, (c)). (C) The lost tissue volumes were measured in each group (n=3). The transplantation of BINCs was associated with a marked reduction in the lost volume enlarged by 5FU injection. (D) In the ‘5FU+BINCs' group brains, marked accumulation of Iba1+ cells in and around ischemic regions. The figure shows the large aggregates of Iba1+ cells occupying the temporal cortex (blue arrowheads). A yellow arrowhead denotes the surface of the brain. In the basal ganglia, astrogliosis occurred where many Iba1+ cells were present. (E) The cells forming the large aggregates were Iba1+/NG2+ cells or BINCs. (F) Many of the BINCs, identified with anti-CD11b antibody, in the BINCs-transplanted brains were highly proliferative, as revealed by Ki67 immunostaining. Scale bars=50 μm.
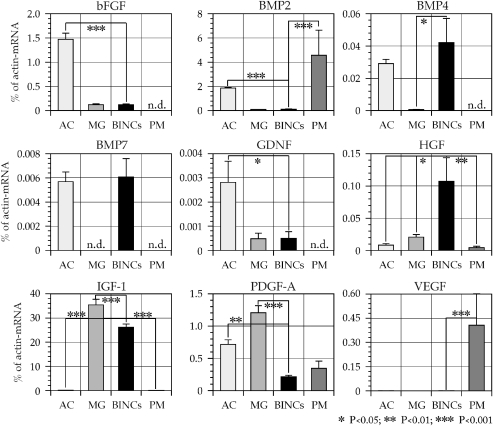
Thus, the abundant BINCs in the lesions appeared to play a protective role by suppressing the progression of ischemic tissue degeneration. To investigate a possible mechanism of how BINCs might be protective, we assessed their production of nine neuroprotective factors, basic fibroblast growth factor (bFGF), bone morphogenetic protein-2 (BMP2), BMP4, BMP7, glial cell line-derived neurotrophic factor (GDNF), hepatocyte growth factor (HGF), IGF-1, platelet-derived growth factor-A (PDGF-A), and vascular endothelial growth factor (VEGF), using quantitative real-time RT-PCR (Figure 4). Shortly after isolating BINCs from ischemic brains at 7 dpr, total RNA was prepared. As control for comparing the expression levels of mRNAs, total RNA was also prepared from primary cultured rat astrocytes and microglia, both of which are well known for their neuroprotective actions (Tanaka et al, 1999; Toku et al, 1998). Furthermore, expression of theses factors by peritoneal macrophages was examined. BINCs expressed all the nine neuroprotective factors. However, due to the very low expression level (0.0001945% of β-actin mRNA) of VEGF mRNA, the graph bar is not visible in Figure 4. Astrocytes expressed mRNAs encoding bFGF and GDNF at the highest level among these three cell types. Microglia expressed IGF-1 and PDGF-A mRNAs at the highest level, peritoneal macrophages BMP2 and VEGF-mRNA and BINCs BMP4, BMP7, and HGF-mRNA. Although microglia, BINCs, and peritoneal macrophages are supposed to belong to the same cell lineage, there are apparent distinctions in the expression of mRNAs encoding these factors. It should be noted that IGF-1 mRNA expression by microglia and BINCs reached around 30% of the β-actin mRNA expression level, which was extraordinarily higher than the expression levels of the other eight factors.
Figure 4.
Isolated BINCs expressed mRNAs encoding a variety of neuroprotective factors at significant levels as shown by quantitative real-time RT-PCR. The vertical axes of the graphs denote the expression level of the mRNA encoding each factor as the percentage of β-actin mRNA expression level. Total RNA was extracted from BINCs shortly after their isolation from ischemic brains at 7 dpr. The levels of the expression by BINCs were compared with primary cultured astrocytes (AC), microglia (MG), and peritoneal macrophages (PM). Note the high expression level of IGF-1 in MG and BINCs, which reached around 30% of the β-actin mRNA level. Among the other factors, HGF mRNA expression was higher in BINCs than in other cells. Data of triplicate experiments from four separate cultures of each cell type are expressed as mean±s.e.m.
