Abstract
The inflammatory response triggered by stroke has been viewed as harmful, focusing on the influx and migration of blood-borne leukocytes, neutrophils, and macrophages. This review hypothesizes that the brain and meninges have their own resident cells that are capable of fast host response, which are well known to mediate immediate reactions such as anaphylaxis, known as mast cells (MCs). We discuss novel research suggesting that by acting rapidly on the cerebral vessels, this cell type has a potentially deleterious role in the very early phase of acute cerebral ischemia and hemorrhage. Mast cells should be recognized as a potent inflammatory cell that, already at the outset of ischemia, is resident within the cerebral microvasculature. By releasing their cytoplasmic granules, which contain a host of vasoactive mediators such as tumor necrosis factor-α, histamine, heparin, and proteases, MCs act on the basal membrane, thus promoting blood–brain barrier (BBB) damage, brain edema, prolonged extravasation, and hemorrhage. This makes them a candidate for a new pharmacological target in attempts to even out the inflammatory responses of the neurovascular unit, and to stabilize the BBB after acute stroke.
Keywords: cerebral ischemia, inflammation, intracerebral hemorrhage, mast cell, neurovascular unit, stroke
Introduction
Inflammatory reaction, a host response to noxious stimuli, has been the focus of research on ischemic brain injury for several decades. It has been viewed as a detrimental process with a wide array of mechanisms, and approaches to harness it are still considered as promising avenues for future stroke treatment (Hallenbeck et al, 2006). The purpose of this review is to provide new evidence supporting a hypothesis that mast cells (MCs), which are resident in several brain structures and meninges, act as early regulators of the immediate blood–brain barrier (BBB) changes and ensuing vasogenic edema that characterize sudden cerebral ischemia. By releasing potent preformed vasoactive mediators that then act on the cerebral vessels, MCs could promote the breakdown of the BBB, edema, extravasation, and hemorrhage.
We review the following in this paper:
Existing data on MC activation and their role in immediate host response;
the multitude of released vasoactive mediators of MC granule contents;
antagonism of inflammatory cell responses as a strategy for clinical stroke therapy;
recent experimental ‘proof-of-concept' studies suggesting that MC stabilization might provide a basis for effective novel therapies in ischemic or hemorrhagic stroke in the future;
evidence on how MCs could participate in a step-by-step degradation of the BBB and promote secondary inflammatory responses in relation to other well-known regulators in this cascade, such as cytokines, matrix metalloproteinases (MMPs), and cathepsins;
the putative interrelationships between MCs and components of the vascular wall, perivascular inflammatory, neuronal, and glial cells assembled to form an integral neurovascular unit (NVU); and
the potential relevance of the reviewed evidence to therapeutic attempts to harness ischemia-dependent inflammation, expansive brain edema, and cerebral hemorrhages.
Mast cells and host response in the brain
The brain has long been considered an immunologically privileged organ, and recent findings have implied an involvement of immune mechanisms in neurologic disease and illness, including ischemic and hemorrhagic stroke. The presence of the BBB and the lack of lymphatic vessels alluded that immunologic host response within the central nervous system (CNS) is limited. However, at present, considerable literature attests the innate participation of several resident brain cells in immune surveillance, e.g., by astrocytes, oligodendrocytes, microglia, various perivascular cells (endothelial cells, smooth muscle cells, pericytes, perivascular macrophages, MCs), and even neurons during health and disease (Griffiths et al, 2009; Price et al, 2003). Although resident cerebral cells do not characteristically express proteins of major histocompatibility complex, microglia and astrocytes as so-called nonprofessional antigen-presenting cells can present novel antigens to naive helper T cells that will infiltrate into the neuropil on appropriate stimuli (Griffiths et al, 2009; Price et al, 2003).
Although inhibition of the inflammatory cascade of blood-borne neutrophil and phagocyte infiltration after cerebral insults has been the subject of serious attempts to establish a new therapeutic avenue in stroke, as reviewed previously (Emerich et al, 2002; Frijns and Kappelle, 2002), recent research on ischemia has not been focused on resident brain cell types that are capable of mounting immediate host responses in the brain and meninges, namely the MCs. Although MCs are present in the brain from the time of birth and earlier investigators had noticed the presence of perivascular MCs in the brain and meninges (Figure 1) (Dropp, 1976; Florenzano and Bentivoglio, 2000; Goldschmidt et al, 1984; Hough, 1988; Manning et al, 1994; Silver et al, 1996; Theoharides, 1990), they have thereafter received insufficient attention.
Figure 1.
Mast cells in human cerebral and meningeal tissues. (A) Antitryptase-stained section of the human cerebral cortex showing predominantly spindle-shaped MCs (arrows) in the characteristic perivascular position. Modified with permission from Ann Med 2009; 41:438–450. (B) A round/oval perivascular mast cell in human cerebral ischemia typically expressing CD117 (arrows). Thin arrows point to the nuclei of endothelial cells. (C) Anti-tryptase staining showing MCs (arrows) in human meningeoma.
Mast cells enter the CNS during development through penetrating blood vessels, with which they remain associated (Lambracht-Hall et al, 1990), as shown by ultrastructural studies showing the predominantly perivascular location (Figures 1 and 2) of MCs (Dimitriadou et al, 1990; Letourneau et al, 2003). Even during development, MCs occur in two locations: the pia and the brain parenchyma. Although the pial MC population in rodents declines rapidly and reaches adult levels by postnatal week 30, the thalamic population does increase during the same period (Asarian et al, 2002; Khalil et al, 2007). Almost 97% of MCs lie on the abluminal (brain) side of the blood vessels (Khalil et al, 2007). In the body, MCs are typically arranged in the proximity of outer layers and barriers, such as the epithelia, mucosal membranes, and vascular walls, where they can protect organs against noxious environmental or blood-borne stimuli. In the CNS, corresponding tissues are the meninges and the blood vessels forming the BBB.
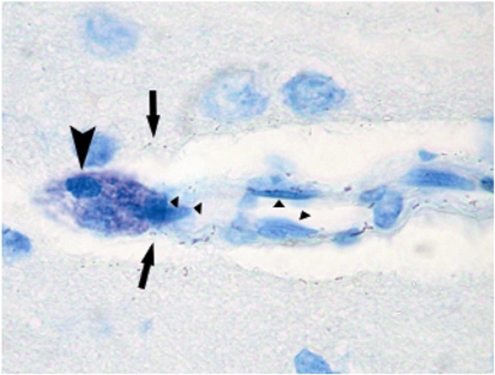
Figure 2.
Toluidine blue staining of rat brain sections. Toluidine blue staining of ischemic rat brain sections highlighting the metachromatic (deep violet) heparin-containing cytoplasmic granules associated solely with MCs in an intensely stained round/oval MC (arrows). The typical perivascular position along a penetrating cortical artery must be noted. Arrows point to exocytosed MC granules, and triangles highlight endothelial cells.
Mechanisms of action of mast cells
Mast cells have been recognized as major cellular mediators of fulminant type-I hypersensitivity responses and systemic anaphylaxis. These responses have also long been known to be biphasic, the immediate phase occurring within minutes and the late phase within several hours. The former is caused by the release of MC granule contents (such as histamine, tumor necrosis factor (TNF)-α, chymase, tryptase, cathepsin-G, heparin, and various chemoattractants). The second phase is characterized by de novo-produced MC mediators, such as leukotrienes, prostaglandins, cytokines, and additional chemotactic factors, and is followed by infiltration of circulating polymorphonuclear leukocytes (PMNLs). One of the most potent mediators involved in PMNL recruitment is probably TNF-α, which represents a distinct type of MC mediator, as it is derived from both preformed and newly synthesized pools (Galli, 1993). Mast cells are probably the only cell type containing preformed stores of TNF-α (Gordon and Galli, 1991), and therefore they likely represent an instantaneous source of TNF-α that triggers generalized tissue inflammation (Costa et al, 1997). Thereafter, more TNF-α is produced by blood-borne cells, neutrophils, eosinophils, T and B cells, and macrophages (Galli et al, 1991).
Should acute cerebral insults trigger an analogous biphasic MC-driven phlogistic response, the predicted result would be an acute increase in vascular permeability, followed by the gradual accumulation of circulating PMNLs facilitated by the released histamine, chemotactic agents, and morphologic alteration of the BBB. Indeed, earlier data support the observation that edematous changes in the brain may be regulated by histamine and its receptors (Abbott, 2000; Esposito et al, 2001; Joo et al, 1994; Yong et al, 1994; Zhuang et al, 1996), as histamine is a main granule constituent of MCs. Even earlier experiments had shown that MC activation by potent enzymes such as phospholipase-A could induce edema in the peripheral vascular bed (Brain et al, 1977). Recent results obtained from experimental models summarized in detail below have provided more data supporting the existence of such an immediate hypersensitivity-type response that opens the BBB and leads to very acute expansive brain edema and secondary PMNL infiltration (Strbian et al, 2006, 2007a, 2007b), which is very analogous to the well-known MC-dependent biphasic phlogistic responses in other organs. These data have renewed interest in the modulation of inflammatory cell responses as a therapeutic strategy in stroke.
Inflammatory cell inhibition as a strategy in acute stroke therapy
There have been three major attempts to test the clinical value of limiting inflammatory cell-dependent injury in ischemic stroke (Becker, 2001; ICOS; Investigators, 2001; Krams et al, 2003). These have targeted the cascade of tissue entry of leukocytes that is well elucidated in various experimental ischemia–reperfusion injury models and in a multitude of organs. Typically, such approaches have targeted adhesion, transmigration, and infiltration of PMNLs that start within hours after the onset of tissue ischemia (Frijns and Kappelle, 2002). Antibodies have been developed to bind to adhesion molecules on the circulating leukocyte membranes (e.g., CD11/CD18) (Rieu et al, 1994) or on their counterpart molecules on the luminal side of the vascular endothelium (e.g., intercellular adhesion molecule-1) (Rothlein et al, 1993).
