Abstract
Cerebral angiogenesis is an important adaptive response to hypoxia. As the αvβ3 integrin is induced on angiogenic vessels in the ischemic central nervous system (CNS), and the suggested angiogenic role for this integrin in other systems, it is important to determine whether the αvβ3 integrin is an important mediator of cerebral angiogenesis. αvβ3 integrin expression was examined in a model of cerebral hypoxia, in which mice were subject to hypoxia (8% O2) for 0, 4, 7, or 14 days. Immunofluorescence and western blot analysis revealed that in the hypoxic CNS, αvβ3 integrin was strongly induced on angiogenic brain endothelial cells (BEC), along with its ligand vitronectin. In the hypoxia model, β3 integrin-null mice showed no obvious defect in cerebral angiogenesis. However, early in the angiogenic process, BEC in these mice showed an increased mitotic index that correlated closely with increased α5 integrin expression. In vitro experiments confirmed α5 integrin upregulation on β3 integrin-null BEC, which also correlated with increased BEC proliferation on fibronectin. These studies confirm hypoxic induction of αvβ3 integrin on angiogenic vessels, but suggest distinct roles for the BEC integrins αvβ3 and α5β1 in cerebral angiogenesis, with αvβ3 having a nonessential role, and α5β1 promoting BEC proliferation.
Keywords: adhesion molecules, animal models, angiogenesis, capillaries, endothelium
Introduction
Cerebral angiogenesis is a well-described adaptive response to cerebral hypoxia (LaManna et al, 1992) or ischemia (Krupinski et al, 1994). When the brain is starved of oxygen, a molecular cascade is initiated that exerts it effect to promote new blood vessel growth. The precise sequence of events underlying this process has yet to be fully elucidated, though evidence suggests it includes the transcription factor hypoxia-inducible factor-1α, and the growth factors vascular endothelial growth factor (VEGF) and angiopoietin-2 (LaManna et al, 1998). Growth of new cerebral blood vessels has been reported in cerebral hypoxia and ischemia, both in animal models (Chen et al, 1994; Hayashi et al, 2003; Wei et al, 2001) and in the human brain (Krupinski et al, 1994). Following focal cerebral ischemia, growing capillaries are first seen 7 days after the insult, and are localized to the area immediately surrounding the ischemic core, or penumbra (Chen et al, 1994; Hayashi et al, 2003; Wei et al, 2001). Neural cells in the ischemic penumbra are hypoxic but still viable, suggesting that these cells could be rescued if adequate support is delivered. Thus, interventions that promote new vessel sprouting in the ischemic penumbra represent an attractive therapeutic target in the treatment of ischemic stroke, making it imperative to identify the key molecular mechanisms promoting angiogenesis in response to hypoxia/ischemia.
The major pathways that promote angiogenesis are well described. These include the growth factors VEGF (Millauer et al, 1993), basic fibroblast growth factor (Klein et al, 1997), transforming growth factor-β1 (Roberts et al, 1986), and the angiopoietins (Sato et al, 1995). The present working model suggests that in the hypoxic central nervous system (CNS), hypoxia triggers hypoxia inducible factor-1α production (Chavez et al, 2000), which leads to increased expression of VEGF (Kuo et al, 1999) and angiopoietin-2 (Pichiule and LaManna, 2002), leading to angiogenic sprouting.
Extracellular matrix (ECM) proteins have a pivotal role in regulating angiogenesis and vascular remodeling (Eliceiri and Cheresh, 2001; Stromblad and Cheresh, 1996). In particular, fibronectin and its cognate integrin receptors are essential for angiogenesis, as shown by a failure of angiogenesis in mice mutants lacking the fibronectin gene (George et al, 1993) or fibronectin-specific integrins (Yang et al, 1993, 1995). In cerebral angiogenesis, the precise role of ECM–integrin interactions has not been fully explored, though several lines of evidence suggest an important role. During development, angiogenic vessels are surrounded by a fibronectin-rich ECM, and in the postnatal CNS, angiogenesis is associated with a switch in the expression of ECM proteins and endothelial cell β1 integrins, from fibronectin to laminin-mediated pathways (Milner and Campbell, 2002a). In addition, we recently showed that in the adult hypoxic CNS, angiogenic vessels show marked upregulation of fibronectin and endothelial cell α5β1 integrin expression (Milner et al, 2008a). Previous studies have described induction of the αvβ3 integrin on cerebral microvessels after cerebral ischemia (Abumiya et al, 1999; Okada et al, 1996; Wei et al, 2001). Furthermore, retroviral delivery of the proangiogenic transcription factor HoxD3 to the CNS resulted in blood vessel sprouting and a concomitant induction of the αvβ3 integrin on angiogenic capillaries (Chen et al, 2004). Taken with our previous observation that fibronectin promotes angiogenic behavior in brain endothelial cells (BEC) through α5β1 and αvβ3 integrins (Wang and Milner, 2006), the data suggest that both these integrins may be important in cerebral angiogenesis. In this study, we aimed to test the hypothesis that the αvβ3 integrin plays an instructive role in cerebral angiogenesis, by (1) examining expression of the αvβ3 integrin and its ECM ligands in a mouse model of hypoxia-induced cerebral angiogenesis, and (2) examining the angiogenic response in β3 integrin-null mice.
Materials and methods
Animals
The studies described have been reviewed and approved by The Scripps Research Institute Institutional Animal Care and Use Committee. Wild-type C57Bl/6 and β3 integrin-null mice (backcrossed >10 times on the C57Bl/6 background) were maintained under pathogen-free conditions in the closed breeding colony of The Scripps Research Institute. Heterozygous β3 integrin-null mice were bred and offspring were genotyped using previously described protocols (Hodivala-Dilke et al, 1999), to generate homozygous β3 integrin-null (β3−/−) mice and wild-type littermate controls (β3+/+).
Chronic Hypoxia Model
β3 integrin-null or wild-type littermate control mice, 8 to 10 weeks of age, were housed 4 to a cage, and placed into a hypoxia chamber (Biospherix, Redfield, NY, USA), maintained at 8% oxygen for up to 14 days. Littermate controls of each strain were also kept in the same room under similar conditions except that they were kept at normal oxygen levels (normoxia) for the duration of the experiment. Every few days, the chamber was opened for cage cleaning and food and water replacement as needed.
