Abstract
Brain edema is frequently shown after cerebral ischemia. It is an expansion of brain volume because of increasing water content in brain. It causes to increase mortality after stroke. Agmatine, formed by the decarboxylation of -arginine by arginine decarboxylase, has been shown to be neuroprotective in trauma and ischemia models. The purpose of this study was to investigate the effect of agmatine for brain edema in ischemic brain damage and to evaluate the expression of aquaporins (AQPs). Results showed that agmatine significantly reduced brain swelling volume 22 h after 2 h middle cerebral artery occlusion in mice. Water content in brain tissue was clearly decreased 24 h after ischemic injury by agmatine treatment. Blood–brain barrier (BBB) disruption was diminished with agmatine than without. The expressions of AQPs-1 and -9 were well correlated with brain edema as water channels, were significantly decreased by agmatine treatment. It can thus be suggested that agmatine could attenuate brain edema by limitting BBB disruption and blocking the accumulation of brain water content through lessening the expression of AQP-1 after cerebral ischemia.
Keywords: agmatine, aquaporin, blood–brain barrier disruption, brain edema, cerebral ischemia
Introduction
Agmatine, formed by the decarboxylation of -arginine by arginine decarboxylase, was first discovered in 1910. It is hydrolyzed to putrescine and urea by agmatinase (Yang and Reis, 1999). Recently, agmatine, arginine decarboxylase, and agmatinase were found in mammalian brain (Li et al, 1994). Agmatine is an endogenous clonidine-displacing substance, an agonist for the two adrenergic and imidazoline receptors, and an antagonist at N-methyl--aspartate receptors (Li et al, 1994; Reynolds, 1990). It has been shown that agmatine may be neuroprotective in trauma and ischemia models (Gilad et al, 1996; Kim et al, 2004; Yang and Reis, 1999; Yu et al, 2000). Agmatine was shown to protect neurons against glutamate toxicity and this effect was mediated through N-methyl--aspartate receptor blockade, with agmatine interacting at a site located within the N-methyl--aspartate channel pore (Olmos et al, 1999). Despite this work, the mode and site(s) of agmatine action in the brain have not been fully understood and yet to be undefined. However, nitric oxide synthases generate nitric oxide by sequential oxidation of the guanidino group in arginine, and agmatine is an arginine analogue with a guanidino group. Being structurally similar to arginine, agmatine has been known as a competitive inhibitor of nitric oxide synthase (Auguet et al, 1995; Galea et al, 1996). This suggests that agmatine may protect the brain from ischemic injury by interfering with nitric oxide signaling (Kim et al, 2004).
Stroke is one of the leading causes of death in most of the developed countries with its incidence slowly increasing worldwide (Bonita, 1992; Chalela et al, 2004). Edema is frequently observed in ischemic stroke. Brain edema, defined as an abnormal increase in brain water content, which leads to an expansion of brain volume, has a crucial impact on morbidity and mortality after stroke, in that it increases intracranial pressure, favors herniations, and contributes to additional ischemic injuries (Klatzo, 1985). Aquaporins (AQPs) are a family of water channel proteins that facilitate the diffusion of water through the plasma membrane (Agre et al, 2002). In the rodent brain, three AQPs have been clearly identified, AQPs-1, -4, and -9 (Agre et al, 2004; Badaut et al, 2002). AQP-1 has been detected in epithelial cells of the choroid plexus (Nielsen et al, 1993), AQP-4 in astrocytes with a polarization on astrocyte endfeet (Nielsen et al, 1997), and AQP-9 in astrocytes of the white matter and in catecholaminergic neurons (Badaut et al, 2001, 2004). AQP-1 and AQP-4 are permeable only to water and are presumed to be involved in cerebrospinal fluid formation and brain water homeostasis (Amiry-Moghaddam and Ottersen, 2003). AQP-9 is an aquaglyceroporin, a subgroup of the AQP family, and is permeable to water and also glycerol, monocarboxylates, and urea (Badaut and Regli, 2004). These three channels may be implicated in water movements occurring during the formation and resolution of cerebral edema after ischemia.
