Abstract
Severity of stroke varies widely among individuals. Whether differences in the extent of the native (preexisting) pial collateral circulation exist and contribute to this variability is unknown. We addressed these questions and probed for potential genetic contributions using morphometric analysis of the collateral circulation in 15 inbred mouse strains recently shown to exhibit wide differences in infarct volume. Morphometrics were determined in the unligated left hemisphere (for native collaterals) and ligated right hemisphere (for remodeled collaterals) 6 days after permanent middle cerebral artery (MCA) occlusion. Variation among strains in native collateral number, diameter, MCA, anterior cerebral artery (ACA), and posterior cerebral artery (PCA) tree territories were, respectively: 56-fold, 3-fold, 42%, 56%, and 61%. Collateral length (P<0.001) and the number of penetrating arterioles branching from them also varied (P<0.05). Infarct volume correlated inversely with collateral number (P<0.0001), diameter (P<0.0001), and penetrating arteriole number (P<0.05) and directly with MCA territory (P<0.05). Relative collateral conductance and MCA territory, when factored together, strongly predicted infarct volume (P<0.0001). Outward remodeling of collaterals in the ligated hemisphere varied ∼3-fold. These data show that the extent of the native pial collateral circulation and collateral remodeling after obstruction vary widely with genetic background, and suggest that this variability, due to natural polymorphisms, is a major contributor to variability in infarct volume.
Keywords: arteriole anastomoses, cerebral circulation, collaterals, infarction, mouse strain
Introduction
Occlusive vascular disease of the brain, heart, and peripheral limbs is the leading cause of morbidity and mortality in developed countries. The presence of an abundant collateral circulation is believed to provide substantial protection. The 18th century English physician, John Hunter, first coined the term ‘collaterals' to describe the presumed presence of native (preexisting) arteriole-to-arteriole anastomoses interconnecting arterial trees of the thigh and calf in a patient with a popliteal aneurism, to account for the significant distal perfusion that remained after femoral artery ligation (Yasargil, 1987). It is now recognized that these unique vessels can serve as ‘endogenous bypass vessels' when present in the heart and limbs of healthy individuals, should occlusive disease develop (Heil et al, 2006).
Heubner (1874) described the presence of similar collaterals, which he termed ‘leptomeningeal anastomoses,' interconnecting some of the outer branches of the anterior (ACA), middle (MCA), and posterior cerebral artery (PCA) trees of the pial circulation supplying the human cerebral cortex. Perfusion of the penumbral cortex after proximal obstruction of an artery (e.g., the MCA) distal to the Circle of Willis is assumed to depend, in part, on the extent (i.e., number and diameter) of these preexisting vessels (Brozici et al, 2003; Liebeskind, 2005; Hossmann, 2006). However, whether the pial collateral circulation significantly impacts infarct volume is a controversial subject that has received relatively little treatment in recent authoritative monographs and textbooks (Brozici et al, 2003; Mohr et al, 2004). Doubts about its contribution may extend from the small caliber and apparent variable presence of these vessels (Mohr et al, 2004) plus the absence of methods—other than recent gene targeting approaches (Clayton et al, 2008; Chalothorn et al, 2009), to experimentally vary their abundance in animal studies.
Meier et al (2007) recently reported indirect evidence suggesting that a large variation exists among healthy humans in the density and/or diameter of native coronary collaterals, as well as the ability of these vessels to enlarge/remodel in patients with obstructive disease (a process termed ‘arteriogenesis' Heil et al, 2006; Hossmann and Buschmann, 2005). Likewise, Liebeskind (2005), Bang and coworkers (2008) and Christoforidis et al (2009) recently found, using dynamic angiography in patients suffering acute obstruction of the MCA, that retrograde perfusion of the MCA tree downstream from the occlusion, which is dependent on collateral extent, varies substantially among patients. Whether differences in collateral number or diameter, secondary to natural genetic variation or environmental factors, exist and contribute to this variability is unknown. We previously reported that the C57BL/6 and BALB/c mouse strains exhibit large differences in the density and diameter of native collaterals in several tissues, including the cerebral cortex, which are accompanied by large differences in infarct volume after occlusion (Chalothorn et al, 2007). We also identified two genes, Vegfa and Clic4, whose level of expression strongly influence—in multiple tissues—the formation of the collateral circulation, which occurs perinatally in mice (collaterogenesis), and severity of stroke (Clayton et al, 2008; Chalothorn et al, 2009). These findings indicate that variation in the expression of genes controlling collaterogenesis can impact the extent of the native collateral circulation.
This study had these three aims: (1) to extend the above analyses to a cohort of in-bred strains to understand the degree to which natural genetic variation can contribute to variation in collateral circulatory function; (2) to determine whether the extent of the native collateral circulation impacts stroke volume; and (3) to provide a mouse strain database for future studies aimed at understanding the source of variation in the collateral circulation, i.e., to identify optimal strains to cross for mapping quantitative trait loci (QTL) and haplotypes for identification of candidate genes regulating native collaterogenesis and collateral remodeling in ischemia. We measured native and remodeled (i.e., after permanent MCA occlusion) pial collateral dimensions in 15 inbred mouse strains derived primarily from the Mouse Diversity Panel (Payseur and Place, 2007). Collateral metrics were correlated against values for infarct volume after permanent MCA occlusion that were reported recently by Keum and Marchuk (2009) for the same strains. Obstruction of the MCA is the most frequently affected artery in human embolic stroke (Mohr et al, 2004). We report that the extent of the native pial collateral circulation exhibits remarkably wide variation, due to natural genetic differences, that closely predicts an equally wide variation in severity of stroke.
Materials and methods
The strains of male 10 to 12-week-old mice (Jackson Laboratories, Bar Harbor, ME, USA) studied were: C57BLKS/J(BLKS), FVB/NJ(FVB/N), CBA/J(CBA), DBA/2J(DBA/2), NOD/ShiLtJ(NOD), SJL/J(SJL), 129S1/SvImJ(129S1), C57BL/6J(B6), NZW/LacJ(NZW), KK/HlJ(KK), C3H/HeJ(C3H), A/J(A), AKR/J(AKR), BALB/cJ(BALB), and SWR/J(SWR). All the procedures were according to the NIH Guidelines.