Immunohistochemistry and Antibodies
Immunohistochemistry was performed as described previously (Milner and Campbell, 2002a) on 10 μm frozen sections of cold saline-perfused brains taken from mice subjected to either normoxia (control) or hypoxia for 4, 7, or 14 days. Each slide contained mouse brains representing the four different time points of hypoxia, to ensure consistent antibody incubation times across different time points. The following monoclonal antibodies were obtained from BD Pharmingen (La Jolla, CA, USA): rat monoclonal antibodies reactive for the integrin subunits α5 (clone 5H10-27; MFR5), αv (clone RMV-7), α6 (clone GoH3), CD31 (PECAM-1, clone MEC13.3), clone MECA-32, the hamster monoclonal antibody reactive for the β3 integrin subunit (clone 2C9.G2), and isotype control antibodies; rat anti-KLH (A110-2), and hamster anti-TNP-KLH (G235-1). The monoclonal anti-β1 integrin antibody (MB1/2) was obtained from Chemicon (Temecula, CA, USA). Rabbit polyclonal antibodies against the following proteins were used in this study: vitronectin (Molecular Innovation, Southfield, MI, USA), fibronectin (Sigma, St Louis, MO, USA), Ki67 (Vector Laboratories, Burlingame, CA, USA), α5 integrin (Chemicon), β3 integrin (Cell Signaling Technology, Danvers, MA, USA), VEGF (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and β-actin (Neomarker, Fremont, CA, USA). The Cy3-conjugated secondary antibodies against rat IgG, mouse IgG, and rabbit IgG were all obtained from Jackson Immunoresearch (West Grove, PA, USA). Alexa Fluor 488-conjugated secondary anti-rabbit and anti-rat IgGs were obtained from Invitrogen (Carlsbad, CA, USA).
Quantification of the number of capillaries positive for the different antigens was performed by taking images using a × 20 objective on a Zeiss Axio Observer A1 microscope and Zeiss Axiocam MRC digital camera (Thornwood, NY, USA). These images were used to determine the number of positive events within this field of view. For each animal, several images of the brain stem and cerebral cortex were taken for each antigen, and the mean number of events was calculated. Each experiment was performed with three different animals per condition, and the results are expressed as the mean±s.e.m. of the number of capillaries positive for each antigen per field of view. Statistical significance was assessed using the Student's t-test, in which P<0.05 was defined as statistically significant.
Western Blot Analysis
Western blot analysis was used to determine levels of the α5 and β3 integrins, vitronectin, and VEGF in brain lysates, as previously described (Milner et al, 2007). Within each brain sample, levels of these proteins were first normalized to the level of β-actin, and then expressed as the fold-increase over the level present within the brain of normoxic animals.
Brain Endothelial Cell Culture
Pure cultures of mouse BEC were obtained as described previously (Milner et al, 2008b), with the modification that puromycin (4 μg/mL; Alexis GmbH, Grunberg, Germany) was included in culture media between days 1 and 3 to remove contaminating cell types. Endothelial cell purity was >99% as determined by CD31 in flow cytometry. BEC were used only for the first passage.
Flow Cytometry
Integrin expression of β3 integrin-null or wild-type littermate control BEC was examined as described previously (Milner et al, 2008b). The fluorescent intensity of labeled cells was analyzed with a Becton Dickinson FACScan machine (San Diego, CA, USA), with 10,000 events recorded for each condition. For each integrin subunit, the mean fluorescent intensity (MFI) of β3 integrin-null BEC was compared with wild-type BEC. Each experiment was repeated three times and the data are expressed as the mean±s.e.m. Statistical significance was assessed using Student's t-test, in which P<0.05 was defined as statistically significant.
Cell Adhesion Assays
Adhesion assays were performed as previously described (Milner and Campbell, 2002b). Adhesion was quantified by phase microscopy by counting all attached cells within five fields of view per condition. Within each experiment each condition was performed in duplicate; the results represent the mean±s.e.m. of three experiments. Statistical significance was assessed using Student's t-test, in which P<0.05 was defined as statistically significant.
Proliferation Assays
Glass coverslips were coated with ECM substrates and BEC cultured until cells reached ∼50% confluence. BEC were then cultured overnight in the presence of 5-bromo-2-deoxyuridine (BrdU; Invitrogen), fixed in acid/alcohol, and analyzed for BrdU incorporation according to the manufacturer's instructions. BrdU-positive cells were expressed as the percentage of total cells (Hoechst staining), and the results expressed as the mean±s.e.m. of three experiments. Statistical significance was assessed using Student's t-test, in which P<0.05 was defined as statistically significant.
Results
Cerebral Hypoxia Induced β3 Integrin Expression on BEC
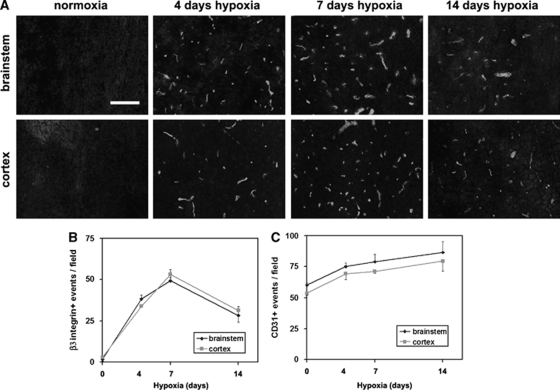
To examine whether BEC expression of the αvβ3 integrin is regulated during angiogenesis in the adult CNS, we examined these events in a mouse model of chronic hypoxia, in which exposure to mild hypoxia (8% O2) induces a strong angiogenic response in the CNS (Kanaan et al, 2006; LaManna et al, 1992). Four groups of mice were studied: normoxia (control), or hypoxia for 4, 7, or 14 days (n=3 animals per group). As shown in Figure 1C, 14 days of cerebral hypoxia induced a marked angiogenic response, resulting in increased capillary density, as measured by CD31 immunofluorescence (IF). β3 integrin-positive vessels were rarely detected within the normoxic CNS. In contrast, hypoxia triggered a striking induction of β3 integrin expression on capillaries throughout the CNS, with the number of β3 integrin-positive capillaries reaching a maximum after 7 days hypoxia, and declining thereafter (Figures 1A and 1B). Seven days hypoxia increased the number of β3 integrin-positive capillaries per field of view, from 1.7±0.6 to 49.2±7.0 in the brainstem (P<0.001), and from 2.9±2.4 to 53.2±2.1 in the cerebral cortex (P<0.005). Western blot analysis of brain lysates confirmed these findings, with β3 integrin protein levels increased after 7 days hypoxia to 2.9±0.1-fold of the normoxic level (P<0.005; Figure 3C).