The first medicament to reach clinical development targeted intercellular adhesion molecule-1, and was shown to be beneficial in several preclinical experimental models and to be tolerated in humans (Bowes et al, 1993, 1995; Schneider et al, 1998). Unfortunately, it led to paradoxical neutrophil activation and hyperthermia. The antibody was found to elicit host antibodies and paradoxically activated neutrophils in later laboratory examinations (Vuorte et al, 1999; Furuya et al, 2001). It activated blood complement (Vuorte et al, 1999), which is common for this isotype of murine IgG2a. However, the trial was completed with the inclusion of 625 patients and resulted in an excess of CNS-related deaths and neurologic worsening in the active treatment arm (Investigators, 2001; Jiang et al, 1998).
Another clinical investigation used the antibody UK-279,276 (neutrophil inhibitory factor), a recombinant glycoprotein with selective binding to the CD11b integrin of CD11b/CD18 (Mac-1), which reduced neutrophil infiltration and infarct volume in transient (2-h) rat MCAo (middle cerebral artery occlusion) models when administered within 4 h after the onset of ischemia (Jiang et al, 1998) and was shown to be tolerated in patients with acute stroke (Lees et al, 2003). The results of this study (N=966) were neutral with regard to therapeutic efficacy, but showed a nonsignificant trend toward favorable outcomes in the subgroup treated with thrombolysis (Krams et al, 2003). The investigators could not reach a conclusion with regard to whether the trial disproves the hypothesis of the efficacy of the antileukocyte approach in acute stroke.
The hypothesis of PMNL-targeted therapies has probably not yet been adequately tested in clinical trials. However, PMNLs as the early wave of phagocytic cells arrive at the scene only after the most immediate ischemic damage has already occurred. It may well be that the tissue volume to be salvaged by blocking the activity of PMNLs is very limited and impossible to detect in clinical outcome measures (Emerich et al, 2002). To continue the debate, translation of preclinical data to clinical trials is considered to be not straightforward (Dirnagl et al, 2007). A key issue in this regard is the need to devote more attention to validating the nominal effect of the interaction between the drug candidate and its molecular target, and its relationship to pharmacodynamic treatment end points. It is equally important to prove that clinical outcomes are linked to the alleged mechanism of action of the compound (Feuerstein et al, 2008).
As the trials targeted to PMNLs have been subjected to criticisms possibly because of being rather late in responding to inflammatory cells in the face of ischemic brain injury (Emerich et al, 2002), more proximal effector cells inciting the inflammation driven by ischemia–reperfusion injury should be sought as pharmacological targets. Immediate hypersensitivity reactions that are well-established physiologic responses to either environmental exogenous or endogenous or direct physico-chemical noxious stimuli constitute an archaic but powerful host defense mechanism. These immediate reactions within various end organs are performed by resident immune cells that are already present in the exterior aspects (such as the epithelia, mucosae, blood vessels) of target organs––tissue MCs––which can also produce an abrupt systemic anaphylactic reaction. This is in contrast to circulating immune cells, which must first be invited by locally released chemotactic substances and anchored by endothelial adhesion molecules before they gradually infiltrate the organs to carry out their purpose.
Mast cells are present and active in the brain
Detection of Tissue Mast Cells
Mast cells are not easily visible in standard histopathological stainings such as hematoxylin and eosin, and therefore they need to be sought after using few specialized stainings. Mast cells arise from a CD34+/CD117+ progenitor cell population (Kirshenbaum et al, 1999) and reside in the bone marrow and peripheral organs, often associated with blood vessels (Figures 1 and 2), nerves, glandular ducts, stromal connective tissue, or mucosa. In contrast to basophils, they characteristically have a round or elongated nucleus and finer but dense cytoplasmic granules ∼1 μm in diameter. Mast cell morphology varies from round or oval to spindle (Figures 1A and 1B). Their abundant cytoplasmic heparin sulfate-containing granules can be shown by metachromatic dyes such as toluidine blue (Figure 2), azures A and B, thionin, and methylene blue, which induce purple or violet staining of the granules. Toluidine blue and Giemsa are the most commonly used staining procedures. Fluorophore-conjugated egg white avidin also binds to heparin and has been used as well. With enzyme cytochemistry, MCs can be detected by the demonstration of Naphthol AS-D chloroacetate esterase activity (Leder staining) (Figure 3), which is lacking in basophils (Horny et al, 2008; Leder, 1964). Safranin and alcian blue stains have been used for showing the immaturity of MCs.
Figure 3.
Chloroacetate esterase (Leder)-stained mast cells in ischemic rat brain. (A) A partially degranulated MC (arrow) located perivascularly; the perivascular edema must be noted. (B) A nondegranulated MC (arrow) within ischemic brain tissue.
In addition to CD117, MCs express various myelomonocytic antigens, including CD68, CD11c, CD 33, CD43, CD45, and C5aR. They can also be immunohistochemically detected by preformed granule-associated mediators such as histamine, heparin, chymase, and tryptase (Figure 1A). In practice, the most sensitive and specific antigen for immunohistochemistry is tryptase; CD117 (Figure 1B) is highly sensitive but is not as specific (Horny et al, 2008).
Mast Cell Trafficking into The Brain
Alterations do appear in the abundance of MCs in the brain, which may be caused by changes in marker-dependent detection, by the rate of both entry and proliferation of precursor cells and the differentiation activity of MCs (Zhuang et al, 1999). It is generally accepted that MCs circulate as devoted precursors rather than as mature cells (Galli et al, 1992; Kitamura et al, 1993). However, what is also possible is that mature MCs translocate from peripheral sources into the CNS (Silverman et al, 2000). In the latter study, migrated donor MCs represent 2% to 20% of the total MC population in the analyzed brain region 1 h after injection, suggesting fast crossing of the BBB. Reconstructions of confocal images showed that MCs were localized deep within the basal lamina, in nests of glial processes. Furthermore, electron microscopic analysis showed that MCs indeed migrate into the CNS (Silverman et al, 1994).
Although fully differentiated MCs and their precursors have been considered to be able to divide (Dvorak et al, 1976), no evidence of MC division was found through 5-bromo-2-deoxyuridine labeling (Silverman et al, 2002), supporting the hypothesis that an increase in end-organ MC population is apparently due to migration from the periphery or entry of new precursors from the circulation. As mature cells MCs do not circulate in the blood, it is suggested that the source of the augmented population is likely to be through the pial sheath of the thalamic blood vessels (Lambracht-Hall et al, 1990; Manning et al, 1994).
Mast Cell Activation in Cerebral Ischemia
In the brain, MCs are typically perivascular (Figures 1 and 2), have substantial granule content, and are frequently observed siding cortical penetrating arterioles (Figure 2). After ischemia, MCs are found more abundantly in the cerebral tissue, where they release their granules, thus leading to permeability change and perivascular edema, as documented in our laboratory in the MCAo model in the Wistar rat (Figure 3A) (Karjalainen-Lindsberg et al, 2001; Strbian et al, 2006). These findings have been confirmed by other laboratories (Hu et al, 2004; Jin et al, 2007). More recently, the rapid recruitment and activation of TNF-α-positive MCs within 1 h after generalized hypoxic–ischemic (HI) challenge has been shown in the neonate rat brain (Jin et al, 2009), although such a rapid MC accumulation has not been documented in other ischemia models. Mast cells recruited into the brain tissue are observed along elongating blood vessels, some of which express the GLUT1 isoform of the glucose transporter protein, indicative of vessels within the BBB (Jin et al, 2007). The potential regulatory role of MCs in vascular permeability is not a novel concept, as this property has been known for several decades (Valent, 2000) and was also shown in the vessels of the CNS by previous studies (Abbott, 2000; Esposito et al, 2001; Yong et al, 1994; Zhuang et al, 1996). However, this literature has focused more on inflammatory, endocrinological, and stress-related disorders in quite distinct animal models.
The Effect of Mast Cells in Focal Ischemic and Hemorrhagic Brain Damage
The finding of the increased presence of MCs and their rapid degranulation in association with morphologic changes of ischemic, edematous brain injury (Figure 3) led us to interventive, ‘proof-of-concept' studies on the role of MCs in the deleterious sequelae of ischemic and hemorrhagic stroke. In the MCAo model, pharmacological MC stabilization with sodium cromoglycate yielded significantly reduced ischemic brain swelling by 40% and BBB leakage by 50%, whereas abrupt MC degranulation induced with a secretagogue compound 48/80 increased these rates by 90 and 50%, respectively (Strbian et al, 2006). These effects probably share pathophysiological mechanisms with traumatic brain injury and its MC-dependent edematous sequelae, which have also been described previously (Lozada et al, 2005; Stokely and Orr, 2008).
The specificity of the above-described effects was supported by investigations using rats born with no MCs, which is a trait induced by a mutation of the c-kit receptor (Kirshenbaum et al, 1999; Tsujimura et al, 1991). In these rats, genetic mutation prevents the action of stem cell factor responsible for MC differentiation. Mast cell deficiency was associated with ∼60% reduction in brain swelling and 50% reduction in BBB leakage to molecules the size of albumin compared with controls (Strbian et al, 2006). These observations have later been replicated in other experiments showing that ischemic brain swelling and BBB leakage are reduced after postischemic administration of tissue plasminogen activator (tPA) by using pharmacological MC stabilization (sodium cromoglycate), and in genotypically MC-deficient rats (Strbian et al, 2007a). Furthermore, in a rat model of intracerebral hemorrhage with autologous blood injection into the basal ganglia, depending on the route of sodium cromoglycate administration, brain edema was reduced by 50% to 75%. Induced MC degranulation (the secretagogue compound 48/80) again produced opposite effects. The dominant role of MCs was supported by congruent findings in genotypically MC-deficient rats (Strbian et al, 2007b).