On the basis of the above available reports, it was hypothesized that agmatine may have neuroprotective effect on brain edema. The purpose of this study was to investigate the protective effect of agmatine, if any, for brain edema in ischemic brain damage and also to investigate the expression of AQPs.
Materials and methods
Animals
ICR mice from Sam (Osan, Korea) were used for this study. All animal procedures were performed according to a protocol approved by the Yonsei University Animal Care and Use Committee in accordance with NIH guidelines.
Stroke Model
Male ICR mice weighing 36±2 g were subjected to transient middle cerebral artery occlusion (MCAO, n=50). Animals were anesthetized with chloral hydrate (400 mg/kg) intraperitoneally. Depth of anesthesia was assessed by toe pinch every 15 mins. In a separate set of animals (n=5, agamatine treated; n=5, control), a femoral arterial line was placed and physiologic parameters including mean arterial blood pressure, arterial blood gases, and rectal temperature were monitored before, during, and after ischemia. Ischemia was induced using an occluding intraluminal suture as described earlier (Lee et al, 2001). In brief, an uncoated 15 mm segment of 6-0 nylon monofilament suture with the tip rounded by a flame was inserted into the arteriotomy and advanced under direct visualization into the internal carotid artery 11 mm from the bifurcation to occlude the ostium of the middle cerebral artery. After 2 h, the suture was withdrawn and surgical incisions were closed. Twenty-two hours later, the animals were decapitated and the brains were removed; 1.5 mm thick blocks were cut in the coronal plane, stained with triphenyl tetrazolium chloride (Sigma, St Louis, MO, USA) to delineate regions of infarction, and embedded in paraffin. After paraffin embedding, 10 μm thick sections were stained with H&E and immunostained (Lee et al, 2001).
Treatment
Agmatine was dissolved in normal saline (100 mg/kg IP, Sigma, St Louis, MO, USA) and administered after the suture was removed (agmatine, n=21). Controls received normal saline in equivalent volumes (EC, n=18).
Assessment of Brain Edema and Infarct Volume
Brain swelling and infarct volumes were determined by triphenyl tetrazolium chloride staining, using a computer-assisted image analysis system (Optimas ver 6.1, Optimas, Bothell, WA, USA), and corrected for the presence of edema using earlier published methods (Kim et al, 2004; Lee et al, 2001). The volume of infarct was expressed as a percentage of the total area of ipsilateral hemisphere.
Brain Water Content
Mice were killed 22 h after reperfusion, at the time point of maximal brain edema formation. Brains were removed. Hemispheres were separated and weighed to assess the wet weight (WW). Thereafter, the hemispheres were dried for 24 h at 110°C and the dry weight (DW) was determined (Groger et al, 2005). Hemispheric water content (%) was calculated using the following formula: ((WW−DW)/WW) × 100 (%).
Blood–Brain Barrier Disruption
The integrity of the blood–brain barrier (BBB) was investigated using Evans blue extravasation (Chan et al, 1991; Uyama et al, 1988). Evans blue dissolved at 2% in saline (100 μL) was injected in the tail vein and allowed to circulate for 90 mins. The chest wall was then opened under chloral hydrate anesthesia (400 mg/kg, intraperitoneally). Blood sample was obtained from the heart. Animals were perfused transcardially with saline until blue color was washed out from the effluent. Brains were removed immediately and were separated into cortex, hippocampus, striatum, and oligemia (the border area of cerebral infarct) with weights taken. They were homogenized in 500 μL of 50% trichloroacetic acid (weight/volume), and centrifuged (10,000 r.p.m., 20 mins). The supernatant obtained was measured at 445 nm using ELISA reader. Evans blue content of the plasma was similarly determined and the ratio of tissue to plasma Evans blue content was calculated as tissue Evans blue (μg/g wet weight)/plasma Evans blue (μg/g).