Middle Cerebral Artery Occlusion
Mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) ip. An ∼4 mm incision was made between the right eye and ear, and the temporal muscle was retracted from its midpoint to partially expose the zygomatic and temporal bones. An ∼2 mm diameter craniotomy was made over the trunk of the MCA with a drill (18000-17, FST Inc, Foster City, CA, USA). The MCA was cauterized (18010-00, FST, modified) and transected, and the incision was closed with suture and Vetbond (3 M). Permanent MCAO occlusion (versus reversible filament or carotid occlusion (Virley, 2005)) was used because it was used previously to study infarct volume in the above strains (Keum and Marchuk, 2009), because we sought to also examine collateral remodeling (requiring sustained increased shear stress), and to prevent known anatomic variation among mouse strains in the anterior and posterior commissural arteries from influencing infarct volume (Wellons et al, 2000; Carmichael, 2005).
Cerebral Collateral Dimensions
Cerebral collateral dimensions were measured 6 days after MCAO on the unligated side (for native collaterals) and ligated side (for remodeled collaterals) (Chalothorn et al, 2009). Mice were anesthetized with ketamine and xylazine, and heparinized. The abdominal aorta was cannulated retrograde and the circulation was cleared and maximally dilated with adenosine (1 mg/mL) and papaverine (40 μg/mL) in phosphate-buffered saline. The dorsal calvarium was removed to expose the pial circulation. The thoracic aorta was cannulated retrograde and polyurethane (PU4ii, Vasqtec, Switzerland) with a viscosity sufficient to restrict capillary transit (1:1 resin:methylethyl ketone) was infused into the cerebral circulation viewed with a stereomicroscope. Paraformaldehyde (4% PFA in phosphate-buffered saline) was applied topically to the cortex, and the polyurethane was allowed to cure for 20 mins. After post-fixation in 4% PFA overnight, the pial circulation was imaged (Leica MZ16FA, Bannockburn, IL, USA). Collaterals were then denoted on the images (Figure 1) during direct viewing using stereomicroscopy for later determination of collateral morphometrics. We confined our analysis to collaterals between the MCA and ACA trees for the following reasons (Chalothorn et al, 2007): (1) in strains with abundant collaterals (e.g., B6) the number interconnecting the ACA and MCA trees is ∼5-fold greater than the several collaterals interconnecting the MCA and PCA trees; (2) the territories of the MCA and ACA trees, together, overlie ∼85% to 95% of the dorsal cerebral cortical area, with the remainder supplied by the PCA; and (3) the full length of the several collaterals connecting to the PCA often cannot be imaged.
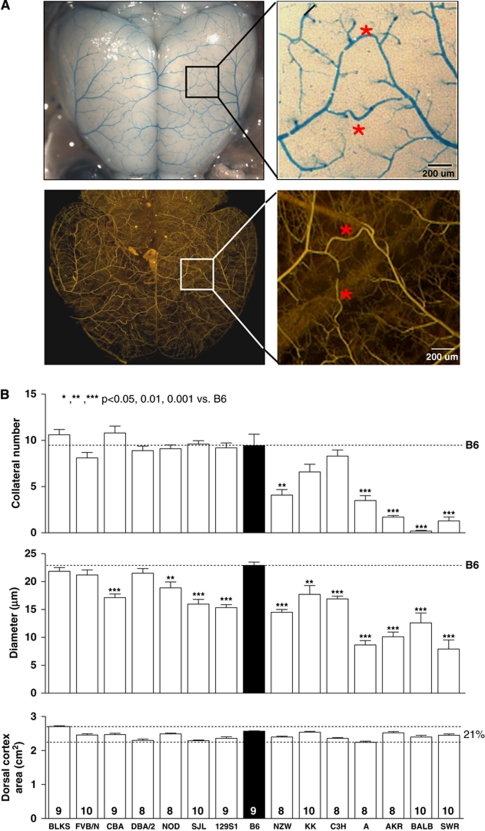
Figure 1.
Wide genetic variance of the native pial collateral circulation exists among 15 inbred mouse strains. (A) Mouse pial arterial circulation. Upper panels show vasculature after clearing, dilation, fixation, and filling with high-viscosity polyurethane restricted from capillary transit. Measurements in (B) and Figures 2, 3, 4, 5, 6 to 7 and Supplementary Figures are for collaterals interconnecting the middle and anterior cerebral artery trees (*). Lower panels show high-viscosity microfil (Flow Tech Inc), followed by optical clearance with methylsalicylate, to highlight penetrating arterioles and confirm that native collaterals are confined to the pial surface. An occasional center-most collateral segment between two penetrating arterioles that escapes filling is easily identified as part of a single collateral (lower enlargement). (B) Variation in collateral number and diameter (per hemisphere) is unrelated to small variation in cortex area (or body weight, Supplementary Figure 1). B6, C57BL/6; number of animals given at base of columns also applies to subsequent figures, unless indicated otherwise.
For each collateral, lumen diameter was obtained as the average at 3 to 4 positions along the middle third of the collateral, and length was determined by tracing using a tablet-PC (Photoshop, Adobe Inc; ImageJ, NIH; see legend of Supplementary Figure 5 for additional details). Cortical territories (surface areas) supplied by the MCA, ACA, and PCA were determined.
Statistics
Data are given as the mean±s.e.m. for n number of mice (n≈10 per strain) and were subjected to ANOVA, unpaired two-tailed t-tests or regression analysis.
