Abstract
Carotid artery stenting (CAS) is currently a standard procedure to treat severe carotid artery stenosis. This procedure causes mechanical plaque rupture, potentially releasing soluble factors into the circulating blood. The purpose of this study is to clarify whether inflammation factors are released from an atherosclerotic plaque after CAS and whether local release of inflammation factors is associated with periprocedural new ischemic lesions. The study consisted of 35 patients with 40 severely stenotic carotid arteries who underwent CAS. Blood samples were obtained from the aorta before the procedure and from the carotid plaque site just after the procedure. Blood levels of interleukin-6 (IL-6), interleukin-18, matrix metalloproteinase (MMP)-2, and tissue inhibitor of MMP-1 were determined. Diffusion-weighted magnetic resonance imaging was performed before and after the procedure. Among inflammatory markers, IL-6 levels markedly increased at the plaque site in comparison to those at the aorta (P<0.001). The IL-6 levels in the local samples were significantly higher in symptomatic lesions than those in asymptomatic lesions. More importantly, higher local IL-6 levels were associated with the appearance of new ischemic lesions (P=0.003). The association remained significant (P=0.030) after controlling for potential risk factors for CAS. Association of local IL-6 levels and periprocedural new ischemic lesions suggests that massive release from the plaque and entry into the cerebral circulation of IL-6 might be one of important factors on periprocedural complications related to CAS.
Keywords: carotid artery stenting, complications, embolism, inflammation, interleukins
Introduction
Inflammation is currently recognized as an important factor in the development, progression, and rupture of atherosclerotic plaques (Libby et al, 2002). The measurements of circulating inflammatory markers, including high sensitivity C-reactive protein, interleukin-6 (IL-6) (Harris et al, 1999; Ridker et al, 2000a; Ridker et al, 2000b), and IL-18 (Blankenberg et al, 2003), carry important information as predictors of future vascular events. Recently, other candidate markers associated with cardiovascular events have been indicated, such as matrix metalloproteinase (MMP) (Kai et al, 1998), and tissue inhibitor of MMP (TIMP) (Cavusoglu et al, 2006). Recent studies of carotid endoarterectomy (CEA) have reported the expression of all of these inflammatory markers, in carotid atherosclerotic plaques (Mallat et al, 2001; Choudhary et al, 2006; Krupinski et al, 2006), thus suggesting that inflammatory markers are transcribed locally. However, transcription itself does not necessarily indicate release from the plaque to the circulating blood (Maier et al, 2005). Carotid artery stenting (CAS), which is a less-invasive percutaneous procedure than CEA, is currently a standard treatment for carotid artery stenosis (Goodney et al, 2006). Although the risk of stroke and death resulting from CEA has been shown to depend on clinical indication (Bond et al, 2003), little is known about the risk factors for periprocedural complications after CAS (Stingele et al, 2008; Theiss et al, 2008). In contrast to CEA, CAS causes mechanical plaque rupture, releasing plaque debris as well as soluble factors from atheromatous plaques. It is obvious that plaque debris, unless trapped safely, causes distal embolism and severe complications. Use of embolism protection device during CAS markedly decreased the rate of periprocedural stroke (Goodney et al, 2006). However, soluble factors, once released from carotid plaque, could be directly transferred into the brain through the internal carotid artery (ICA). It could be helpful for safe approach of this procedure to know whether all or a part of soluble factors within the carotid plaque is released into the circulating blood during mechanical rupture. Therefore, the purpose of this study is to clarify (i) whether soluble factors of inflammation are released locally after CAS, and (ii) whether the release of the inflammatory markers is associated with periprocedural new ipsilateral diffusion-weighted cerebral MRI lesions.
Materials and methods
Subjects
Between December 2005 and November 2007, 37 patients underwent CAS for symptomatic or asymptomatic stenosis in Osaka University Hospital. Among them, two patients with laryngeal carcinoma (n=1) or aortic aneurysm (n=1) were excluded from this study. Thus, this study included 35 patients with 40 severely stenotic carotid vessels, including five patients with bilateral carotid artery stenosis, who were to undergo CAS. Patients with a history of ipsilateral ischemic events in the preceding 12 months were defined as ‘symptomatic' patients, and ‘asymptomatic' was defined by the absence of such ischemic events. Eight of the patients underwent CAS within 4 weeks after acute cerebral ischemic events. All patients were assessed for stenotic lesions using B-mode and color Doppler imaging before CAS. This study was approved by the Ethics Committee of Osaka University Hospital (# 06055). Written informed consent was obtained from all patients.
