Abstract
Tinnitus is often defined as the perception of sounds or noise in the absence of any external auditory stimuli. The pathophysiology of subjective idiopathic tinnitus remains unclear. The aim of this study was to investigate the functional brain activities and possible involved cerebral areas in subjective idiopathic tinnitus patients by means of single photon emission computerized tomography (SPECT) coincidence imaging, which was fused with magnetic resonance imaging (MRI). In this cross-sectional study, 56 patients (1 subject excluded) with subjective tinnitus and 8 healthy controls were enrolled. After intravenous injection of 5 mCi F18-FDG (fluorodeoxyglucose), all subjects underwent a brain SPECT coincidence scan, which was then superimposed on their MRIs. In the eight regions of interest (middle temporal, inferotemporal, medial temporal, lateral temporal, temporoparietal, frontal, frontoparietal, and parietal areas), the more pronounced values were represented in medial temporal, inferotemporal, and temporoparietal areas, which showed more important proportion of associative auditory cortices in functional attributions of tinnitus than primary auditory cortex. Brain coincidence SPECT scan, when fused on MRI is a valuable technique in the assessment of patients with tinnitus and could show the significant role of different regions of central nervous system in functional attributions of tinnitus.
Keywords: magnetic resonance imaging, SPECT coincidence imaging, tinnitus
Introduction
Tinnitus is ‘the conscious experience of a sound that originates in an involuntary manner in the head of its owner or may appear to him to be so' (McFadden, 1982). In addition, tinnitus is a common symptom of disorders of the auditory system that usually is associated with sensory neural hearing loss (Lockwood et al, 2002; Adams et al, 1999). The prevalence of chronic tinnitus in general population was reported between 5% and 15%, and in 1% to 3%, it causes severe impairment of the quality of life (Plewnia et al, 2007; Davis and Rafaie, 2000). Most of tinnitus patients make a successful adaptation to the presence of this phantom sound. For those who fail to adapt, tinnitus may become a source of significant disability.
Idiopathic subjective tinnitus may occur during a malfunction of feedback loops or hyperactivity in the periphery (cochlea and eighth cranial nerve) (Zenner and Ernst, 1993; Coles, 1997). In recent years, it has been widely accepted that maladaptation of central information processing are critically responsible in tinnitus perception and generation (Plewnia et al, 2007). In addition, abnormal activity at higher levels of the auditory pathways (auditory nuclei, auditory cortex, associative cortices) may contribute significantly or considerably to the generation of tinnitus. Despite this profusion of assumed locations in the generation of tinnitus, most current hypotheses agree that abnormal neural activity is interpreted and perceived as tinnitus in higher cortical centers (e.g., auditory cortex). The interpretation of an aberrant auditory signal as troublesome tinnitus is because of conscious sound processing in auditory centers and also association of the signal with unpleasantness and distress. Memory, attention, and the emotional state of the patients are factors that may be involved in this reaction. Thus, this neurophysiological process may not be detected by an evaluation of the cerebral function in tinnitus sufferers (Mirz et al, 1999).
Although the psychophysical characteristics of tinnitus have been described in some details, the neural loci and mechanisms that cause tinnitus and associated disabilities are poorly understood in the absence of suitable techniques for assessing the abnormal neural activation in human (Murai et al, 1992). Advances in brain function imaging techniques have made it possible to identify the brain regions associated with the production of transient, subjective sensations, such as phantom limb pain or hallucination, and also perception and processing of sound and tinnitus and associating characteristics such as loudness of tinnitus (Lockwood et al, 1998; Plewnia et al, 2007; Flor et al, 1995; Silbersweig et al, 1995; Mirz et al, 1999; Giraud et al, 1999; Shulman et al, 2004; Lockwood et al, 1998, 2001).
It could be a critical step in the task of defining the factors that create these phantom sensations and developing rational treatments for this chronic and disabling condition (Lockwood et al, 1998, 2001).
There is not enough investigation in functional brain scan of tinnitus patients with large sample size to define exact association of tinnitus and brain abnormality and involved region in comparison with a control group. The purpose of our study was to investigate the functional brain abnormalities by single photon emission computerized tomography (SPECT) coincidence imaging of positron-emitter fluorodeoxyglucose (FDG) to visualize abnormal metabolic brain activity in patients with chronic tinnitus. The association of tinnitus laterality and brain abnormality was evaluated.
