Abstract
The mechanisms responsible for vascular autoregulation in the brain during changes in mean arterial blood pressure are ambiguous. Potentially, adenosine, a purine nucleoside and potent vasodilator, may be involved as earlier studies have documented an increase in brain adenosine concentrations with cerebral ischemia and hypotension. Consequently, we tested the hypothesis that adenosine is involved in vasodilatation during hypotension within the autoregulatory range (>50 mm Hg) by exposing adenosine 2a receptor (A2aR) knockout and wild type (WT) mice to short (2 to 5 mins) periods of hypotension. We found that autoregulation was significantly (P<0.05) impaired by 29% in A2a knockout mice as compared with WT animals. Furthermore, the A2R antagonist (A2a>A2b:10–85>1), ZM-241385, in a dose (1, 5, 10 mg/kg, intraperitoneally)-related manner, attenuated autoregulation in WT mice. In knockout mice treated with ZM-2413585 (5 and 10 mg/kg), autoregulation was further impaired indicating that A2b receptors also participated in cerebral vasodilatation. Treatment with dipyridamole (1.0 mg/kg) that increases extracellular concentrations of adenosine improved autoregulation in the A2aR knockout mice. We would conclude that adenosine through both A2a and A2b receptors is involved in physiologic vascular regulation during hypotension even within the autoregulatory range.
Keywords: A2 receptor, autoregulation, brain adenosine, CBF, hypotension, knockout mice
Introduction
The brain has the capacity to modulate cerebral blood flow (CBF) during changes in blood pressure. This phenomenon is known as autoregulation (Lassen, 1959). The mechanism(s) responsible for the regulation of cerebral arteriolar tone during autoregulation is unclear, but a number of theories have been proposed (Paulson et al, 1990), including the myogenic (Heistad and Kontos, 1983), neural (Hamel, 2006), and metabolic hypotheses (Heistad and Kontos, 1983). All three hypotheses entail biochemical (or transmitter) signal(s) whose action leads to a change in arterial diameter. A number of biochemical factors have been proposed to have such a role. For example, in vivo and in vitro studies have suggested involvement of arachidonic acid metabolites (Harder et al, 2000; Roman, 2002), nitric oxide (Jones et al, 2003), calcitonin gene-related peptide (Hong et al, 1994; Wei et al, 1992), to mention a few, in vasoreactivity during alteration in mean arterial blood pressure (MABP) or intraluminal pressure.
Another potential signaling compound is the purine nucleoside adenosine, which is a potent vasodilator (Berne et al, 1974; Ngai et al, 2001). Brain adenosine concentrations are increased during hypoxia (Winn et al, 1981; Zetterstrom et al, 1982), ischemia (Winn et al, 1979) and neuronal activation (Winn et al, 1980a). However, there is controversy as to whether adenosine is elevated in brain during hypotension. To resolve this controversy, we performed studies to test the hypothesis that adenosine is involved in autoregulation during decreases in MABP and to determine the relative contribution of the adenosine 2a receptor (A2aR) and the 2b receptor (A2bR). We restricted our analysis to changes in MABP within the autoregulatory range and used short periods of decreases in MABP in wild type (WT) and A2aR knockout (KO) mice treated with increasing doses of the A2a antagonist ZM-241385 [4-(2-{7-amino-2-[2-furyl][3,2,4]triazolol [2,3-a] [1,3,5]triazin-5-yl-amino}ethyl)phenol]. In addition, we analyzed the effects of dipyridamole (DIP) in A2aR knockout mice. Dipyridamole at low concentrations (<10−5 mol/L) inhibits cellular uptake of adenine nucleosides, thus exaggerating extracellular concentrations of adenosine (Heistad et al, 1981).
Materials and methods
All procedures in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of Mount Sinai School of Medicine.
Animals
A2aR knockout mice were generated by Chen et al (1999). These investigators demonstrated that absolute blood flows were similar in both WT and A2aR knockout mice (Chen et al, 1999). Further details about animal preparation are outlined in Miekisiak et al (2008, 2009).
Surgical Procedures
In brief, mice weighing 22 to 31 g were intubated, ventilated, and anesthetized with 1.1% to 1.7% isoflurane in 40% O2 balanced with nitrogen (Miekisiak et al, 2008). End-tidal CO2 was monitored with a microcapnograph. Rectal temperature was continuously monitored and maintained at 37°C. The right femoral artery was cannulated with a narrowed PE-10 tube for continuous blood pressure monitoring and to obtain arterial blood gases and determine the pH. The left femoral artery was also cannulated for withdrawal and return of arterial blood. Throughout the entire experiment pancuronium bromide (0.4 mg/kg, q45 mins) was administered intraperitoneally. After securing the arterial lines, the animal's head was fixed in the stereotactic frame and the scalp was incised along the midline to expose the bone. Then a laser-Doppler probe was placed over the intact frontal skull to allow measurement of CBF. Alpha-chloralose was administered intraperitoneally (75 mg/kg, q1 h) and, as described earlier (Miekisiak et al, 2008), isoflurane was gradually discontinued and the animals maintained stable for 45 to 60 mins before initiating hypotensive challenges to avoid confounding effects of isoflurane on the CBF response (Miekisiak et al, 2008). Level of anesthesia was monitored through assessment of physiologic parameters and alpha-chloralose supplemented to assure that the animal was not in pain.