Immunohistochemical Staining for AQPs
Brains were fixed with 4% paraformaldehyde, and embedded in paraffin. Brain sections were microtomed into 10 μm. Sections were immunostained with antibodies against AQP-1 (Abcam, Cambridgeshire, UK), AQP-4, or AQP-9 (Chemicon, Temecula, CA, USA), followed by an appropriate fluorescein-conjugated secondary antibody for double-labeled fluorescent immunohistochemistry. Double-labeled immunostaining was evaluated using a fluorescence microscope (LSM 510 META, Carl Zeiss, Jena, Germany). Immunostained controls were also prepared simultaneously without primary antibodies. All incubation steps were performed in a humidified chamber.
Immunoblotting of AQPs
The expressions of AQPs-1, -4, and -9 proteins in ischemic injured brain were estimated by immunoblotting. Immunoblotting was performed using anti-AQPs and anti-actin (Santa Cruz, Santa Cruz, CA, USA) antibodies. Equal amounts of protein (100 μg) per condition were separated on an 8% polyacrylamide gel and electrotransferred onto Immobilon-NC membrane (Millipore, Bedford, MA, USA). Immunoreactive bands were visualized with the ECL detection system using Kodak X-AR film (Kim et al, 2004).
Statistical Analysis
Statistical tests to determine the differences between groups were performed with Student's t-test using SAS ver. 8.01 (SAS Institute Inc., Cary, NC, USA). P-value <0.05 was considered significant. Data were expressed as mean±standard deviation (s.d.).
Results
Agmatine Reduced the Brain Edema and the Infarct Volume After Cerebral Ischemia
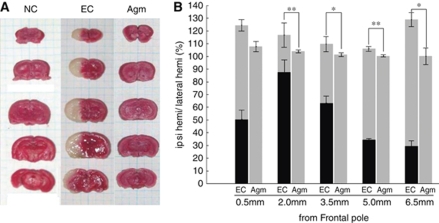
To investigate the effect of agmatine in ischemic damage, brain swelling volume was assessed in serial coronal sections of the mouse brain. The data were summarized in Figure 1. The average total brain swelling volume (cortical plus subcortical areas) in experimental control group was 117.11%±2.37% after 2 h MCAO and 22 h reperfusion. In agmatine treatment group, the average total brain swelling volume was 102.73%±0.16% after 2 h MCAO and 22 h reperfusion. Agmatine significantly reduced brain swelling volume (14.38%±2.21%, P<0.01; Figure 1).
Figure 1.
Brain edema and infarct volume on cerebral ischemia. Agmatine reduced infarct volume and brain swelling after ischemic injury. (A) Serial coronal sections (1.5 mm of thickness) of mouse brain stained with 2% triphenyl tetrazolium chloride solution. (B) Graph of brain swelling percent (%) 22 h after 2 h MCAO (*P<0.05; **P<0.01 versus EC). Data were expressed as mean±s.d. Gray bar, noninfarct area; black bar, infarct area; EC (n=7), experimental control group; NC (n=4), normal control group; Agm (n=9), agmatine treatment group
Agmatine Decreased the Water Content in Ischemic Injured Brain
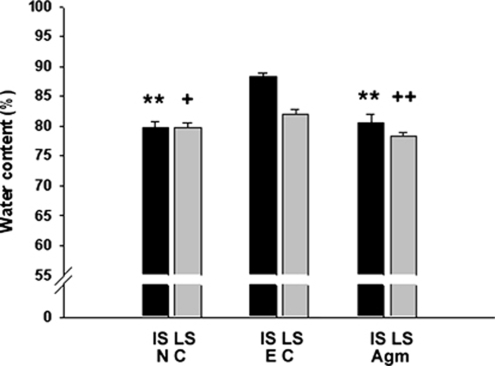
The water content in ischemic injured mouse brains at 22 h reperfusion was shown in Figure 2. In normal control group, water content averaged 79.63%±1.10% in the ipsilateral hemispheres. Ischemia led to a significant increase in water content in the ipsilateral hemispheres (88.23%±0.59% Figure 2). However, in agmatine treatment group, water content was significantly decreased in the ipsilateral hemispheres (80.61%±1.33%, P<0.01; Figure 2).
Figure 2.