Procedures
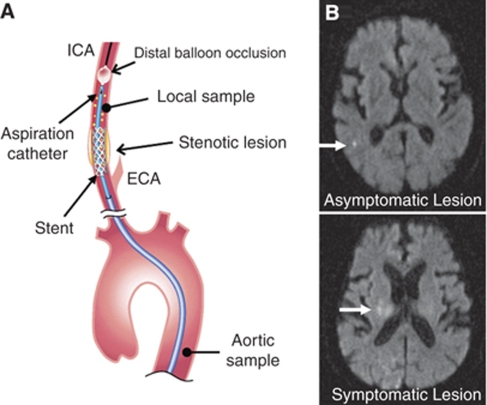
All patients received antiplatelet therapy at least for a week before CAS. Among 35 patients, 12 received aspirin alone (100 mg per day), and 21 received the combination of aspirin (100 mg per day) with ticlopidine (200 mg/day, n=11), clopidogrel (75 mg/day, n=4) or cilostazol, inhibitor of cyclic adenosine monophosphate phosphodiesterase (200 mg/day, n=6). The other two patients received clopidogrel or cilostazol alone. In all patients, CAS was performed by the same medical teams (TF and MS) with the PercuSurge GuardWire cerebral protection device based on balloon occlusion of the distal ICA and debris aspiration. An 8-Fr-long sheath was initially placed into the descending aorta by a femoral approach. The patients were administered 5000 IU heparin before CAS to increase the activated clotting time to as long as 250 to 300 secs. Systemic blood samples were obtained from the descending aorta. An 8-Fr guiding catheter was inserted into the common carotid artery, and the GuardWire was then slowly advanced through the guiding catheter and cross the ICA lesion. After passing the stenotic lesion, the distal protection balloon was inflated, and the stenotic vessels were predilated with a slightly smaller balloon. The stent was placed into the stenotic lesion, followed by post dilatation. Any generated debris was removed from the ICA with an aspiration catheter. The first sampling of debris-laden blood from the culprit lesion was used as the postprocedural local sample (Figure 1A). When aspiration was complete, the distal balloon was deflated, re-establishing normal blood flow. The procedure lasted 10 to 15 mins. Sera and plasma were separated, and samples were frozen at −80oC until use.
Figure 1.
(A) A schematic drawing of aortic and local sampling during carotid artery stenting and aspiration. ICA, internal carotid artery; ECA, external carotid artery. (B) Representative DWI-MRI imaging after CAS. New ischemic lesions are shown with white arrows.
Measurement of Blood Inflammatory Markers
The serum concentrations of IL-6 and IL-18, and the plasma concentrations of TIMP-1 and MMP-2 were measured. The serum IL-6 levels and plasma levels of TIMP-1 and MMP-2 were measured by enzyme-linked immunosorbent assays, (ELISA; R&D Systems, Minneapolis, MN, USA). Serum IL-18 levels were also measured by an ELISA (MBL Co, Ltd., Nagoya, Japan).
Carotid Ultrasonography
All examinations were performed with the use of Phillips SONOS 5500 equipped with a 7.5 MHz linear-array transducer (Phillips Medical Systems, Tokyo, Japan). A single trained operator (YA) performed all carotid scans without clinical detail information of patients. The common and ICAs were assessed cross-sectionally and longitudinally by B-mode and color Doppler imaging. The degrees of the stenotic lesion were measured by the method used in the European Carotid Surgery Trial (Rothwell et al, 1994).
MRI Assessments
MR imaging of the brain was performed before and after treatment with a 1.5 T Signa Horizon (GE Healthcare Japan, Hino, Tokyo, Japan) or 1.5 T magnetom Vision (Siemens-Asahi Medical Technology, Tokyo, Japan). The whole brain was scanned, and 20 axial images were produced; slice thickness of 5 mm and interslice gap of 2 mm. The imaging protocol consisted of a T2-weighted fluid-attenuated inversion recovery and diffusion-weighted MRI imaging (DWI) (Figure 1B). MR imaging was performed within 3 days after CAS. New cerebral ischemic lesions were assessed by experienced neuroradiologists, who were blinded for all clinical details.