Figure 1.
Hypoxic induction of β3 integrin expression on cerebral blood vessels. (A) Frozen sections of brainstem or cerebral cortex taken from mice exposed to normoxia or 4, 7, or 14 days hypoxia were immunostained for the β3 integrin. Scale bar=50 μm. (B) Quantification of β3 integrin-positive vessels. (C) Quantification of CD31-positive events. Experiments were performed with three different animals per condition, and the results expressed as the mean±s.e.m. of the number of capillaries positive for each antigen per field of view. Note that hypoxia promoted an increased capillary density that continued to increase over the 14-day time-course. In contrast, hypoxia induced a transient increase in β3 integrin expression, which peaked after 7 days hypoxia.
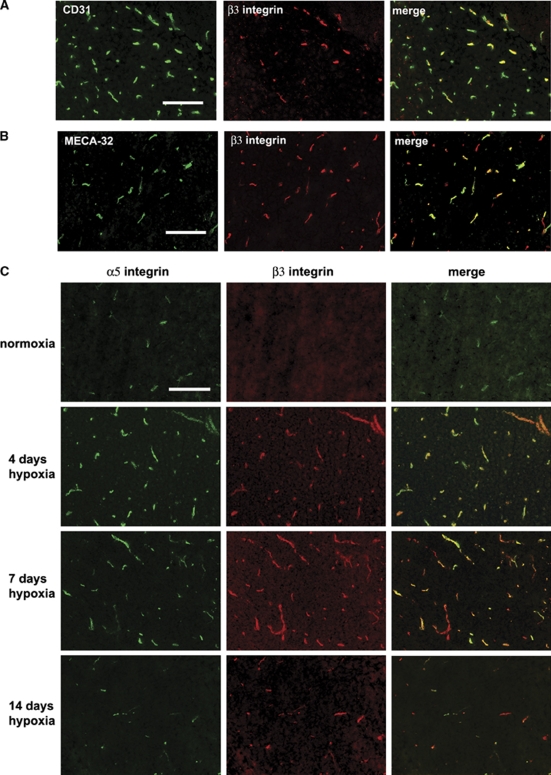
In the Hypoxic CNS, the αvβ3 Integrin is Expressed by Angiogenic BEC
Close examination of the number of blood vessels positive for CD31 or β3 integrin suggested that at any one time, only a fraction of the capillaries express β3 integrin. To confirm this relationship, we performed dual-color IF for CD31 and β3 integrin in the 4-day hypoxic CNS (Figure 2A). This revealed two points: first, that colocalization of CD31 and β3 integrin identifies BEC as the cells expressing β3 integrin, and second, not all CD31-positive structures are β3 integrin positive, showing that the αvβ3 integrin is expressed by only a subset of capillaries in the hypoxic CNS. The MECA-32 antigen is expressed by endothelial cells in the CNS up to embryonic day 15, and then switched off. After this time it cannot be detected on BEC, implying that MECA-32 labels only immature BEC (Hallman et al, 1995). Previously, we showed that MECA-32 is absent in the normoxic adult brain, and then re-expressed briefly after hypoxia (peaked at 4 days hypoxia), consistent with the idea that MECA-32 can be used as a marker of angiogenic immature BEC (Milner et al, 2008a). To examine whether angiogenic capillaries in the hypoxic CNS express the β3 integrin, we next performed dual-IF staining for MECA-32 and β3 integrin. As shown in Figure 2B, all capillaries expressing MECA-32 antigen also expressed β3 integrin, suggesting that angiogenic capillaries specifically induce the αvβ3 integrin.
Figure 2.
Expression of the β3 integrin on angiogenic capillaries in the hypoxic central nervous system (CNS). Dual-immunofluorescence (IF) was performed on frozen sections of cerebral cortex from normoxic or hypoxia-exposed mice using monoclonal antibodies specific for: (A) CD31 (Alexa Fluor 488) or β3 integrin (Cy-3), (B) MECA-32 (Alexa Fluor 488) or β3 integrin (Cy-3), and (C) α5 integrin (Alexa Fluor 488) or β3 integrin (Cy-3). Scale bar=50 μm. Note that after 4 days hypoxia, only a fraction of the CD31-positive capillaries stained positive for β3 integrin, but all MECA-32-positive capillaries stained positive for β3 integrin, confirming that β3 integrin is expressed by angiogenic endothelial cells within the hypoxic CNS. Also note that hypoxia promoted strong induction of both α5 and β3 integrins on capillaries, though the timing of expression of the two subunits was different; α5 integrin expression peaked at 4 days hypoxia, whereas β3 expression peaked at 7 days hypoxia.
Both α5β1 and αvβ3 Integrins are Upregulated on Angiogenic Endothelial Cells but the Temporal and Spatial Expression Patterns only Partially Overlap
As α5β1 integrin is also induced by angiogenic BEC in the hypoxia model (Milner et al, 2008a), we next performed dual-IF to examine whether the α5 and β3 integrin subunits show similar expression profiles. As shown in Figure 2C, in the normoxic brain, a few α5 integrin-positive capillaries were always present, whereas β3 integrin-positive capillaries were rarely detected. Hypoxia promoted strong induction of both α5 and β3 integrins on capillaries, and largely speaking, the two receptors were co-expressed by the same capillaries. However, consistent with previous findings, capillary expression of the α5 integrin was quickly induced, peaked at day 4, and then declined. In contrast, expression of the β3 integrin followed a slower time-course, reaching a peak after 7 days hypoxia. This shows that although hypoxia increases BEC expression of α5β1 and αvβ3 integrins, these responses follow a different time-course.