These results provide compelling evidence of the involvement of MCs in augmenting postischemic BBB leakage and vasogenic edema formation, and also in the regulation of the latter after intracerebral hemorrhage. In the past, the pathophysiology of these phenomena was extensively studied by many research groups in an effort to identify the underlying molecular mechanisms. However, the reviewed data suggested that MCs represent a cell type that might set forth a multitude of downstream pathways. Thus, there are several pathobiologic rationales that would explain a critical role for MCs in these cascades.
Ischemic blood–brain barrier opening—magic bullets or shotgun fire?
Although a prevailing strategy in research devoted to cerebral ischemia has been focused on showing pivotal roles for particular single molecules or receptors in producing direct or delayed vasculopathic brain injury, identification of the cellular sources of molecules such as MMPs and substances that cleave pro-MMPs needs careful clarification. Clearly, astrocytes, macrophages, and pericytes have been identified as dominant sources or activators of several members of the MMP family (Cunningham et al, 2005; Rosenberg et al, 1998). However, instead of dissecting isolated activities of specific molecular mediators in a ‘seamless web of intricately interwoven relationships' (Ratnoff, 1969) produced in ischemic brain injury, a compound effect mounted by an immune cell capable of releasing a multitude of deleterious, vasoactive, and proteolytic mediators might lead us to develop more fruitful therapeutic concepts. In other words, rather than considering brain edema and secondary hemorrhages to be a result of excessive production of single proteins (such as MMP-2 and MMP-9), we believe that a composite effect of biphasic MC activation could regulate a multitude of those vasoactive molecular responses that have been viewed to be central in ischemia–reperfusion injury. To this end, in the following paragraphs, we review data that place MCs within the context of well-known vasculopathic consequences of acute cerebral ischemia.
Mast Cell Proteases and Integrity of the Basal Lamina
Several investigations have linked inflammation to the production of MMPs and other proteases capable of degrading major proteins of the basal lamina (laminin, collagen type IV, and fibronectin), which loses its integrity rapidly after the onset of ischemia (Hamann et al, 1995; Wagner et al, 1997). At present, other investigators have already provided us with ample evidence showing that MMPs (especially MMP-9 and MMP-2) have a pivotal role in BBB damage and acute dissolution of the basal lamina (Asahi et al, 2001; Hamann et al, 1995, 1996; Heo et al, 1999; Lo et al, 2002; Rosenberg et al, 1998; Rosenberg, 2002; Sumii and Lo, 2002). Later research by other investigators has implicated MMP-9 as a biomarker of ischemic brain injury that leads to BBB failure and hemorrhagic transformation (Montaner et al, 2003; Rosell et al, 2006; Tejima et al, 2007). In addition to MMPs, other types of proteases, e.g., cathepsins (Fukuda et al, 2004), were suggested to have a role.
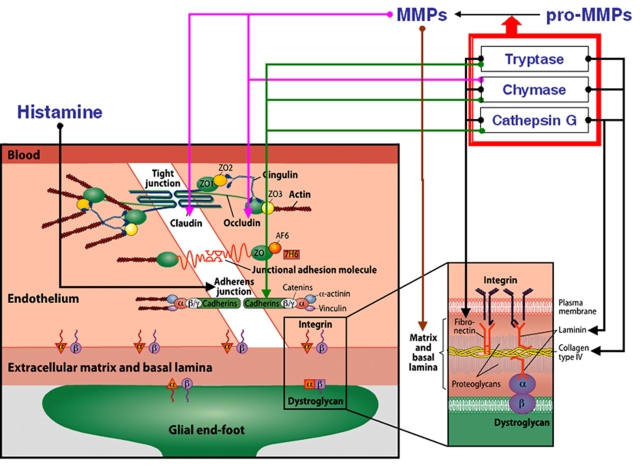
How can MCs disrupt the BBB and degrade components of the basal lamina? Several possible mechanisms are involved, in which the vasoactive and matrix-degrading components of MCs might act in concert. These proposed mechanisms are outlined in Figure 4. Mast cell activation produces a potent vasodilatory mediator, histamine, and contributes up to 90% of its content in the thalamus and up to 50% in the whole brain (Goldschmidt et al, 1985). Hence, of the resident cells in the brain and meninges, only MCs acutely release massive amounts of preformed vasodilatory mediators, such as histamine. Mast cell-borne heparin markedly accelerates the generation of another potent vasoactive substance, bradykinin (Reddigari et al, 1995). Under experimental conditions in vitro, histamine binding to its specific receptors on the endothelial surface leads to increased phosphorylation of the adherens junction and loosening of vascular-endothelial-cadherin-mediated adhesion of adjacent endothelial cells (Andriopoulou et al, 1999; Winter et al, 2004). The same effect can be induced by MC-derived protease tryptase (Itoh et al, 2005), a process that can be aggravated by other MC neutral proteases—chymase and cathepsin G (Schechter et al, 1998). Again, all three of these MC proteases are acutely released from activated MCs along with histamine. Cerebral MC-derived chymase (Dimitriadou et al, 1990) is a potent protease that cleaves fibronectin and activates procollagenases (Saarinen et al, 1994; Tetlow et al, 1998), even in the presence of the MMP-inhibiting protein TIMP-1 (Frank et al, 2001). Indeed, MC-derived chymase can degrade TIMP-1 (Di Girolamo et al, 2006), and a key role for MC chymase in the activation of pro-MMP-2 and pro-MMP-9 was shown by Pejler and colleagues (Tchougounova et al, 2005). Similarly, MC tryptase also activates MMPs (Lohi et al, 1992) and degrades TIMP-1 (Di Girolamo et al, 2006). Once activated, MMPs can degrade most of the protein constituents of the neurovascular matrix, such as collagen, elastin, fibronectin, vitronectin, and gelatin (Yong et al, 2001). Furthermore, MMP-mediated disruption of the tight junction proteins occludin and claudin (Figure 4) was reported in rats (Rosenberg et al, 1998; Yang et al, 2007).
Figure 4.
Illustration of the components of the blood–brain barrier (left) and the basal lamina (right). Arrows represent the possible involvement of MC-derived mediators in blood–brain barrier and basal lamina damage. ECM, extracellular matrix; ZO, zona occludens; AF6, afadin; 7H6, tight junction-associated phosphoprotein; MMP, matrix metalloproteinase. Modified with permission from Ann Med 2009; 41:438–450.
Importantly, MCs themselves can release gelatinase A (MMP-2) and gelatinase B (MMP-9) (Fang et al, 1999). Cathepsin G, a neutral protease present in some MCs, cleaves many components of the extracellular matrix and pericellular matrix, including fibronectin and vitronectin (Helske et al, 2006; Mäyränpää et al, 2006). The role of cathepsins in microvascular matrix degradation after focal cerebral ischemia has been shown (Fukuda et al, 2004). Finally, apart from proteases, MCs can release TNF-α instantly from its preformed granules and interleukin (IL)-1; both of these cytokines are involved in BBB failure and in ischemic brain edema formation (Kim et al, 1992; Yamasaki et al, 1992). Indeed, MCs do possess a potent armamentarium for targeting the key constituents of the BBB and basal lamina (Figure 4) shortly after their activation, whereas de novo production of these and additional mediators can reactivate and maintain this process that is originally aimed at serving as a host response against intruding exogenous agents.
Mast Cells and Fibrinolysis
Hemorrhage formation, either spontaneous or iatrogenic (in association with thrombolytic therapy with tPA), can devastate the outcome after successful vessel recanalization. Investigators searching for improved control of unwanted fibrinolysis during blood-clot-lysing therapy may have to take a closer look at MCs, which have been considered essentially as a fibrinolytic cell type (Valent, 2000). To this end, in vivo experiments in rats that underwent focal cerebral ischemia–reperfusion and postischemic tPA administration and no MC modulation showed a 70- to 100-fold increase in the area of hemorrhage formation compared with rats treated with vehicle (Strbian et al, 2007a). Pharmacological MC stabilization with sodium cromoglycate led to a significant reduction in tPA-mediated hemorrhage formation at 3 (97%), 6 (76%), and 24 h (96%) compared with controls (Strbian et al, 2007a). Moreover, genotypically MC-deficient rats also showed a robust reduction in tPA-mediated hemorrhage compared with their wild-type MC-competent littermates. Finally, the clinically used tPA preparation was found to cause by itself a robust degranulation of MCs in vitro, as shown by histamine release (Strbian et al, 2007a), which is a finding not reported for any other fibrinolytic drug so far.
The mechanism of postthrombolytic hemorrhage formation still awaits full understanding. It is interesting to note that it occurs with any of the fibrinolytic substances used clinically, namely tPA, streptokinase, prourokinase, and reteplase (Furlan et al, 1999; Qureshi et al, 2001). They all are potent serine proteases, which, in addition to their local plasminogen-activating effect to dissolve fibrin locally, also induce systemic plasminemia (Sobel, 1994). Plasmin, again, has an independent proinflammatory activity that causes direct brain tissue damage (Asahi et al, 2000; Xue and Del Bigio, 2001) and, in addition, by activating MMPs it degrades a range of extracellular matrix proteins (Castellino, 1998). Interestingly, inhibition of MMPs reduced tPA-mediated mortality in experimental ischemia–reperfusion (Pfefferkorn and Rosenberg, 2003).