Experimental Protocols
For purposes of this study, the term ‘hypotension' represents a MABP below the baseline (initial) MABP. We achieved hypotension by lowering MABP by withdrawal of blood (52 μL/secs) from the cannulated right femoral artery while measuring simultaneously the MABP in the contralateral femoral artery. Blood was withdrawn and re-infused by means of a roller pump. The withdrawn blood was kept in gas impermeable tubing and at body temperature by a heating light.
During hypotension, CBF (as measured by laser Doppler) decreased and then stabilized at a plateau. The plateau value (Figure 1A) was used to calculate cerebrovascular resistance (CVR). After the hypotensive period (120 to 180 secs), the withdrawn blood was re-infused to re-establish baseline MABP and the animals stabilized for a minimum of 5 mins before being subjected to another hypotensive period. Each animal was subjected to 4 to 6 hypotensive challenges of varying degree (5% to 40% of baseline) in a random order. Animals having an initial MABP (i.e., preblood withdrawal) less than 75 mm Hg (∼90 mm Hg at the internal carotid, see below) were excluded.
Figure 1.
Percentage change in MABP (white) and CBF (black) in WT (A) and A2aR knockout (B) mice. Change in MABP was achieved by femoral arterial blood withdrawal while MABP was recorded from cannulated contralateral femoral artery. CBF was measured through the intact skull by laser Doppler flowmetry. Dash arrow=60 secs. Both CBF and MABP achieved a stable value (‘plateau'), which was subsequently used to calculate change in cerebral vascular resistance (CVR).
All animals were challenged with 7% CO2 before hypotensive periods to assess their arterial reactivity. Animals having <20% increase in CBF were euthanized as outlined earlier (Miekisiak et al, 2009). We initially obtained arterial blood (100 μL) for assessment of CO2, O2, and pH and subsequently continuously monitored end-tidal CO2 as described earlier (Miekisiak et al, 2009).
We assessed the genotype by PCR (JF Chen) in many of our animals, but as homozygous interbreeding bypasses the need to assess genotype, we also confirmed the genotype by a functional test using CGS21680 (0.1 mg/kg), a highly selective A2aR agonist at the end of the experiment (Miekisiak et al, 2008). As reported earlier, with administration of CGS21680, WT animals with functional A2aR and in the absence of ZM-241385 became hypotensive and tachycardic, whereas knockout and animals treated with ZM-241385 maintained their MABP and heart rate relatively stable (Miekisiak et al, 2008).
In mice administered dipyridamole (1 mg/kg), we obtained 100 μL of blood at the end of the procedure for determination of serum concentration of this compound.
Preliminary Studies
Validation of Model
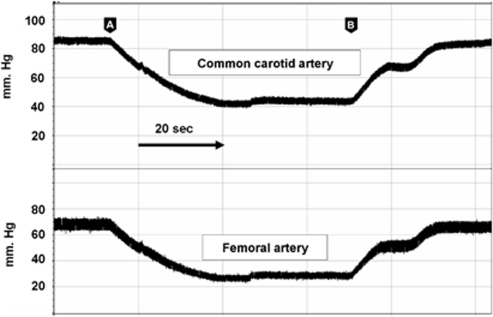
To confirm that the MABP recorded in the femoral artery reflected MABP in the root of the aorta and internal carotid arteries, we measured MABP in the common carotid artery (cannulated through the external carotid) during blood withdrawal from the right femoral artery while also measuring MABP in the contralateral femoral artery. As shown in Figure 2, the changes, both absolute and temporal, in MABP in the femoral artery reflected the changes MABP in the carotid artery (carotid artery MABP=1.1 (femoral MABP) +13.7; R2=0.99; n=24). Dalkara et al (1995) measured MABP in the femoral artery and documented that the lower limit of autoregulation in the mouse was ∼40 mm Hg. This is, in general, lower than that found in other species. However, as demonstrated by our simultaneous measurements, femoral MABP is ∼14 mm Hg lower than in the root of the aorta and carotid artery. Thus, the ‘low' limit of autoregulation in the mouse noted by Dalkara et al (1995) reflects the site of BP measurement and the attenuation of resting MABP in the femoral artery rather than a unique aspect of the mouse cerebral circulation.
Figure 2.