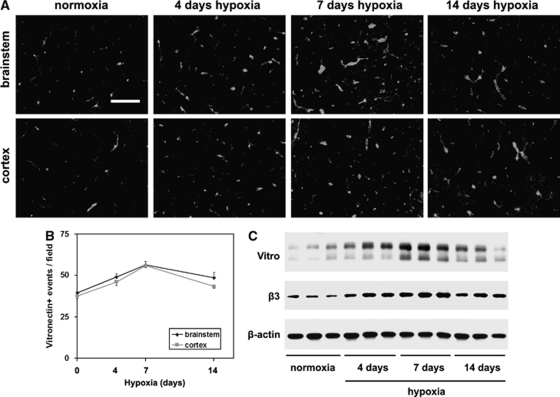
Hypoxia also Induces Brain Capillary Expression of the αvβ3 Ligand Vitronectin
We have shown that hypoxia leads to increased expression of fibronectin around cerebral capillaries, reaching maximal level after 4 days hypoxia, before declining at later time points (Milner et al, 2008a). Now, we examined whether expression of other αvβ3 ligands also shows changes in expression. The αvβ3 integrin is a promiscuous receptor, binding vitronectin, osteopontin, and fibronectin (Eliceiri and Cheresh, 2001; Stromblad and Cheresh, 1996). As shown in Figure 3, although vitronectin-positive capillaries were weakly present in the normoxic brain in both brainstem and cortex, cerebral hypoxia markedly increased expression levels. Quantification showed that in both the brainstem and cerebral cortex, the number of vitronectin-positive capillaries was maximal after 7 days hypoxia, and then declined at later time points. Seven days hypoxia increased the number of vitronectin-positive vessels per field of view, from 39.5±0.6 to 56.3±2.3 in the brainstem (P<0.005) and from 37.5±1.5 to 55.8±1.3 in the cerebral cortex (P<0.001). No osteopontin-positive events were detected in the brain, under any condition (not shown). Western blot analysis of brain lysates confirmed these findings, with vitronectin levels in the 7-day hypoxic brain increased 10.8±1.6-fold compared with normoxic conditions (P<0.005). These results show that vascular expression of vitronectin is upregulated by cerebral hypoxia in a similar manner to fibronectin, though with a slightly later time-course, which coincides with induction of the β3 integrin.
Figure 3.
Hypoxic induction of vitronectin on cerebral blood vessels. (A) Frozen sections of brainstem or cerebral cortex taken from mice exposed to normoxia or 4, 7, or 14 days hypoxia were immunostained for vitronectin. Scale bar=50 μm. (B) Quantification of vitronectin-positive vessels. Experiments were performed with three different animals per condition, and the results expressed as the mean±s.e.m. of the number of capillaries positive for each antigen per field of view. (C). Western blotting confirmation of hypoxic induction of β3 integrin and vitronectin in brain lysates. Note that cerebral hypoxia promoted increased expression of vitronectin and the β3 integrin subunit, with the greatest effect after 7 days hypoxia.
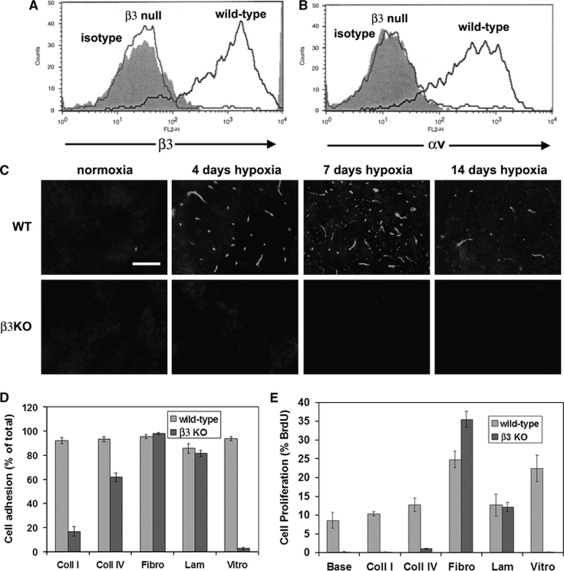
BEC from β3 Integrin-Null Mice Express no αv Integrins
To investigate the potential angiogenic role of the αvβ3 integrin, we studied β3 integrin-null mice. First we characterized the αv integrin expression profile of BEC derived from β3 integrin-null or wild-type littermate control mice by flow cytometry. As shown in Figure 4A, BEC from wild-type mice expressed high levels of the β3 integrin subunit, whereas in contrast, no β3 integrin expression was detected on β3 integrin-null BEC. Next, to exclude the possibility that BEC express other αv integrins, or that β3 integrin-null BEC show compensatory induction of other αv integrins, we examined the expression of the αv integrin subunit (Figure 4B). While wild-type BEC expressed the αv subunit, β3 integrin-null BEC showed absolutely no expression. This confirms that BEC express just one αv integrin, αvβ3, and also shows that β3 integrin-null BEC show no compensatory upregulation of other αv integrins. To confirm the absence of the β3 integrin subunit in vivo, we performed IF on brain sections. This showed that in contrast to the hypoxic induction of β3 integrin on cerebral blood vessels in wild-type mice, those in β3 integrin-null mice showed absence of β3 integrin expression (Figure 4C).
Figure 4.
Characterization of brain endothelial cells (BEC) derived from β3 integrin-null mice. (A and B) Wild-type or β3 integrin-null BEC were cultured on fibronectin and expression of the β3 (A) or αv (B) integrin subunits analyzed using flow cytometry. Note that in contrast to wild-type BEC, β3 integrin-null BEC showed a total absence of both the β3 and αv subunits. (C) Confirmation of the absence of the hypoxia induction of β3 integrin expression in β3 integrin-null mice. Scale bar=50 μm. Note that in contrast to the wild-type central nervous system (CNS), where strong hypoxia induction of the β3 integrin subunit was observed, vessels in the β3 integrin-null CNS showed a total lack of β3 integrin. (D) One-hour adhesion assays were performed, with adhesion expressed as the percentage of cells adherent to each substrate; all points represent the mean±s.e.m. of three experiments. Note that wild-type BEC attached well to all extracellular matrix (ECM) proteins. In contrast, β3 integrin-null BEC showed marked adhesive defects on vitronectin and collagen I, and a mild defect on collagen IV. (E) BEC proliferation on different ECM substrates was examined over 16 h, and expressed as the percentage of BEC that incorporated 5-bromo-2-deoxyuridine (BrdU); all points represent the mean±s.e.m. of three experiments. Note that fibronectin and vitronectin significantly promoted proliferation of wild-type BEC, relative to uncoated glass. Proliferation of β3 integrin-null BEC was almost totally abolished on vitronectin, collagen I, and collagen IV, but significantly increased on fibronectin, relative to wild-type cells.