Perhaps even more important in the setting of thrombolytic therapy of ischemic stroke is the observation that the development of a hemorrhage may be further augmented and prolonged by heparin. This mediator is acutely released by activated MCs, which are the only source of this strong anticoagulant (Lane and Björk, 1992; Rosenberg and Bauer, 1994). Heparin release from perivascularly positioned MCs might impair hemostasis and prevent plugging of BBB breaches. In theory, endogenously released heparin may accelerate erythrocyte extravasation and contribute to the formation of frank hemorrhages. In dogs bearing MC tumors, intense activation of the fibrinolytic system has been observed after injection of minute amounts of the MC-degranulating agent 48/80 (Ende and Auditore, 1964). In addition to the fact that besides heparin, tPA also belongs to the palette of MC-derived anticoagulant mediators (Valent, 2000), these data confirm the antithrombotic and fibrinolytic potential of MCs. We believe that the reviewed data support a hypothesis that MC activation could participate in the formation of vicious, space-occupying intracerebral hematomas (spontaneous or tPA-mediated), as well as less dangerous hemorrhagic transformations.
Mast cells and brain protection
Effects in the Immature Brain
In the rodent brain with excitotoxic lesions mimicking those described in human HI brain injury (cerebral palsy), IL-9 pretreatment exacerbated brain damage produced by intracerebral injections of the glutamatergic analog ibotenate (Dommergues et al, 2000). Among its different cell targets, the Th2 cytokine IL-9 is an MC growth and differentiation factor that can cause MC degranulation. Interleukin-9 pretreatment had no significant effect on ibotenate-induced excitotoxic brain lesions in genotypically MC-deficient mice, whereas IL-9 exacerbated these lesions in wild-type littermates. Cromoglycate or antihistamine drugs significantly reduced ibotenate-induced brain lesions in IL-9-treated mice (Patkai et al, 2001). In a retrospective study of children with cerebral palsy, higher levels of several perinatal circulating cytokines, including IL-9, were detected (Nelson et al, 1998).
Hypoxic–ischemic brain damage in immature rats led to a rapid increase in the cerebral population of MCs along with their activation (Jin et al, 2007). In that study, activated MCs were shown in the pia, brain parenchyma, and, importantly, in regions showing neuronal loss. Mast cell stabilization with cromoglycate reduced the MC count in the latter region and limited brain damage by more than 50% compared with controls. In the same study, it was noted that only HI—but not hypoxia alone—led to brain damage, suggesting that a tissue-level metabolic or inflammatory challenge is required. Another study (Biran et al, 2008) showed the contribution of MC-derived histamine to ischemia-induced neuronal death in P7 newborn rats. Another important finding was that MC-associated genes were upregulated after HI brain damage (Hedtjarn et al, 2004). Most recently, cerebral MC counts and their activation were shown to be elevated immediately after HI before detection of cleaved caspase-3 in neurons (NeuN+ 2 h after HI), astroglial activation (GFAP+ with swollen cell body, 4 h after HI), or microglial activation (OX42+, 4 h after HI) (Jin et al, 2009). Tumor necrosis factor-α-positive MCs were present in a subpopulation of MCs in control animals, and the fraction of TNF-α-positive MCs increased dramatically ipsilaterally immediately after HI. Microglial TNF-α was evident at 4 h, but endothelial cells had no detectable TNF-α until 48 h after HI. Tumor necrosis factor-α was implicated in the generation of early inflammatory and neurotoxic effects. Cromoglycate prevented MC migration, reduced brain damage/neuronal loss, glial activation, and brain atrophy through 4 weeks of recovery (Jin et al, 2009). In summary, these data support the observation that MC activation may have a significant detrimental effect during neonatal HI brain damage. In these models, MCs seem to be early responders with their preformed TNF-α pools, and MC stabilizing treatments seem a promising novel neuroprotective avenue to prevent neonatal brain injuries.
Effects in the Adult Brain
Although the features of cerebral palsy share the morphologic characteristics of ischemic and HI cortical damage (Gressens et al, 1997; Marret et al, 1995), similar evidence of neuroprotection by MC stabilization in ischemic brain damage is scarce. Sodium cromoglycate probably does not possess any intrinsic neuroprotective activity. Any neuroprotective net effect would probably be mediated by prevention of the release of neurotoxic mediators, such as TNF-α, and reduced inflammation and vasculopathic effects, such as BBB damage and edema. Importantly, inhibition of tPA-mediated hemorrhage formation with this drug led to better neurologic outcomes and reduced mortality up to 24 h after MCAo (Strbian et al, 2006, 2007a, 2007b). Genotypically MC-deficient rats also had significantly better neurologic outcomes than did wild-type littermates. In line, a beneficial effect of MC stabilization and MC deficiency was also found in the model of intracerebral hemorrhage (Strbian et al, 2007b). Despite these findings, infarct sizes were unchanged with and without cromoglycate treatment (Strbian et al, 2006), as well as in gene-manipulated rats.
To reconcile the above evidence, we believe that there are differences between immature and adult rodent brain in vulnerability to ischemic or HI damage, and in their responsiveness to MC stabilization. Furthermore, intraperitoneal cromoglycate administration was shown to be neuroprotective in the immature mice brain (Jin et al, 2007, 2009), whereas in adult rats, the drug had to be administered intraventricularly to be efficacious. An exception was the model of intracerebral hemorrhage, in which also the intravenous route of administration was effective in reducing edema, mortality, and neurologic recovery (Strbian et al, 2007b). Obviously, immature BBB and permeability to circulating macromolecules in the immature brain can account for this difference. Future studies should be directed to the therapeutic potential of MC stabilization, regardless of whether it is dominantly dependent on direct neuroprotection or secondary brain protection through reduction of microcirculatory failure and expansive edema.
Mast cells in cerebral micromilieu
Interplay and Crosstalk With Endothelial and Perivascular Cells
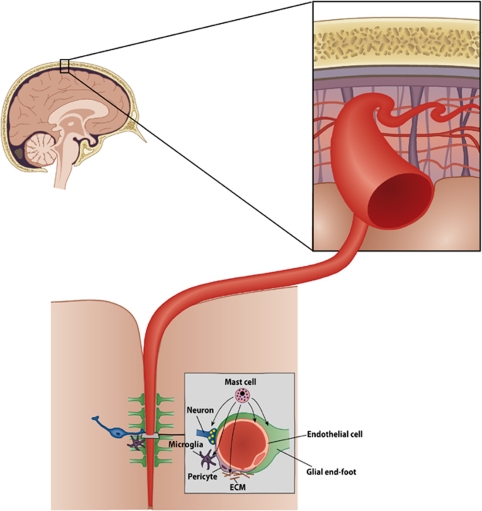
A conceptual framework, ‘the neurovascular unit' (Figure 5), has been proposed, which consists of vascular and perivascular structures and cells; microvessels (endothelial cells––basal lamina matrix––astrocyte end-feet and pericytes), astrocytes, neurons and their axons, in addition to other supporting cells (e.g., microglia and oligodendroglia) (del Zoppo, 2009) (Figure 5). The endothelial cell is viewed central to the NVU, but its function is regulated by crosstalk between neighboring astrocyte end-feet, neurons, and pericytes (del Zoppo, 2009; Simard et al, 2003). The extracellular matrix and the matrix-degrading enzymes and their inhibitors have a key role at the basal lamina and the cell surface in the regulation of cell signaling (Cunningham et al, 2005).
Figure 5.
Schematic illustration of the cellular elements of the neurovascular unit. Modified with permission from Ann Med 2009; 41:438–450.
In addition, MCs have recently been viewed to be involved in several types of interactions within the NVU (del Zoppo, 2009), the various components of which together with their complex crosstalk secure the integrity and homeostasis of the microvasculature. Mast cells possess a palette of mediators that could participate in the fine-tuning of the microcirculatory and metabolic milieu nurtured within an NVU—histamine in regulating the degree of vasodilation and bidirectional permeability to circulating or extracellular substances—heparin and tPA in regulating the balance between hemostasis and fibrinolysis—and TNF-α in regulating inflammatory changes, such as expression of adhesion molecules and chemotactic signaling. Early release of chemotactic signals and facilitation of BBB permeation could pave the way for circulating phagocytic cells necessary for clearance of noxious substances and cell debris. Theories such as these need to be addressed in studies yet to come, but in the following paragraphs, we review shortly what is presently known of MCs and their cellular interactions with dominant cells within the NVU (Figure 5).
Astrocytes
During development, association of yet undifferentiated MCs with the vascular bed (preferentially at branching points) is dependent on the contact of the blood vessel wall with astroglial processes, which involves α4-integrins expressed by MCs (Khalil et al, 2007). Adjacent astrocytes can influence the differentiation, the eventual tissue-specific phenotype, and the migration of MCs (Dimitriadou et al, 1996), presumably by synthesizing MC growth factors such as IL-3 (Farrar et al, 1989; Frei et al, 1985) and nerve growth factor (Carman-Krzan et al, 1991). Furthermore, mature MCs can be grown on astrocytes (Johnson and Krenger, 1992; Shalit et al, 1993), and astroglial processes elongate in close proximity to MCs (Shanas et al, 1998).
Neurons
Mast cell products enter neurons in at least three ways (transgranulation) in the dove brain: (Wilhelm et al, 2005) (1) direct fusion of the granule and plasma membranes of both MCs and neurons, (2) engulfment of MC processes containing granules or capture of released granule remnants, and (3) receptor-mediated endocytosis. Interestingly, the frequency of transgranulation events is related to the activity status of the MCs.