Brain water content on cerebral ischemia. Brain water content was analyzed as a measure of brain edema of the ischemic hemisphere 22 h after 2 h MCAO. Agmatine decreased the total water content of ischemic injured brain to normal level (**P<0.01 versus IS of EC; +P<0.05 and ++P<0.01 versus LS of EC). Data are expressed as mean±s.d. EC (n=3), experimental control group; NC (n=5), normal control group; Agm (n=4), agmatine treatment group; IS, ipsilateral ischemic side; LS, contralateral ischemic side.
Agmatine Limited BBB Disruption
It is needed to confirm whether the brain edema is vasogenic accompanied by BBB disruption or cytotoxic without BBB disruption. At 24 h after ischemic injury, Evans blue contents in the striatal, hippocampal, and cerebral cortical area of agmatine treatment group (2.74±0.307; 3.08±0.134; 1.14±0.042) were significantly less than that of experimental control group (4.90±0.120 in striatum; 4.24±0.135 in hippocampus; 1.96±0.110 in cerebral cortex, P<0.05; Table 1). However, in the oligemia area (the border area of cerebral infarct), there is no difference observed between experimental control (2.44±0.157) and agmatine treatment group (2.50±0.145; Table 1).
Table 1. EB ratio (Evans blue (μg/g wet weight)/plasma Evans blue (μg/g)).
| Oligemia | Hippo | Striatum | Cortex | |
|---|---|---|---|---|
| EC | 2.44±0.157 | 4.24±0.135 | 4.90±0.120 | 1.96±0.110 |
| Agm | 2.50±0.145 | 3.08±0.134* | 2.74±0.307* | 1.14±0.042* |
Agmatine treatment reduced the BBB disruption in hippo, striatum, and cortex. (*P<0.05 versus EC.) Agm (n=3), agmatine treatment group; EB, Evans blue; EC (n=3), experimental control group; Oligemia, the border area of cerebral infarct.
Agmatine Lessened the Expression of AQPs 22 h After 2 h of MCAO
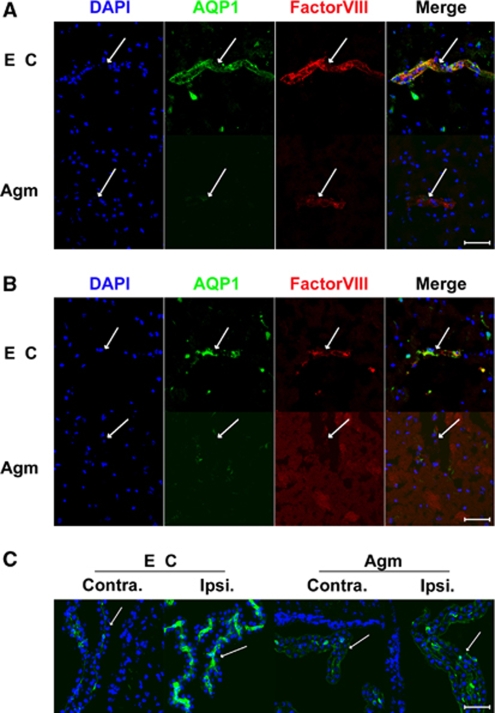
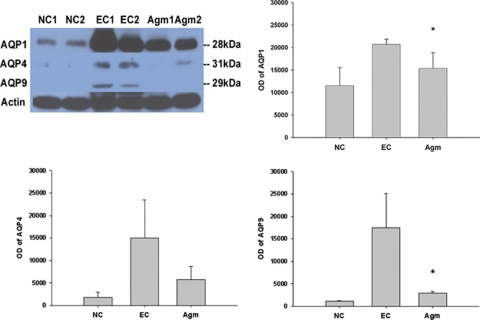
It was well known that AQP-1 was expressed only in epithelial cells of choroid plexus under normal condition, but AQP-1 was strongly expressed in endothelial cells 22 h after MCAO without agmatine. AQP-1-positive endothelial cells were shown in cortex (Figure 3A) and striatum (Figure 3B) except oligemia (data not shown). The expression of AQP-1 was reduced in endothelial cells of cortex (Figure 3A) and striatum (Figure 3B) in agmatine treatment group. Agmatine treatment also decreased the expression of AQP-1 in blood vessels of the choroid plexus (Figure 3C). Immunoblotting results showed significantly less expression of AQP-1 in agmatine treatment group compared with experimental control at 22 h after 2 h MCAO (P<0.05; Figure 4). The other type of AQP, AQP-4 also showed less expression but the obtained value was not significantly different between with and without agmatine treatment. However, the expression of AQP-9 was clearly decreased in agmatine treatment group than experimental control group (Figure 4), the obtained value was significant statistically (P<0.05).