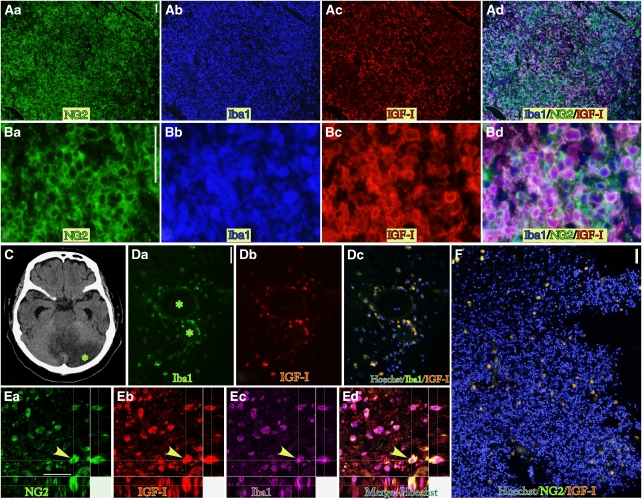
In fact, BINCs in the ischemic core at 7 dpr expressed the IGF-1 protein, as revealed by immunohistochemical staining using anti-IGF-1 antibody (Figures 5A and 5B). IGF-1-expressing BINCs were found not only in ischemic rat brains, but also in ischemic human brains (Figures 5D–5F). However, the density and number of human BINCs was much less than those of rat BINCs. This might be attributable to the old age (76 years) of the patient.
Figure 5.
Strong expression of IGF-1 in BINCs in rat and human ischemic brain lesions as revealed by IGF-1 immunohistochemistry. (A) Weakly magnified view of the parietal cortex of ischemic rat at 7 dpr shows dense accumulation of BINCs, most of which expressed IGF-1. NG2, Iba1, and IGF-1 immunoreactivities were detected using fluorescein isothiocyanate-, AMCA-, and Cy3-labeled secondary antibodies, respectively. Triple stained cells appear pink or violet. Not all, but most BINCs, expressed IGF-1. (B) High magnification view. (C–F) IGF-1+ BINCs are also present in human ischemic brains. The specimen was from a cerebellar infarction in a patient and was resected 4 days after the onset of symptoms. (C) Brain CT scan at the level of the cerebellum. The infarcted region was markedly edematous. The asterisk denotes the portion examined immunohistochemically. (D) Many BINCs were found around blood vessels (asterisks denote the lumen of the vessels). Iba1+ amoeboid cells express IGF-1. (E) Examination using a laser-scanning microscope clearly shows that the same cell denoted with arrowheads expresses NG2 (green), IGF-1 (red), and Iba1 (pink). Nuclear staining with Hoechst 33258 is shown in blue in the merged picture (Ed). (F) A weakly magnified micrograph shows the density of BINCs in the lesion. Compared with the ischemic rat brains, the density is much less. Scale bars=50 μm in panels A, B, D, and F; 20 μm in panel E.
Discussion
BINCs are characterized by simultaneous expression of Iba1 and NG2. Iba1 is a well-known marker for microglia in the brain, and NG2 in the brain is recognized as a marker of NG2 cells, a distinct class of glial cells (Nishiyama et al, 1999); at least a certain population of NG2 cells function as oligodendrocyte progenitor cells. It is generally accepted that NG2 cells or oligodendrocyte progenitor cells are neuroectodermal and microglia are mesodermal. BINCs, therefore, appear to be special in terms of cell lineage. This study shows that this special type of cell is beneficial in ischemic brains.