Materials and methods
Subjects
This cross-sectional study was performed on a population of subjects referred to the Otolaryngology; Head and Neck Research Center of Iran University of Medical Sciences (IUMS) for evaluation and treatment of their tinnitus, from September 2005 to February 2007. All subjects gave written informed consent in accord with the declaration of Helsinki Principles, National Committee of Ethics in Medical Research (Technology and Research Deputy of Ministry of Health and Medical Education) and the Committee on Ethics at the ENT and Head and Neck Research Center of Iran University of Medical Sciences, radiation safety, and radioactive drug research committee before participating in this study.
Brain coincidence SPECT scan was performed on 56 tinnitus subjects and 8 normal controls. From 56 tinnitus subjects enrolled in this study, 1 female was excluded because of thrombophlebitis after intravenous injection of FDG. All subjects and controls were over 20 years old, and mean age of subjects was 48 (s.d.=13) years old and for the control group it was 35 years (s.d.=12). Subjects had permanent chronic (over 6 months) unilateral or bilateral moderate-to-severe subjective idiopathic tinnitus (loss of steady state tinnitus could lead to loss of coherency of subjects and differences between subjects' imaging results). Subjects reported subjective tinnitus and there was no evidence of evoked tinnitus. Subjects had mild-to-moderate high frequency sensorineural hearing loss (higher than 2 kHz) ranging from 30 to 70 dB HL. Each participant was healthy and not under any specific medications from at least 3 months ago. None of the subjects had any invasive therapeutic interventions on brain or ears before or after tinnitus.
All types of intervention (acoustico-physical, pharmacological, and so on), which could potentially interfere with the tinnitus sensation and the central auditory function, were avoided. There is sufficient evidence in the literature that those kind of interventions can actually alter brain functional study outcomes. In addition, subjects were considered as homogenous because of the constant and steady state feature of their tinnitus.
Subjects with the following characteristics were not included: pregnancy or any decision for pregnancy, psychiatric disorders or its history (according to psychiatrist verification), any treatment for tinnitus during past 3 months, dementia, seizure, or alcohol/drug abuse in past 6 months, head and neck diseases or space occupying lesions, any organic disease that cause tinnitus. Subjects were examined by an ENT specialist for head and neck disorders, neurologist for neurological disorders, internist for other medical diseases and psychiatrist for psychological disorders. Standard laboratory tests that are needed for tinnitus workup were performed, such as CBC and Platelets count, General Biochemestry, Electrolytes, TFT, Clotting tests, VDRL. Complete audiological and electrophysiological assessments were performed for all subjects. Also magnetic resonance imaging (MRI) with and without gadolinium injection were performed to rule out any neurodegenerative or other space-occupying lesions in all subjects.
Tinnitus Assessment
Pitch and loudness matching of tinnitus were evaluated in tinnitus subjects in the affected ear to an external tone presented to the contralateral ear. This task was accomplished using tinnitus evaluation device (TinED, Otolaryngology, Head & Neck Research Center, Tehran, Iran), which includes six channels to reconstruct the most troublesome tinnitus with a similar frequency and intensity. The accuracy of the calibrating equipment shall be sufficient to determine that the TinED is within the tolerances permitted by American Standard Specification for Audiometers, S3.6-2004. The subjects had to have loudness matching of tinnitus more than 6-decibel sensation level (dB SL) to be included in this study.
Using tinnitus questionnaire (Hallam et al, 1988), the severity of tinnitus in subjects was rated to six scales. We used Persian version of tinnitus questionnaire, which was translated and validated (Daneshi et al, 2005; Farhadi et al, 2005). Subjects with tinnitus questionnaire score of 44 or more were proposed to have moderate-to-severe tinnitus.