Figure 3.
Macrographs of AQP-1 immunofluorescence in the ischemic injured brain 22 h after 2 h MCAO with or without agmatine. Blood vessel marked with factorVIII (red) was stained with AQP-1 (green) in cortex (A) and striatum (B) of experimental control (EC), but was not merged with AQP-1 (green) in cortex (A) and striatum (B) of agmatine treatment group (Agm). AQP-1 (green) was boldly detected at blood vessel of the choroid plexus of ipsilateral side (Ipsi) in experimental control (EC) but was not in agmatine treatment group (Agm) (C). Scale bar is 50 μm. Contra, contralateral side.
Figure 4.
The expression of AQPs in the ischemic injured brain 22 h after 2 h MCAO with or without agmatine by immunoblotting. Optical densites (OD) of AQPs-1, -4, and -9 are expressed as the band density of each group. Data are expressed as mean±s.d. Agmatine treatment significantly decreased the expression of AQPs-1 and -9 compared with experimental control (*P<0.05 versus EC). NC (n=3), normal control group; EC (n=4), experimental control group; Agm (n=3), agmatine treatment group.
Discussion
It was shown that agmatine reduced brain swelling and brain edema in experimental stroke in this study. This effect of agmatine on the brain edema is associated with the decrease in AQP-1 expression and with the limitation of BBB disruption. It also appears that agmatine lessens the expression of AQP-1 in endothelial cells in cerebral ischemia.
There are two types of brain edema, cytotoxic edema and vasogenic edema after brain injury, such as brain trauma and stroke. Cytotoxic edema occurs without the BBB disruption, but vasogenic edema with the BBB disruption (The Korean Neurosurgical Society, 2005). The disruption of BBB was observed 22 h after 2 h MCAO in this study (Table 1). AQP-1 and AQP-4 are the two members of AQP family known to be expressed in the central nervous system, and it is possible that these proteins contribute to water transport across the BBB (Dolman et al, 2005). In a study of AQP-1 expression in human brain, a small number of microvessels were positively stained, but they were markedly upregulated in endothelium in astrocytomas and metastatic carcinomas (Verkman, 2002). Function of BBB is known to be impaired in such brain tumors, leading to formation of edema (Saadoun et al, 2002). Downregulation of the tight-junction proteins claudin and occludin has also been demonstrated in microvessels in glioblastoma multiforme (Liebner et al, 2000). Thus, loss of BBB function and the expression of AQP-1 may both be regarded as downregulation of BBB phenotype (Dolman et al, 2005). BBB disruption was reduced with agmatine treatment compared with experimental control. Earlier we had reported that agmatine decreased the expression of matrix metalloproteinase-2 and the expression of matrix metalloproteinase-9 in cerebral ischemia (Kim et al, 2008) and it was known that the early expression of matrix metalloproteinases-2 or -9 is associated with BBB disruption and the formation of vasogenic edema after transient focal cerebral ischemia (Fujimura et al, 1999; Gasche et al, 1999; Heo et al, 1999; Rosenberg et al, 1998). These reports can explain the possible mechanism of agmatine to limit BBB disruption in this study.