β3 Integrin-Null BEC Show Reduced Adhesion and Proliferation on Vitronectin and the Collagens I and IV, but Enhanced Proliferation on Fibronectin
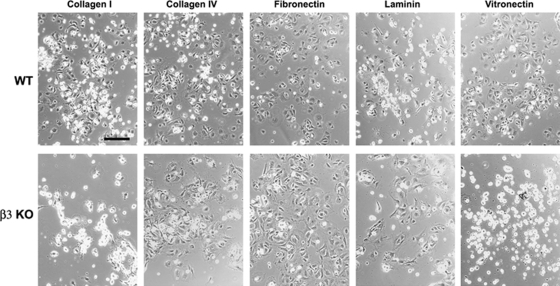
Next, we examined whether β3 integrin-null BEC showed any defects in their response to ECM ligands. Wild-type BEC attached to all ECM proteins tested, including collagens I and IV, fibronectin, laminin, and vitronectin. In contrast, β3 integrin-null BEC showed marked adhesive defects to vitronectin and collagen I, and a mild defect to collagen IV, though adhesion to fibronectin and laminin was no different from wild-type cells (Figures 4D and 5). The adhesive defect of β3 integrin-null BEC was most profound on vitronectin, with almost no cells adhering to vitronectin (2.6%±1.1% compared with 93.8%±1.7% wild-type, P<0.001). On collagen I, adhesion of β3 integrin-null BEC was markedly reduced (16.7%±3.9% compared with 92.0%±2.3% wild-type, P<0.005), whereas adhesion to collagen IV showed a slight reduction (61.5%±3.7% compared with 93.2%±2.2% wild-type, P<0.02).
Figure 5.
Analysis of the adhesion defects in β3 integrin-null brain endothelial cells (BEC). Wild-type or β3 integrin-null BEC were plated onto the extracellular matrix (ECM) proteins, collagen I, collagen IV, fibronectin, laminin, or vitronectin. After 3 h, photographs were taken. Scale bar=50 μm. Note that wild-type BEC attached and spread on all ECM proteins tested. In contrast, β3 integrin-null BEC showed marked adhesive defects on vitronectin and collagen I, and a mild defect on collagen IV, though adhesion to fibronectin and laminin was no different from wild-type cells.
Next, the role of αvβ3 integrin in mediating BEC proliferation on different ECM proteins was examined using BrdU incorporation. Consistent with previous findings (Wang and Milner, 2006), fibronectin (24.8%±2.2%) and vitronectin (22.4%±3.3%) significantly promoted proliferation of wild-type BEC, relative to uncoated glass (8.6%±2.1%, P<0.05 for both ECM proteins; Figure 4E). As expected in light of the adhesive defects, proliferation of β3 integrin-null BEC on vitronectin, collagen I, and collagen IV was almost totally abolished, as it was on uncoated glass. In contrast, proliferation of β3 integrin-null BEC on laminin was no different from wild-type cells, and unexpectedly, proliferation on fibronectin was increased relative to wild-type cells (35.5%±2.1% versus 24.8%±2.2%, P<0.05).
β3 Integrin-Null Mice Show an Altered Angiogenic Response to Cerebral Hypoxia
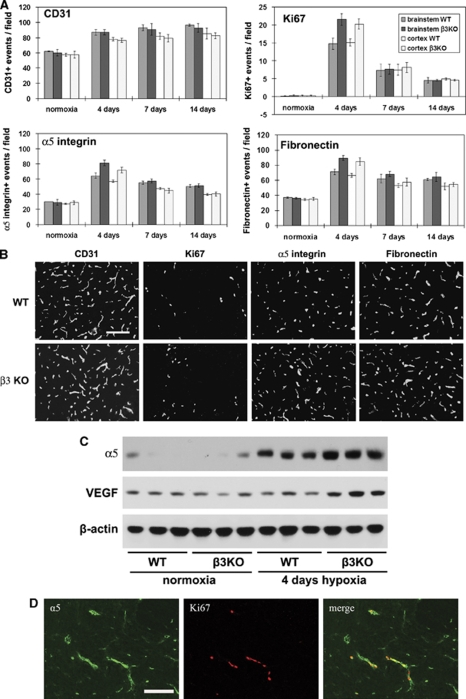
To determine whether the αvβ3 integrin has an important role in mediating the angiogenic response to cerebral hypoxia, we examined this response in β3 integrin-null mice. As an increased capillary density is the end product of angiogenesis, we first examined capillary density at the different time points. CD31 IF showed that cerebral hypoxia promoted an increased capillary density in the brains of β3 integrin-null and wild-type littermate control mice, with no significant differences observed between the two groups at any time point (Figures 6A and 6B). Next we examined cell proliferation by Ki67 IF, as this is a sensitive early indicator of angiogenesis that peaks after 4 days cerebral hypoxia (Li et al, unpublished observations). Contrary to our expectations, after 4 days hypoxia, β3 integrin-null brains showed a significantly increased Ki67 index relative to wild-type mice, an effect consistently observed both in brainstem (21.6±1.6 versus 14.8±1.5, P<0.05) and cerebral cortex (20.2±1.4 versus 15.1±1.0, P<0.05). These results show that although the αvβ3 integrin is not essential for hypoxia-induced cerebral angiogenesis, its absence leads to an accelerated BEC proliferation response at an early stage of angiogenesis. The most likely reason for this is that loss of αvβ3 integrin triggers compensatory upregulation of other proangiogenic molecules, and that this directly promotes BEC proliferation. To investigate this possibility, we examined expression levels of the α5 integrin and VEGF in these mice. IF revealed that after 4 days hypoxia, β3 integrin-null mice brains showed a significantly increased number of α5 integrin-positive vessels (Figures 6A and 6B), both in the brainstem (81.2±3.9 versus 64.5±3.2, P<0.05) and cerebral cortex (71.9±4.0 versus 57.1±1.4, P<0.05), though the effect was relatively short-lived and not apparent at later time points. Western blot analysis of 4-day hypoxic brain lysates confirmed this finding, with α5 integrin levels in the β3 integrin-null lysates increased 2.3±0.1-fold over the wild-type level (P<0.002; Figure 6C). In addition, western blot analysis revealed that at the same time point, VEGF levels in the β3 integrin-null lysates increased 3.4±0.8-fold over the wild-type level (P<0.05). Examination of vitronectin and fibronectin expression showed that although there were no difference in hypoxic induction of vitronectin, the number of fibronectin-positive capillaries after 4 days hypoxia was significantly increased in β3 integrin-null mice, both in the brainstem (89.3±3.2 versus 71.0±3.5, P<0.02) and cerebral cortex (85.0±4.8 versus 66.3±2.7, P<0.05), compared with wild-type mice. To substantiate a role for α5 integrin in BEC proliferation, brains from 4-day hypoxic wild-type mice were examined for colocalization of α5 integrin and Ki67. This revealed a very close colocalization pattern, showing that BEC expressing high levels of α5 integrin were the most mitogenic (Figure 6D). In summary, these data show that in the in vivo hypoxia model, β3 integrin-null mice showed no major defect in cerebral angiogenesis. However, contrary to expectations, compared with wild-type mice, BEC in β3 integrin-null mice showed a significantly increased mitotic index at an early stage (day 4) of the angiogenic process, which correlated closely with increased BEC expression of the α5 integrin and increased VEGF.