SPECT Coincidence Imaging
For SPECT coincidence study, 4 h fasting with no glucose intake were mandatory and the recorded serum level was required to be less than 6 mmol/dL. All subjects had an intravenous line established while they were lying down, with their eyes closed (or covered) and ears unplugged, in a quiet dark room with low ambient sound and light. We asked them to focus on their tinnitus and instructed them not to speak, read, or move for at least five mins before and five mins after injection. No sedation was applied. Approximately 30 mins after 5 mCi intravenous injection of F18-FDG, provided by department of nuclear medicine via cyclotron nearby the nuclear medicine department, and in accordance with USP/NF 2005 standard (Williams, 2006). They were positioned supine and then SPECT coincidence scans were performed. SPECT coincidence procedure was performed with a dual head SMV camera (SMV-Sopha, France), with a pair of low energy, high-resolution collimators. Standard head position was based on uniform alignment of the external auditory meatus using automated table positioning and camera-to-head-detector ratio values. The projections data were processed with a filtered-backprojection algorithm, butherworth 5-0.5 to show a three dimensional view of the brain. Anatomical tissue images were generated and standard circular regions of interest were created for each subject in the study. Standard uptake values were calculated for each regions of interest. After subtracting areas with normal uptake from SPECT coincidence images of subjects, an average normal scaning of control group were used. Regions with abnormal uptake were identified. All these abnormal regions constituted eight regions of interests, which were found to be related to tinnitus. These regions consisted of middle temporal, inferotemporal, medial temporal, lateral temporal, temporoparietal, frontal, frontoparietal, and parietal areas. These areas were separated by anatomical sulcus in three-dimensional images and were localized by one expert visually and semiquantitatively.
MRI
Magnetic resonance imagings were obtained using tool marked Siemens (Erlangen, Germany), 1.5 Tesla, Avanto 18 channels. The participants were kept approximately 8 mins without any movement. The images were stored in Dicom format to be applied in Brain Anatomical Functional Images Co-registration Software (BrainAFICS, Otolaryngology, Head & Neck Research Center, Tehran, Iran) software.
Image Fusion
Software for image fusion
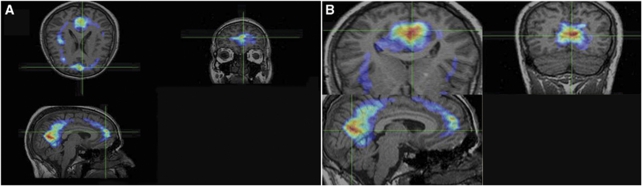
Single photon emission computerized tomography coincidence imaging is inherently a functional modality, so it is sometimes difficult to exactly interpret the anatomical area of disturbed function. Therefore, it is helpful to correlate the relatively coarse functional images to high-resolution anatomical MRI (Figure 1).
Figure 1.
Fusion of single photon emission computerized tomography coincidence imaging coincidence imaging and magnetic resonance imaging in tinnitus-related brain abnormalities in one subject. regions of interests (ROIs) were used for semiquantitative analysis of hyperactivity. (A and B) Abnormalities in frontal and inferotemporal lobes. Hyperactivity of brain was defined visually and semiquantitatively analysis. Activity ratio was calculated as follows: for unilaterally involved subjects, after localized abnormal area visually, regions of interests (1pixel size) were drawn and the count per voxel were compared with the ROIs in the other side. In bilaterally involved subjects, counts per voxel of the abnormal areas were compared with similar ROIs on cerebellum (as reference). We also classify all activity ratios as below: 1=normal, 1 to 1.5=mild, 1.5 to 2=moderate, and >2 as sever hyperactivity.
BrainAFICS, a software system designed in Otolaryngology; Head and Neck Research Center of IUMS, is capable of registering and fusing unsynchronized PET and SPECT with MRIs with different dimensions, in same subjects.
Statistical Analysis
For finding the association and difference between side and site of SPECT coincidence imaging abnormality with tinnitus and in different groups, χ2 and Fisher's exact test were used. Subjects and controls were compared by χ2-test. Comparison of quantitative variables was performed by t-test. A probability value less than 0.05 was considered significant.
Results
Subjects consisted of 42 males (76.4%) and 8 normal controls included 5 males (62.5%) who underwent imaging process. In subjects, 16 (29%) had left, 12 (21%) had right tinnitus, and 28 (50%) subjects had tinnitus in both sides (Table 1).