The precise cell lineage of BINCs has not yet been determined. The present bone marrow transplantation experiments showed that they were bone marrow-derived. Still, BINCs may be the activated form of resident microglia, because microglia are also said to originate in bone marrow or other hematogenous tissues during embryonic development. Furthermore, irradiation preceding bone marrow transplantation might cause impairment of blood–brain barrier, resulting in enhanced recruitment of blood-borne cells into ischemic lesions (Davoust et al, 2008). However, as has been shown in our previous study (Matsumoto et al, 2007), most resident microglia in and around the core of ischemic lesions of MCAO rats degenerated by 2 dpr, and therefore BINCs are unlikely derivatives of resident microglia. Alternatively, BINCs appeared to be descendants of monocyte-like Iba1+ cells infiltrating through blood vessels into the brain parenchyma. In fact, BINCs were mainly found around blood vessels in the human case, 4 days after the onset of stroke (Figure 5). In the rat model, during the initial phase around 3 dpr BINCs in the ischemic lesions were not abundant. However, proliferation appeared to be rapid and vigorous, as shown in Figure 1 and described previously (Matsumoto et al, 2008). On the basis of these observations, it is likely that a small number of Iba1+ cells derived from bone marrow cells infiltrate ischemic lesions during the initial phase of ischemia and vigorously proliferate in the ischemic core, leading to massive accumulation of BINCs in the lesion's core. As there are normally no NG2+ circulating cells (Neudenberger et al, 2006), NG2 may be expressed in the Iba1+ cells after they infiltrate the ischemic lesions. Additional studies are needed to determine what kinds of factors in ischemic lesions induce the expression of NG2 in Iba1+/NG2− cells. Such factors may be clinically useful to increase the number of BINCs in these lesions to produce better outcomes.
There has been a long-lasting debate on the role of macrophage-like cells, including microglia, specifically regarding whether they are harmful or helpful to damaged brains (Bessis et al, 2007; Kreutzberg, 1996; Stoll et al, 2002). As activated microglia release various detrimental factors, such as reactive oxygen species and cytotoxic cytokines, some researchers have insisted that they aggravate various kinds of neural tissue damage (Banati et al, 1993; Lippoldt et al, 2005). In contrast, some studies using genetic techniques have clearly shown that microglia protect damaged neural tissue against ischemic insults (Bruce et al, 1996; Lalancette-Hebert et al, 2007). Lalancette-Hebert et al (2007) selectively ablated proliferating microglia in MCAO brains using a combination of ganciclovir and transgenic mice in which the herpes simplex virus type-1 thymidine kinase (HSV-tk) gene is expressed under the control of the CD11b gene promoter. On the basis of the notion that microglia or macrophages start proliferating shortly after the onset of an ischemic insult, they administered ganciclovir to the transgenic mice for several days, starting 48 h before MCAO, to kill proliferating CD11b+ cells. They concluded that proliferating microglia in the ischemic brain are beneficial.
However, CD11b is predominantly expressed by neutrophils rather than microglia, as shown in our previous study (Matsumoto et al, 2007). Therefore, ganciclovir probably eliminates proliferating progenitor cells of neutrophils in the bone marrow and this effect likely alters the outcome of the ischemic event. Conversely, in this study, we used a simpler method to eliminate proliferating cells, that is, administering a single injection of 5FU at 2 dpr when resident microglia and astrocytes in the lesion core have diminished, and BINCs or blood borne-progenitors of BINCs start to proliferate. When the rats were intraperitoneally injected with BrdU at 2 dpr and killed at 3 dpr, most BrdU-labeled cells were found to be BINCs (Figure 1). This observation suggests that 5FU is selectively taken up by BINCs and kills them. The selectivity may be similar to or better than the combination of the HSV-tk transgenic mice and ganciclovir. Although single injection of 5FU at 2 dpr markedly reduces the number of accumulating BINCs seen at 7 dpr in the ischemic core, this injection does not affect the health of normal rats. Therefore, our 5FU administration model may be suitable for analyzing the effects of BINCs in the ischemic brain. Considering the scarcity of BINCs in the lesion of our elderly human patient, the 5FU-treated MCAO model might be better than the normal model for simulating human cases.
The results obtained by the 5FU-injection experiment showed that elimination of BINCs caused aggravation of ischemic insults, leading to frequent death of ischemic rats. Conversely, when BINCs were transplanted into the 5FU model, the lesions were markedly ameliorated. Thus, the accumulating BINCs appear to be beneficial in suppressing further spread of degeneration. BINCs appeared and proliferated in ischemic lesions after the period of acute neuronal death, indicating that BINCs cannot prevent ischemia-induced neuronal death. Considering the observation that transplantation of BINCs at 5 dpr, when almost all neurons and glial cells disappeared from the ischemic core, effectively suppressed the spreading of ischemic lesions and death of ischemic rats, BINCs can be said to play beneficial roles to prevent progressive secondary degeneration caused by the primary lesions (Fujie et al, 1990; Iizuka et al, 1990).