Simultaneous blood pressure recordings in the femoral and carotid arteries. Blood was withdrawn (arrow head A) and replaced (arrow head B) at a rate of 52 μL/secs from the contralateral femoral artery simulating the hypotensive challenge administered to WT and knockout mice. The changes, both absolute and temporal, in blood pressure in the femoral artery reflected the changes in the carotid artery in the following manner: carotid artery MABP=1.1 (femoral MABP) +13.7; R2=0.99; (n=24).
Determination of the lower limit of autoregulation
As our goal was to study the cerebrovascular response in the physiologic autoregulatory range, we initially analyzed the CBF changes during decreases in MABP in WT, WT treated with ZM-241385 (10 mg/kg), and knockout mice to identify the blood pressure (‘inflection point') where compensatory alteration in CBF began to fail during hypotension. In all three groups, the inflection point was found to be below 40 mm Hg as measured at the femoral artery (∼55 mm Hg at the carotid artery). Consequently, our studies of autoregulation (see below) were performed above 45 mmHg.
Groups Studied
The following groups of male mice were subject to the experimental protocol outlined above:
Group I: WT and A2aR knockout mice.
Group II: WT mice treated with 1.0, 5.0, and 10.0 mg/kg ZM-241385.
Group III: Knockout mice treated with 5 mg and 10 mg/kg ZM-241385.
Group IV: WT and knockout mice treated with di-pyridamole (0.2 and 1.0 mg/kg).
Pharmacological Protocol
ZM-241385
To analyze the relative contributions of A2aR compared to A2bR, we used the relatively specific A2aR antagonist, ZM-241385. This compound has an ∼10- to 85-fold greater affinity for the A2a versus the A2b subtype receptor and is 300- and 10,000-fold more active at the A2a than at the A1 and A3 receptor, respectively (Fredholm et al, 2001; Meno et al, 2001). We chose ZM-241385 (1, 5, and10 mg/kg) because an earlier in vitro study by Ngai et al (2001) demonstrated that adenosine induced vasodilatation appeared to be predominantly related to stimulation of the A2a receptor although, with high concentrations of adenosine in the presence of ZM-241385 (1 μmol/L), Ngai et al, found residual vasodilatation presumably related to A2b receptor activity; in vivo studies in both rat and mouse found that ZM-241385 (1.0 and 5.0 mg/kg) was effective in attenuating CBF during topical glutamate application (Iliff et al, 2003), hypoxia (Miekisiak et al, 2008), and neuronal stimulation (Meno et al, 2001) caused by sciatic nerve stimulation (although some investigators were unable to diminish CBF hyperemia induced during whisker stimulation with ZM-241385 (Liu et al, 2008)); and finally, there are no highly specific A2b receptor antagonists that cross the blood–brain barrier. To analyze the role of A2bR activity in cerebral vasodilatation, we studied the effects of ZM-241385 in A2aR knockout animals based on the rationale that in absence of functional a2aR (as would occur in A2aR knockout mice), ZM-241385 would exclusively antagonize A2b receptors.
Dipyridamole
Dipyridamole at concentrations ⩽10−6 mol/L impairs uptake of adenosine from the extracellular space and diminishes adenosine kinase activity (Ko et al, 1990). This drug may also inhibit adenosine deaminase, attenuate cyclic phosphodiesterase activity (thereby increasing cyclic GMP), relax directly smooth muscle, and interact with the prostaglandin system (Ko et al, 1990). However, all of these latter activities occur at concentration greater then 10–5 mol/L (Ko et al, 1990). Consequently, in preliminary studies, we measured serum concentrations of dipyridamole (Kim et al, 2008) and found that dosage of dipyridamole ⩽1 mg/kg, intraperitoneally resulted in serum concentrations ⩽10−6 mol/L. In addition, we measured dipyridamole in mice subjected to hypotensive challenges.
Data analysis
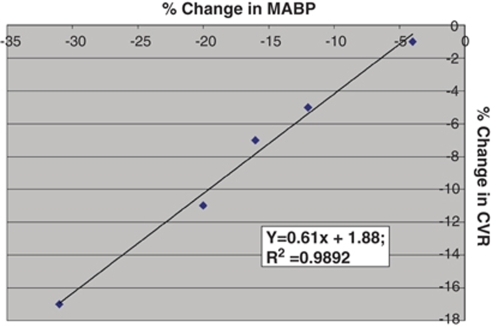
The data were acquired with a Digidata 1322A digitizer (Axon CNS, Union City, CA, USA) and analyzed with the SPSS 14.0 data analysis package (SPSS Inc., Chicago, IL, USA). As outlined above, CVR was calculated for each hypotensive trial and then percent change in CVR was plotted against the percentage change in MABP. For each animal, scatter plots were constructed, linear regression analysis performed, and a slope calculated (Figure 3). We then derived the means (±s.e.m.) of the slopes for each group (i.e., WT, knockout, etc.) and analyzed the means by independent t-tests and analysis of covariance for statistical comparisons. When multiple simultaneous comparisons were made, Bonferroni correction was applied. A P value of <0.05 was used to indicate statistically significant differences.
