Abstract
Activation of endogenous stem cells has been proposed as a novel form of therapy in a variety of neurologic disorders including traumatic brain injury (TBI). Vascular endothelial growth factor (VEGF) is expressed in the brain after TBI and serves as a potent activator of angiogenesis and neurogenesis. In this study, we infused exogenous VEGF into the lateral ventricles of mice for 7 days after TBI using mini-osmotic pumps to evaluate the effects on recovery and functional outcome. The results of our study show that VEGF significantly increases the number of proliferating cells in the subventricular zone and in the perilesion cortex. Fate analysis showed that most newborn cells differentiated into astrocytes and oligodendroglia and only a few cells differentiated into neurons. Functional outcome was significantly better in mice treated with VEGF compared with vehicle-treated animals after TBI. Injury size was significantly smaller at 90 days after TBI in VEGF-treated animals, suggesting additional neuroprotective effects of VEGF. In conclusion, VEGF significantly augments neurogenesis and angiogenesis and reduces lesion volumes after TBI. These changes are associated with significant improvement in recovery rates and functional outcome.
Keywords: angiogenesis, neurogenesis, neuroprotection, traumatic brain injury, vascular endothelial growth factor
Introduction
At least 1.4 million Americans have traumatic brain injury (TBI) each year according to the Center for Disease Control (Langlois et al, 2004). Of these, 50,000 die, 235,000 are admitted to hospital, and 90,000 are left with permanent disability (Langlois et al, 2004). Neuroprotective strategies aimed at preventing cellular death in the affected brain have been largely disappointing, and to date, no neuroprotective agent is clinically available (Beauchamp et al, 2008; Leker and Shohami, 2002).
Repair mechanisms based on the proliferation of endogenous cells have been identified in the posttraumatic brain, suggesting that precursors at the ventricular surface proliferate, migrate to sites of injury, and differentiate into neurons (Chirumamilla et al, 2002; Dash et al, 2001). However, spontaneous neurogenesis is not sufficient to induce meaningful recovery (Shetty et al, 2004). Therefore, augmenting neurogenesis is a reasonable approach to improve functional outcome after TBI. Owing to the many similarities in response to injury that exist between cerebral trauma and ischemia (Leker and Shohami, 2002) important insights into the development of clinically significant therapies can be learned from our experience in stroke. Preliminary results in a model of cerebral ischemia show that neurogenesis can be significantly enhanced with the continuous delivery of basic fibroblast growth factor (FGF2) into the injured cortex (Leker et al, 2007). However, merely augmenting the number of newborn cells in the brain may not suffice as these cells depend on newly formed blood vessels for their metabolic and trophic needs (Gage et al, 1998; Horner and Palmer, 2003; Palmer et al, 2000).
Vascular endothelial growth factor (VEGF) is a trophic factor that is expressed in the CNS after injury (Dore-Duffy et al, 2007; Hayashi et al, 1997; Skold et al, 2005; Wang et al, 2005) and induces angiogenesis (Nag et al, 1997; Nieto et al, 2001). VEGF may also have beneficial effects on the survival of newborn neuronal precursors (Widenfalk et al, 2003) and has been implicated in neurogenesis (Jin et al, 2002) after cerebral ischemia (Sun et al, 2003; Wang et al, 2007) and neurite outgrowth (Jin et al, 2006). Furthermore, VEGF has important implications in increasing the size of the subventricular zone (SVZ) (Gotts and Chesselet, 2005), and inhibition of VEGF expression after injury may actually exacerbate outcome (Skold et al, 2006). However, VEGF could be a double-edged sword as it may increase vascular permeability and exacerbate cerebral edema, thus causing unwanted effects (Hayashi et al, 1997). Furthermore, as previously shown, VEGF antagonists can reduce brain edema formation after brain insult (van Bruggen et al, 1999). Therefore, this study was designed to explore the effect of continuous infusion of VEGF into the lateral ventricles of mice on neurogenesis, differentiation, and functional recovery of mice after TBI.