Table 1. Frequency of side of tinnitus in subjects (N (%)).
| Side | Frequency (%) | Side details | Frequency (%) |
|---|---|---|---|
| Right | 11 (20) | — | — |
| Left | 16 (29) | — | — |
| Bilateral | 28 (51) | Right=left | 17 (30) |
| Right>left | 6 (12) | ||
| Left >right | 5 (9) | ||
| Total | 55 (100.0) | — | 28 (51) |
Abnormalities (defined as hyperactivity detected by brain coincidence SPECT scan) were seen in 49 subjects (87%) and 4 controls (50%). Most subjects (N=41, 84%) had multifocal abnormalities in SPECT coincidence scan. There was more than one abnormal area in one hemisphere in eight males. In control group, two persons (25%) had abnormality in both hemispheres (no one had multiple abnormality in one hemisphere).
Difference between subjects and control group in functional abnormality was statistically significant (abnormal to normal ratio was 49/6 in subjects versus 4/4 in controls, P-value=0.017). This difference was significant in every hemisphere (right hemisphere: abnormal to normal ratio was 42/13 in subjects versus 3/5 in controls, P-value=0.037 and left hemisphere: abnormal to normal ratio was 47/8 in subjects versus 3/5 in controls, P-value=0.007).
There was no significant association between side of tinnitus and side of SPECT coincidence scan abnormality in the brain (P-value=0.211) (Table 2). The most common site of abnormality seen in both right and left hemisphere scanning was middle temporal (53% and 60%) and temporoparietal (15% and 15%) (Table 3).
Table 2. Association of side of tinnitus and single photon emission computerized tomography (SPECT) coincidence image abnormality (N (%).
| The side of tinnitus | SPECT coincidence scanning results | Total | |||
|---|---|---|---|---|---|
| Normal (%) | Right sided abnormality (%) | Left sided abnormality (%) | Abnormality in both sides (%) | ||
| Right | 1 (1.8) | 2 (3.6) | 1 (1.8) | 7 (12.6) | 11 |
| Left | 1 (1.8) | 0 | 2 (3.6) | 13 (23.4) | 16 |
| Bilateral | 4 (7.2) | 0 | 4 (7.2) | 20 (36.3) | 28 |
| Total | 6 (10.8) | 2 (3.6) | 7 (12.6) | 40 (72.7) | 55 (100%) |
Table 3. Site of abnormalities in single photon emission computerized tomography (SPECT) coincidence imaging (N (%)).
| Site of abnormality | N (%) of frequency (%) | Control group |
|---|---|---|
| Right side hemisphere involvement in SPECT coincidence imaging | ||
| Middle temporal | 29 (53) | 3 (37.5%) |
| Inferotemporal | 5 (9) | 0 |
| Medial temporal | 2 (3.6) | 0 |
| Lateral temporal | 1 (1.8) | 0 |
| Temporoparietal | 8 (15) | 0 |
| Frontal | 2 (3.6) | 0 |
| Frontoparietal | 1 (1.8) | 0 |
| Parietal | 1 (1.8) | 0 |
| No abnormality | 13 (24) | 5 (62.5%) |
| Left side hemisphere involvement in SPECT coincidence imaging | ||
| Middle temporal | 33 (60) | 3 (37.5%) |
| Inferotemporal | 6 (10.9) | 0 |
| Medial temporal | 0 | 0 |
| Lateral temporal | 1 (1.8) | 0 |
| Temporoparietal | 8 (15) | 0 |
| Frontal | 1 (1.8) | 0 |
| Frontoparietal | 1 (1.8) | 0 |
| Parietal | 2 (3.6) | 0 |
| All normal | 8 (14.5) | 5 (62.5%) |
No abnormality was found in other areas of the brain. The quantitative values showed more pronounced increased cortical activity in middle temporal according to the Atlas of Regional Anatomy of the Brain using MRI (2004), Brodmann area 21 and inferomedial-temporal, Brodmann area 37 and temporoparietal area, Brodmann area 22. Table 3 shows frequency of cerebral areas that indicated increased FDG uptake. In left hemisphere scanning, eight (14%) subjects showed normal FDG uptake, whereas in right hemisphere 13 (24%) subjects had normal uptake. Most of the subjects had a focal increase in FDG uptake in the middle and inferior temporal lobe in both right and left hemispheres (53% and 60%). The next frequent areas with increased FDG uptake were temporoparietal and inferomedial temporal areas in both hemispheres. There was no significant relation between subject's gender and number of involved zones in the right and left sides with P-value=0.760 and 0.843, respectively.