Figure 3.
Slope. Relationship between percentage change in MABP versus percentage change in CVR for one WT mouse subject to five hypotensive challenges (in random order). Linear regression is illustrated. Mean (and s.e.m.) of all slope values (‘x') for each group of mice was then calculated.
Drugs
ZM-241385 (Sigma Aldrich, St Louis, MO, USA); CGS-21680 (Sigma Aldrich); pancuronium bromide (Gensia Sicor Pharmaceuticals, Irvine, CA, USA); Lidocaine (Hospira Inc., Lake Forest, IL, USA); Isoflurane (Baxter Corp., Deerfield, IL, USA); and Alpha-chloralose (Fischer Scientific, St Louis, MO, USA).
Results
Physiologic Parameters
Table 1 displays the MABP in each group of mice before hypotensive challenge. In addition, there were no differences in mean arterial blood gases (CO2 and O2) and pH, percentage increase in CBF after hypercarbic challenge, baseline end-tidal CO2, and animal weight (surrogate for age). In addition, we noted a lack of change in end-tidal CO2 during hypotensive challenge in all groups. Finally, there was no difference in time to nadir in MABP and CBF among the different experimental groups.
Table 1. Baseline mean arterial blood pressure (MABP).
| Group | (n) | MABP±s.e.m. |
|---|---|---|
| WT | 6 | 82±3 |
| WT+1 ZM | 5 | 82±3 |
| WT+5 ZM | 5 | 84±2 |
| WT+10 ZM | 6 | 83±3 |
| WT+1 DIP | 4 | 81±2 |
| KO | 6 | 91±5 |
| KO+5 ZM | 5 | 93±6 |
| KO+10 ZM | 4 | 86±4 |
| KO+1 DIP | 4 | 86±6 |
Analysis of the Autoregulatory Response
Group I (WT and A2aR Knockout Mice)
Figure 1 illustrates examples of the changes in CBF during 20% to 30% decrease in MABP and contrasts the response of CBF in WT animals (Figure 1A) and A2aR knockout mice (Figure 1B). In calculating the slope of (% Δ MABP versus % Δ CVR), the determination of CBF was made when CBF established a stable plateau (see Figure 1A). Note the different response in the A2aR knockout mice, where CBF follows the changes in MABP in a more passive manner. In addition, with restoration of the extracted blood, a slight temporary elevation above baseline MABP occurs. Although not a focus of this study, this relative ‘hypertensive' challenge reveals that CBF in the WT remains below the increase in MABP whereas in the A2aR knockout mice, CBF parallels or exceeds the changes in MABP.
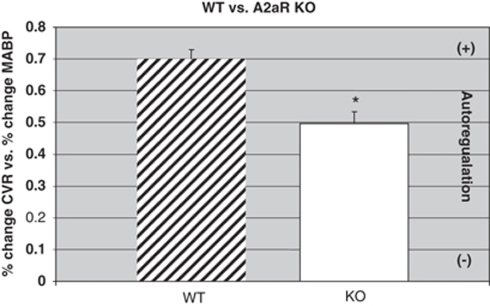
As indicated in Figure 4, the slope (% Δ MABP versus % Δ CVR) in WT male mice (n=6) was 0.70±0.03. In comparison (Figure 4), knockout animals (n=6) had a significant impairment of their autoregulatory response. For example, the slope of the % Δ MABP versus % Δ CVR (Figure 3) in the knockout mice (n=6) averaged 0.50±0.04, significantly different (P<0.05) from the WT (0.70±0.03) animals. The decrease in the autoregulatory response in the A2aR knockout mice as compared to the response in WT animals was 29%.
Figure 4.
Slope of WT (n=6) compared with A2aR knockout (KO) (n=6) mice. Mean and s.e.m.; *P<0.05, WT versus KO.
Group II (WT+ZM-241385)
As illustrated in Figure 5, there was a dose-related effect of ZM-241385. As compared to WT mice, 1 mg/kg of ZM-241385 did not alter the relationship of Δ MABP versus % ΔCVR, whereas treatment with 5 mg/kg resulted in a downward trend (P=0.06) to 0.50±0.07. The slope in the WT+10 mg/kg mice (0.39±0.04) decreased 44%, which was significantly (P<0.05) less than in untreated mice.
Figure 5.
Autoregulatory response in WT mice treated with ZM-241385. Mean±s.e.m.; *P=0.04; **P<0.05 versus 0.