Results
Wide Genetic Variation in the Extent of the Native Pial Collateral Circulation
Retrograde perfusion of the distal MCA in patients suffering acute obstruction of its trunk varies substantially (Bang et al, 2008; Christoforidis et al, 2009), presumably in part from differences in the extent of the preexisting pial collateral circulation. The contribution of genetic differences to such variation is unknown. We performed morphometry on the pial collateral circulation of the left hemisphere 6 days after right MCAO. Occlusion of the right MCA does not affect collateral number or diameter in the left hemisphere [data not shown; see last paragraph of the figure legend for Supplementary Figure 5 for additional information; note also that the number and diameter for B6 and BALB agree with previously obtained values wherein MCAO was not performed, and that collateral number does not differ significantly between the hemispheres (Chalothorn et al, 2007, 2009)]. Also, penetrating arterioles branching from the cerebral artery trees (Figure 1) end in capillaries; thus, cortical collaterals in mouse are confined to the pial circulation (Chalothorn et al, 2007; Chalothorn and Faber, 2009) in agreement with rat and human (Coyle, 1984; Duvernoy, 1999; Wei et al, 2001). Therefore, data given in Figure 1 represent the number and diameter of native collaterals interconnecting the MCA and ACA trees per hemisphere. Pronounced variation exists, indicating a significant effect of genetic background. Diameters are smaller in strains with fewer collaterals (P<0.0001, Figure 2B). Thus, variation in collateral number and diameter could arise from a polymorphism in a gene(s) involved in specifying formation of the native collateral circulation. Dorsal cortex area showed modest variation (21%) that did not correlate with collateral number or diameter (Figure 1). Body weight (Supplementary Figure 1) also showed no correlation with collateral number or diameter (or other parameters measured below).
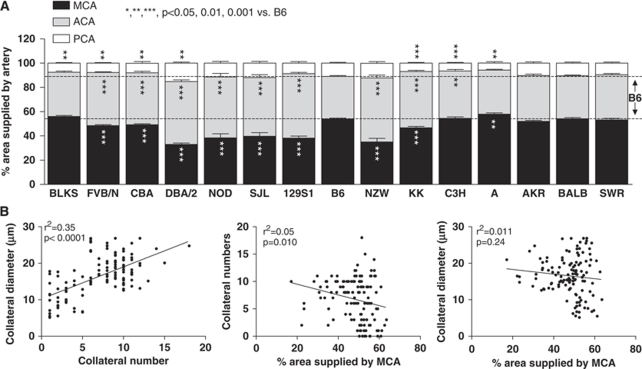
Figure 2.
Genetic variation in cerebral artery tree territory. (A) MCA, ACA, PCA, middle, anterior, and posterior cerebral artery tree areas, expressed as a percentage of dorsal cortex surface area. (B) Collateral number and diameter correlated closely. Collateral number correlated weakly with MCA area.
Wide Genetic Variability in Cerebral Artery Territories
Differences in the native collateral density and diameter could be influenced by differences in the size of the cerebral artery trees. Maeda et al (1998) reported that the MCA and ACA territories of B6 mice are larger and smaller, respectively, than of 129S1 mice. Figure 2A confirms this finding (but note that collateral number is the same for B6 and 129S1; Figure 1) and shows that wide genetic variation in tree size exists. For all strains, territories of the MCA and ACA trees together cover ∼90% of the dorsal cortical area and, as expected, vary inversely with each other. The MCA territory positively correlated with cortex area (Supplementary Figure 2). Collateral number and diameter showed virtually no correlation with MCA territory (Figure 2B). These data suggest that differences in the extent of the collateral circulation are not simply a consequence of differences in cerebral artery tree sizes. A study comparing formation of the collateral circulation in BALB and B6 embryos and neonates confirms this conclusion (Chalothorn and Faber, 2009).
Infarct Volume Strongly Correlates with the Extent of the Native Collateral Circulation
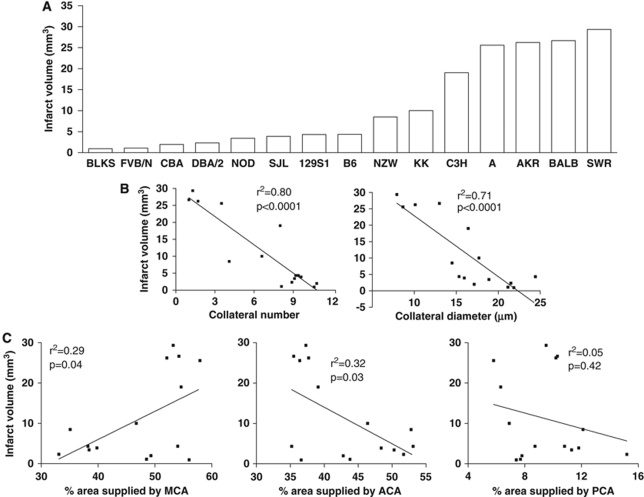
Whether differences in the number and diameter (and length, see below) of native cerebral collaterals influence infarct volume is an important question because the significance of the pial collateral circulation to stroke severity remains controversial (Brozici et al, 2003; Mohr et al, 2004). As recently reported by Keum and Marchuk (2009), marked variability in infarct volume after permanent MCAO is evident among the 15 strains (Figure 3A). Correlation analysis of mean values for infarct volume, derived from Figure 1 of their study, with collateral metrics measured herein showed that infarct volume is strongly correlated inversely with collateral number and diameter (Figure 3B). Infarct volume was also directly and inversely correlated, although less strongly, with MCA and ACA territories, respectively (Figure 3C). This is expected, as the sizes of these territories are inversely related to each other and because infarction was induced by occlusion of the MCA.
Figure 3.
Infarct volume correlates inversely with collateral number and diameter. (A) Infarct volumes (24 h after MCA occlusion) were estimated from Figure 1 of an earlier report (Keum and Marchuk, 2009). (B) Infarct volumes correlated closely and inversely with mean values for collateral number and diameter obtained for the 15 strains in this study. (C) Infarct volume correlated less tightly with MCA (directly) and ACA (inversely) tree territories.
We calculated the heritability for collateral number and diameter. Heritability [h2=genetic (interstrain) variance divided by the sum of the genetic variance and environmental (intrastrain) variance × 100] for collateral number and diameter is 85% and 76%, respectively. Thus, genetic differences account for the majority of the variability in collateral extent among the 15 strains, which agrees with the heritability of infarct volume for the same strains (88%, Keum and Marchuk, 2009).