Materials and methods
Animals and Traumatic Brain Injury Model
The study was conducted according to the Institutional Animal Care and Use Committee guidelines in compliance with National Institutes of Health guidelines. Adult Sabra male mice weighing 40 g (n=51) were used for these experiments and treated with VEGF or vehicle and 5 sham-operated animals served as further controls. Food and water were provided ad libitum. Traumatic brain injury was induced in mice using the closed head injury (CHI) model (Shapira et al, 1988, 1993). This is a highly reproducible model of TBI that results in isolated frontoparietal cortical injury. Briefly, after induction of isoflurane anesthesia, a midline longitudinal incision was performed, the skin was retracted, and the skull was exposed. The left anterior frontal area was identified and a tipped Teflon cone was placed 1 mm lateral to the midline. The head was fixed and a 95 g weight was dropped on the cone from a height of 18 cm (Shapira et al, 1988, 1993). At the time of surgery and while anesthetized, the animals were implanted with an Alzet mini-osmotic pump secreting vehicle or VEGF for 7 days at a rate of 0.5 μl/h for a total dose of 0.84 μg (n=15 per group) into the right lateral ventricle. After recovery from anesthesia, the mice were returned to their home cages with postoperative care and free access to food and water. Sham controls received anesthesia and skin incision only but no head injury was induced.
BrdU Injections
From day 4 after injury or sham operation, and during 6 days, all animals received i.p. injection of the tracer 5-bromo-2-deoxyuridine (BrdU; 50 mg/kg, q12 h) to label dividing cells.
Evaluations
Neurobehavioral evaluation
Animals were weighed and examined with a standardized neurologic severity score (NSS) during 3 months after injury. During the first week, testing was performed every other day, then until the end of the second month every week, and finally, at 90 days after TBI. Ten different tasks were used to evaluate motor ability, balancing, and alertness of the tested animal and points were given for failure to perform a task (Beni-Adani et al, 2001). The pathologic scores correlate very well with clinical disability scores and with the degree of brain edema (Shapira et al, 1988). The first NSS was obtained at 1 h after TBI, and it reflects the initial severity of injury. Neurologic severity score at 1 h is predictive of both mortality and morbidity and it also correlates well with the extent of damage seen on MRI (Tsenter et al, 2008). Therefore, we selected only animals with an NSS=6 at 1 h for this test. The extent of recovery is calculated as the difference between the NSS at 1 h and at any subsequent time point (ΔNSS).
Injury size
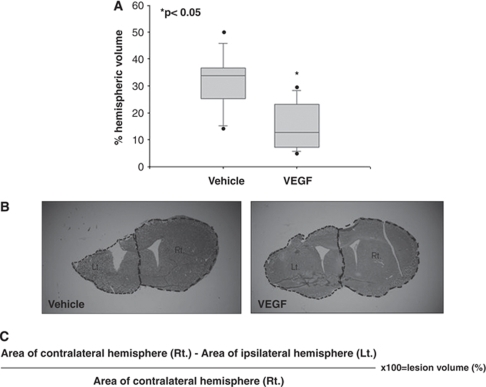
At 90 days after the surgery, the animals were deeply anesthetized and perfusion fixed with 4% paraformaldehyde. Their brains were frozen-sectioned at 10 μm. Brain slices 200 μm apart between bregma +1.42 and bregma −0.8 were stained with Giemsa stain-modified solution (1:1; Fluka, Sigma-Aldrich Corporation, St Louis, MO, USA) and digitally photographed. The volume of injured tissue was measured with ImageJ.40 g software (National Institutes of Health, Bethesda, MD, USA). Damaged tissue volume was calculated by dividing the volume of the injured hemisphere by that of the nonlesioned hemisphere (as shown in Figure 2C; Swanson et al, 1990) The results are expressed as a percentage of hemispheric tissue.
Immunohistochemistry
Overall, 7 evenly spaced slices were counted for each brain between bregma +1.42 and bregma −0.8, and in each slice, 10 evenly spaced high-power magnification fields ( × 400) in the entire area surrounding the infarct were counted. As the lesion core had already liquefied by 90 days after CHI, the area forming the outer boundary of the brain represents the border zone that survived. This area included mainly the cortex and subcortical tissue but not the striatum. Brain slices were double or triple stained for immunohistochemical evaluation using fate-specific antibodies that included rat anti-BrdU (marker for cell proliferation; 1:200; Accurate, New York, New York, USA), goat anti-doublecortin (DCX, marker for migrating neuroblasts, 1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-GFAP (marker for astrocytes, 1:200; Dako, Glostrup, Denmark), mouse anti-NeuN (marker for mature neurons; 1:100), rabbit anti Gal-C (marker for oligodendrocytes; 1:200) were all from Chemicom (Temecula, CA, USA), mouse anti-rat RECA1 (marker for blood vessels, 1:200; AbD Serotec, Oxford, UK) and mouse anti-CD31 (marker for endothelial cells, 1:100; Abcam Inc., Cambridge, MA, USA). Alexa 488 and Alexa 555 conjugates were used as secondary antibodies (1:200; Molecular Probes, Leiden, the Netherlands) and 46-diamidino-2-phenyl indole (Sigma Israel) was used to visualize nuclei.