Group III (A2aR Knockout+ZM-241385)
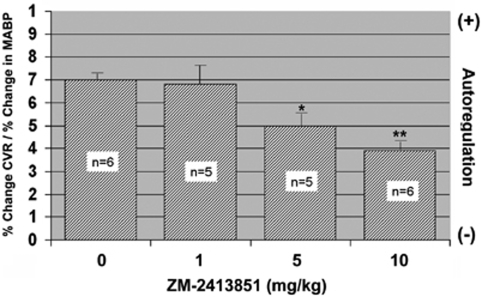
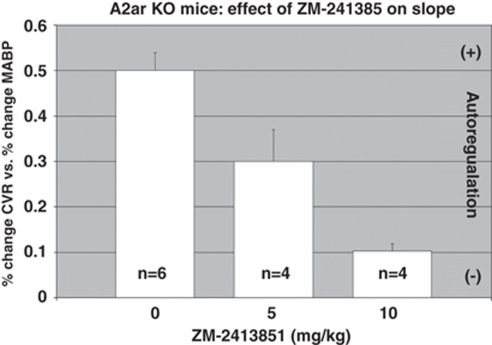
These studies were designed to analyze the contribution of A2b receptors. As ZM-241385 at high doses will also affect A2b receptors, we evaluated the autoregulatory response in knockout mice treated with ZM-241385. As illustrated in Figure 6, we found that the autoregulatory response in the A2aR mice was further and significantly (P<0.05) impaired by 40% in the presence of 5 mg/kg of ZM-241385 and by 80% with 10 mg/kg (80%).
Figure 6.
Effect of ZM-2413851 on slope (% change CVR/%change in MABP). As A2aR knockout (KO) lack functional A2a receptors, the effect of ZM-2413851 is exclusively on A2b receptors. Mean±s.e.m.; *P<0.05; 0 versus 5 and 10 mg/kg (Bonferroni correction).
Group IV, Dipyridamole
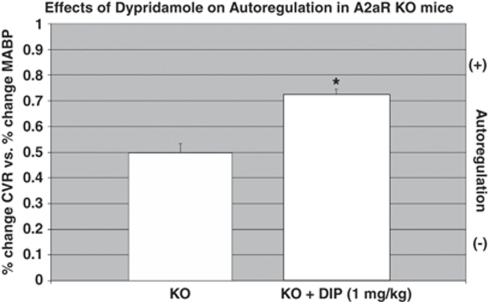
As indicated in Figure 7, dipyridamole (1 mg/kg) significantly (P<0.05) improved the autoregulatory response in both WT (27% from 0.70±0.03 to 0.89±0.007) and the A2aR knockout mice (38% from 0.50±0.04 to 0.69±0.04). Lower doses of dipyridamole (0.2 mg/kg) did not result in a significant improvement in vasodilatation. Dipyridamole in all serum samples was determined to be less than 10−6 mol/L.
Figure 7.
Effect of dipyridamole (DIP) on autoregulation in A2aR knockout (KO) mice. As A2aR KO lack functional A2a receptors, the effect of dipyridamole on vasodilatation occurs by means of A2b receptors. Mean±s.e.m.; n=6 in KO; n=4 in KO+DIP:=P<0.05; KO versus KO+DIP.
Response to CGS 21680
WT animals had a previously described hypotensive and tachycardic response to CGS 21680 (0.1 mg/kg), whereas this response was blocked in knockout animals and animals treated with ZM-241385 (data not shown).
Discussion
In this study, we tested the hypothesis that adenosine is involved in autoregulation during hypotension in the mouse. Our studies were focused on changes in MABP within the autoregulatory range. During short periods of hypotension, we found that A2aR knockout mice had a significant reduction in their capacity to vasodilate their cerebral circulation as compared to WT animals. Furthermore, in WT mice, autoregulation was reduced by treatment with a semiselective A2aR antagonist, ZM-241385. In A2aR knockout mice, ZM-241385 further attenuated the autoregulatory capacity, whereas dipyridamole improved autoregulation. In the absence of functioning A2a receptor, these latter observations in A2aR knockout mice indicate that A2b receptors are capable of participating in cerebral arteriolar vasodilatation. In an earlier study, changes in brain adenosine concentrations (Winn et al, 1979) were found to occur with seconds (⩽5 secs) of the onset of ischemia and thus with sufficient rapidity to account for the swift (∼7 secs) response of cerebral arterioles to hypotension (Kontos et al, 1978a).
There are four adenosine receptors(R): A1R; A2aR, A2bR, and A3R (Fredholm et al, 2001). A1R and A3R are not involved in cerebral vasodilatation (Ngai et al, 2001), Ngai et al (2001) has demonstrated that the A2 receptor is the main adenosine receptor involved in cerebral vasodilatation with the A2aR having a higher affinity (∼300 × ) for adenosine than the less sensitive A2bR (Fredholm et al, 2001). Unfortunately, there are no highly specific pharmacological antagonists for A2aR and A2bR, thus limiting the pharmacological ability to uncover the relative contributions of these two receptors to cerebral vasodilatation (Fredholm et al, 2001). The creation of A2aR knockout mice provides an experimental technique to assess not only the role of adenosine in CBF regulation, but potentially to determine the relative contributions of the A2aR and A2bR to vasodilatation.