Endothelial Cells
Changes in endothelial cell–matrix interactions may be influenced by TNF-α and IL-1β (del Zoppo and Mabuchi, 2003), most likely by the downregulation of integrin receptors of the β1 subfamily (Defilippi et al, 1992). Accordingly, TNF-α reduces the integrin α1β1 expression of ECs, leading to decreased adhesion to laminin (Defilippi et al, 1992), whereas IL-1β contributes to early ischemic brain edema, presumably by altering β1 expression (Yamasaki et al, 1992). Both cytokines can be released by different cells, but are included among MC mediators as well.
Basal Lamina and Extracellular Matrix
Mast cells can attach to and migrate on laminin- and fibronectin-coated surfaces (Thompson et al, 1993). Furthermore, surface receptors of MCs (one of them for laminin) regulate MC trafficking and distribution by engaging extracellular matrix components, including the classical integrin receptors (Metcalfe, 1995).
These data suggest that biologically and pathobiologically meaningful crosstalk exists between MCs and the NVU. Finally, one should recognize the large pool of MCs resident within the meninges (largely pial in the developing CNS and dural in the adult). The effects of MCs within this compartment might be dominant in the early phase of catastrophic space-occupying or lacerating ischemic, hemorrhagic, and traumatic brain injuries.
What Triggers Mast Cell Responses in the Ischemic Brain?
Ischemic and hemorrhagic stroke are catastrophic situations to be managed merely locally by the NVU (Figure 5), which subserves its ‘neurosphere' under physiologic conditions. We raise the possibility that the sudden cessation of blood circulation and rapid accumulation of waste products and reduction of pH probably trigger MCs to degranulate, and they act as a ‘fast response force' to tackle the noxious, nonhomeostatic micromilieu within the NVU. Mast cells are well known to respond and degranulate rather stereotypically on a multitude of physico-chemical challenges, such as ionizing irradiation, trauma, toxic substances, allergens, complement activation, thermal challenges, hyperosmolality, and pH changes. Therefore, sudden dense ischemia, the release of, e.g., toxic-free hemoglobin or activated serum complement proteins, such as anaphylatoxins (C3a, C5a) into the abluminal interstitial space, could well represent one candidate for sufficient noxious stimuli to which MCs in the affected brain area respond by rapid degranulation. This is an interesting subject for further study.
Although brain tissues are known to possess MCs scattered within the cerebral cortex and basal ganglia, they are found much more numerously in the meningeal tissues (Figure 1C). This pool of MCs has been shown to lead to edematous cerebral changes in models of traumatic brain injury (Stokely and Orr, 2008). Less disrupting metabolic or irritating challenges such as stress and migraine-type models are associated with spontaneous MC degranulation within leptomeningeal or cerebral tissue (Esposito et al, 2001; Reuter et al, 2001). We find it plausible that, after a major stroke or hemorrhage, early activation and degranulation of dural and pial MCs ensue and participate in the process occurring within the intracranial vault, leading to aggravation of BBB damage and expansive brain edema. Collateral circulation from the leptomeninges could pass MC-derived mediators humorally into the brain tissue proper to exert the deleterious effects described above. However, at present, these suggestions remain exciting research areas for future investigations.
Conclusions
The main aim of this review is to shed light on the potentially significant, yet not sufficiently realized, role of MCs in mounting rapid host responses within different structures of the CNS, especially the cerebral cortex, basal ganglia, and the meninges. Through several decades, similar responses have been amply described in other organs, and we are aware of the potent, acute effects of MC degranulation and activation apart from the CNS. We have now reviewed a body of recent literature that provides rather compelling yet largely experimental evidence for hypothesizing a role for cerebral MCs in influencing BBB permeability, space-occupying brain edema, neuroprotection, and important outcome variables such as preservation of neurologic function and survival after HI, ischemic, and hemorrhagic brain damage. However, the bulk of the reviewed material remains at the level of ‘proof-of-concept' study.
Future studies on this subject should include a more detailed dissection of the pathobiological mechanisms underlying MC-mediated BBB disruption, as well as more rigorous elucidation of potential beneficial effects of MC-stabilizing agents given in therapeutic, postinsult protocols. Given the promiscuity of MC-dependent responses in allergic, inflammatory, and additional hypersensitivity reactions throughout the body, vast literature has been accumulated to support the stabilization of MCs in helping various therapeutic regimens. Stabilization of MCs is obviously amenable also for novel purposes within the CNS using familiar drugs used for several decades, such as sodium cromoglycate. Perhaps the most immediate area of further work in stroke research would be the development of pharmacological compositions to be entered in clinical assessment of fibrinolytic therapies to minimize the risk of threatening hemorrhagic complications. In the field of basic research, a more thorough understanding of MC-related phenomena in the crosstalk with components of the NVU will probably reveal exciting novel perspectives on physiologic and pathobiologic regulation of the microvascular milieu in the CNS.
Acknowledgments
Financial support has been received from the Finnish Academy (PJL), Sigrid Juselius foundation (PJL) and the Helsinki University Hospital District (EVO) funds (PJL).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Abbott NJ. Inflammatory mediators and modulation of blood–brain barrier permeability. Cell Mol Neurobiol. 2000;20:131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriopoulou P, Navarro P, Zanetti A, Lampugnani MG, Dejana E. Histamine induces tyrosine phosphorylation of endothelial cell-to-cell adherens junctions. Arterioscler Thromb Vasc Biol. 1999;19:2286–2297. doi: 10.1161/01.atv.19.10.2286. [DOI] [PubMed] [Google Scholar]
- Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarian L, Yousefzadeh E, Silverman AJ, Silver R. Stimuli from conspecifics influence brain mast cell population in male rats. Horm Behav. 2002;42:1–12. doi: 10.1006/hbeh.2002.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KB. Anti-leukocyte antibodies: LekArrest (Hu23F2G) and Enlimomab (R6.5) in acute stroke. Curr Med Res Opin. 2001;18:s18–s22. doi: 10.1185/030079902125000688. [DOI] [PubMed] [Google Scholar]
- Biran V, Cochois V, Karroubi A, Arrang JM, Charriaut-Marlangue C, Heron A. Stroke induces histamine accumulation and mast cell degranulation in the neonatal rat brain. Brain Pathol. 2008;18:1–9. doi: 10.1111/j.1750-3639.2007.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes MP, Rothlein R, Fagan SC, Zivin JA. Monoclonal antibodies preventing leukocyte activation reduce experimental neurologic injury and enhance efficacy of thrombolytic therapy. Neurology. 1995;45:815–819. doi: 10.1212/wnl.45.4.815. [DOI] [PubMed] [Google Scholar]
- Bowes MP, Zivin JA, Rothlein R. Monoclonal antibody to the ICAM-1 adhesion site reduces neurological damage in a rabbit cerebral embolism stroke model. Exp Neurol. 1993;119:215–219. doi: 10.1006/exnr.1993.1023. [DOI] [PubMed] [Google Scholar]
- Brain S, Lewis GP, Whittle BJR. Actions of phospolipase-A on mast-cell histamine release and paw oedema in the rat. Br J Pharmacol. 1977;59 (Suppl:440–441. [PMC free article] [PubMed] [Google Scholar]
- Carman-Krzan M, Vige X, Wise BC. Regulation by interleukin-1 of nerve growth factor secretion and nerve growth factor mRNA expression in rat primary astroglial cultures. J Neurochem. 1991;56:636–643. doi: 10.1111/j.1471-4159.1991.tb08197.x. [DOI] [PubMed] [Google Scholar]
- Castellino FJ.1998Plasmin Handbook of Proteolytic Enzymes(Barrett AJ, Rawlings ND, Woessner JF, eds),San Diego: California Academic Press; 190–199. [Google Scholar]
- Costa JJ, Weller PF, Galli SJ. The cells of the allergic response: mast cells, basophils, and eosinophils. JAMA. 1997;278:1815–1822. [PubMed] [Google Scholar]
- Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50:329–339. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- Defilippi P, Silengo L, Tarone G. Alpha 6.beta 1 integrin (laminin receptor) is down-regulated by tumor necrosis factor alpha and interleukin-1 beta in human endothelial cells. J Biol Chem. 1992;267:18303–18307. [PubMed] [Google Scholar]