Cell counting
Immunopositive cells were counted using an epifluorescent Olympus (Tokyo, Japan) microscope in prespecified regions of interest including the SVZ, corpus callosum, and the area surrounding the lesioned cortex. Overall, 10 equidistant fields were counted per region of interest (30 fields per slide and 210 fields per brain at × 400 magnification). Confocal Z sections on an Olympus system were used to determine colocalization.
Western blot analysis
An additional 21 mice underwent CHI as described above, were treated with VEGF (n=11) or vehicle (n=10) with an Alzet mini-osmotic pump as described above, and killed 6 days later. Sham-operated mice served as controls (n=5). After decapitation, the brains were quickly removed and the left cortex was dissected and frozen at −70°C until analysis. Cytosolic extracts were prepared from each sample. Tissue segments were incubated separately in ice-cold buffer containing the following: 50 mmol/L Tris-HCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 0.5 mmol/L Na3VO4, 0.1% 2-mercaptoethanol, 1% Triton X-100, 50 mmol/L NaF, 5 mmol/L sodium pyrophosphate, 10 mmol/L sodium β-glyceropyrophosphate, 0.1 mmol/L phenylmethanesulfonyl fluoride, and protease inhibitor mixture (Roche Diagnostics, Mannheim, Germany). After a 15 min incubation period on ice, the extracts were centrifuged at 10,000 r.p.m. for 20 min at 4°C and protein content in the supernatant was determined according to the Bradford method (Bio-Rad Laboratories, Munich, Germany). Total extract protein (50 μg) of each sample was mixed with SDS sample buffer and boiled at 95°C for 5 min before loading. Proteins were separated by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrotransferred onto polyvinylidene difluoride membranes (Biotrace Ltd). The membranes were blocked in 5% non-fat dry milk in Tris-buffered saline (pH 7.4), with 0.1% Tween 20 (Tris-buffered saline–Tween) for 1 h at room temperature. To assess the signal transduction pathways of VEGF, we performed a detection of proteins using primary antibodies: total AKT and phospho-AKT-Ser473 (both 1:1,000; Cell Signaling Technology, Danvers, MA, USA), which were diluted in 5% bovine serum albumin solution, and membranes were incubated overnight at 4°C. The primary antibody was removed, and the blots were washed in Tris-buffered saline–Tween and incubated for 1 h at room temperature in horseradish peroxidase-conjugated secondary antibodies (1:10,000; Jackson Immunoresearch, Soham, Cambridgeshire, UK). Reactive proteins were visualized using an enhanced chemiluminescence reagent (Biological Industries, Beit Haemek, Israel). Optical density was determined using Tina software (Raytest, Straubenhardt, Germany). All membranes were also incubated with a rabbit polyclonal antibody for β-actin (1:1,000; Cell Signaling Technology). Protein level was expressed as relative optical density, representing the optical density of pAkt divided by the optical density of β-actin within the same lane.
Statistical Analysis
Analysis was performed with the SigmaStat software package (Systat, Richmond, CA, USA). Data are presented as mean±s.e.m. as indicated in the legends. Values were compared using one-way analysis of variance followed by Student–Newman–Keuls posttest method. P-values ⩽0.05 were considered significant for all comparisons.