Discussion
Availability of an objective and noninvasive technique by which auditory function can be readily showed and measured is of significant medical and medico-legal importance.
The neural origin and mechanisms of tinnitus are largely unclear. However, recent evidences point to a pivotal role of the central nervous system, particularly cortical functions in the pathophysiology of tinnitus. Central mechanisms suggested as the origin of tinnitus have been confirmed by tinnitus developing after surgical transection of the auditory nerve, or ablation of the cochlea as main peripheral parts of hearing system (Norena and Eggermont, 2003; Lenarz et al, 1993; Eggermont and Sininger, 1995).
Although the functional imaging data in tinnitus subjects are sparse, it is nevertheless worth recognizing that the results are broadly compatible with a single view of auditory cortical activity in tinnitus subjects. In this view, the tinnitus percept would correspond to abnormally high levels of cortical activity (i.e., ‘tinnitus-related' activity). There have been several additional preliminary reports of tinnitus-related brain activity detected by functional MRI (Cacace et al, 1999; Langguth et al, 2006; Mirz et al, 2000).
There was significant difference between subjects and controls in brain functional abnormalities, which were defined as hyperfunctioning areas detected by functional imaging. This finding has been confirmed by other studies (Mirz et al, 1999; Mirz et al, 2000; Arnold et al, 1996). But as a bias in our methodology, the subjects and control group had not been matched about hearing loss that could have some effects on tinnitus and brain function abnormality. In the study performed by Wang et al (2000), the subjects with comorbidity of hearing loss and tinnitus were shown to have abnormalities in bilateral hemispheres associated with hearing loss while foci site were located at the posterior superior temporal gyrus, medial portion of middle temporal gyrus, and so on. Further investigation to evaluate the independent association of hearing loss with tinnitus and brain functional abnormalities is strongly suggested.
In this research, no increase was seen in FDG uptake in both control and subject group in the primary auditory center located on superior temporal gyrus; compatible with Brodmann areas 41, 42. The most increase in FDG uptake was found to be in associative auditory cortex of tinnitus patients. It was mainly located at the middle temporal gyrus (compatible with Brodmann areas 21 and 22) and inferior temporal and temporoparietal areas.
It has been speculated that chronic tinnitus may be a consequence of maladaptive neuroplastic changes of the central nervous system after injury to the periphery, analogous to the pathophysiological model of chronic pain (Moller, 2000). In this pathophysiological model of tinnitus, ‘auditory phantom perception' is the consequence of differentiation-induced disinhibition in the central auditory system, reflected in irregular hyperactivity of neuronal networks integrated in the processing of auditory information (Eggermont and Roberts, 2004).
Our results strengthened the conceptual link of chronic tinnitus with abnormally enhanced cortical activation.
Positron emitter scanning such as SPECT coincidence scan and functional MRI assess the brain on the basis of the number and activity of neurons and synaptic events in cortices of tinnitus subjects. ‘Activity' means excitatory and inhibitory synaptic events, and neural discharges which correspond to an increase in neural metabolism leading to a local increase in blood flow, blood oxygenation (Fox et al, 1988; Kwong et al, 1992; Ogawa et al, 1992; Nudo and Masterton, 1986; Proshansky et al, 1980; Moratti et al, 2008; Jastreboff and Hazell, 1993). Brain functional assessment, using a positron emitter scanning permits acquisition of data from inferior frontal and anterior temporal areas, can be used for subjects with cochlear and other implants (such as pacemakers) and can be used to map neurochemical pathways and receptors (Cacace, 1997). On the basis of our results, inferior regions of temporal lobe including middle and inferior temporal lobes and temporoparietal cortex show the most abnormallity in FDG uptake. There is controversy about the most common abnormal foci in the tinnitus subject's left primary, secondary, and integrative auditory brain areas, as well as the right paralimbic areas, which were suggested in one investigation (Eggermont and Sininger, 1995). Increased abnormality in the primary auditory cortex in tinnitus subjects, irrespective of tinnitus laterality was found by Arnold et al (1996). Andersson et al (2000) believed that increases in rCBF were found in the left temporoparietal cortex, left parietal cortex, right frontal cortex, and cerebellum. Mirz et al (1999) stated that of particular interest were the lateralization of tinnitus at the cortical level and activation of the right lateralized paralimbic area.