Our study is the first to analyze cerebral autoregulation using adenosine knockout mice and indicates that adenosine contributes to CBF regulation during hypotension. Furthermore, this study resolves the dispute as to whether adenosine has a role in cerebral arterial vasodilatation even within the autoregulatory range. Previous investigators suggested that adenosine concentration become elevated only when MABP decreases below 50 mm Hg (Van Wylen et al, 1988), whereas others, using a rapid freeze-blow brain sampling technique (Winn et al, 1980b), found that adenosine doubled from resting levels (10−7 mol/L) even within the autoregulatory range. The former study used brain dialysis techniques, which may not have sufficient spatial and/or temporal resolution to discern changes in adenosine concentrations (Ngai et al, 1998). This study also extends an earlier work that indicated that adenosine receptor antagonism can limit the lower extent of the autoregulatory plateau (Shin et al, 2000), but is in contrast to the findings by Phillis and DeLong (1986), who failed to alter autoregulation with application of caffeine. Caffeine affects both A1 and A2 receptors (Fredholm et al, 2001). Stimulation of the former receptor may confound interpretation of CBF response during hypotension because stimulation of the A1 receptor will increase neural activation and brain metabolism (Fredholm et al, 2001). In addition, Phillis and DeLong used the retroglenoid venous outflow method in rat to measure CBF. Alteration in the venous outflow of the brain with resultant increase in venous pressure and transmural pressure may have perturbed the autoregulatory response (Heistad and Kontos, 1983).
Our studies were based on changes in CBF as determined by laser Doppler, which do not measure absolute CBF, but indicate only comparable changes. As absolute baseline blood flow in WT and A2aR knockout mice has been shown to similar (Chen et al, 1999), the use of laser Doppler is a valid technique to compare the changes in CBF in WT, A2aR knockout and WT mice treated with Zm-241385.
On the basis of the difference between the WT and A2aR knockout response to hypotension, adenosine's contribution to vasodilatation through A2a receptors appears to be 29% of the change in CVR during hypotension. This 29% may be an underestimation because of the potential of adaptation in the A2aR knockout mice (such as increased expression of A2b receptors). The dose response to ZM-241385 in WT mice also supports the role of Ado in autoregulation and suggest that the contribution of the A2a receptor is 30% to 44% of total vasodilatation. Finally, the very steep (80%) attenuation of vasodilatation during hypotension observed in the A2aR knockout mice treated with ZM-241385 (10 mg/kg) may represent the total contribution of Ado (i.e., A2aR+A2bR) to vasodilatation during hypotension. However, this 80% may be exaggerated by upregulation of A2b receptors in A2aR knockout mice, although Sitkovsky and his colleagues have demonstrated the absence of a compensatory increase in A2bR activity in A2aR knockout mice (Armstrong et al, 2001). The ability of A2bR to participate in vasodilatation is evident from our studies in knockout animals treated with ZM-241385 and dipyridamole, where we observed deterioration with ZM-241385 and improvement with dipyridamole.
Other circulations such as heart, skeletal muscle, intestine, and kidney display autoregulation and investigators have demonstrated the involvement of adenosine in the response to hypotension (Morff and Granger, 1983) in some organs. However, the role of adenosine in the regulation of CBF during hypotension should be placed in context with other factors, such as arachidonic acid metabolites (Harder et al, 2000; Roman, 2002), nitric oxide (Jones et al, 2003), or calcitonin gene-related peptide (Hong et al, 1994; Wei et al, 1992), which have been demonstrated to contribute to vasoregulation.
What mechanisms are responsible for the release of brain adenosine during hypotension? Earlier studies suggested that with hypotension, even within the autoregulatory range, brain tissue becomes hypoxic and that local hyperoxia can attenuate vasodilatation observed with hypotension (Kontos et al, 1978b). Hypoxia, in turn, as noted above, can rapidly increase cerebral concentrations of adenosine (Winn et al, 1981). A recent study indicates that functioning A2 receptors are critical for hypoxic hyperemia (Miekisiak et al, 2008). In addition, Gordon et al (2008) have suggested a new role for astrocytic-derived adenosine in hypoxia: switching function is operational in vasodilatation during hypotension. Furthermore, astrocytes, which are highly sensitive to changes in tissue oxygenation as determined by the production of adenosine, are the likely cellular source for the rapid production of adenosine during hypotension (Kulik et al, 2008).
In summary, using both knockout technique and pharmacological methods, we have demonstrated that adenosine is involved in cerebral vasodilatation during hypotension within the autoregulatory blood pressure range. Vasodilatation occurs by means of both A2a and A2b receptors. Adenosine along with other factors either acting in concert or in an integrative manner appears to be critical for autoregulation. However, the source and regulatory mechanisms involved in adenosine production during hypotension remain to be elucidated.