Results
VEGF Significantly Increased Functional Gain after TBI
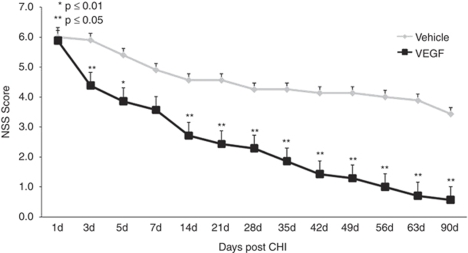
Neurologic deficits were measured at predetermined time points after CHI with the NSS. A more rapid and pronounced improvement in NSS was observed in VEGF-treated animals, compared with those treated with vehicle. The absolute NSS was decreased and the ΔNSS from days 1 to 90 was significantly greater in animals treated with VEGF (Figure 1). The differences in NSS were apparent as early as day 3 after TBI, suggesting a neuroprotective effect, and the difference continued to increase with time until the conclusion of the study. It is noted that the slope in NSS increased significantly after 14 days and the curves diverged significantly more at this time point similar to what was seen after cerebral ischemia (Shetty et al, 2004).
Figure 1.
Vascular endothelial growth factor (VEGF) improves neurologic disability. Animals underwent closed head injury (CHI) and were treated with VEGF or vehicle. Neurologic disability was evaluated at different time points after CHI with the neurologic severity score (NSS). Mice treated with VEGF showed significantly larger improvement beginning at day 3 after CHI and the curves continued to diverge until the end of the study.
VEGF Decreases Lesion Volume after TBI
In this set of experiments, exogenous VEGF was administered through mini-osmotic pumps for 1 week after TBI. A significant 50% reduction in lesion size was identified at 90 days after TBI in VEGF-treated mice (Figures 2A and 2B), implying a neuroprotective effect for exogenous VEGF.
Figure 2.
Vascular endothelial growth factor (VEGF) treatment significantly reduces lesion size as measured at 90 days after traumatic brain injury (TBI). Seven equidistant brain slices were stained with Giemsa and the volume of injured tissue was measured (A). (B) A sample showing that VEGF-treated mice had significantly smaller lesions at each level. The dotted line marks the lesioned brain as calculated according to the equation shown in (C).
VEGF Increases the Number of Newborn Cells after TBI
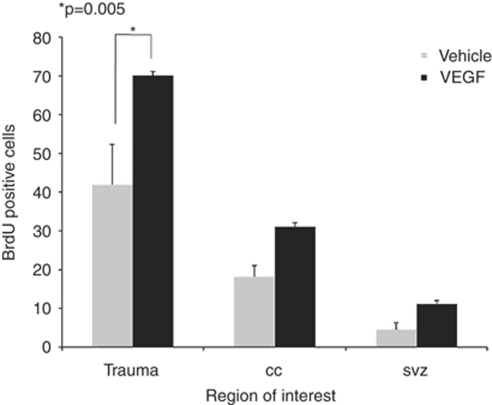
To assess the effect of VEGF on cell division, we measured the total number of newborn BrdU+ cells was measured at 90 days after injury. An increase in the number of BrdU+ cells was found in mice treated with VEGF at all regions of interest including the SVZ (2.5-fold increase), the corpus callosum (1.7-fold increase), and the area surrounding the lesioned cortex (1.7-fold increase) (Figure 3). At 90 days after TBI, most BrdU+ cells were localized to the immediate perilesion area suggesting accumulation of newborn cells around the injured brain area. These results suggest a possible mitogenic or prosurvival effect of VEGF on dividing cells.
Figure 3.
Vascular endothelial growth factor (VEGF) increases cell proliferation after traumatic brain injury (TBI). Animals were injected with 5-bromo-2-deoxyuridine (BrdU) after closed head injury (CHI). The absolute number of BrdU+ cells was measured at 90 days after TBI at various regions of interest including the subventricular zone, the corpus callosum, and the cortex on the side of injury. Note that VEGF led to significantly more BrdU+ cells in the cortex surrounding the injury.