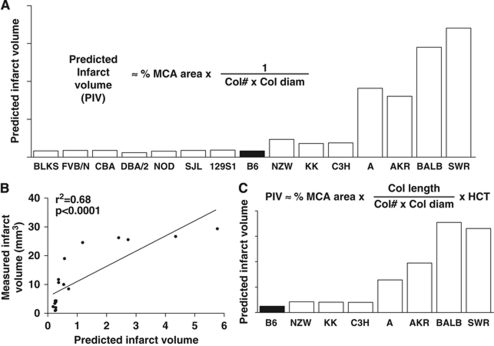
Polymorphisms affecting nonvascular genes contributing to stroke severity, such as proteins involved in pathways affecting sensitivity of neurons and glia to hypoxia and apoptosis, distal thrombosis/lysis, reperfusion injury, and endogenous neuroprotective mechanisms, also likely contribute to variation in infarct volume (Hossmann, 2006). To estimate the relative contribution of genetic variation in vascular determinants of infarct volume, we assembled MCA territory plus collateral number and diameter into a deterministic ‘equation' (Figure 4A). Diameter was not raised to the 4th power to avoid amplification of measurement errors. Comparison of Figures 3A and 4A shows a similar pattern of predicted and measured infarct volumes among the 15 strains. According to the Hagen–Poiseuille's equation, flow is also dependent on vessel length, blood viscosity (where hematocrit accounts for ∼77% of viscosity), and arterial pressure. Therefore, we measured the length of each collateral in the eight strains with the largest differences in collateral dimensions and infarct volume. (Length was not measured in the other strains due to the difficulty involved.) Length varied with strain (P<0.001), and correlated positively (though weakly) with collateral number and diameter (Supplementary Figure 3). Length showed a weak inverse correlation with infarct volume. We also obtained hematocrit from the Mouse Phenome Database (MPD) (http://phenome.jax.org) (Supplementary Figure 4) and inserted it and collateral length into an expanded deterministic equation (Figure 4B). Comparison of the predicted and measured infarct volumes (Figures 4B versus 3A) showed a similar prediction to that obtained in Figure 4A.
Figure 4.
Extent of collateral circulation and MCA territory predict infarct volume. Predicted relative infarct volumes (PIV) based on deterministic ‘equations' without (A) and with (C) inclusion of collateral length and hematocrit (HCT). Linear correlation analysis (B) is for data in panel (A) and Figure 3A.
We did not account for any effect of differences in mean arterial pressure, which is a determinant of collateral flow after MCAO, because data in the MPD for arterial pressure were collected using methods (e.g., tail cuff in the conscious, heated, restrained state) that may not predict arterial pressure under the conditions of our study. Acquisition of such data would require continuous measurement of arterial pressure under ketamine/xylazine anesthesia during the MCAO procedure, followed by 24 h of continuous measurement in the unrestrained state. However, as cerebral blood flow exhibits robust autoregulation and given that differences in arterial pressure reported in the MDP for these strains are small, the predicted values in Figure 5B are unlikely to vary greatly with inclusion of arterial pressure. Of note, arterial pressures reported in the MPD (e.g., Sugiyama et al, 2007) show no correlation with infarct volume in the above 15 strains.
Figure 5.
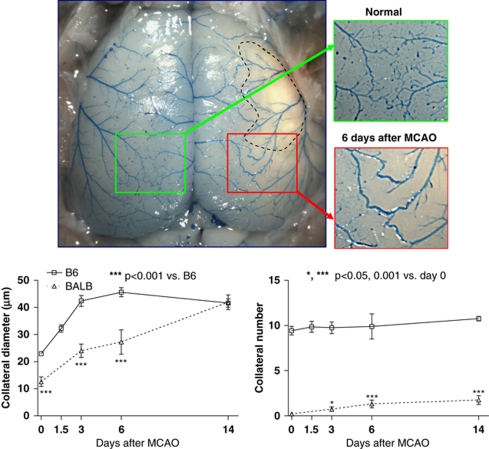
Collateral remodeling is slower in BALB/c than C57Bl/6 strain. Top panels, enlargement of collaterals on side that received MCAO (methods same as in Figure 1). Lower panels, collateral diameter and number measured over time after MCAO. BALB had slower collateral remodeling and a small increase in collateral number. n=4 to 30 mice for the various time points (most BALB lacked native collaterals, thus n-sizes for BALB bars are 4 to 8).
We measured collateral tortuosity and the number of penetrating arterioles branching off the collaterals on the nonligated side in the above eight strains, as these parameters could impact penumbra area and subsequent infarct volume after vascular obstruction. Tortuosity did not differ among the strains (Supplementary Figure 5D, white bars). However, the number of penetrating arterioles was slightly though significantly lower in strains exhibiting larger infarct volumes (Supplementary Figure 5E). The greater number of penetrating arterioles in strains with increased collateral length may offset the latter's effect to decrease collateral conductance and, in turn, increase infarct volume.