Acknowledgments
This work was supported by a grant from the NIH: NS—021076 (26) to HRW.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Armstrong JM, Chen JF, Schwarzschild MA, Apasov S, Smith PT, Caldwell C, Chen P, Figler H, Sullivan G, Fink S, Linden J, Sitkovsky M. Gene doseeffect reveals no Gs-coupled A2A adenosine receptor reserve in murine T-lymphocytes: studies of cells from A2A-receptor-gene-deficient mice. Biochem J. 2001;354:123–130. doi: 10.1042/0264-6021:3540123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne RM, Rubio R, Curnish R. Release of adenosine from ischemic brain. Circ Res. 1974;35:262–271. [Google Scholar]
- Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalkara T, Irikura K, Huang Z, Panahian N, Moskowitz MA. Cerebrovascular responses under controlled and monitored physiological conditions in the anesthetized mouse. J Cereb Blood Flow Metab. 1995;15:631–638. doi: 10.1038/jcbfm.1995.78. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, Macvicar BA. Brain metabolism dictates the polarity of astrocyte control overarterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- Harder DR, Roman RJ, Gebremedhin D. Molecular mechanisms controlling nutritive blood flow: role of cytochrome P450 enzymes. Acta Physiol Scand. 2000;168:543–549. doi: 10.1046/j.1365-201x.2000.00707.x. [DOI] [PubMed] [Google Scholar]
- Heistad D, Kontos H.1983Cerebral circulation.2nd ed.Bethesda, MD: American Physiological Society; 137–182. [Google Scholar]
- Heistad DD, Marcus ML, Gourley JK, Busija DW. Effect of adenosine and dipyridamole on cerebral blood flow. Am J Physiol. 1981;240:H775–H780. doi: 10.1152/ajpheart.1981.240.5.H775. [DOI] [PubMed] [Google Scholar]
- Hong KW, Pyo KM, Lee WS, Yu SS, Rhim BY. Pharmacological evidence thatcalcitonin gene-related peptide is implicated in cerebral autoregulation. Am J Physiol. 1994;266:H11–H16. doi: 10.1152/ajpheart.1994.266.1.H11. [DOI] [PubMed] [Google Scholar]
- Iliff JJ, D'Ambrosio R, Ngai AC, Winn HR. Adenosine receptors mediate glutamate-evoked arteriolar dilation in the rat cerebral cortex. Am J Physiol Heart Circ Physiol. 2003;284:H1631–H1637. doi: 10.1152/ajpheart.00909.2002. [DOI] [PubMed] [Google Scholar]
- Jones SC, Easley KA, Radinsky CR, Chyatte D, Furlan AJ, Perez-Trepichio AD. Nitric oxide synthase inhibition depresses the height of the cerebral blood flow-pressure autoregulation curve during moderate hypotension. J Cereb Blood Flow Metab. 2003;23:1085–1095. doi: 10.1097/01.WCB.0000081202.00668.FB. [DOI] [PubMed] [Google Scholar]
- Kim HH, Sawada N, Soydan G, Lee HS, Zhou Z, Hwang SK, Waeber C, Moskowitz MA, Liao JK. Additive effects of statin and dipyridamole on cerebral blood flow and stroke protection. J Cereb Blood Flow Metab. 2008;28:1285–1293. doi: 10.1038/jcbfm.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko KR, Ngai AC, Winn HR. Role of adenosine in regulation of regional cerebral blood flow in sensory cortex. Am J Physiol. 1990;259:H1703–H1708. doi: 10.1152/ajpheart.1990.259.6.H1703. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Wei EP, Navari RM, Levasseur JE, Rosenblum WI, Patterson JL., Jr Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol. 1978a;234:H371–H383. doi: 10.1152/ajpheart.1978.234.4.H371. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Wei EP, Raper AJ, Rosenblum WI, Navari RM, Patterson JL., Jr Role of tissue hypoxia in local regulation of cerebral microcirculation. Am J Physiol. 1978b;234:H582–H591. doi: 10.1152/ajpheart.1978.234.5.H582. [DOI] [PubMed] [Google Scholar]
- Kulik T, Aronhime S, Beylin A, Kusano Y, Sandler A, Winn HR. Are astrocytes the cerebral source of adenosine. Purinergic Signal. 2008;4:S162–S163. [Google Scholar]
- Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959;39:183–238. doi: 10.1152/physrev.1959.39.2.183. [DOI] [PubMed] [Google Scholar]