Data Analysis
Samples of aspirated stenotic lesions were diluted slightly in 0.9% sodium chloride solution and iodinated contrast medium. To correct for dilution, the concentration was normalized for total protein. In addition, the serum and plasma levels of albumin, aspartate amino transferase, and total cholesterol were measured to ensure consistency. All analyses were performed with SPSS 11.5J software (SPSS Japan Inc., Tokyo, Japan). The Wilcoxon signed-rank test was used for pair wise comparisons. Comparisons between two different groups were carried out with the Mann–Whitney U-test. Subsequently, the association of the local IL-6 levels with periprocedural new ischemic lesions was examined by a logistic regression model together with age, sex, prior symptoms, and contralateral carotid stenosis—potential risk factors for CAS—(Stingele et al, 2008). Values of P<0.05 were considered to be statistically significant.
Results
Patient Characteristics
The baseline characteristics of the patients are summarized in the Table 1. The study included 16 symptomatic and 19 asymptomatic patients for CAS. Among the 40 vessels subjected to CAS, 17 vessels were symptomatic. Although the prevalence of atherosclerotic risk factors was relatively high in the subjects, they were generally well controlled by medication.
Table 1. Baseline patient characteristics (n=35 patients, 40 vessels).
| Age, year | 69.4±6.15 |
| Sex, n (%) Male/female | 30 (86)/5 (14) |
| Body mass index, kg/m2 | 22.6±1.9 |
| Hypertension/ACEI or ARB use, n (%) | 32 (91) /18 (51) |
| Systolic/diastolic BP, mm Hg | 134.7±13.3/73.6±9.7 |
| Diabetes mellitus/medical treatment, n (%) | 19 (54)/8 (23) |
| Fasting blood glucose, mmol/L | 6.1±1.1 |
| Hemoglobin A1c, % | 5.8±1.0 |
| Dyslipidemia/statin use, n (%) | 24 (69)/18 (58) |
| Total/LDL cholesterol, mmol/L | 4.7±0.9/2.7±0.7 |
| Triglyceride, mmol/L | 1.6±1.3 |
| HDL cholesterol, mmol/L | 1.3±0.7 |
| Smokers, n (%) | 28 (68) |
| Carotid stenosis, % | 81.8±5.7 |
| History of CVD | |
| Stroke/transient ischemic attack, n (%) | 16 (45)/6 (17) |
| Ischemic heart disease, n (%) | 22 (63) |
| ASO, n (%) | 9 (26) |
| Antiplatelet/aspirin use, n (%) | 35 (100)/33 (94) |
| Aspirin (100 mg/day) alone | 12 (34) |
| Aspirin and other antiplatelet drugs | 21 (60) |
| Clopidogrel (75 mg/day) alone | 1 (3) |
| Cilostazol (200 mg/day) alone | 1 (3) |
ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin type II receptor blockers; ASO, arteriosclerosis obliterans; CVD, cerebrovascular disease. Values indicate mean±s.d. NS indicates not significant.
Blood Inflammatory Marker Levels in Aortic Versus Local Samples
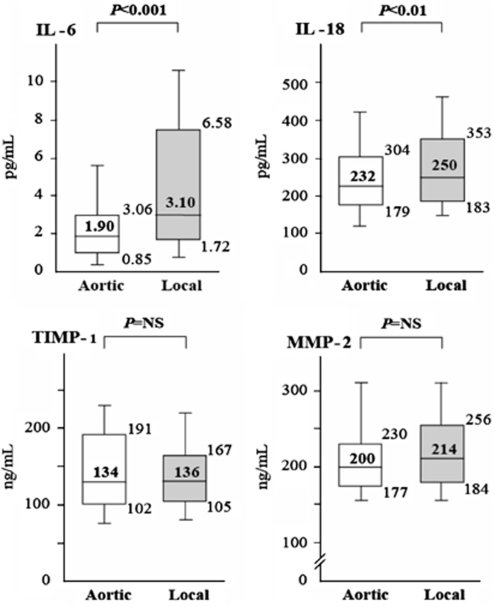
The local IL-6 levels (median, 3.10 pg/mL; interquartile range (IQR), 1.72 to 6.58 pg/mL) markedly increased at the plaque site in comparison to the aortic values (median, 1.90 pg/mL; IQR, 0.85 to 3.06 pg/mL) (P<0.001) (Figure 2). The local IL-18 level (median, 250 pg/mL; IQR, 183 to 353 pg/mL) was slightly increased over the aortic values (median, 232 pg/mL; IQR, 179 to 304 pg/mL) (P<0.01). In contrast, the local levels of TIMP-1, and MMP-2 were similar to their aortic ones (P=NS).