With considering our results, associative cortices are the most common involved areas in this disorder. It could be hypothesized that numerous processing applied on auditory pathway are more important in tinnitus sensation. Aberrant increase in function of neural networks or enhancement of stimulatory synapses function or decreased function of inhibitory synapses may be responsible for these impaired pathways. It may be more appropriate to focus treatment mostly on these processing neural networks.
In some brain function studies in tinnitus subjects, it was proposed that cortical activation occurs primarily in the left hemisphere, unrelated to the laterality of the tinnitus (Arnold et al, 1996; Kleinjung et al, 2005), while others reported the right side as more involved side (Mirz et al, 1999; Mirz et al, 2000). In our investigation, most of the subjects had bilateral abnormal brain function. We found that left sided tinnitus was more commonly represented by left hemisphere abnormality in SPECT coincidence imaging and vice versa, but this association was not statistically significant.
Loss of multiple involvement of one hemisphere in females may be explained by endocrine, psychological, and occupational effects.
Probably, because of a high potential cost of brain function imaging procedures, and so few volunteers, sample sizes in majority of the related articles were very small. Small sample size has been addressed as a main cause of false negative in functional imaging studies by Andreasen et al (1996). In our research, the sample size was rather large in comparison with others, so the results were more reliable.
Conclusion
There is association between tinnitus and brain function abnormality. On the basis of findings in this research, detection of different levels of cortical activation represents central involvement in pathophysiology of tinnitus. In these cortical centers, most activation is noticed in areas compatible with associative cortices and not main primary auditory cortex. Therefore, it seems reasonable to look for pathophysiology of tinnitus in processing of sound perception and not just the sound itself.
Further studies are needed to determine neuroanatomy and pathophysiology of tinnitus more precisely. More can be learned regarding pathophysiology and the true mechanisms of tinnitus by using brain topognostic neural electrical activity mapping and event-related potential mapping. In addition, efficacy of various modalities of treatment may be evaluated by these methods. For example, effect of targeted drugs or electrical stimulation on cortical functionality can be assessed in tinnitus subjects through an interventional study using functional scanning by FDG and MRIs before and after treatment. By this way, more effective modalities of treatment can be described.
Acknowledgments
This work was financially supported by the Iran National Science Foundation (INSF) and ENT and Head and Neck Research Center of Iran University of Medical Sciences and Agriculture, Medical and Industrial Research School. We are grateful to acknowledge Miss Maryam Darbeheshti and Miss Arefeh Afkhami who kindly cooperated in performing audiological tests.
Footnotes
Disclosure/conflict of interest
All authors declare no conflict of interest.