Genetic Variability in Outward Remodeling of Collateral Diameter After Middle Cerebral Artery Occlusion
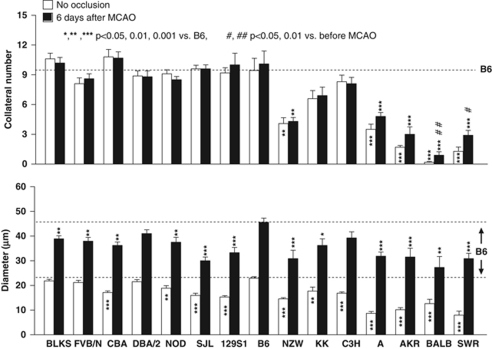
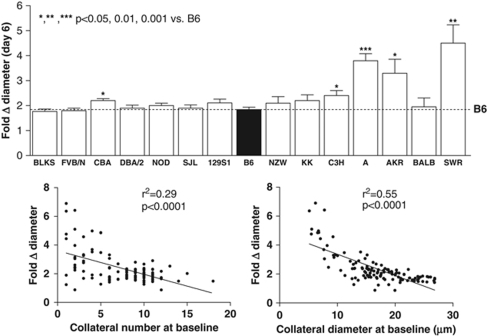
Besides dimensions of native collaterals, amount of outward remodeling (i.e., lumen enlargement or ‘arteriogenesis') in obstructive vascular disease of the heart and peripheral limbs is a critical determinant of severity of ischemic tissue injury (Heil et al, 2006). Whether this is also true in brain is not clear, because the time course for this process, which occurs over weeks in striated muscle, has not been examined in pial collaterals. If it is as slow as in skeletal muscle, the impact on penumbra may be minimal. To address this question and determine whether remodeling exhibits genetic variability, we determined the time course in additional groups of B6 and BALB, as reference strains for backgrounds possessing large and small collateral circulations (Figure 2). Remodeling was remarkably rapid in B6. However, it was considerably slower in BALB, although it eventually achieved the same diameter (Figure 5). A similar doubling of collateral diameter was observed in rats 30 days after occlusion (Wei et al, 2001). Collateral number increased in BALB but not in B6 mice (Figure 5). On the basis of these data, and because dural fibrosis often occurred in the region overlying the infarction (especially when large)—leading to damage of the pia when the dorsal calvarium was removed >6 days after MCAO—we examined collateral metrics 6 days after MCAO in the 15 strains (Figure 6). Collateral number was unaffected in all but BALB and SWR mice, where it increased fivefold and twofold, respectively. Diameter increased between twofold and fivefold in all strains (Figure 7). Strains with fewer collaterals or smaller-diameter collaterals before MCAO underwent—with the exception of BALB—greater collateral remodeling after occlusion.
Figure 6.
Genetic variation in collateral remodeling. Collateral number and diameter before (open bars) and 6 days after MCAO (black bars). BALB and SWR had small increases in collateral number after MCAO.
Figure 7.
Fold change in diameter (from baseline=no occlusion, Figure 6) after MCAO is greater in strains with fewer or smaller collaterals at baseline. Infarct volume correlated positively with fold change in diameter (r2=0.61, P=0.0006), although BALB was outlier (values calculated from Figure 6 data).
We also measured collateral tortuosity and the number of penetrating arterioles branching from the collaterals on the ligated side in the above 8 strains. Tortuosity increased significantly only in C57 and C3H strains, and there was no correlation with infarct volume (Supplementary Figure 5D, black bars). The number of penetrating arterioles did not change (i.e., no sprouting or pruning) 6 days after MCAO in any strain (Supplementary Figure 5E, black versus white bars). Thus, there is no evidence that increased tortuosity or reduced penetrating arteriole numbers occur after MCAO in a manner that associates them with strains evidencing greater infarct volumes.
Discussion
The pial collateral circulation is the sole source of perfusion after proximal cerebral artery occlusion, yet treatments for acute embolic stroke focus on vessel recanalization and penumbral neuroprotection without evaluation of pial collateral capacity (Mohr et al, 2004; Liebeskind, 2005). Recent studies have evaluated whether differences in collateral conductance influence recovery from stroke. Ringelstein et al (1992) found that early recanalization of MCA occlusions within up to 8 h, if in conjunction with good pial collateral blood flow, significantly reduced infarct size and improved outcome. This observation was recently confirmed by Bang and co-workers (2008) and Christoforidis and colleagues (2009). Besides lysis of the obstruction, thrombolytics also reverse stasis-induced thrombosis distal to the occlusion. However, access depends on the extent of the pial collateral circulation (Brozici et al, 2003; Liebeskind, 2005), yet patients with acute occlusion evidence wide variation in the extent of retrograde perfusion of the MCA tree (Bang et al, 2008; Christoforidis et al, 2009). Whether this is due to individual variation in the pial collateral circulation or thrombotic or other mechanisms is unclear because current imaging methods cannot resolve these small vessels in humans, and until now, no animal studies had been done. This study suggests that genetic variation in native collateral number and diameter is a major contributor to severity of embolic stroke.
We report several significant new findings: (1) The extent of the pial collateral circulation exhibits surprisingly wide differences due to genetic variation. (2) These differences closely predict the severity of ischemic injury. Although obtained in murine brain, these results likely extend to the other tissues, as collateral densities in skeletal muscle and intestine agree qualitatively with density in the pial circulation (Chalothorn et al, 2007; Chalothorn and Faber, 2009). They also may extend to other species including humans (Meier et al, 2007). (3) Remodeling of collaterals in the brain is nearly an order of magnitude faster than in skeletal muscle—a difference that may serve to more rapidly reduce ischemia and cell death in the high-risk penumbral cortex. (4) Collateral remodeling varies substantially among strains, but with a pattern distinct from the variation in the extent of the collateral circulation. (5) Cerebral artery tree size is subject to wide genetic variation that, again, does not correlate with collateral extent. These findings suggest that variants in different genes may govern collateral formation, collateral remodeling and cerebral artery tree size. (6) We also found that most mouse strains (excepting BALB and SWR) are unable to form additional collaterals after MCAO.
An unexpected finding was that full collateral remodeling occurred within 3 days after MCAO in B6 mice (Figure 5), whereas 3 to 4 weeks is required in skeletal muscle (Heil et al, 2006; Chalothorn et al, 2007). This is not due to differences in baseline collateral diameter, which averages ∼25 microns in both tissues. It is possible that location adjacent to the subarachnoid space eliminates the requirement in other tissues for reorganization of the surrounding matrix and cells before outward remodeling can proceed. Significant smooth muscle tone, if present in collaterals at baseline, could also contribute to faster remodeling. Diameters of pial collaterals of B6 mice (measured under ketamine and xylazine anesthesia with intravital microscopy through an open cranial window) increase up to fivefold after topical adenosine or sudden increase in shear stress following acute MCAO (Chalothorn and Faber, 2009). Others have reported that pial collaterals exhibit basal tone (Wei et al, 2001; Tomita et al, 2005). Inhibition of substantial basal tone in pial collaterals after MCAO could contribute to the rapid remodeling we observed, as chronic vasodilation is known to stimulate outward remodeling of arterioles (van Gieson et al, 2003). Inhibition of basal collateral tone could also contribute to the pronounced ‘steal' of flow from other cortical regions during increases in regional neuronal activity.