Figure 2.
Aortic (white bars) and local (shaded bars) levels of IL-6, IL-18, MMP-2, and TIMP-1. Medians and 10th, 25th, 75th, and 90th percentiles are shown.
Symptomatic Versus Asymptomatic Stenosis
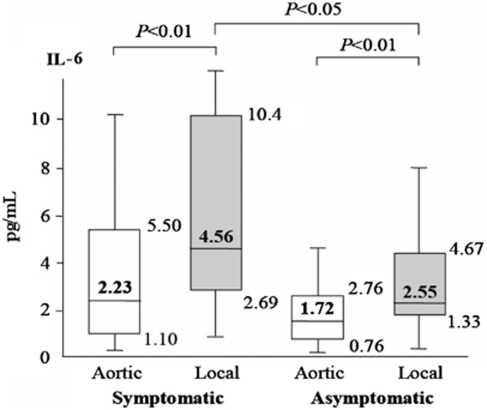
The local IL-6 levels significantly increased both in symptomatic vessels and in asymptomatic lesions (Figure 3). In symptomatic vessels, the IL-6 levels increased from 2.23 pg/mL (IQR, 1.10 to 5.50 pg/mL) in the aortic samples to 4.56 pg/mL (IQR, 2.69 to 10.4 pg/mL) in the local samples (P<0.01). In the asymptomatic lesions, they increased from 1.72 pg/mL (IQR, 0.76 to 2.76 pg/mL) in the aortic samples to 2.55 pg/mL (IQR, 1.33 to 4.67 pg/mL) in the local samples (P<0.01). However, the local IL-6 levels in the symptomatic vessels were significantly higher than those in the asymptomatic vessels (P<0.05).
Figure 3.
Aortic (white bars) and local (shaded bars) levels of IL-6 in symptomatic and asymptomatic vessels. Medians and 10th, 25th, 75th, and 90th percentiles are shown.
Periprocedural New Ipsilateral DWI-MRI Lesions
Among 40 cases, new ischemic lesions in DWI-MRI were found in the ipsilateral hemispheres of 12 cases (30%) after CAS. Among them, only one patient had major stroke (Table 2). Local IL-6 levels were significantly higher in cases with new ischemic lesions (6.33 pg/mL; range, 0.68 to 23.8 pg/mL) than in cases without (2.48 pg/mL; range, 0.25 to 10.97 pg/mL) (P=0.003), whereas aortic IL-6 levels tended to be higher in cases with new ischemic lesions than in those without, but it did not reach to statistical difference (P=0.056). The levels of other inflammatory markers in the local and aortic samples were almost similar between cases with and without new ischemic lesions (Table 2). Potential risk factors for CAS (Stingele et al, 2008)—age, sex, prior symptoms, contralateral carotid stenosis (>90% or occlusion)—did not differ between cases with and without new ischemic lesions in this study (Table 2). The difference in local IL-6 levels between cases with and without new ischemic lesions remained significant (P=0.030) when we controlled for these potential risk factors for CAS.
Table 2. Aortic and local inflammatory markers, and other variables according to the periprocedural new DWI-MRI ischemic lesions.
| Lesions (n=12) | No lesions (n=28) | P-value | ||
|---|---|---|---|---|
| IL-6 (pg/mL) | Aortic | 3.06 (0.56–7.53) | 1.58 (0.16–10.00) | 0.056 |
| Local | 6.33 (0.68–23.8) | 2.48 (0.25–10.97) | 0.003 | |
| IL-18 (pg/mL) | Aortic | 231 (154–451) | 234 (87–654) | 0.610 |
| Local | 274 (200–429) | 238 (92–760) | 0.273 | |
| TIMP-1 (ng/mL) | Aortic | 134 (76.8–294) | 141 (33.3–342) | 0.678 |
| Local | 145 (64.7–255) | 138 (64.7–289) | 0.652 | |
| MMP-2 (ng/mL) | Aortic | 208 (130–394) | 200 (133–366) | 0.963 |
| Local | 219 (119–346) | 203 (142–431) | 0.896 | |
| Age, year | 71.8±4.1 | 68.3±6.6 | 0.202 | |
| Male, % | 75 | 93 | 0.389 | |
| Symptomatic lesions, % | 58 | 36 | 0.273 | |
| Contralateral stenosis, % | 33 | 14 | 0.358 |
CAS, carotid artery stenting; DWI-MRI, diffusion-weighted MRI imaging; IL, interleukin; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of MMP.