References
- Adams PF, Hendershot GE, Marano MA. Current estimates from the National Health Interview Survey, 1996. Hyattsville, Md.: National Center for Health Statistics; 1999. [PubMed] [Google Scholar]
- Andersson G, Lyttkens L, Hirvelä C, Furmark T, Tillfors M, Fredrikson M. Regional cerebral blood flow during tinnitus: a PET case study with lidocaine and auditory stimulation. Acta Otolaryngol. 2000;120:967–972. doi: 10.1080/00016480050218717. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Arndt S, Cizadlo T, O'Leary DS, Watkins GL, Ponto LLB, Hichwa RD. Sample size and statistical power in [15O] H2O studies of human cognition. J Cereb Blood Flow Metab. 1996;16:804–816. doi: 10.1097/00004647-199609000-00005. [DOI] [PubMed] [Google Scholar]
- Arnold W, Bartenstein P, Oesstreicher E, RÖmer W, Schwaiger M. Focal metabolic activation in the predominant left auditory cortex in patients suffering from tinnitus. A PET study with [18F] Deoxyglucose. ORL J Otorhinolaryngol Relat spec. 1996;58:195–199. doi: 10.1159/000276835. [DOI] [PubMed] [Google Scholar]
- Cacace AT. Imaging tinnitus with fMRI. Assoc Res Otolaryngol Abstr. 1997;2:20. [Google Scholar]
- Cacace AT, Cousibs JP, Parnes SM, et al. Cutaneous-evoked tinnitus. Audiol Neurootol. 1999;4:247–257. doi: 10.1159/000013848. [DOI] [PubMed] [Google Scholar]
- Coles RRA.1997Tinnitus Adult audiology1st ed. (Stephens D, eds),Oxford: butterworth-heinemann; 1–34. [Google Scholar]
- Daneshi A, Mahmoudian S, Farhadi M, Hasanzadeh S, Ghalebaghi B. Auditory electrical tinnitus suppression in patients with & without implants. Int Tinnitus J. 2005;11:85–91. [PubMed] [Google Scholar]
- Davis AC, Rafaie EA.2000Epidemiology of tinnitus Tinnitus Handbook(Tyler RS, eds),San Diego CA: Singular; 1–24. [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Sininger Y.1995Correlated neural activity and tinnitus Mechanisems of tinnitus(Vernon JA, Moller AR, eds),Boston: Allyn and Bacon; 21–34. [Google Scholar]
- Farhadi M, Mahmoudian S, Yazdanparasti V, Daneshi A.2005Effects of auditory electrical stimulation (AES) on tinnitus improvement and associated complaints by using Persian Tinnitus Questionnaire (PTQ) Hakim 81–8.ISSN: 1561-252X www.SID.ir [Google Scholar]
- Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, Larbig W, Taub E. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375:482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Chery-Croze S, Fischer G, Fischer C, Vighetto A, Gregoire MC, Lavenne F, Collet I. A selective imaging of tinnitus. Neuroreport. 1999;10:1–5. doi: 10.1097/00001756-199901180-00001. [DOI] [PubMed] [Google Scholar]
- Hallam RS, Jakes SC, Hinchcliffe R. Cognitive variables in tinnitus annoyance. Br J Clin Psychol. 1988;27:213–222. doi: 10.1111/j.2044-8260.1988.tb00778.x. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Hazell JWP. A neurophysiological approach to tinnitus: clinical implications. Br J Audiology. 1993;27:7–l7. doi: 10.3109/03005369309077884. [DOI] [PubMed] [Google Scholar]
- Kleinjung T, Eichhammer P, Langguth B, Jacob P, Marienhagen J, Hajak G, Wolf SR, Strutz J. Long-term effects of repetitive transcranial magnetic stimulation (rTMS) in patients with chronic tinnitus. Otolaryngol Head Neck Surg. 2005;132:566–569. doi: 10.1016/j.otohns.2004.09.134. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weiskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, Cheng HM, Brady TJ, Rosen BR. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Sci Proc Nat Acad. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langguth B, Eichhammer P, Kreutzer A, Maenner P, Marienhagen J, Kleinjung T, Sand P, Hajak G. The impact of auditory cortex activity on characterizing and treating patients with chronic tinnitus--first results from a PET study. Acta Otolaryngol Suppl. 2006;556:84–88. doi: 10.1080/03655230600895317. [DOI] [PubMed] [Google Scholar]
- Lenarz T, Schreiner C, Snyder RL, Ernest A. Neural mechanisms of tinnitus. Eur Arch Otorhinolaryngol. 1993;249:441–446. doi: 10.1007/BF00168851. [DOI] [PubMed] [Google Scholar]