Three possible mechanisms underlining the neuroprotective effects of BINCs may be proposed: (1) phagocytosis, (2) transdifferentiation, and (3) secretion of neuroprotective factors. BINCs may be involved in the removal of degenerated tissue debris by phagocytosis, since they bear phagosomes that internalize degenerated materials, as has been shown by immunoelectronmicroscopic observations (Matsumoto et al, 2008). Removal of cell or tissue debris is shown to facilitate the regeneration of neural processes (Tanaka et al, 2009). BINCs or highly proliferative microglia-like cells can differentiate into new neurons and glial cells, as described in several reports (Butovsky et al, 2007; Niidome et al, 2008; Yokoyama et al, 2006), although it is difficult to evaluate the significance of this for regeneration of damaged brain tissue. Finally, microglial cells or other types of macrophage-like cells have been known to secrete several kinds of neuroprotective factors (Lai and Todd, 2006). Microglia have been recognized as a major source of IGF-1 (Lalancette-Hebert et al, 2007; Thored et al, 2009). In addition to IGF-1, BINCs expressed mRNAs encoding bFGF (Bethel et al, 1997), BMP2 (Gratacos et al, 2001), BMP4 (Niidome et al, 2008; Xin et al, 2006), BMP7 (Chang et al, 2003), GDNF (Kitagawa et al, 1999), HGF (Hayashi et al, 2001; Zhao et al, 2006), PDGF-A (Vana et al, 2007), and VEGF (Svensson et al, 2002), as shown by the quantitative real-time RT-PCR experiments in this study, and all of these factors have been shown to have neuroprotective effects through various mechanisms. BINCs were distinct from microglia or peritoneal macrophages in the expression patterns of each mRNA encoding these factors, suggesting that they are distinct cell types in spite of common expression of macrophage markers.
Among the four types of cells, BINCs most strongly expressed HGF mRNA. HGF has many favorable functions in damaged neural tissue, such as antiapoptosis, angiogenesis, tissue regeneration, and the enhancement of neurite outgrowth (Matsumoto and Nakamura, 1997). To ameliorate ischemic neural damage, gene transfer techniques, such as transfection of the HGF gene or transplantation of cells transfected with the HGF-gene (Hayashi et al, 2001; Zhao et al, 2006), have been attempted. However, this study showed that HGF-expressing BINCs are already present in the ischemic lesions. Rather than gene transfer techniques, safer methods might be devised to activate or proliferate BINCs to stimulate HGF production in ischemic lesions. It should be evaluated through future study whether BINCs actually prevent the progression of ischemic damage by producing and releasing HGF protein to ischemic brain tissue, because only mRNA encoding HGF was detected in this study.
The expression level of IGF-1 mRNA in BINCs was at nearly 30% of the β-actin mRNA level. This level was extraordinarily higher than those of other factors expressed by BINCs, the levels all of which were less than 1% of the β-actin mRNA level. Immunofluorescence of IGF-1 in BINCs was quite bright, suggesting strong expression of IGF-1 not only at the mRNA level but also at the protein level. Due to this high expression, among the factors presently investigated, IGF-1 is thought to play a pivotal role in the amelioration of ischemic damage. The amount of the BINC-derived IGF-1, however, may be insufficient to maximize its neuroprotective ability, in view of several reports showing that exogenously administrated IGF-1 still can give marked amelioration of ischemic brain damage (Kooijman et al, 2009). As shown in Figure 5, the cell density of BINCs in the infarcted cerebellar tissue from the elderly patient was far less than that in ischemic lesions of 8-week-old rats, even though the human tissue was obtained 4 days after symptom onset when the accumulation of BINCs was not yet at its maximum. Administration of IGF-1 itself or IGF-1 gene transfer seems to be still difficult to apply in human stroke patients for reasons of safety and efficacy. Alternatively, it may be worthwhile to invent ways to increase the number of BINCs or to activate BINCs to secrete more IGF-1 and other neuroprotective factors in ischemic lesions. To increase the cell numbers, one might accelerate mobilization of BINC progenitors from the bone marrow and stimulate proliferation of BINCs in the ischemic lesions. To activate the cells, one might try the various agents that have been shown to activate microglial cells or macrophages. There may be some cytokines and growth factors that stimulate mobilization, proliferation, and activation of BINCs at the same time, leading to increased secretion of IGF-1 and the other factors in brain lesions of aged patients.