Collaterals in BALB mice remodeled more slowly that those in B6 but eventually reached the same diameter (Figure 5). By contrast, BALB collaterals remodel to half the diameter of B6 in skeletal muscle (Chalothorn et al, 2007). The inability of neurons to withstand prolonged hypoxia may assign little impact of additional remodeling beyond 3 days to eventual infarct volume (Mohr et al, 2004). Strains with fewer or smaller collaterals (excepting BALB) had greater remodeling (Figures 6 and 7). This may reflect the higher velocity and thus shear stress that smaller collaterals experience after MCAO. However, BALB do not follow this pattern, and the pattern of variability across different genetic backgrounds does not correspond to that for native collateral diameter (Figure 1). These data suggest perhaps not surprisingly that, besides hemodynamic relationships, different genes may regulate collateral formation and remodeling. Impaired remodeling by BALB mice may extend from their reduced vascular endothelial growth factor (VEGF) induction (Clayton et al, 2008) and/or altered T-helper and natural killer cell responsiveness (van Meel et al, 2007). In the rat, enlargement of the ipsilateral posterior cerebral artery (which supplies the posterior commissural collateral artery) after bilateral vertebral and ipsilateral carotid artery ligations was first demonstrated by Busch et al (2003), and subsequently shown by same group to be augmented by GM-CSF treatment in association with increased CD68-positive macrophage recruitment (Buschmann et al, 2003). Understanding mechanisms that promote and restrain remodeling is important for the development of therapeutic approaches (Hossmann and Buschmann, 2005).
An interesting observation was that new collaterals (neo-collaterals) formed, albeit in small numbers, in SWR and BALB strains, with A and AKR showing a similar trend. This does not appear to be from failure to fill (and thus detect) small capillary-like interconnections in the normal pia that then remodel into collaterals after MCAO, for several reasons: (1) we have repeatedly been unable to find such vessels using different filling or staining methods in the present (e.g., Figure 1) or earlier studies (Clayton et al, 2008; Chalothorn et al, 2009); (2) pressure and infusion time are adjusted during filling, while viewing the pial circulation with a stereomicroscope, to make sure that the penetrating arterioles (including those in the collateral zone) and beginning of their capillaries are filled; (3) the adult pial circulation in humans lacks capillaries (Duvernoy, 1999). Inspection of high resolution images in Heinzer et al (2006) appear to extend this absence to the mouse. And we confirmed this in this study—no capillaries were detected in the pia of any of the 15 strains. We also do not see pial capillaries in adult B6 and BC strains using isolectin B4 staining (Zhang and Faber, unpublished).
We cannot be certain that an occasional capillary exists in the pia of the A, BC, AKR, and SWR strains that remodels into a large collateral after MCAO. However, that neo-collaterals were detected only in these strains argues against this. Whether neo-collaterals can form in adult tissues after occlusion, in the absence of preexisting connections, is uncertain and controversial (Coyle, 1984; Wei et al, 2001). The unique position of the pial circulation ‘outside' of the tissue it supplies, together with its lack of capillaries, may limit neo-collateral formation, compared with other tissues, to the small number that we observed. Nevertheless, the present results are to our knowledge the first evidence suggesting that neo-collaterals can form in the adult after arterial obstruction. Although we do not know the mechanism of formation, ischemia may be involved, rather than formation simply being a feature of strains with small native collateral density (the above four stains have this in common). This speculation is based on our failure to observe neo-collaterals in the other strain with low native collateral density—NZW. This strain has a small MCA tree territory and thus sustains small infarctions after MCAO despite small collateral number and diameter.
Our finding of strong correlations between measured infarct volume and collateral number, collateral diameter, and predicted infarct volume suggest that variation in collateral extent has a major effect on stroke. Heritable differences in ischemic angiogenesis or neuronal/glial sensitivity to oxidative stress could also contribute to infarct volume, along with differences in ischemia–reperfusion injury (retrograde via collaterals), thrombosis/lysis, and neural/glial regeneration (Mohr et al, 2004; Hossmann, 2006). Nakai et al (2009) have identified differences in inbred mice and genetic loci affecting angiogenic responsiveness to pellet-delivered VEGF in cornea and in laser-induced retinal ischemia. However, the pattern (129S1>DBA>BALB>FVB>CBA,C3H,B6>A) does not agree with that in Figure 4A vis-a-vis infarct volume. Neither does sensitivity to excitotoxic-induced cell damage/death in hippocampus (FVB>129S1>BALB>B6) (Carmichael, 2005), ischemia-reperfusion injury (albeit in lung) (SWR>C3H>A>SV129>B6>CBA>SJL>BALB) (Dodd-o et al, 2006), or inflammatory leukocyte recruitment (B6>A/J) (Hoover-Plow et al, 2008). However, increased rates of hemostasis/thrombosis (BALB/c,A>B6>129S1) (Hoover-Plow et al, 2006; Sa et al, 2008; White et al, 2009) and slower rebleeding time (A/J slower than C57BL/6; however, PT, aPPT and bleeding time were comparable) (Hoover-Plow et al, 2006) favor less retrograde perfusion after MCAO. This could contribute to the greater infarct volume in the BALB/cBy and A/J versus the other above strains. It could also explain the similar infarct volumes in BALB/c and A/J despite collateral number being greater and diameter less in A/J (collateral number has a greater effect than diameter on overall conductance of the collateral network). These findings, together with these data showing good agreement with predicted and measured infarct volume, support the concept that variation in pial collateral extent is a major determinant of the variation in infarct volume.
Several earlier reports are relevant to this study. In two strains of rats (Wistar, Fischer-344) with comparable MCA diameters but known to sustain large differences in infarct volume after MCAO, no difference was detected in flow velocity 2 h after MCAO using a 0.5-mm diameter probe placed between branches in the distal MCA tree (Herz et al, 1998). However, collateral metrics were not obtained, and measures of highly localized velocity may not correlate with overall collateral conductance. Compared with young cats, aged cats experienced greater reductions in cerebral blood flow and encephalographic disturbances after MCAO, in association with increased collateral resistance (Yamaguchi et al, 1988).