- del Zoppo GJ. Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience. 2009;158:972–982. doi: 10.1016/j.neuroscience.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- Di Girolamo N, Indoh I, Jackson N, Wakefield D, McNeil HP, Yan W, Geczy C, Arm JP, Tedla N. Human mast cell-derived gelatinase B (matrix metalloproteinase-9) is regulated by inflammatory cytokines: role in cell migration. J Immunol. 2006;177:2638–2650. doi: 10.4049/jimmunol.177.4.2638. [DOI] [PubMed] [Google Scholar]
- Dimitriadou V, Lambracht-Hall M, Reichler J, Theoharides TC. Histochemical and ultrastructural characteristics of rat brain perivascular mast cells stimulated with compound 48/80 and carbachol. Neuroscience. 1990;39:209–224. doi: 10.1016/0306-4522(90)90234-u. [DOI] [PubMed] [Google Scholar]
- Dimitriadou V, Rouleau A, Tuong MD, Ligneau X, Newlands GF, Miller HR, Schwartz JC, Garbarg M. Rat cerebral mast cells undergo phenotypic changes during development. Brain Res Dev Brain Res. 1996;97:29–41. doi: 10.1016/s0165-3806(96)00127-7. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Klehmet J, Braun JS, Harms H, Meisel C, Ziemssen T, Prass K, Meisel A. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38:770–773. doi: 10.1161/01.STR.0000251441.89665.bc. [DOI] [PubMed] [Google Scholar]
- Dommergues MA, Patkai J, Renauld JC, Evrard P, Gressens P. Proinflammatory cytokines and interleukin-9 exacerbate excitotoxic lesions of the newborn murine neopallium. Ann Neurol. 2000;47:54–63. [PubMed] [Google Scholar]
- Dropp JJ. Mast cells in mammalian brain. Acta Anat. 1976;94:1–21. doi: 10.1159/000144540. [DOI] [PubMed] [Google Scholar]
- Dvorak AM, Mihm MC, Jr, Dvorak HF. Morphology of delayed-type hypersensitivity reactions in man.II.Ultrastructural alterations affecting the microvasculature and the tissue mast cells. Lab Invest. 1976;34:179–191. [PubMed] [Google Scholar]
- Emerich DF, Dean RL, Bartus RT. The role of leukocytes following cerebral ischemia: pathogenic variable or bystander reaction to emerging infarct. Exp Neurol. 2002;173:168–181. doi: 10.1006/exnr.2001.7835. [DOI] [PubMed] [Google Scholar]
- Ende N, Auditore JV. Activation of a fibrinolytic system in a dog with mast cell tumor. Am J Physiol. 1964;206:567–572. doi: 10.1152/ajplegacy.1964.206.3.567. [DOI] [PubMed] [Google Scholar]
- Esposito P, Gheorghe D, Kandere K, Pang X, Connoly R, Jacobson S, Theoharides TC. Acute stress increases permeability of the blood-brain barrier through activation of brain mast cells. Brain Res. 2001;888:117–127. doi: 10.1016/s0006-8993(00)03026-2. [DOI] [PubMed] [Google Scholar]
- Fang KC, Wolters PJ, Steinhoff M, Bidgol A, Blount JL, Caughey GH. Mast cell expression of gelatinases A and B is regulated by kit ligand and TGF-beta. J Immunol. 1999;162:5528–5535. [PubMed] [Google Scholar]
- Farrar WL, Vinocour M, Hill JM. In situ hybridization histochemistry localization of interleukin-3 mRNA in mouse brain. Blood. 1989;73:137–140. [PubMed] [Google Scholar]
- Feuerstein GZ, Zaleska MM, Krams M, Wang X, Day M, Rutkowski JL, Finklestein SP, Pangalos MN, Poole M, Stiles GL, Ruffolo RR, Walsh FL. Missing steps in the STAIR case: a translational medicine perspective on the development of NXY-059 for treatment of acute ischemic stroke. J Cereb Blood Flow Metab. 2008;28:217–219. doi: 10.1038/sj.jcbfm.9600516. [DOI] [PubMed] [Google Scholar]
- Florenzano F, Bentivoglio M. Degranulation, density, and distribution of mast cells in the rat thalamus: a light and electron microscopic study in basal conditions and after intracerebroventricular administration of nerve growth factor. J Comp Neurol. 2000;424:651–669. [PubMed] [Google Scholar]
- Frank BT, Rossall JC, Caughey GH, Fang KC. Mast cell tissue inhibitor of metalloproteinase-1 is cleaved and inactivated extracellularly by alpha-chymase. J Immunol. 2001;166:2783–2792. doi: 10.4049/jimmunol.166.4.2783. [DOI] [PubMed] [Google Scholar]
- Frei K, Bodmer S, Schwerdel C, Fontana A. Astrocytes of the brain synthesize interleukin 3-like factors. J Immunol. 1985;135:4044–4047. [PubMed] [Google Scholar]
- Frijns CJ, Kappelle LJ. Inflammatory cell adhesion molecules in ischemic cerebrovascular disease. Stroke. 2002;33:2115–2122. doi: 10.1161/01.str.0000021902.33129.69. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Fini CA, Mabuchi T, Koziol JA, Eggleston LL, Jr, del Zoppo GJ. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke. 2004;35:998–1004. doi: 10.1161/01.STR.0000119383.76447.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan AJ, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, Pessin M, Ahuja A, Callahan F, Clark WM, Silver F, Rivera F. Intra-arterial prourokinase for acute ischemic stroke: the PROACT II study-A randomized controlled trial: prolyse in acute cerebral thromboembolism. JAMA. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- Furuya K, Takeda H, Azhar S, McCarron RM, Chen Y, Ruetzler CA, Wolcott KM, DeGraba TJ, Rothlein R, Hugli TE, del Zoppo GJ, Hallenbeck JM. Examination of several potential mechanisms for the negative outcome in a clinical stroke trial of enlimomab, a murine anti-human intercellular adhesion molecule-1 antibody: a bedside-to-bench study. Stroke. 2001;32:2665–2674. doi: 10.1161/hs3211.098535. [DOI] [PubMed] [Google Scholar]
- Galli SJ. New concepts about the mast cell. N Engl J Med. 1993;328:257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Gordon JR, Wershil BK. Cytokine production by mast cells and basophils. Curr Opin Immunol. 1991;3:865–873. doi: 10.1016/s0952-7915(05)80005-6. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Wershil BK, Gordon JR, Tsai M, Hammel I.1992Insight into mast cell development and function derived from analyses of mice carrying mutations at beige, W/c-kit, or SI/SCF (c-kit ligand) loci The Mast Cell in Health and Disease(Kaliner MA, Metcalfe DD, eds)New York: Marcel Dekker Inc; 109–128. [Google Scholar]
- Goldschmidt RC, Hough LB, Glick SD. Rat brain mast cells: contribution to brain histamine levels. J Neurochem. 1985;44:1943–1947. doi: 10.1111/j.1471-4159.1985.tb07191.x. [DOI] [PubMed] [Google Scholar]
- Goldschmidt RC, Hough LB, Glick SD, Padawer J. Mast cells in rat thalamus: nuclear localization, sex difference and left-right asymmetry. Brain Res. 1984;323:209–217. doi: 10.1016/0006-8993(84)90291-9. [DOI] [PubMed] [Google Scholar]
- Gordon JR, Galli SJ. Release of both preformed and newly synthesized tumor necrosis factor alpha (TNF-alpha)/cachectin by mouse mast cells stimulated via the Fc epsilon RI. A mechanism for the sustained action of mast cell-derived TNF-alpha during IgE-dependent biological responses. J Exp Med. 1991;174:103–107. doi: 10.1084/jem.174.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressens P, Marret S, Hill JM, Brenneman DE, Gozes I, Fridkin M, Evrard P. Vasoactive intestinal peptide prevents excitotoxic cell death in the murine developing brain. J Clin Invest. 1997;100:390–397. doi: 10.1172/JCI119545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths MR, Gasque P, Neal JW. The multiple roles of the innate immune system in the regulation of apoptosis and inflammation in the brain. Neuropathol Exp Neurol. 2009;68:217–226. doi: 10.1097/NEN.0b013e3181996688. [DOI] [PubMed] [Google Scholar]
- Hallenbeck J, Del Zoppo G, Jacobs T, Hakim A, Goldman S, Utz U, Hasan A. Immunomodulation strategies for preventing vascular disease of the brain and heart: workshop summary. Stroke. 2006;37:3035–3042. doi: 10.1161/01.STR.0000248836.82538.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann GF, Okada Y, del Zoppo GJ. Hemorrhagic transformation and microvascular integrity during focal cerebral ischemia/reperfusion. J Cereb Blood Flow Metab. 1996;16:1373–1378. doi: 10.1097/00004647-199611000-00036. [DOI] [PubMed] [Google Scholar]
- Hamann GF, Okada Y, Fitridge R, del Zoppo GJ. Microvascular basal lamina antigens disappear during cerebral ischemia and reperfusion. Stroke. 1995;26:2120–2126. doi: 10.1161/01.str.26.11.2120. [DOI] [PubMed] [Google Scholar]
- Hedtjarn M, Mallard C, Hagberg H. Inflammatory gene profiling in the developing mouse brain after hypoxia-ischemia. J Cereb Blood Flow Metab. 2004;24:1333–1351. doi: 10.1097/01.WCB.0000141559.17620.36. [DOI] [PubMed] [Google Scholar]
- Helske S, Syväranta S, Kupari M, Lappalainen J, Laine M, Lommi J, Turto H, Mäyränpää M, Werkkala K, Kovanen PT, Lindstedt KA. Possible role for mast cell-derived cathepsin G in the adverse remodelling of stenotic aortic valves. Eur Heart J. 2006;27:1495–1504. doi: 10.1093/eurheartj/ehi706. [DOI] [PubMed] [Google Scholar]
- Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:624–633. doi: 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- Horny H-P, Metcalfe DD, Bennett JM, Bain BJ.2008Mastocytosis WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues(Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, eds), 4th edn, Lyon: WHO Press, IARC, pp. 54–63 [Google Scholar]