Data for inflammatory marker levels are presented as medians (ranges).
The association between local IL-6 levels and new ischemic lesions remained significant (P=0.030) after controlling for potential risk factors for CAS—age, sex, prior symptom, and contralateral stenosis.
Discussion
This study showed that IL-6 is instantly released by a mechanical plaque rupture resulting from CAS. Meanwhile, the local level of IL-18 was slightly but significantly increased, and there were no significant patterns of change in TIMP-1 and MMP-2 levels. As previous studies reported expression of all of these proteins in samples of CEA (Mallat et al, 2001; Choudhary et al, 2006; Krupinski et al, 2006), it is surprising to find that among the inflammatory markers, only IL-6 is markedly released from atheromatous carotid plaques after CAS. Our study suggested that IL-6 was released into the ICA by mechanical rupture after CAS and it could enter the brain, which would not occur in the case of CEA.
Prominent secretion of IL-6 from symptomatic lesions supported the close association of plaque instability and IL-6 production in vulnerable plaques. Rus and Niculescu (1999) reported 200-fold higher levels of IL-6 in atherosclerotic lesions than in blood. IL-6 expression level is significantly elevated in carotid lesions collected from patients who had suffered from a recent stroke (Peeters et al, 2009). Moreover, higher levels of IL-6 are associated with lower echo-lucent carotid plaque (Yamagami et al, 2004), a sign of vulnerable plaque (Grønholdt et al, 2001). These results suggest that the amount of local IL-6 is related to plaque instability. Locally produced IL-6 may have roles in the expression of high sensitivity C-reactive protein, other proinflammatory cytokines, MMP, and tissue factor within atherosclerotic lesions as a messenger cytokine (Packard and Libby, 2008).
Surgical and endovascular approaches for severe carotid stenosis are recommended to prevent ischemic stroke, particularly in symptomatic patients. However, about 3% of patients incidentally suffer from ipsilateral stroke in both techniques (Goodney et al, 2006). Distal embolism and cerebral hyperperfusion syndrome are serious adverse effects. For CAS, development of device such as balloon protection and filter system lessened the incidence of distal embolism. However, because of mechanical rupture of atheromatous plaque within the vessel, CAS could cause massive release of soluble factors into the circulating blood. Little attention has been paid to this aspect in CAS. This study clearly suggested the possibility that IL-6 could be released into the ICA and transferred into the brain circulation. Close association between local IL-6 levels and periprocedural new ischemic lesions (Table 2), suggests that transfer of IL-6 into the cerebral circulation after CAS might have important functions on periprocedural complications. There are growing evidence that circulating IL-6 levels are associated with intracranial atherosclerosis (Hoshi et al, 2008) and cerebral small vessel disease (Hoshi et al, 2005). At least two potential mechanisms could be proposed for relation of IL-6 release and periprocedural complications. First, IL-6 accelerates cytokine production and oxygen radical production within the vessels (Kofler et al, 2005). In smooth muscle cells, acceleration of free radical production was reported after exposure to IL-6 (Wassmann et al, 2004). Second, IL-6 decreases expression of endothelial nitric oxide synthase and causes endothelial dysfunction (Saura et al, 2006; Schrader et al, 2007). Production of oxygen-free radicals and endothelial dysfunction were recognized as one of key components for cerebral hyperperfusion after carotid revascularization (van Mook et al, 2005). However, there is possibility that other soluble factors released, such as hemostatic factors, lipid peroxide, and vasoactive substances, are more important for periprocedural stroke than IL-6. To prevent the soluble factors from entering the brain, balloon protection device seems better than filter device during CAS. However, after CAS, plaque component displaced outside the stent could slowly release soluble factors into the ICA.
There are several limitations to this study. First, aspirated samples of stenotic lesions were slightly diluted in 0.9% sodium chloride solution. To correct for this dilution, the concentration was normalized for total protein. However, small amounts of protein may be secreted from the plaque to the aspirate. Therefore, it is possible that the levels of inflammatory markers in the local samples were underestimated. Second, the concentrations of inflammatory markers may be affected by stress or invasive procedures and could be elevated in response to CAS. However, this point may have little influence on the results because the aim was to compare levels of inflammatory markers between aortic blood samples before CAS and local blood samples just after CAS. Finally, this study was a small case study. The association between release of IL-6 and the risk of periprocedural stroke will have to be examined with a large sample size in the future study.