Our present investigation showed that AQP-1 was expressed in endothelial cells but not in endfoot of astrocytes surrounding capillary vessel in ischemic injured brain (Supplementary Figure 1). As of now, this might be the first report that AQP-1 is highly expressed in endothelial cells of choroid plexus in the case of vasogenic edema after cerebral ischemia to our knowledge. In normal rat brain, AQP-1 transcript and protein expression are restricted to the ventricular facing surface of choroid plexus and AQP-1 is related to cerebrospinal fluid secretion (Hasegawa et al, 1993; Masseguin et al, 2000). The expression of AQP-1 in the ventricular facing surface of choroid plexus was reduced in contralateral side but was not reduced in ipsilateral side of ischemic injured hemisphere compared with normal brain. This phenomenon may be because of the controlled cerebrospinal fluid secretion through dropping the expression of AQP-1 in the ventricular facing surface of choroid plexus to decrease the ascension of intracranial pressure caused by ipsilateral swelling. In agmatine treatment group, its expression in ipsilateral and contralateral side was similar to normal group, but the expression of AQP-1 was little increased in endothelial cells of choroid plexus in ipsilateral side compared with contralateral side of ischemic injured hemisphere. On the basis of these results, agmatine treatment decreased the expression of AQP-1 in endothelial cells of whole injured brain tissue, such as cortex, striatum, and choroid plexus.
The expression of AQP-4 mRNA is strongly induced when BBB is preserved. However, the expression of AQP-4 mRNA is reduced in astrocytes when BBB is disrupted after brain trauma (Kim et al, 2006). It explains that the expression of AQP-4 is much less than that of AQP-1 in the brain tissue after MCAO injury in this study (Figure 4 and Supplementary Figure 2). The expression of AQP-4 was not significantly changed but a reducing trend was obtained in agmatine treatment group than in experimental control group. Gunnarson et al (2008) reported that AQP-4 phosphorylation is related to nitric oxide in astrocyte. It is possible that agmatine would suppress the expression of AQP-4 in astrocyte by inhibiting nitric oxide synthesis (Auguet et al, 1995; Galea et al, 1996).
It was published that AQP-9 protein is upregulated on reactive astrocytes in the border of the infarct after transient MCAO in mice (Badaut et al, 2001) and AQP-9 showed a significant induction at 24 h, with expression increasing gradually with time, without correlation to swelling (Ribeiro et al, 2006). These reports suggest that the decreased expression of AQP-9 in agmatine treatment group is not directly related to the reduced brain edema after cerebral ischemia by agmatine treatment.
In conclusion, agmatine attenuates brain edema by limiting BBB disruption and blocking the accumulation of brain water content through lessening the expression of AQP-1 in endothelial cells in cortex, striatum, and choroid plexus after cerebral ischemia, suggesting that agmatine may have therapeutic potential in treatment of brain edema after brain injury, such as stroke and brain trauma.
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST) (R01-2007-000-10357-0).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow and Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Agre P, King LS, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y, Engel A, Nielsen S. Aquaporin water channels--from atomic structure to clinical medicine. J Physiol. 2002;542:3–16. doi: 10.1113/jphysiol.2002.020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agre P, Nielsen S, Ottersen OP. Towards a molecular understanding of water homeostasis in the brain. Neuroscience. 2004;129:849–850. doi: 10.1016/j.neuroscience.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci. 2003;4:991–1001. doi: 10.1038/nrn1252. [DOI] [PubMed] [Google Scholar]
- Auguet M, Viossat I, Marin JG, Chabrier PE. Selective inhibition of inducible nitric oxide synthase by agmatine. Jpn J Pharmacol. 1995;69:285–287. doi: 10.1254/jjp.69.285. [DOI] [PubMed] [Google Scholar]