VEGF Increases Gliogenesis and Neurogenesis after TBI
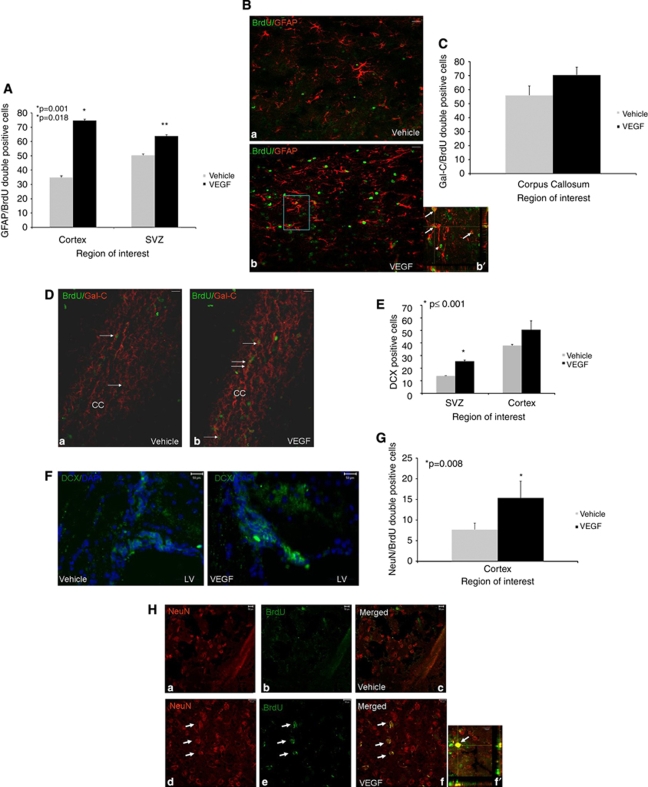
To determine the fates of newborn cells after TBI, we used immunohistochemical methods with double and triple labeling and confocal microscopy with Z sectioning for colocalization. Most newborn cells in VEGF-treated mice differentiated into astrocytes (GFAP+ Figures 4A and 4B) or oligodendroglia (GalC+ Figures 4C and 4D) and a smaller amount differentiated into newborn neurons around the SVZ region (DCX+ Figures 4E and 4F) and into mature neurons (NeuN+ Figures 4G and 4H). Most newborn astrocytes and neurons were located around the injured cortex and most newborn oligodendroglia were located in white matter tracts such as the corpus callosum suggesting site-appropriate differentiation of the newborn cells. Overall, the total numbers of newborn astrocytes and neurons at the lesion border were significantly higher in VEGF-treated mice (twofold increase compared with vehicle-treated mice, P=0.001 and 1.3-fold increase compared with vehicle-treated mice, P=0.008, respectively), whereas most newborn cells in vehicle controls did not differentiate into neurons or glia and remained undifferentiated.
Figure 4.
Effects of vascular endothelial growth factor (VEGF) on newborn cell differentiation. Animals underwent closed head injury (CHI) and were killed 90 days later. Newborn cell fate was determined with immunohistochemistry. Most newborn cells in animals treated with VEGF differentiated into GFAP+ astrocytes in the perilesion cortex (A, B) and at the subventricular zone (SVZ), and the number of newborn astrocytes was significantly larger in these areas in VEGF-treated mice. The inset in (b′) is a Z section at × 400 taken from the rectangle in (B) showing colocalization of 5-bromo-2-deoxyuridine (BrdU) and GFAP in many of the positive cells (white arrows). Arrowheads point to BrdU+ GFAP− cells. At the corpus callosum, most newborn cells differentiated into newborn Gal-C+ oligodendroglia (C), but the differences between the groups were not significant. (D) A photomicrograph ( × 200) from the corpus callosum area (CC in D) showing an abundance of BrdU (green) and Gal-C (red) double-positive cells (white arrows) in both vehicle- (a) and VEGF (b)-treated mice. Significantly more doublecortin-positive (DCX+) cells were detected at the SVZ and surrounding the damaged cortex in VEGF-treated mice (E). (F) Sample photomicrographs ( × 400) from the SVZ area of vehicle- or VEGF-treated mice showing an abundance of DCX+ cells (green) in VEGF-treated animals. The numbers of NeuN/BrdU double-positive newborn neurons were significantly larger in the cortical area surrounding the lesion of VEGF-treated mice (G). (H) A sample of photomicrographs ( × 400) showing that NeuN (red) and BrdU (green) double-positive cells in the cortex surrounding the lesion of VEGF- (white arrows in panels d–f) but not vehicle (panels a–c)-treated mice. (f′) A confocal Z section showing the colocalization of BrdU (green) and NeuN (red) in a newborn neuron at the lesion border (bar graph=10 μm). Bar graphs in all figures=50 μm unless otherwise stated.
Although more newborn oligodendroglia were found in the corpus callosum of the VEGF-treated mice, compared with vehicle-treated animals, this difference did not reach statistical significance (70% versus 56%, respectively).
Taken together, these results suggest that VEGF may have an instructive role toward glial and neuronal differentiation but does not significantly affect oligodendroglial differentiation.