- Liu X, Li C, Falck JR, Roman RJ, Harder DR, Koehler RC. Interaction of nitric oxide, 20-HETE, and EETs during functional hyperemia in whisker barrel cortex. Am J Physiol Heart Circ Physiol. 2008;295:H619–H631. doi: 10.1152/ajpheart.01211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meno JR, Crum AV, Winn HR. Effect of adenosine receptor blockade on pial arteriolar dilation during sciatic nerve stimulation. Am J Physiol Heart Circ Physiol. 2001;281:H2018–H2027. doi: 10.1152/ajpheart.2001.281.5.H2018. [DOI] [PubMed] [Google Scholar]
- Miekisiak G, Kulik T, Kusano Y, Kung D, Chen JF, Winn HR. Cerebral blood flow response in adenosine 2a receptor knockout mice during transient hypoxic hypoxia. J Cereb Blood Flow Metab. 2008;28:1656–1664. doi: 10.1038/jcbfm.2008.57. [DOI] [PubMed] [Google Scholar]
- Miekisiak G, Yoo K, Sandler AL, Kulik TB, Chen JF, Winn HR. The role of adenosine in hypercarbic hyperemia: in vivo and in vitro studies in adenosine 2(A) receptor knockout and wild-type mice. J Neurosurg. 2009;110:981–988. doi: 10.3171/2008.8.JNS08460. [DOI] [PubMed] [Google Scholar]
- Morff RJ, Granger HJ. Contribution of adenosine to arteriolar autoregulation in striated muscle. Am J Physiol. 1983;244:H567–H576. doi: 10.1152/ajpheart.1983.244.4.H567. [DOI] [PubMed] [Google Scholar]
- Ngai AC, Coyne EF, Meno JR, West GA, Winn HR. Receptor subtypes mediating adenosine-induced dilation of cerebral arterioles. Am J Physiol Heart Circ Physiol. 2001;280:H2329–H2335. doi: 10.1152/ajpheart.2001.280.5.H2329. [DOI] [PubMed] [Google Scholar]
- Ngai AC, Meno JR, Ko KR, Winn HR. Role of adenosine in cerebral vasodilator responses to sciatic nerve stimulation. J Cereb Blood Flow Metab. 1998;18:580–581. doi: 10.1097/00004647-199805000-00012a. [DOI] [PubMed] [Google Scholar]
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- Phillis JW, DeLong RE. The role of adenosine in cerebral vascular regulation during reductions in perfusion pressure. J Pharm Pharmacol. 1986;38:460–462. doi: 10.1111/j.2042-7158.1986.tb04610.x. [DOI] [PubMed] [Google Scholar]
- Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- Shin HK, Park SN, Hong KW. Implication of adenosine A2A receptors in hypotension-induced vasodilation and cerebral blood flow autoregulation in rat pial arteries. Life Sci. 2000;67:1435–1445. doi: 10.1016/s0024-3205(00)00737-2. [DOI] [PubMed] [Google Scholar]
- Van Wylen DG, Park TS, Rubio R, Berne RM. Cerebral blood flow and interstitial fluid adenosine during hemorrhagic hypotension. Am J Physiol. 1988;255:H1211–H1218. doi: 10.1152/ajpheart.1988.255.5.H1211. [DOI] [PubMed] [Google Scholar]
- Wei EP, Moskowitz MA, Boccalini P, Kontos HA. Calcitonin gene-related peptide mediates nitroglycerin and sodium nitroprusside-induced vasodilation in feline cerebral arterioles. Circ Res. 1992;70:1313–1319. doi: 10.1161/01.res.70.6.1313. [DOI] [PubMed] [Google Scholar]
- Winn HR, Rubio R, Berne RM. Brain adenosine production in the rat during 60 secs of ischemia. Circ Res. 1979;45:486–492. doi: 10.1161/01.res.45.4.486. [DOI] [PubMed] [Google Scholar]
- Winn HR, Welsh JE, Rubio R, Berne RM. Changes in brain adenosine during bicuculline-induced seizures in rats. Effects of hypoxia and altered systemic blood pressure. Circ Res. 1980a;47:568–577. doi: 10.1161/01.res.47.4.568. [DOI] [PubMed] [Google Scholar]
- Winn HR, Welsh JE, Rubio R, Berne RM. Brain adenosine production in rat during sustained alteration in systemic blood pressure. Am J Physiol. 1980b;239:H636–H641. doi: 10.1152/ajpheart.1980.239.5.H636. [DOI] [PubMed] [Google Scholar]
- Winn HR, Rubio R, Berne RM. Brain adenosine concentration during hypoxia in rats. Am J Physiol. 1981;241:H235–H242. doi: 10.1152/ajpheart.1981.241.2.H235. [DOI] [PubMed] [Google Scholar]
- Zetterstrom T, Vernet L, Ungerstedt U, Tossman U, Jonzon B, Fredholm BB. Purine levels in the intact rat brain. Studies with an implanted perfused hollow fibre. Neurosci Lett. 1982;29:111–115. doi: 10.1016/0304-3940(82)90338-x. [DOI] [PubMed] [Google Scholar]