Acknowledgments
We are grateful to Staffs in Animal Center of INCS, Ehime University. This study was partly supported by grants from the Setsuro Fujii Memorial, The Osaka Foundation for Promotion of Fundamental Medical Research, and Ehime University.
Footnotes
Disclosure/conflict of interest
The authors declare no conflict of interest.
References
- Banati RB, Gehrmann J, Schubert P, Kreutzberg GW. Cytotoxicity of microglia. Glia. 1993;7:111–118. doi: 10.1002/glia.440070117. [DOI] [PubMed] [Google Scholar]
- Bessis A, Bechade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia. 2007;55:233–238. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- Bethel A, Kirsch JR, Koehler RC, Finklestein SP, Traystman RJ. Intravenous basic fibroblast growth factor decreases brain injury resulting from focal ischemia in cats. Stroke. 1997;28:609–615. doi: 10.1161/01.str.28.3.609. [DOI] [PubMed] [Google Scholar]
- Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, Holtsberg FW, Mattson MP. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. 1996;2:788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Bukshpan S, Kunis G, Jung S, Schwartz M. Microglia can be induced by IFN-gamma or IL-4 to express neural or dendritic-like markers. Mol Cell Neurosci. 2007;35:490–500. doi: 10.1016/j.mcn.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Chang CF, Lin SZ, Chiang YH, Morales M, Chou J, Lein P, Chen HL, Hoffer BJ, Wang Y. Intravenous administration of bone morphogenetic protein-7 after ischemia improves motor function in stroke rats. Stroke. 2003;34:558–564. doi: 10.1161/01.str.0000051507.64423.00. [DOI] [PubMed] [Google Scholar]
- Davoust N, Vuaillat C, Androdias G, Nataf S. From bone marrow to microglia: barriers and avenues. Trends Immunol. 2008;29:227–234. doi: 10.1016/j.it.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- Fujie W, Kirino T, Tomukai N, Iwasawa T, Tamura A. Progressive shrinkage of the thalamus following middle cerebral artery occlusion in rats. Stroke. 1990;21:1485–1488. doi: 10.1161/01.str.21.10.1485. [DOI] [PubMed] [Google Scholar]
- Gratacos E, Checa N, Alberch J. Bone morphogenetic protein-2, but not bone morphogenetic protein-7, promotes dendritic growth and calbindin phenotype in cultured rat striatal neurons. Neuroscience. 2001;104:783–790. doi: 10.1016/s0306-4522(01)00122-1. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Morishita R, Nakagami H, Yoshimura S, Hara A, Matsumoto K, Nakamura T, Ogihara T, Kaneda Y, Sakai N. Gene therapy for preventing neuronal death using hepatocyte growth factor: in vivo gene transfer of HGF to subarachnoid space prevents delayed neuronal death in gerbil hippocampal CA1 neurons. Gene Ther. 2001;8:1167–1173. doi: 10.1038/sj.gt.3301498. [DOI] [PubMed] [Google Scholar]
- Iizuka H, Sakatani K, Young W. Neural damage in the rat thalamus after cortical infarcts. Stroke. 1990;21:790–794. doi: 10.1161/01.str.21.5.790. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Sasaki C, Sakai K, Mori A, Mitsumoto Y, Mori T, Fukuchi Y, Setoguchi Y, Abe K. Adenovirus-mediated gene transfer of glial cell line-derived neurotrophic factor prevents ischemic brain injury after transient middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 1999;19:1336–1344. doi: 10.1097/00004647-199912000-00007. [DOI] [PubMed] [Google Scholar]