- Hough L. Cellular localization and possible functions for brain histamine: recent progress. Prog Neurobiol. 1988;30:469–505. doi: 10.1016/0301-0082(88)90032-9. [DOI] [PubMed] [Google Scholar]
- Hu W, Xu L, Pan J, Zheng X, Chen Z. Effect of cerebral ischemia on brain mast cells in rats. Brain Res. 2004;1019:275–280. doi: 10.1016/j.brainres.2004.05.109. [DOI] [PubMed] [Google Scholar]
- ICOS Press release 9/26/97, 1/12/00, 4/20/00
- Investigators Use of anti-ICAM-1 therapy in ischemic stroke: results of the enlimomab acute stroke trial. Neurology. 2001;57:1428–1434. doi: 10.1212/wnl.57.8.1428. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Sendo T, Oishi R. Physiology and pathophysiology of proteinase-activated receptors (PARs): role of tryptase/PAR-2 in vascular endothelial barrier function. J Pharmacol Sci. 2005;97:14–19. doi: 10.1254/jphs.fmj04005x3. [DOI] [PubMed] [Google Scholar]
- Jiang N, Chopp M, Chahwala S. Neutrophil inhibitory factor treatment of focal cerebral ischemia in the rat. Brain Res. 1998;788:25–34. doi: 10.1016/s0006-8993(97)01503-5. [DOI] [PubMed] [Google Scholar]
- Jin Y, Silverman AJ, Vannucci SJ. Mast cell stabilization limits hypoxic-ischemic brain damage in the immature rat. Dev Neurosci. 2007;29:373–384. doi: 10.1159/000105478. [DOI] [PubMed] [Google Scholar]
- Jin Y, Silverman AJ, Vannucci SJ. Mast cells are early responders after hypoxia-ischemia in immature rat brain. Stroke. 2009;40:3107–3112. doi: 10.1161/STROKEAHA.109.549691. [DOI] [PubMed] [Google Scholar]
- Johnson D, Krenger W. Interactions of mast cells with the nervous system—recent advances. Neurochem Res. 1992;9:939–951. doi: 10.1007/BF00993271. [DOI] [PubMed] [Google Scholar]
- Joo F, Kovacs J, Szerdahelyi P, Temesvari P, Tosaki A. The role of histamine in brain oedema formation. Acta Neurochir Suppl (Wien) 1994;60:76–78. doi: 10.1007/978-3-7091-9334-1_19. [DOI] [PubMed] [Google Scholar]
- Karjalainen-Lindsberg M-L, Tatlisumak T, Lindsberg PJ.2001Mast cells in ischemic rat brain Soc Neurosci AbstrNo:330.11
- Khalil M, Ronda J, Weintraub M, Jain K, Silver R, Silverman AJ. Brain mast cell relationship to neurovasculature during development. Brain Res. 2007;1171:18–29. doi: 10.1016/j.brainres.2007.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Wass CA, Cross AS, Opal SM. Modulation of blood-brain barrier permeability by tumor necrosis factor and antibody to tumor necrosis factor in the rat. Lymphokine Cytokine Res. 1992;11:293–298. [PubMed] [Google Scholar]
- Kirshenbaum AS, Goff JP, Semere T, Foster B, Scott LM, Metcalfe DD. Demonstration that human mast cells arise from a progenitor cell population that is CD34+, c-kit+, and expresses aminopeptidase N (CD13) Blood. 1999;94:2333–2342. [PubMed] [Google Scholar]
- Kitamura Y, Kasugai T, Arizono N, Matsuda H. Development of mast cells and basophils: processes and regulation mechanisms. Am J Med Sci. 1993;306:185–191. doi: 10.1097/00000441-199309000-00011. [DOI] [PubMed] [Google Scholar]
- Krams M, Lees K, Hacke W, Grieve A, Orgogozo J, Ford G. Acute stroke therapy by inhibition of neutrophils (ASTIN): an adaptive dose-response study of UK-279,276 in acute ischemic stroke. Stroke. 2003;34:2543–2548. doi: 10.1161/01.STR.0000092527.33910.89. [DOI] [PubMed] [Google Scholar]
- Lambracht-Hall M, Dimitriadou V, Theoharides TC. Migration of mast cells in the developing rat brain. Brain Res Dev Brain Res. 1990;56:151–159. doi: 10.1016/0165-3806(90)90077-c. [DOI] [PubMed] [Google Scholar]
- Lane DA, Björk I.1992Heparin and Related PolysaccharidesVol. 313Springer: New York; p.388 [Google Scholar]
- Leder L-D. Ueber die selective fermentcytochemische Darstellung neutrophiller myeloischer Zellen und Gewebsmastzellen im Parafinschnitt. Wien Klin Wochenschr. 1964;42:553. doi: 10.1007/BF01486688. [DOI] [PubMed] [Google Scholar]
- Lees KR, Diener H-C, Asplund K, Krams M. UK-279,276, a neutrophil inhibitory glycoprotein, in acute stroke: tolerability and pharmacokinetics. Stroke. 2003;34:1704–1709. doi: 10.1161/01.STR.0000078563.72650.61. [DOI] [PubMed] [Google Scholar]
- Letourneau R, Rozniecki JJ, Dimitriadou V, Theoharides TC. Ultrastructural evidence of brain mast cell activation without degranulation in monkey experimental allergic encephalomyelitis. J Neuroimmunol. 2003;145:18–26. doi: 10.1016/j.jneuroim.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Lo EH, Wang X, Cuzner ML. Extracellular proteolysis in brain injury and inflammation: role for plasminogen activators and matrix metalloproteinases. J Neurosci Res. 2002;69:1–9. doi: 10.1002/jnr.10270. [DOI] [PubMed] [Google Scholar]
- Lohi J, Harvima I, Keski-Oja J. Pericellular substrates of human mast cell tryptase: 72,000 Dalton gelatinase and fibronectin. J Cell Biochem. 1992;50:337–349. doi: 10.1002/jcb.240500402. [DOI] [PubMed] [Google Scholar]
- Lozada A, Maegele M, Stark H, Neugebauer EMA, Panula P. Traumatic brain injury results in mast cell increase and changes in regulation of central histamine receptors. Neuropathol Appl Neurobiol. 2005;31:150–162. doi: 10.1111/j.1365-2990.2004.00622.x. [DOI] [PubMed] [Google Scholar]
- Manning KA, Pienkowski TP, Uhlrich DJ. Histaminergic and non-histamine-immunoreactive mast cells within the cat lateral geniculate complex examined with light and electron microscopy. Neuroscience. 1994;63:191–206. doi: 10.1016/0306-4522(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Marret S, Mukendi R, Gadisseux JF, Gressens P, Evrard P. Effect of ibotenate on brain development: an excitotoxic mouse model of microgyria and posthypoxic-like lesions. J Neuropathol Exp Neurol. 1995;54:358–370. doi: 10.1097/00005072-199505000-00009. [DOI] [PubMed] [Google Scholar]
- Mäyränpää MI, Heikkilä HM, Lindstedt KA, Walls AF, Kovanen PT. Desquamation of human coronary artery endothelium by human mast cell proteases: implications for plaque erosion. Coron Artery Dis. 2006;17:611–621. doi: 10.1097/01.mca.0000224420.67304.4d. [DOI] [PubMed] [Google Scholar]
- Metcalfe DD. Interaction of mast cells with extracellular matrix proteins. Int Arch Allergy Immunol. 1995;107:60–62. doi: 10.1159/000236931. [DOI] [PubMed] [Google Scholar]
- Montaner J, Molina CA, Monasterio J, Abilleira S, Arenillas JF, Ribo M, Quintana M, Alvarez-Sabin J. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107:598–603. doi: 10.1161/01.cir.0000046451.38849.90. [DOI] [PubMed] [Google Scholar]
- Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665–675. doi: 10.1002/ana.410440413. [DOI] [PubMed] [Google Scholar]
- Patkai J, Mesples B, Dommergues MA, Fromont G, Thornton EM, Renauld JC, Evrard P, Gressens P. Deleterious effects of IL-9-activated mast cells and neuroprotection by antihistamine drugs in the developing mouse brain. Pediatr Res. 2001;50:222–230. doi: 10.1203/00006450-200108000-00010. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn T, Rosenberg GA. Closure of the blood-brain barrier by matrix metaloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 2003;34:2025–2030. doi: 10.1161/01.STR.0000083051.93319.28. [DOI] [PubMed] [Google Scholar]
- Price CJS, Warburton EA, Menon DK. Human cellular inflammation in the pathology of acute cerebral ischaemia. J Neurol Neurosurg Psychiatry. 2003;74:1476–1484. doi: 10.1136/jnnp.74.11.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi AI, Ali Z, Suri MF, Kim SH, Shatla AA, Ringer AJ, Lopes DK, Guterman LR, Hopkins LN. Intra-arterial third-generation recombinant tissue plasminogen activator (reteplase) for acute ischemic stroke. Neurosurgery. 2001;49:41–50. doi: 10.1097/00006123-200107000-00006. [DOI] [PubMed] [Google Scholar]
- Ratnoff OD. Some relationships among hemostasis, fibrinolytic phenomena, immunity, and the inflammatory responses. Adv Immunol. 1969;10:146–227. doi: 10.1016/s0065-2776(08)60417-4. [DOI] [PubMed] [Google Scholar]
- Reddigari S, Silverberg M, Kaplan AP. Assembly of the human plasma kinin-forming cascade along the surface of vascular endothelial cells. Int Arch Allergy Immunol. 1995;107:93–94. doi: 10.1159/000236941. [DOI] [PubMed] [Google Scholar]
- Reuter U, Bolay H, Jansen-Olesen I, Chiarugi A, Sanchez del Rio M, Letourneau R, Theoharides TC, Waeber C, Moskowitz MA. Delayed inflammation in rat meninges: implications for migraine pathophysiology. Brain. 2001;124:2490–2502. doi: 10.1093/brain/124.12.2490. [DOI] [PubMed] [Google Scholar]