Figure 6.
Comparison of the hypoxic-induced angiogenic response, in the wild-type and β3 integrin-null central nervous system (CNS). β3 integrin-null and wild-type littermate control mice were maintained at normoxia or exposed to 8% hypoxia for 4, 7, or 14 days before frozen brain sections immunostained to assess blood vessel density (CD31), cell proliferation (Ki67), and expression of α5 integrin and fibronectin. (A) Quantification of analysis. Brain sections were examined for the number of vessels that stained positive for the different antigens. Experiments were performed with three different animals per condition, and the results expressed as the mean±s.e.m. of the number of capillaries positive for each antigen per field of view. (B) Representative images of immunofluorescence (IF) of brainstem after 4 days hypoxia. Scale bar=50 μm. Note that cerebral hypoxia promoted an increased capillary density (CD31) in the brains of wild-type and β3 integrin-null mice, with no significant differences observed between the two groups at any time point. In contrast, compared with wild-type mice, after 4 days hypoxia, brain endothelial cells (BEC) in β3 integrin-null mice showed a significantly increased mitotic index (Ki67-positive events), with parallel increases in the number of vessels positive for α5 integrin and fibronectin. (C) Western blot analysis. Note that after 4 days hypoxia, levels of the α5 integrin and vascular endothelial growth factor (VEGF) were significantly higher in the β3 integrin-null mice compared with wild-type controls (n=3 mice per condition). (D) Colocalization of α5 integrin and the proliferation marker Ki67 in the 4-day hypoxic CNS. Scale bar=50 μm.
β3 Integrin-Null BEC also Show Upregulated Expression of β1 Integrins In Vitro
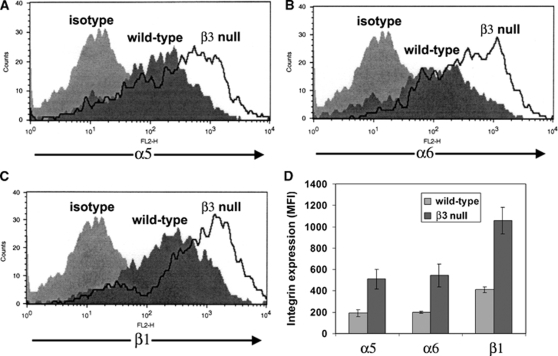
To quantify the compensatory upregulation of α5 integrin in β3 integrin-null BEC, and also examine whether this mechanism operates in vitro, we performed flow cytometry to quantify expression levels of different integrin subunits on BEC derived from β3 integrin-null or wild-type littermate mice. As shown in Figure 7, β3 integrin-null BEC expressed significantly higher levels of the integrin subunits, α5 (511.0±92.1 MFI compared with 192.0±31.8 MFI in wild-type cells, P<0.05), α6 (544.7±106.2 MFI compared with 197.7±9.5 MFI in wild-type cells, P< 0.05), and β1 (1,058.3±122.8 MFI compared with 411.0±27.9 MFI in wild-type cells, P<0.02). Thus, β3 integrin-null BEC showed strong compensatory upregulation of the α5, α6, and β1 integrin subunits.
Figure 7.
Compensatory upregulation of β1 integrin expression on β3 integrin-null brain endothelial cells (BEC). Wild-type or β3 integrin-null BEC were cultured on fibronectin. On reaching confluence, flow cytometry was used to quantify BEC expression of the integrin subunits α5 (A), α6 (B), or β1 (C). (D) Quantification of expression of β1 integrins by β3 integrin-null BEC. All points represent the mean±s.e.m. of the mean fluorescent intensity (MFI) of three separate experiments. Note that relative to wild-type cells, β3 integrin-null BEC showed a marked upregulation of the α5, α6, and β1 integrin subunits.
Discussion
In this study, we examined the expression and potential angiogenic role of the αvβ3 integrin in the hypoxic CNS. We found that the αvβ3 integrin and its ECM ligand vitronectin were strongly upregulated on angiogenic capillaries, with the effect maximal after 7 days hypoxia. Surprisingly, in the cerebral hypoxia model, β3 integrin-null mice showed no obvious defect in cerebral angiogenesis. However, at an early stage of the angiogenic process, BEC in these mice showed an increased rate of proliferation, which correlated closely with increased expression of α5 integrin. Flow cytometry confirmed strong compensatory upregulation of α5 integrin on β3 integrin-null BEC, which correlated with an increased BEC proliferation rate on fibronectin. These studies confirm hypoxic induction of αvβ3 integrin on angiogenic vessels, and show that in β3 integrin-null BEC, parallel increases occur in α5 integrin expression and cell proliferation, both in vitro and in vivo. Taken together, this suggests distinct roles for the BEC integrins αvβ3 and α5β1 in cerebral angiogenesis, with αvβ3 having a nonessential role, and α5β1 promoting BEC proliferation.
Induction of the αvβ3 Integrin on Angiogenic Cerebral Blood Vessels
In this study, we showed that αvβ3 integrin was strongly upregulated on angiogenic capillaries in the hypoxia CNS. This observation is consistent with previous findings from studies of focal cerebral ischemia, both in the primate (Abumiya et al, 1999; Okada et al, 1996) and in the rat (Wei et al, 2001). Previously, we described upregulation of the α5β1 integrin and its ligand fibronectin in the cerebral hypoxia model (Milner et al, 2008a), and upregulation of α5 integrin on hypoxic BEC in vitro (Milner et al, 2008b), thus showing that hypoxia exerts the same effect on BEC, both in vivo and in vitro. Similar observations highlighting parallel in vivo and in vitro responses to hypoxic stress have also been reported for hypoxia inducible factor-1α expression in the intact brain (Chavez et al, 2000) and in isolated neural cells in vitro (Ruscher et al, 2002). It is interesting that cerebral hypoxia induces expression of the microvascular integrins α5β1 and αvβ3, but not others, such as α1β1. This specific upregulation of these two integrins in cerebral hypoxia is consistent with angiogenic studies in other systems, including wound-healing (Clark et al, 1996) and tumor-induced angiogenesis (Kim et al, 2000). What determines this specificity is currently unknown, but it seems likely that the α5 and β3 genes contain hypoxia-response elements. It has also been shown that retroviral delivery of the transcription factor HoxD3 to the brain promotes angiogenic sprouting, and strong induction of αvβ3 integrin on angiogenic capillaries (Chen et al, 2004).