- Badaut J, Hirt L, Granziera C, Bogousslavsky J, Magistretti PJ, Regli L. Astrocyte-specific expression of aquaporin-9 in mouse brain is increased after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:477–482. doi: 10.1097/00004647-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Badaut J, Lasbennes F, Magistretti PJ, Regli L. Aquaporins in brain: distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab. 2002;22:367–378. doi: 10.1097/00004647-200204000-00001. [DOI] [PubMed] [Google Scholar]
- Badaut J, Petit JM, Brunet JF, Magistretti PJ, Charriaut-Marlangue C, Regli L. Distribution of Aquaporin 9 in the adult rat brain: preferential expression in catecholaminergic neurons and in glial cells. Neuroscience. 2004;128:27–38. doi: 10.1016/j.neuroscience.2004.05.042. [DOI] [PubMed] [Google Scholar]
- Badaut J, Regli L. Distribution and possible roles of aquaporin 9 in the brain. Neuroscience. 2004;129:971–981. doi: 10.1016/j.neuroscience.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Bonita R. Epidemiology of stroke. Lancet. 1992;339:342–344. doi: 10.1016/0140-6736(92)91658-u. [DOI] [PubMed] [Google Scholar]
- Chalela JA, Merino JG, Warach S. Update on stroke. Curr Opin Neurol. 2004;17:447–451. doi: 10.1097/01.wco.0000137536.06986.f9. [DOI] [PubMed] [Google Scholar]
- Chan PH, Yang GY, Chen SF, Carlson E, Epstein CJ. Cold-induced brain edema and infarction are reduced in transgenic mice overexpressing CuZn-superoxide dismutase. Ann Neurol. 1991;29:482–486. doi: 10.1002/ana.410290506. [DOI] [PubMed] [Google Scholar]
- Dolman D, Drndarski S, Abbott NJ, Rattray M. Induction of aquaporin 1 but not aquaporin 4 messenger RNA in rat primary brain microvessel endothelial cells in culture. J Neurochem. 2005;93:825–833. doi: 10.1111/j.1471-4159.2005.03111.x. [DOI] [PubMed] [Google Scholar]
- Fujimura M, Gasche Y, Morita-Fujimura Y, Massengale J, Kawase M, Chan PH. Early appearance of activated matrix metalloproteinase-9 and blood-brain barrier disruption in mice after focal cerebral ischemia and reperfusion. Brain Res. 1999;842:92–100. doi: 10.1016/s0006-8993(99)01843-0. [DOI] [PubMed] [Google Scholar]
- Galea E, Regunathan S, Eliopoulos V, Feinstein DL, Reis DJ. Inhibition of mammalian nitric oxide synthases by agmatine, an endogenous polyamine formed by decarboxylation of arginine. Biochem J. 1996;316 (Part 1:247–249. doi: 10.1042/bj3160247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasche Y, Fujimura M, Morita-Fujimura Y, Copin JC, Kawase M, Massengale J, Chan PH. Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: a possible role in blood-brain barrier dysfunction. J Cereb Blood Flow Metab. 1999;19:1020–1028. doi: 10.1097/00004647-199909000-00010. [DOI] [PubMed] [Google Scholar]
- Gilad GM, Salame K, Rabey JM, Gilad VH. Agmatine treatment is neuroprotective in rodent brain injury models. Life Sci. 1996;58 PL:41–46. doi: 10.1016/0024-3205(95)02274-0. [DOI] [PubMed] [Google Scholar]
- Groger M, Lebesgue D, Pruneau D, Relton J, Kim SW, Nussberger J, Plesnila N. Release of bradykinin and expression of kinin B2 receptors in the brain: role for cell death and brain edema formation after focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005;25:978–989. doi: 10.1038/sj.jcbfm.9600096. [DOI] [PubMed] [Google Scholar]
- Gunnarson E, Zelenina M, Axehult G, Song Y, Bondar A, Krieger P, Brismar H, Zelenin S, Aperia A. Identification of a molecular target for glutamate regulation of astrocyte water permeability. GLIA. 2008;56:587–596. doi: 10.1002/glia.20627. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Zhang R, Dohrman A, Verkman Tissue-specific expression of mRNA encoding rat kidney water channel CHIP28k by in situ hybridization. Am J Physiol. 1993;264:C237–C245. doi: 10.1152/ajpcell.1993.264.1.C237. [DOI] [PubMed] [Google Scholar]
- Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:624–633. doi: 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee YW, Kim JY, Lee WT, Park KA, Lee JE. The effect of agmatine on expression of MMP2 and MMP9 in cerebral ischemia. Korean J Anat. 2008;41:97–104. [Google Scholar]