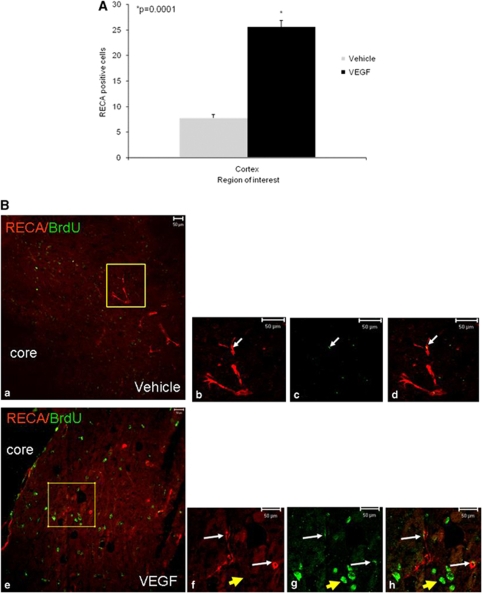
VEGF Increases Angiogenesis after TBI
At least part of the benefit afforded by VEGF could be attributed to its effect on angiogenesis. To examine the effect of exogenous VEGF on angiogenesis, we stained newly formed blood vessels with an antibody against the endothelial marker RECA1. A significant increase in the number of blood vessels (3.3-fold increase compared with vehicle-treated mice, P=0.0001) in the cortex around the lesion was seen in the VEGF group (Figure 5), suggesting that treatment either protected existing blood vessels or led to a proangiogenic effect resulting in the formation of new blood vessels. Similar results were observed when we used the antiendothelial marker CD31 (Supplementary Figure 1).
Figure 5.
Vascular endothelial growth factor (VEGF) increases angiogenesis after traumatic brain injury (TBI). Animals were killed 90 days after TBI and the number of blood vessels was counted in the perilesion cortex. Significantly more vessels were seen in the cortical area surrounding the damage in VEGF-treated mice (A). (B) A photomicrograph ( × 200) showing that many of the blood vessels in this area had BrdU+ cells colocalizing with the endothelial marker RECA1 (white arrows) in vehicle- (a–d) and VEGF (e–h)-treated animals, but the number of vessels with colocalization was significantly larger in VEGF-treated animals. Note that in VEGF-treated mice, 5-bromo-2-deoxyuridine (BrdU)/RECA1 double-positive cells (white arrows) have a different morphology from that of BrdU+ RECA1 cells (yellow arrow).
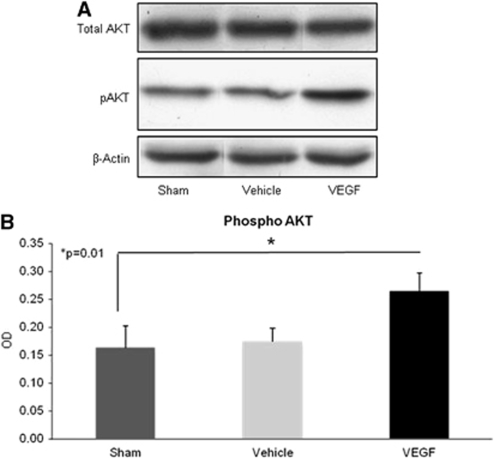
VEGF Activates the Survival Signaling Pathway by Increasing Phospho-Akt Levels
To elucidate the molecular mechanisms responsible for the salutary effects of VEGF, we tested treatment effects on the expression of several mediators of survival in vivo using western blots. As the intracellular signaling of VEGF involves Akt-phosphorylation, we examined the Akt-mediated signaling pathway. Significantly increased levels of the phosphorylated Akt (at Ser473), but not total, were found in VEGF-treated mice (1.6-fold increase compared with vehicle-treated mice, P=0.01; Figure 6). In contrast, we found that VEGF treatment did not lead to further changes in downstream signals such as mTOR and IκB (data not shown), suggesting that the protective mechanisms were related to pAKT-activated pathways that were not related to mTOR or NFκB signaling but rather through different pathways.
Figure 6.
Vascular endothelial growth factor (VEGF) upregulates pAKT after traumatic brain injury (TBI). Animals underwent TBI, were treated with VEGF or vehicle for 7 days administered through mini-osmotic pumps, and were killed within minutes of end of therapy on day 7. pAKT levels were measured with western blots on cortical tissue. (A) A sample of blots from vehicle- or VEGF-treated brains showing larger increments in pAKT in VEGF-treated mice. (B) Quantification of pAKT optical density.