Behavior of β3 Integrin-Null BEC In Vitro
Flow cytometry revealed that β3 integrin-null BEC express no αv integrins. This makes two points: first, consistent with our previous biochemical studies (Wang and Milner, 2006), BEC appear to express only one αv integrin, αvβ3, and second, β3 integrin-null BEC show no compensatory induction of other αv integrins. Interestingly however, β3 integrin-null BEC showed strong upregulation of β1 integrins, including the α5, α6, and β1 subunits, all of which showed a 2.5-fold increase. This is the first demonstration that β3 integrin-null cells show compensatory upregulation of other integrins, though it is in keeping with findings from other systems that show increased α2 integrin levels in renal cells of α8 integrin-null mice (Haas et al, 2003), and elevated levels of α6 integrin in cerebral vessels from α7 integrin-null mice (Flintoff-Dye et al, 2005). Upregulation of the α5 integrin subunit is particularly significant, as aside from αvβ3, the α5β1 integrin is the main fibronectin receptor expressed by BEC (Wang and Milner, 2006), thus it is logical that β3 integrin-null BEC would upregulate α5β1 integrin in an attempt to maintain their responses to fibronectin. Consistent with this, proliferation of β3 integrin-null BEC on fibronectin was greater than wild-type cells, highlighting a tight correlation between BEC α5 integrin expression and proliferation rate. β3 integrin-null BEC did show marked adhesive defects on vitronectin and the collagens, culminating in a lack of proliferation on these substrates. The lack of adhesion to vitronectin is consistent with studies in other cell types (Reynolds et al, 2002, 2005), and highlights the importance of αvβ3 as a major vitronectin receptor.
Role of αvβ3 in Angiogenesis In Vivo
Induction of αvβ3 integrin on angiogenic endothelial cells is well established (Abumiya et al, 1999; Eliceiri and Cheresh, 2001; Okada et al, 1996; Wei et al, 2001). What is less clear is whether this integrin is essential for angiogenesis. Studies examining the angiogenic role of αvβ3 integrin in other systems have generated conflicting results, with function-blocking approaches supporting an angiogenic role (Brooks et al, 1994a, 1994b), but studies in β3 integrin-null mice refute this role (Hodivala-Dilke et al, 1999; Reynolds et al, 2002). The results of our study show that the αvβ3 integrin is not essential for hypoxia-induced cerebral angiogenesis. In fact its absence led to accelerated BEC proliferation, which correlated with elevated levels of VEGF and BEC expression of the alternate fibronectin receptor, α5β1 integrin. These observations are consistent with other studies, which describe increased tumor-induced angiogenesis, and accelerated wound-healing in β3 integrin-null mice, events that correlate with compensatory increases in VEGF and transforming growth factor-β1-mediated signaling, in these two respective models (Reynolds et al, 2002, 2005). Exactly what accelerates BEC proliferation in β3 integrin-null mice is unclear. The most likely reason is that the αvβ3 integrin has an important, though redundant adhesive function on BEC, and that absence of this function leads to upregulation of other angiogenic mechanisms, including the α5β1 integrin and VEGF, which exert their effects to promote BEC proliferation. Compensatory upregulation of the α5 integrin was observed both in vivo and in vitro, suggesting a fundamental mechanism. An alternative reason previously suggested is that αvβ3 might function as a transdominant regulator of other integrins, and that in the absence of this integrin, suppression is relieved, leading to enhanced α5β1 function and increased rate of BEC proliferation (Silva et al, 2008).
Implications for the Role of α5β1 Integrin in Cerebral Angiogenesis
The results of our study point to the conclusion that of the two BEC fibronectin receptors, αvβ3 is not essential for cerebral angiogenesis, whereas the tight relationship between α5β1 and BEC proliferation strongly suggests a proangiogenic role for this integrin. This conclusion is consistent with recent data obtained in our laboratory, which show a very close correlation between α5β1-fibronectin expression levels and BEC proliferation, during the angiogenic response to hypoxia (Li et al, unpublished observations). This interpretation also predicts that artificially increasing the expression level of α5β1 integrin on BEC might be an effective way of stimulating blood vessel growth in the adult CNS. In future experiments we will examine hypoxia-induced cerebral angiogenesis in transgenic mice that lack α5 integrin expression in endothelial cells. On the basis of our current findings, we predict that loss of α5 integrin will lead to reduced BEC proliferation and attenuation of cerebral angiogenesis.
The authors declare no conflict of interest.
References
- Abumiya T, Lucero J, Heo JH, Tagaya M, Copeland BR, Koziol JA, del Zoppo GJ. Activated microvessels express vascular endothelial growth factor and integrin alpha (v) beta3 during focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:1038–1050. doi: 10.1097/00004647-199909000-00012. [DOI] [PubMed] [Google Scholar]
- Brooks P, Clark RAF, Cheresh DA. Requirement for vascular integrin αvβ3 for angiogenesis. Science. 1994a;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Montgomery AMP, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin αvβ3 antagonists promote tumour regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994b;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Chavez JC, Agani F, Pichiule P, LaManna JC. Expression of hypoxic inducible factor 1α in the brain of rats during chronic hypoxia. J Appl Physiol. 2000;89:1937–1942. doi: 10.1152/jappl.2000.89.5.1937. [DOI] [PubMed] [Google Scholar]
- Chen HH, Chien CH, Liu HM. Correlation between angiogenesis and basic fibroblast growth factor expression in experimental brain infarct. Stroke. 1994;25:1651–1657. doi: 10.1161/01.str.25.8.1651. [DOI] [PubMed] [Google Scholar]
- Chen Y, Xu B, Arderiu G, Hashimoto T, Young WL, Boudreau NJ, Yang GY. Retroviral delivery of homeobox D3 gene induces cerebral angiogenesis in mice. J Cereb Blood Flow Metab. 2004;24:1280–1287. doi: 10.1097/01.WCB.0000141770.09022.AB. [DOI] [PubMed] [Google Scholar]