- Kooijman R, Sarre S, Michotte Y, De Keyser J. Insulin-like growth factor I: a potential neuroprotective compound for the treatment of acute ischemic stroke. Stroke. 2009;40:e83–e88. doi: 10.1161/STROKEAHA.108.528356. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Lai AY, Todd KG. Microglia in cerebral ischemia: molecular actions and interactions. Can J Physiol Pharmacol. 2006;84:49–59. doi: 10.1139/Y05-143. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippoldt A, Reichel A, Moenning U. Progress in the identification of stroke-related genes: emerging new possibilities to develop concepts in stroke therapy. CNS Drugs. 2005;19:821–832. doi: 10.2165/00023210-200519100-00002. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Kumon Y, Watanabe H, Ohnishi T, Shudou M, Chuai M, Imai Y, Takahashi H, Tanaka J. Accumulation of macrophage-like cells expressing NG2 proteoglycan and Iba1 in ischemic core of rat brain after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2008;28:149–163. doi: 10.1038/sj.jcbfm.9600519. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Kumon Y, Watanabe H, Ohnishi T, Shudou M, Ii C, Takahashi H, Imai Y, Tanaka J. Antibodies to CD11b, CD68, and lectin label neutrophils rather than microglia in traumatic and ischemic brain lesions. J Neurosci Res. 2007;85:994–1009. doi: 10.1002/jnr.21198. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Nakamura T. Hepatocyte growth factor (HGF) as a tissue organizer for organogenesis and regeneration. Biochem Biophys Res Commun. 1997;239:639–644. doi: 10.1006/bbrc.1997.7517. [DOI] [PubMed] [Google Scholar]
- Nakagomi T, Taguchi A, Fujimori Y, Saino O, Nakano-Doi A, Kubo S, Gotoh A, Soma T, Yoshikawa H, Nishizaki T, Nakagomi N, Stern DM, Matsuyama T. Isolation and characterization of neural stem/progenitor cells from post-stroke cerebral cortex in mice. Eur J Neurosci. 2009;29:1842–1852. doi: 10.1111/j.1460-9568.2009.06732.x. [DOI] [PubMed] [Google Scholar]
- Neudenberger J, Hotfilder M, Rosemann A, Langebrake C, Reinhardt D, Pieters R, Schrauder A, Schrappe M, Rottgers S, Harbott J, Vormoor J. Lack of expression of the chondroitin sulphate proteoglycan neuron-glial antigen 2 on candidate stem cell populations in paediatric acute myeloid leukaemia/abn(11q23) and acute lymphoblastic leukaemia/t(4;11) Br J Haematol. 2006;133:337–344. doi: 10.1111/j.1365-2141.2006.06013.x. [DOI] [PubMed] [Google Scholar]
- Niidome T, Matsuda S, Nonaka H, Akaike A, Kihara T, Sugimoto H. A molecular pathway involved in the generation of microtubule-associated protein 2-positive cells from microglia. Biochem Biophys Res Commun. 2008;370:184–188. doi: 10.1016/j.bbrc.2008.03.068. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Chang A, Trapp BD. NG2+ glial cells: a novel glial cell population in the adult brain. J Neuropathol Exp Neurol. 1999;58:1113–1124. doi: 10.1097/00005072-199911000-00001. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Schroeter M. Detrimental and beneficial effects of injury-induced inflammation and cytokine expression in the nervous system. Adv Exp Med Biol. 2002;513:87–113. doi: 10.1007/978-1-4615-0123-7_3. [DOI] [PubMed] [Google Scholar]
- Streit WJ.2005Microglial cells Neuroglia(Kettenmann H, Ransom BR, eds). 2nd ed.New York: Oxford University Press; 60–71. [Google Scholar]
- Svensson B, Peters M, Konig HG, Poppe M, Levkau B, Rothermundt M, Arolt V, Kogel D, Prehn JH. Vascular endothelial growth factor protects cultured rat hippocampal neurons against hypoxic injury via an antiexcitotoxic, caspase-independent mechanism. J Cereb Blood Flow Metab. 2002;22:1170–1175. doi: 10.1097/01.wcb.0000037988.07114.98. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Matsumoto H, Smirkin A, Itai T, Nishimura Y, Tanaka J. Involvement of heparanase in migration of microglial cells. Biochim Biophys Acta. 2008;1780:709–715. doi: 10.1016/j.bbagen.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Toku K, Matsuda S, Sudo S, Fujita H, Sakanaka M, Maeda N. Induction of resting microglia in culture medium devoid of glycine and serine. Glia. 1998;24:198–215. [PubMed] [Google Scholar]