Although the genetic polymorphisms responsible for the variation in collateral extent are unknown, some leads are surfacing. Two genes, Vegfa and Clic4, have been shown by targeted alterations in their expression to affect density and diameter during formation of the native collateral circulation in both pia and skeletal muscle (Clayton et al, 2008; Chalothorn et al, 2009). Keum and Marchuk (2009) recently identified a major QTL (LOD=11.9) for infarct volume on chromosome 7 in a B6xBALB-F2 intercross. Moreover, using the same intercross, we have mapped variation in native pial collateral density and diameter to this same locus (LOD=21.2), as well as to several other QTL (Wang et al, 2010). Interestingly, none of those loci includes Vegfa or Clic4. These and the current study set the stage for future work to identify the pathways that specify formation of the collateral circulation, the genetic variants responsible for its wide variation, and the environmental factors that impact these mechanisms. These findings may have significance not only for cerebral collaterals, but also for other tissues where formation and remodeling of the collateral circulation may be governed by similar molecular mechanisms. In support of this, van den Borne et al (2009) recently reported that the severity of congestive heart failure after ligation of the left anterior descending artery followed a pattern of strain sensitivity among five mouse strains (SW and BALB/cJ worse than C57BL/6J, FVB/NJ, and 129S6/SvEv) that is similar to our findings for the extent of the native pial collateral circulation.
Acknowledgments
We thank S Keum and D Marchuk, Department of Molecular Genetics and Microbiology, Duke University, for advice on middle cerebral artery occlusion, sharing their infarct volume data before publication, and valuable discussions.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B, Kim D, Jahan R, Duckwiler GR, Yoon SR, Vinuela F, Liebeskind DS. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2008;79:625–629. doi: 10.1136/jnnp.2007.132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozici M, van der Zwan A, Hillen B. Anatomy and functionality of leptomeningeal anastomoses: a review. Stroke. 2003;34:2750–2762. doi: 10.1161/01.STR.0000095791.85737.65. [DOI] [PubMed] [Google Scholar]
- Busch HJ, Buschmann IR, Mies G, Bode C, Hossmann KA. Arteriogenesis in hypoperfused rat brain. J Cereb Blood Flow Metab. 2003;23:621–628. doi: 10.1097/01.WCB.0000057741.00152.E4. [DOI] [PubMed] [Google Scholar]
- Buschmann IR, Busch HJ, Mies G, Hossmann KA. Therapeutic induction of arteriogenesis in hypoperfused rat brain via granulocyte-macrophage colony-stimulating factor. Circulation. 2003;108:610–615. doi: 10.1161/01.CIR.0000074209.17561.99. [DOI] [PubMed] [Google Scholar]
- Carmichael ST. Rodent models of focal stroke: size, mechanism and purpose. NeuroRx. 2005;2:396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalothorn D, Clayton JA, Zhang H, Pomp D, Faber JE. Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol Genomics. 2007;30:179–191. doi: 10.1152/physiolgenomics.00047.2007. [DOI] [PubMed] [Google Scholar]
- Chalothorn D, Faber JE. Differences in collateral formation in the embryo are associated with genetic variation in patterning and maturation of the cerebral cortical circulation. Atheroscler Thromb Vasc Biol. 2009;29:e57. [Google Scholar]
- Chalothorn D, Zhang H, Smith JE, Edwards JE, Faber JE. Chloride intracellular channel-4 is a determinant of native collateral formation in skeletal muscle and brain. Circ Res. 2009;105:89–98. doi: 10.1161/CIRCRESAHA.109.197145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis GA, Karakasis C, Mohammad Y, Caragine LP, Yang M, Slivka AP. Predictors of hemorrhage following intra-arterial thrombolysis for acute ischemic stroke: the role of pial collateral formation. AJNR Am J Neuroradiol. 2009;30:165–170. doi: 10.3174/ajnr.A1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia. Circ Res. 2008;103:1027–1036. doi: 10.1161/CIRCRESAHA.108.181115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle P. Diameter and length changes in cerebral collaterals after middle cerebral artery occlusion in the young rat. Anat Rec. 1984;210:357–364. doi: 10.1002/ar.1092100211. [DOI] [PubMed] [Google Scholar]
- Dodd-o JM, Hristopoulos ML, Welsh-Servinsky LE, Tankersley CG, Pearse DB. Strain-specific differences in sensitivity to ischemia-reperfusion lung injury in mice. J Appl Physiol. 2006;100:1590–1595. doi: 10.1152/japplphysiol.00681.2005. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. Vascularization of the cerebral cortex. Rev Neurol (Paris) 1999;155:684–687. [PubMed] [Google Scholar]
- Heil M, Eitenmuller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med. 2006;10:45–55. doi: 10.1111/j.1582-4934.2006.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzer S, Krucker T, Stampanoni M, Abela R, Meyer EP, Schuler A, Schneider P, Muller R. Heirarchical microimaging for multiscale analysis of large vascular networks. Neuroimage. 2006;32:626–636. doi: 10.1016/j.neuroimage.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Herz RC, Hillen B, Versteeg DH, De Wildt DJ. Collateral hemodynamics after middle cerebral artery occlusion in Wistar and Fischer-344 rats. Brain Res. 1998;793:289–296. doi: 10.1016/s0006-8993(98)00187-5. [DOI] [PubMed] [Google Scholar]
- Heubner O. Die leutischen Erkrankungen der Hirnarterien. Leipzig, Germany: FC Vogel; 1874. pp. 170–214. [Google Scholar]