In conclusion, the IL-6 levels markedly increased in stenotic lesions in vivo in comparison to the aortic values after CAS. It is highly likely that IL-6 is produced within carotid atherosclerosis plaques and then released into the circulating blood. Furthermore, the local IL-6 level was found to be significantly increased in patients with periprocedural new ischemic lesions after CAS. The significance of soluble factors released from atheromatous plaque after CAS on periprocedural stroke should be further examined.
Acknowledgments
We thank Ms C Kurano and Ms M Nishiyama for their secretarial and technical assistances.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Blankenberg S, Luc G, Ducimetière P, Arveiler D, Ferrières J, Amouyel P, Evans A, Cambien F, PRIME Study Group Interleukin-18 and the risk of coronary heart disease in European men: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) Circulation. 2003;108:2453–2459. doi: 10.1161/01.CIR.0000099509.76044.A2. [DOI] [PubMed] [Google Scholar]
- Bond R, Rerkasem K, Rothwell PM. Systematic review of the risks of carotid endarterectomy in relation to the clinical indication for and timing of surgery. Stroke. 2003;34:2290–2303. doi: 10.1161/01.STR.0000087785.01407.CC. [DOI] [PubMed] [Google Scholar]
- Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, Clark LT, Pinsky DJ, Marmur JD. Tissue inhibitor of metalloproteinase-1 (TIMP-1) is an independent predictor of all-causemortality, cardiac mortality, and myocardial infarction. Am Heart J. 2006;151:1101.e1101–1101.e1108. doi: 10.1016/j.ahj.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Choudhary S, Higgins CL, Chen IY, Reardon M, Lawrie G, Vick GW, 3rd, Karmonik C, Via DP, Morrisett JD. Quantitation and localization of matrix metalloproteinases and their inhibitors in human carotid endarterectomy tissues. Arterioscler Thromb Vasc Biol. 2006;26:2351–2358. doi: 10.1161/01.ATV.0000239461.87113.0b. [DOI] [PubMed] [Google Scholar]
- Goodney PP, Schermerhorn ML, Powell RJ. Current status of carotid artery stenting. J Vasc Surg. 2006;43:406–411. doi: 10.1016/j.jvs.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Grønholdt ML, Nordestgaard BG, Schroeder TV, Vorstrup S, Sillesen H. Ultrasonic echolucent carotid plaques predict future strokes. Circulation. 2001;104:68–73. doi: 10.1161/hc2601.091704. [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Hoshi T, Kitagawa K, Yamagami H, Furukado S, Hougaku H, Hori M. Relations between serum high-sensitivity C-reactive protein and interleukin-6 levels and silent brain infarction. Stroke. 2005;36:768–772. doi: 10.1161/01.STR.0000158915.28329.51. [DOI] [PubMed] [Google Scholar]
- Hoshi T, Kitagawa K, Yamagami H, Furukado S, Hougaku H, Hori M. Relation between interleukin-6 level and subclinical intracranial large-artery atherosclerosis. Atherosclerosis. 2008;197:326–332. doi: 10.1016/j.atherosclerosis.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Kai H, Ikeda H, Yasukawa H, Kai M, Seki Y, Kuwahara F, Ueno T, Sugi K, Imaizumi T. Peripheral blood levels of metalloproteases-2 and -9 are elevated in patients with acute coronary syndromes. Am J Cardiol. 1998;32:368–372. doi: 10.1016/s0735-1097(98)00250-2. [DOI] [PubMed] [Google Scholar]
- Kofler S, Nickel T, Weis M. Role of cytokines in cardiovascular diseases: a focus on endothelial responses to inflammation. Clin Sci (Lond) 2005;108:205–213. doi: 10.1042/CS20040174. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Turu MM, Martinez-Gonzalez J, Carvajal A, Juan-Babot JO, Iborra E, Slevin M, Rubio F, Badimon L. Endogenous expression of C-reactive protein is increased in active (ulcerd noncomplicated) human carotid artery plaques. Stroke. 2006;37:1200–1204. doi: 10.1161/01.STR.0000217386.37107.be. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1134. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Maier W, Altwegg LA, Corti R, Gay S, Hersberger M, Maly FE, Sutsch G, Roffi M, Neidhart M, Eberli FR, Tanner FC, Gobbi S, von Eckardstein A, Luscher TF. Inflammatory markers at the site of ruptured plaque in acute myocardial infarction: locally increased interleukin-6 and serum amyloid A but decreased C-reactive protein. Circulation. 2005;111:1355–1361. doi: 10.1161/01.CIR.0000158479.58589.0A. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Corbaz A, Scoazec A, Besnard S, Lesèche G, Chvatchko Y, Tedgui A. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation. 2001;104:1598–1603. doi: 10.1161/hc3901.096721. [DOI] [PubMed] [Google Scholar]
- Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54:24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- Peeters W, Hellings WE, de Kleijn DPV, de Vries JPPM, Moll FL, Vink A, Pasterkamp G. Carotid atherosclerotic plaques stabilizes after stroke. Insights into the natural process of atherosclerotic plaque stabilization. Arterioscler Thromb Vasc Biol. 2009;29:128–133. doi: 10.1161/ATVBAHA.108.173658. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000a;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000b;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Gibson RJ, Slattery J, Sellar RJ, Warlow CP. Equivalence of measurements of carotid stenosis. A comparison of three methods on 1001 angiogram. European Carotid Surgery Trialists' Collaborative Group. Stroke. 1994;25:2435–2439. doi: 10.1161/01.str.25.12.2435. [DOI] [PubMed] [Google Scholar]
- Rus H, Niculescu F. Inflammatory response in unstable angina. Circulation. 1999;100:e98. doi: 10.1161/01.cir.100.19.e98. [DOI] [PubMed] [Google Scholar]
- Saura M, Zaragoza C, Bao C, Herranz B, Rodriguez-Puyol M, Lowenstein CJ. Stat3 mediates interleukin-6 inhibition of human endothelial nitric-oxide synthase expression. J Biol Chem. 2006;281:30057–30062. doi: 10.1074/jbc.M606279200. [DOI] [PubMed] [Google Scholar]
- Schrader LI, Kinzenbaw DA, Johnson AW, Faraci FM, Didion SP. IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler Thromb Vasc Biol. 2007;27:2576–2581. doi: 10.1161/ATVBAHA.107.153080. [DOI] [PubMed] [Google Scholar]
- Stingele R, Berger J, Alfke K, Eckstein HH, Fraedrich G, Allenberg J, Hartmann M, Ringleb PA, Fiehler J, SPACE investigators. Bruckmann H, Hennerici M, Jansen O, Klein G, Kunze A, Marx P, Niederkorn K, Schmiedt W, Solymosi L, Zeumer H, Hacke W. Clinical and angiographic risk factors for stroke and death within 30 days after carotid endarterectomy and stent-protected angioplasty: a subanalysis of the SPACE study. Lancet Neurol. 2008;7:216–222. doi: 10.1016/S1474-4422(08)70024-3. [DOI] [PubMed] [Google Scholar]
- Theiss W, Hermanek P, Mathias K, Brückmann H, Dembski J, Hoffmann FJ, Kerner R, Leisch F, Mudra H, Schulte KL, Sievert H. Predictors of death and stroke after carotid angioplasty and stenting. A subgroup analysis of the Pro-CAS data. Stroke. 2008;39:2325–2330. doi: 10.1161/STROKEAHA.108.514356. [DOI] [PubMed] [Google Scholar]
- van Mook WN, Rennenberg RJ, Schurink GW, van Oostenbrugge RJ, Mess WH, Hofman PA, de Leeuw PW. Cerebral hyperperfusion syndrome. Lancet Neurol. 2005;4:877–888. doi: 10.1016/S1474-4422(05)70251-9. [DOI] [PubMed] [Google Scholar]
- Wassmann S, Stumpf M, Strehlow K, Schmid A, Schieffer B, Böhm M, Nickenig G. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ Res. 2004;94:534–541. doi: 10.1161/01.RES.0000115557.25127.8D. [DOI] [PubMed] [Google Scholar]
- Yamagami H, Kitagawa K, Nagai Y, Hougaku H, Sakaguchi M, Kuwabara K, Kondo K, Masuyama T, Matsumoto M, Hori M. Higher levels of interleukin-6 are associated with lowerechogenicity of carotid artery plaques. Stroke. 2004;35:677–668. doi: 10.1161/01.STR.0000116876.96334.82. [DOI] [PubMed] [Google Scholar]