- Kim JH, Yenari MA, Giffard RG, Cho SW, Park KA, Lee JE. Agmatine reduces infarct area in a mouse model of transient focal cerebral ischemia and protects cultured neurons from ischemia-like injury. Exp Neurol. 2004;189:122–130. doi: 10.1016/j.expneurol.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Kim KW, Ahn BZ, Kim JH, Lee HJ.2006The regulation of water homeostasis by aquaporins in CNS BioWave 8 http://bric.postech.ac.kr/myboard/read.php?Board=review0&id=1248 [Google Scholar]
- Klatzo I. Brain oedema followingbrain ischaemia and the influence of therapy. Br J Anaesth. 1985;57:18–22. doi: 10.1093/bja/57.1.18. [DOI] [PubMed] [Google Scholar]
- Lee JE, Yenari MA, Sun GH, Xu L, Emond MR, Cheng D, Steinberg GK, Giffard RG. Differential neuroprotection from human heat shock protein 70 overexpression in in vitro and in vivo models of ischemia and ischemia-like conditions. Exp Neurol. 2001;170:129–139. doi: 10.1006/exnr.2000.7614. [DOI] [PubMed] [Google Scholar]
- Li G, Regunathan S, Barrow CJ, Eshraghi J, Cooper R, Reis DJ. Agmatine: an endogenous clonidine-displacing substance in the brain. Science. 1994;263:966–969. doi: 10.1126/science.7906055. [DOI] [PubMed] [Google Scholar]
- Liebner S, Fischmann A, Rascher G, Duffner F, Grote EH, Kalbacher H, Wolburg H. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol (Berl) 2000;100:323–331. doi: 10.1007/s004010000180. [DOI] [PubMed] [Google Scholar]
- Masseguin C, Corcoran M, Carcenac C, Daunton NG, Gell A, Verkman , Gabrion J. Altered gravity downregulates aquaporin-1 protein expression in choroid plexus. J Appl Physiol. 2000;88:843–850. doi: 10.1152/jappl.2000.88.3.843. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Smith BL, Christensen EI, Agre P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci USA. 1993;90:7275–7279. doi: 10.1073/pnas.90.15.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos G, DeGregorio-Rocasolano N, Paz Regalado M, Gasull T, Assumpcio Boronat M, Trullas R, Villarroel A, Lerma J, Garcia-Sevilla JA. Protection by imidazol(ine) drugs and agmatine of glutamate-induced neurotoxicity in cultured cerebellar granule cells through blockade of NMDA receptor. Br J Pharmacol. 1999;127:1317–1326. doi: 10.1038/sj.bjp.0702679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds IJ. Arcaine uncovers dual interactions of polyamines with the N-methyl-D-aspartate receptor. J Pharmacol Exp Ther. 1990;255:1001–1007. [PubMed] [Google Scholar]
- Ribeiro MdeC, Hirt L, Bogousslavsky J, Regli L, Badaut J. Time course of aquaporin expression after transient focal cerebral ischemia in mice. J Neurosci Res. 2006;83:1231–1240. doi: 10.1002/jnr.20819. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Davies DC, Bell BA, Krishna S. Increased aquaporin 1 water channel expression in human brain tumours. Br J Cancer. 2002;87:621–623. doi: 10.1038/sj.bjc.6600512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Korean Neurosurgical Society 2005Head injury Neurosurgery3rd ed.(Jung YK, ed)Seoul: Jungang Moonhwa Co.391–460. [Google Scholar]
- Uyama O, Okamura N, Yanase M, Narita M, Kawabata K, Sugita M. Quantitative evaluation of vascular permeability in the gerbil brain after transient ischemia using Evans blue fluorescence. J Cereb Blood Flow Metab. 1988;8:282–284. doi: 10.1038/jcbfm.1988.59. [DOI] [PubMed] [Google Scholar]
- Verkman Aquaporin water channels and endothelial cell function. J Anat. 2002;200:617–627. doi: 10.1046/j.1469-7580.2002.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XC, Reis DJ. Agmatine selectively blocks the N-methyl-D-aspartate subclass of glutamate receptor channels in rat hippocampal neurons. J Pharmacol Exp Ther. 1999;288:544–549. [PubMed] [Google Scholar]
- Yu CG, Marcillo AE, Fairbanks CA, Wilcox GL, Yezierski RP. Agmatine improves locomotor function and reduces tissue damage followingspinal cord injury. Neuroreport. 2000;11:3203–3207. doi: 10.1097/00001756-200009280-00031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.