- Hoover-Plow J, Shchurin A, Hart E, Sha J, Hill AE, Singer JB, Nadeau JH. Genetic background determines response to hemostasis and thrombosis. BMC Blood Disord. 2006;6:6–18. doi: 10.1186/1471-2326-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover-Plow JL, Gong Y, Shchurin A, Busuttil SJ, Schneeman TA, Hart E. Strain and model dependent differences in inflammatory cell recruitment in mice. Inflamm Res. 2008;57:457–463. doi: 10.1007/s00011-008-7062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossmann KA. Pathophysiology and therapy of experimental stroke. Cell Mol Neurobiol. 2006;26:1057–1083. doi: 10.1007/s10571-006-9008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossmann KA, Buschmann IR. Granulocyte-macrophage colony-stimulating factor as an arteriogenic factor in the treatment of ischaemic stroke. Expert Opin Biol Ther. 2005;5:1547–1556. doi: 10.1517/14712598.5.12.1547. [DOI] [PubMed] [Google Scholar]
- Keum S, Marchuk DA. A locus mapping to murine chromosome 7 determines infarct volume in a mouse model of ischemic stroke. Circ Cardiovasc Genet. 2009;2:591–598. doi: 10.1161/CIRCGENETICS.109.883231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebeskind DS. Collaterals in acute stroke: beyond the clot. Neuroimaging Clin N Am. 2005;15:553–573. doi: 10.1016/j.nic.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Maeda K, Hata R, Hossmann KA. Differences in the cerebrovascular anatomy of C57black/6 and SV129 mice. Neuroreport. 1998;9:1317–1319. doi: 10.1097/00001756-199805110-00012. [DOI] [PubMed] [Google Scholar]
- Meier P, Gloekler S, Zbinden R, Beckh S, de Marchi SF, Zbinden S, Wustmann K, Billinger M, Vogel R, Cook S, Wenaweser P, Togni M, Windecker S, Meier B, Seiler C. Beneficial effect of recruitable collaterals: a 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation. 2007;116:975–983. doi: 10.1161/CIRCULATIONAHA.107.703959. [DOI] [PubMed] [Google Scholar]
- Mohr JP, Choi DW, Grottra JC, Weir B, Wolf PA. Stroke. Pathophysiology, Diagnosis, and Management. Philadelphia, PA: Churchill Livingstone; 2004. [Google Scholar]
- Nakai K, Rogers MS, Baba T, Funakoshi T, Birsner AE, Luyindula DS, D'Amato RJ. Genetic loci that control the size of laser-induced choroidal neovascularization. FASEB J. 2009;23:2235–2243. doi: 10.1096/fj.08-124321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payseur BA, Place M. Prospects for association mapping in classical inbred mouse strains. Genetics. 2007;175:1999–2008. doi: 10.1534/genetics.106.067868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringelstein EB, Biniek R, Weiller C, Ammeling B, Nolte PN, Thron A. Type and extent of hemispheric brain infarctions and clinical outcome in early and delayed middle cerebral artery recanalization. Neurology. 1992;42:289–298. doi: 10.1212/wnl.42.2.289. [DOI] [PubMed] [Google Scholar]
- Sa Q, Hart E, Hill AE, Nadeau JH, Hoover-Plow JL. Quantitative trait locus analysis for hemostasis and thrombosis. Mamm Genome. 2008;19:406–412. doi: 10.1007/s00335-008-9122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita Y, Kubis N, Calando Y, Dinh AT, Meric P, Seylaz J, Pinard E. Long-term in vivo investigation of mouse cerebral microcirculation by fluorescence confocal microscopy in the area of focal ischemia. J Cereb Blood Flow Metab. 2005;25:858–867. doi: 10.1038/sj.jcbfm.9600077. [DOI] [PubMed] [Google Scholar]
- van den Borne SW, van de Schans VA, Strzelecka AE, Vervoort-Peters HT, Lijnen PM, Cleutjens JP, Smits JF, Daemen MJ, Janssen BJ, Blankesteijn WM. Mouse strain determines the outcome of wound healing after myocardial infarction. Cardiovasc Res. 2009;84:273–282. doi: 10.1093/cvr/cvp207. [DOI] [PubMed] [Google Scholar]
- Van Gieson EJ, Murfee WL, Skalak TC, Price RJ. Enhanced smooth muscle cell coverage of microvessels exposed to increased hemodynamic stresses in vivo. Circ Res. 2003;92:929–936. doi: 10.1161/01.RES.0000068377.01063.79. [DOI] [PubMed] [Google Scholar]
- Van Meel V, Toes REM, Seghers L, Deckers MML, de Vries MR, Eilers PH, Sipkens J, Schepers A, Eefting D, van Hinsbergh VWM, van Bockel JH, Quax PHA. Natural killer cells and CD4+ T-cells modulate collateral artery development. Atheroscler Thromb Vasc Biol. 2007;27:2310–2318. doi: 10.1161/ATVBAHA.107.151407. [DOI] [PubMed] [Google Scholar]
- Virley D. Choice, methodology, and characterization of focal ischemic stroke models: the search for clinical relevance. Methods Mol Med. 2005;104:19–48. doi: 10.1385/1-59259-836-6:019. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang H, Dai X, Sealock RW, Faber JE.2010Genetic architecture underlying the native collateral circulation FASEB J 24(Suppl 4(in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Erinjeri JP, Rovainen CM, Woolsey TA. Collateral growth and angiogenesis around cortical stroke. Stroke. 2001;32:2179–2184. doi: 10.1161/hs0901.094282. [DOI] [PubMed] [Google Scholar]
- Wellons JC, Sheng H, Laskowitz DT, Machensen GB, Peralstein RD, Warner DS. A comparison of strain-related susceptibility in two murine recovery models of global cerebral ischemia. Brain Res. 2000;868:14–21. doi: 10.1016/s0006-8993(00)02216-2. [DOI] [PubMed] [Google Scholar]
- White TA, Pan S, Witt TA, Simari RD.2009Murine strain differences in hemostasis and thrombosis and tissue factor pathway inhibitor Thromb Resdoi: 10.1016/.thromres.2009.03.006 [DOI] [PMC free article] [PubMed]
- Yamaguchi S, Kobayashi S, Murata A, Yamashita K, Tsunematsu T. Effect of aging on collateral circulation via pial anastomoses in cats. Gerontology. 1988;34:157–164. doi: 10.1159/000212946. [DOI] [PubMed] [Google Scholar]
- Yasargil MG.1987AVM of the brain—history MicroneurosurgeryVol IIIA (Yasargil MG, ed),New York, NY: Thieme Medical Publ Inc; 12–22. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.