- Rieu P, Ueda T, Haruta I, Sharma CP, Arnaout MA. The A-domain of beta 2 integrin CR3 (CD11b/CD18) is a receptor for the hookworm-derived neutrophil adhesion inhibitor NIF. J Cell Biol. 1994;127:2081–2091. doi: 10.1083/jcb.127.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell A, Ortega-Aznar A, Alvarez-Sabin J, Fernandez-Cadenas I, Ribo M, Molina CA, Lo EH, Montaner J. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke. 2006;37:1399–1406. doi: 10.1161/01.STR.0000223001.06264.af. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39:279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- Rosenberg RD, Bauer KA.1994The heparin-antithrombin system: a natural anticoagulant mechanism Hemostasis and Thrombosis: Basic Principles and Clinical Practice(Colman RW, Hirsh J, Marder VJ, Salzman EW, eds), 4th edn.Philadelphia: Lippincott; 837–860. [Google Scholar]
- Rothlein R, Kennedy C, Czajkowski M, Barton RW. Generation and characterization of an anti-idiotypic antibody specific for intercellular adhesion molecule-1. Int Arch Allergy Immunol. 1993;100:121–127. doi: 10.1159/000236398. [DOI] [PubMed] [Google Scholar]
- Saarinen J, Kalkkinen N, Welgus H, Kovanen P. Activation of human interstitial procollagenase through direct cleavage of the Leu83-Thr84 bond by mast cell chymase. J Biol Chem. 1994;269:18134–18140. [PubMed] [Google Scholar]
- Schechter NM, Brass LF, Lavker RM, Jensen PJ. Reaction of mast cell proteases tryptase and chymase with protease activated receptors (PARs) on keratinocytes and fibroblasts. J Cell Physiol. 1998;176:365–373. doi: 10.1002/(SICI)1097-4652(199808)176:2<365::AID-JCP15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Schneider D, Berrouschot J, Brandt T, Hacke W, Ferbert A, Norris SH, Polmar SH, Schäfer E. Safety, pharmacokinetics and biological activity of enlimomab (anti-ICAM-1 antibody): an open-label, dose escalation study in patients hospitalized for acute stroke. Exp Neurol. 1998;40:78–83. doi: 10.1159/000007962. [DOI] [PubMed] [Google Scholar]
- Shalit M, Brenner T, Shohami E, Levi-Schaffer F. Interaction between mast cells and glial cells: an in vitro study. J Neuroimmunol. 1993;43:195–199. doi: 10.1016/0165-5728(93)90092-d. [DOI] [PubMed] [Google Scholar]
- Shanas U, Bhasin R, Sutherland AK, Silverman AJ, Silver R. Brain mast cells lack the c-kit receptor: immunocytochemical evidence. J Neuroimmunol. 1998;90:207–211. doi: 10.1016/s0165-5728(98)00137-4. [DOI] [PubMed] [Google Scholar]
- Silver R, Silverman AJ, Vitkovic L, Lederhendler II. Mast cells in the brain: evidence and functional significance. Trends Neurosci. 1996;19:25–31. doi: 10.1016/0166-2236(96)81863-7. [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Asarian L, Khalil M, Silver R. GnRH, brain mast cells and behavior. Prog Brain Res. 2002;141:315–325. doi: 10.1016/S0079-6123(02)41102-8. [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Millar RP, King JA, Zhuang X, Silver R. Mast cells with gonadotropin-releasing hormone-like immunoreactivity in the brain of doves. Proc Natl Acad Sci USA. 1994;91:3695–3699. doi: 10.1073/pnas.91.9.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman AJ, Sutherland AK, Wilhelm M, Silver R. Mast cells migrate from blood to brain. J Neurosci. 2000;20:401–408. doi: 10.1523/JNEUROSCI.20-01-00401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel BE. Intracranial bleeding fibrinolysis and anticoagulation: causal connections and clinical implications. Circulation. 1994;90:2147–2152. doi: 10.1161/01.cir.90.4.2147. [DOI] [PubMed] [Google Scholar]
- Stokely ME, Orr EL. Acute effects of calvarial damage on dural mast cells, pial vascular permeability, and cerebral cortical histamine levels in rats and mice. J Neurotrauma. 2008;25:52–61. doi: 10.1089/neu.2007.0397. [DOI] [PubMed] [Google Scholar]
- Strbian D, Karjalainen-Lindsberg ML, Kovanen PT, Tatlisumak T, Lindsberg PJ. Mast cell stabilization reduces hemorrhage formation and mortality after administration of thrombolytics in experimental ischemic stroke. Circulation. 2007a;116:411–418. doi: 10.1161/CIRCULATIONAHA.106.655423. [DOI] [PubMed] [Google Scholar]
- Strbian D, Karjalainen-Lindsberg ML, Tatlisumak T, Lindsberg PJ. Cerebral mast cells regulate early ischemic brain swelling and neutrophil accumulation. J Cereb Blood Flow Metab. 2006;26:605–612. doi: 10.1038/sj.jcbfm.9600228. [DOI] [PubMed] [Google Scholar]
- Strbian D, Kovanen PT, Karjalainen-Lindsberg M-L, Tatlisumak T, Lindsberg PJ. An emerging role of mast cells in cerebral ischemia and hemorrhage. Ann Med. 2009;41:438–450. doi: 10.1080/07853890902887303. [DOI] [PubMed] [Google Scholar]
- Strbian D, Tatlisumak T, Ramadan UA, Lindsberg PJ. Mast cell blocking reduces brain edema and hematoma volume and improves outcome after experimental intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007b;27:795–802. doi: 10.1038/sj.jcbfm.9600387. [DOI] [PubMed] [Google Scholar]
- Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33:831–836. doi: 10.1161/hs0302.104542. [DOI] [PubMed] [Google Scholar]
- Tchougounova E, Lundequist A, Fajardo I, Winberg JO, Abrink M, Pejler G. A key role for mast cell chymase in the activation of pro-matrix metalloprotease-9 and pro-matrix metalloprotease-2. J Biol Chem. 2005;280:9291–9296. doi: 10.1074/jbc.M410396200. [DOI] [PubMed] [Google Scholar]
- Tejima E, Zhao BQ, Tsuji K, Rosell A, van Leyen K, Gonzalez RG, Montaner J, Wang X, Lo EH. Astrocytic induction of matrix metalloproteinase-9 and edema in brain hemorrhage. J Cereb Blood Flow Metab. 2007;27:460–468. doi: 10.1038/sj.jcbfm.9600354. [DOI] [PubMed] [Google Scholar]
- Tetlow LC, Harper N, Dunningham T, Morris MA, Bertfield H, Woolley DE. Effects of induced mast cell activation on prostaglandin E and metalloproteinase production by rheumatoid synovial tissue in vitro. Ann Rheum Dis. 1998;57:25–32. doi: 10.1136/ard.57.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC. Mast cells: the immune gate to the brain. Life Sci. 1990;46:607–617. doi: 10.1016/0024-3205(90)90129-f. [DOI] [PubMed] [Google Scholar]
- Thompson HL, Thomas L, Metcalfe DD. Murine mast cells attach to and migrate on laminin-, fibronectin-, and matrigel-coated surfaces in response to Fc epsilon RI-mediated signals. Clin Exp Allergy. 1993;23:270–275. doi: 10.1111/j.1365-2222.1993.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Tsujimura T, Hirota S, Nomura S, Niwa Y, Yamazaki M, Tono T, Morii E, Kim H-M, Kondo K, Nishimune Y, Kitamura Y. Characterization of Ws Mutant allele of rats: a 12-base deletion in tyrosine kinase of c-kit gene. Blood. 1991;78:1942–1946. [PubMed] [Google Scholar]
- Valent P.2000Role of mast cells in endogeneous fibrinolysis and related (patho)physiological processes Mast Cells and Basophils(Marone G, Lichtenstein LM, Galli SJ, eds),London: Academic Press; 497–505. [Google Scholar]
- Vuorte J, Lindsberg PJ, Kaste M, Meri S, Jansson SE, Rothlein R, Repo H. Anti-ICAM-1 monoclonal antibody R6.5 (Enlimomab) promotes activation of neutrophils in whole blood. J Immunol. 1999;162:2353–2357. [PubMed] [Google Scholar]
- Wagner S, Tagaya M, Koziol JA, Quaranta V, del Zoppo GJ. Rapid disruption of an astrocyte interaction with the extracellular matrix mediated by integrin alpha 6 beta 4 during focal cerebral ischemia/reperfusion. Stroke. 1997;28:858–865. doi: 10.1161/01.str.28.4.858. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Silver R, Silverman AJ. Central nervous system neurons acquire mast cell products via transgranulation. Eur J Neurosci. 2005;22:2238–2248. doi: 10.1111/j.1460-9568.2005.04429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter MC, Shasby SS, Ries DR, Shasby DM. Histamine selectively interrupts VE-cadherin adhesion independently of capacitive calcium entry. Am J Physiol Lung Cell Mol Physiol. 2004;287:L816–L823. doi: 10.1152/ajplung.00056.2004. [DOI] [PubMed] [Google Scholar]
- Xue M, Del Bigio MR. Acute tissue damage after injections of thrombin and plasmin into rat striatum. Stroke. 2001;32:2164–2169. doi: 10.1161/hs0901.095408. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y, Suzuki T, Yamaya H, Matsuura N, Onodera H, Kogure K. Possible involvement of interleukin-1 in ischemic brain edema formation. Neurosci Lett. 1992;142:45–47. doi: 10.1016/0304-3940(92)90616-f. [DOI] [PubMed] [Google Scholar]
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- Yong T, Bebo BFJ, Sapatino BV, Welsh CJ, Orr EL, Linthicum DS. Histamine-induced microvascular leakage in pial venules: differences between the SJL/J and BALB/c inbred strains of mice. J Neurotrauma. 1994;11:161–171. doi: 10.1089/neu.1994.11.161. [DOI] [PubMed] [Google Scholar]
- Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Silverman AJ, Silver R. Brain mast cell degranulation regulates blood-brain barrier. J Neurobiol. 1996;31:393–403. doi: 10.1002/(SICI)1097-4695(199612)31:4<393::AID-NEU1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Silverman AJ, Silver R. Distribution and local differentiation of mast cells in the parenchyma of the forebrain. J Comp Neurol. 1999;408:477–488. doi: 10.1002/(sici)1096-9861(19990614)408:4<477::aid-cne3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]