- Lockwood AH, Salvi RJ, Burkard RF. Tinnitus. N Engl J Med. 2002;347:904–910. doi: 10.1056/NEJMra013395. [DOI] [PubMed] [Google Scholar]
- Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, Murphy BW. The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology. 1998;50:114–120. doi: 10.1212/wnl.50.1.114. [DOI] [PubMed] [Google Scholar]
- Lockwood AH, Wack DS, Burkard RF, Coad ML, Reyes SA, Arnold SA, Salvi RJ. The functional anatomy of gaze-evoked tinnitus and sustained lateral gaze. Neurology. 2001;56:472–480. doi: 10.1212/wnl.56.4.472. [DOI] [PubMed] [Google Scholar]
- McFadden D.1982Tinnitus: Facts, Theories and Treatments. Report of working group 89, Committee on Hearing, Bioacoustics and BiomechanicsNational Research CouncilWashington DC: National Academy Press [Google Scholar]
- Mirz F, Pedersen CB, Ishizu K, Johannsen P, Ovesen T, Stødkilde-Jørgensen H, Gjedde A. Positron emission tomography of cortical centers of tinnitus. Hear Res. 1999;134:133–144. doi: 10.1016/s0378-5955(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Mirz F, Gjedde A, Ishizu K, Pedersen CB. Cortical networks subserving the perception of tinnitus-a PET study. Acta Otolaryngol Suppl. 2000;543:241–243. doi: 10.1080/000164800454503. [DOI] [PubMed] [Google Scholar]
- Moller AR. Similarities between severe tinnitus and chronic pain. J Am Acad Audiol. 2000;11:115–124. [PubMed] [Google Scholar]
- Moratti S, Rubio G, Campo P, Keil A, Ortiz T. Hypofunction of right temporoparietal cortex during emotional arousal in depression. Arch Gen Psychiatry. 2008;65:532–541. doi: 10.1001/archpsyc.65.5.532. [DOI] [PubMed] [Google Scholar]
- Murai K, Tyler RS, Harker LA, Stouffer JL. Review of pharmacologic treatment of tinnitus. Am J Otol. 1992;13:454–464. [PubMed] [Google Scholar]
- Norena AJ, Eggermont JJ. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neuronal correlates of tinnitus. Hear Res. 2003;183:137–153. doi: 10.1016/s0378-5955(03)00225-9. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Masterton RB. Stimulation-induced [14C]2-deoxyglucose labeling of synaptic activity in the central auditory system. J Comp Neurol. 1986;245:553–565. doi: 10.1002/cne.902450410. [DOI] [PubMed] [Google Scholar]
- Ogawa S, TankANK DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plewnia C, Reimold M, Najib A, Brehm B, Reischl G, Plontke SK, Gerloff C. Dose-dependent attenuation of auditory phantom perception (Tinnitus) by PET-Guided Repetitive Transcranial Magnetic Stimulation. Hum Brain Mapp. 2007;28:238–246. doi: 10.1002/hbm.20270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proshansky E, Kauer JS, Stewart WB, Egger D. 2-deoxyglucose uptake in the cat spinal cord during sustained and habituated activity in the plantar cushion reflex pathway. J Comp Neurol. 1980;194:505–517. doi: 10.1002/cne.901940303. [DOI] [PubMed] [Google Scholar]
- Shulman A, Strashun AM, Avitable MJ, Lenhardt ML, Goldstein BA. Ultra-high frequency acoustic stimulation and tinnitus control: a positron emission tomography study. Int Tinnitus J. 2004;10:113–125. [PubMed] [Google Scholar]
- Silbersweig DA, Stern E, Frith C, Cahill C, Holmes A, Grootoonk S, Seaward J, McKenna P, Chua SE, Schnorr L, Jones T, Frackowiak RSJ. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378:176–179. doi: 10.1038/378176a0. [DOI] [PubMed] [Google Scholar]
- Wang H, Tian J, Yin D. Positron emission tomography of tinnitus-related brain areas. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2000;35:420–424. [PubMed] [Google Scholar]
- Williams R. US pharmacopeia council of experts 2005–2010: Work plans, new revision approaches, and other enhancements. AAPS J. 2006;8:E661–E664. doi: 10.1208/aapsj080475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenner HP, Ernst A. A cochlear-motor, transduction and signal transfer tinnitus: model for three types of cochlear tinnitus. Eur Arch Otorhinolaryngol. 1993;149:447–454. doi: 10.1007/BF00168852. [DOI] [PubMed] [Google Scholar]