- Clark RA, Tonneson MG, Gaillit J, Cheresh DA. Transient functional expression of αvβ3 on vascular cells during wound repair. Am J Pathol. 1996;148:1407–1421. [PMC free article] [PubMed] [Google Scholar]
- Eliceiri BP, Cheresh D. Adhesion events in angiogenesis. Curr Opin Cell Biol. 2001;13:563–568. doi: 10.1016/s0955-0674(00)00252-0. [DOI] [PubMed] [Google Scholar]
- Flintoff-Dye NL, Welser J, Rooney J, Scowen P, Tamowski S, Hatton W, Burkin DJ. Role for the alpha7beta1 integrin in vascular development and integrity. Dev Dyn. 2005;234:11–21. doi: 10.1002/dvdy.20462. [DOI] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Haas CS, Amann K, Schittny J, Blaser B, Müller U, Hartner A. Glomerular and renal vascular structural changes in alpha8 integrin-deficient mice. J Am Soc Nephrol. 2003;14:2288–2296. doi: 10.1097/01.asn.0000082999.46030.fe. [DOI] [PubMed] [Google Scholar]
- Hallman R, Mayer DN, Berg EL, Broermann R, Butcher EC. Novel mouse endothelial cell surface marker is suppressed during differentiation of the blood brain barrier. Dev Dyn. 1995;202:325–332. doi: 10.1002/aja.1002020402. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23:166–180. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan A, Farahani R, Douglas RM, LaManna JC, Haddad GC. Effect of chronic continuous or intermittent hypoxia and reoxygenation on cerebral capillary density and myelination. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1105–R1R14. doi: 10.1152/ajpregu.00535.2005. [DOI] [PubMed] [Google Scholar]
- Kim S, Bell K, Mousa SA, Varner JA. Regulation of angiogenesis in vivo by ligation of integrin α5β1 with the central cell-binding domain of fibronectin. Am J Pathol. 2000;156:1345–1362. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S, Roghani M, Rifkin DB. Fibroblast growth factors as angiogenesis factors. EXS. 1997;79:159–192. doi: 10.1007/978-3-0348-9006-9_7. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- Kuo N-T, Benhayon D, Przybylski RJ, Martin RJ, LaManna JC. Prolonged hypoxia increases vascular endothelial growth factor mRNA and protein in adult mouse brain. J Appl Physiol. 1999;86:260–264. doi: 10.1152/jappl.1999.86.1.260. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Kuo NT, Lust WD. Hypoxia-induced brain angiogenesis. Signals and consequences. Adv Exp Med Biol. 1998;454:287–293. doi: 10.1007/978-1-4615-4863-8_34. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Vendel LM, Farrell RM. Brain adaptation to chronic hypobaric hypoxia in rats. J Appl Physiol. 1992;72:2238–2243. doi: 10.1152/jappl.1992.72.6.2238. [DOI] [PubMed] [Google Scholar]
- Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ulrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Milner R, Campbell IL. Developmental regulation of β1 integrins during angiogenesis in the central nervous system. Mol Cell Neurosci. 2002a;20:616–626. doi: 10.1006/mcne.2002.1151. [DOI] [PubMed] [Google Scholar]
- Milner R, Campbell IL. Cytokines regulate microglial adhesion to laminin and astrocyte extracellular matrix via protein kinase C-dependent activation of the α6β1 integrin. J Neurosci. 2002b;22:1562–1572. doi: 10.1523/JNEUROSCI.22-05-01562.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R, Crocker SJ, Hung S, Wang X, Frausto RF, Del Zoppo GJ. Fibronectin- and vitronectin-induced microglial activation and matrix metalloproteinase-9 expression is mediated by integrins α5β1 and αvβ5. J Immunol. 2007;178:8158–8167. doi: 10.4049/jimmunol.178.12.8158. [DOI] [PubMed] [Google Scholar]
- Milner R, Hung S, Erokwu B, Dore-Duffy P, LaManna JC, del Zoppo GJ. Increased expression of fibronectin and the alpha 5 beta 1 integrin in angiogenic cerebral blood vessels of mice subject to hypobaric hypoxia. Mol Cell Neurosci. 2008a;38:43–52. doi: 10.1016/j.mcn.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R, Hung S, Wang X, Berg G, Spatz M, del Zoppo G. Responses of endothelial cell and astrocyte matrix-integrin receptors to ischemia mimic those observed in the neurovascular unit. Stroke. 2008b;39:191–197. doi: 10.1161/STROKEAHA.107.486134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Copeland BR, Hamann GF, Koziol JA, Cheresh DA, del Zoppo GJ. Integrin αvβ3 is expressed in selective microvessels following focal cerebral ischemia. Am J Pathol. 1996;149:37–44. [PMC free article] [PubMed] [Google Scholar]
- Pichiule P, LaManna JC. Angiopoietin-2 and rat brain capillary remodeling during adaptation and de-adaptation to prolonged mild hypoxia. J Appl Physiol. 2002;93:1131–1139. doi: 10.1152/japplphysiol.00318.2002. [DOI] [PubMed] [Google Scholar]
- Reynolds LE, Conti FJ, Lucas M, Grose R, Robinson S, Stone M, Saunders G, Dickson C, Hynes RO, Lacy-Hulbert A, Hodivala-Dilke K. Accelerated re-epithelialization in beta3-integrin-deficient-mice is associated with enhanced TGF-beta1 signaling. Nat Med. 2005;11:167–174. doi: 10.1038/nm1165. [DOI] [PubMed] [Google Scholar]
- Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke K. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield U, Heine I, Liotta A, Falanga J, Kehrl JH, Fauci AS. Transforming growth factor type β: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscher K, Freyer D, Karsch M, Isaev N, Megow D, Sawitzki B, Priller J, Dirnagl U, Meisel A. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002;22:10291–10301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- Silva R, D'Amico G, Hodivala-Dilke KM, Reynolds LE. Integrins: the keys to unlocking angiogenesis. Arterioscler Thromb Vasc Biol. 2008;28:1703–1713. doi: 10.1161/ATVBAHA.108.172015. [DOI] [PubMed] [Google Scholar]
- Stromblad S, Cheresh DA. Integrins, angiogenesis and vascular cell survival. Chem Biol. 1996;3:881–885. doi: 10.1016/s1074-5521(96)90176-3. [DOI] [PubMed] [Google Scholar]
- Wang J, Milner R. Fibronectin promotes brain capillary endothelial cell survival and proliferation through α5β1 and αvβ3 integrins via MAP kinase signaling. J Neurochem. 2006;96:148–159. doi: 10.1111/j.1471-4159.2005.03521.x. [DOI] [PubMed] [Google Scholar]
- Wei L, Erinjeri JP, Rovainen CM, Woolsey TA. Collateral growth and angiogenesis around cortical stroke. Stroke. 2001;32:2179–2184. doi: 10.1161/hs0901.094282. [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in α5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by α4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]