- Tanaka J, Toku K, Zhang B, Ishihara K, Sakanaka M, Maeda N. Astrocytes prevent neuronal death induced by reactive oxygen and nitrogen species. Glia. 1999;28:85–96. doi: 10.1002/(sici)1098-1136(199911)28:2<85::aid-glia1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Tanaka R, Komine-Kobayashi M, Mochizuki H, Yamada M, Furuya T, Migita M, Shimada T, Mizuno Y, Urabe T. Migration of enhanced green fluorescent protein expressing bone marrow-derived microglia/macrophage into the mouse brain following permanent focal ischemia. Neuroscience. 2003;117:531–539. doi: 10.1016/s0306-4522(02)00954-5. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Ueno M, Yamashita T. Engulfment of axon debris by microglia requires p38 MAPK activity. J Biol Chem. 2009;284:21626–21636. doi: 10.1074/jbc.M109.005603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi K, Haga S, Matsuyoshi H, Inoue M, Manabe T, Makinodan M, Wanaka A. Characterization of cells with proliferative activity after a brain injury. Neurochem Int. 2005;46:381–389. doi: 10.1016/j.neuint.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Thored P, Heldmann U, Gomes-Leal W, Gisler R, Darsalia V, Taneera J, Nygren JM, Jacobsen SE, Ekdahl CT, Kokaia Z, Lindvall O. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–849. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- Toku K, Tanaka J, Fujikata S, Hamamoto Y, Horikawa Y, Miyoshi K, Tateishi N, Suzuki Y, Maeda N. Distinctions between microglial cells and peripheral macrophages with regard to adhesive activities and morphology. J Neurosci Res. 1999;57:855–865. [PubMed] [Google Scholar]
- Toku K, Tanaka J, Yano H, Desaki J, Zhang B, Yang L, Ishihara K, Sakanaka M, Maeda N. Microglial cells prevent nitric oxide-induced neuronal apoptosis in vitro. J Neurosci Res. 1998;53:415–425. doi: 10.1002/(SICI)1097-4547(19980815)53:4<415::AID-JNR3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Vana AC, Flint NC, Harwood NE, Le TQ, Fruttiger M, Armstrong RC. Platelet-derived growth factor promotes repair of chronically demyelinated white matter. J Neuropathol Exp Neurol. 2007;66:975–988. doi: 10.1097/NEN.0b013e3181587d46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Li Y, Chen X, Chopp M. Bone marrow stromal cells induce BMP2/4 production in oxygen-glucose-deprived astrocytes, which promotes an astrocytic phenotype in adult subventricular progenitor cells. J Neurosci Res. 2006;83:1485–1493. doi: 10.1002/jnr.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama A, Sakamoto A, Kameda K, Imai Y, Tanaka J. NG2 proteoglycan-expressing microglia as multipotent neural progenitors in normal and pathologic brains. Glia. 2006;53:754–768. doi: 10.1002/glia.20332. [DOI] [PubMed] [Google Scholar]
- Zhao MZ, Nonoguchi N, Ikeda N, Watanabe T, Furutama D, Miyazawa D, Funakoshi H, Kajimoto Y, Nakamura T, Dezawa M, Shibata MA, Otsuki Y, Coffin RS, Liu WD, Kuroiwa T, Miyatake S. Novel therapeutic strategy for stroke in rats by bone marrow stromal cells and ex vivo HGF gene transfer with HSV-1 vector. J Cereb Blood Flow Metab. 2006;26:1176–1188. doi: 10.1038/sj.jcbfm.9600273. [DOI] [PubMed] [Google Scholar]