Discussion
The results of this study show that the administration of exogenous VEGF after TBI increases cell proliferation at the SVZ and leads to migration of cells through white matter toward the lesioned area. Larger proportions of newborn cells differentiated into astrocytes and neurons in VEGF-treated animals compared with controls. As expected, VEGF also significantly increased angiogenesis as shown by the increase in the number of blood vessels in proximity to the lesion. Importantly, the rates of functional motor and sensory recovery after TBI were significantly higher in animals treated with VEGF already at the early postinjury period (days 3 to 7) and increased even further from day 14 till the end of the follow-up period at 90 days. Thus, exogenous VEGF had a significant neuroprotective effect as evidenced by the early improvement in NSS, which proceeded up to the time of neurogenesis-related events. Moreover, the significantly decreased lesion volume observed at 90 days after TBI attests to the early neuroprotective effects of VEGF. However, the continuous improvement in functional deficits, namely, the decrease of NSS in the VEGF-treated mice, in contrast to the near-plateau levels reached in the control seen from day 14 onward, suggests an effect that is typically seen when neurogenesis and angiogenesis take place (Shetty et al, 2004).
Cells in the SVZ continue to proliferate for long periods of time after ischemic lesions (Leker et al, 2007; Thored et al, 2007), and this appears to be also true for TBI as shown in this work.
One of the intracellular pathways that are activated after VEGF binding is phosphorylation of the protein kinase Akt, a central player in a variety of cellular and physiologic events (Parcellier et al, 2008). It is now well established that the prosurvival effects of Akt after brain injury include antiapopototic, proangiogenic, and neuroprotective effects (Kilic et al, 2006; Shein et al, 2007). In this set of experiments, we found an early increase in pAkt in the brain shortly after VEGF administration, suggesting that the early neuroprotective effects of exogenous VEGF in TBI are probably mediated, at least in part, through induction of survival-signaling through pAkt, and a reduction in apoptosis (Kilic et al, 2006; Parcellier et al, 2008; Shein et al, 2007).
The functional status of TBI mice continued to improve at the later postinjury phase, although only a small fraction of the newly born cells differentiated into neurons. This implies that functional status does not entirely depend on neuronal replacement by newborn cells and could have several explanations. First, it was shown that newborn cells secrete a plethora of growth- and survival-promoting factors (Forsberg-Nilsson et al, 1998; Ogunshola et al, 2000; Shetty et al, 2005) and that these cells also have immunomodulatory effects that may improve the outcome (Ben-Hur, 2007) by influencing surviving neurons in the area surrounding the damage. Second, VEGF is an important modulator of angiogenesis (Morgan et al, 2007; Ogunshola et al, 2000), and it is therefore probable that at least part of the late beneficial effects on functionality are related to better perfusion in the area immediately adjacent to the trauma, which could lead to a better metabolic state in this area and thus to a better functional state. Third, it is possible that local factors within the area surrounding the lesion in TBI diminish neuronal differentiation. Such potential factors include myelin-associated factors and other signaling molecules such as NOGO (Zhao et al, 2007) and netrins (Cirulli and Yebra, 2007).
Vascular endothelial growth factor was shown to increase both neurogenesis and angiogenesis in the SVZ and to increase the size of the stem cell niche (Gotts and Chesselet, 2005). The niche is essential for stem cell survival and maturation by vascular recruitment and remodeling (Palmer et al, 2000). Surrounding the niche, neural stem cells were shown to proliferate and differentiate to a lesser extent in the absence of VEGF (Skold et al, 2006). Similar to what has been described in ischemic brain injury (Leker et al, 2007), we noticed an accumulation of newborn cells in the cortex surrounding the injury in treated animals, suggesting that VEGF also promoted migration of these cells toward their target site. The larger absolute numbers of newborn neurons and glia, as well as the percentage of BrdU+ cells that differentiated into these cell types in VEGF-treated animals, suggest that VEGF may have an instructive role in the differentiation of neural progenitors. In future experiments, it would be important to test whether the use of VEGF could be combined with that of a proneuronal differentiating agent such as FGF2 or erythropoietin to yield even better outcomes as the result of further increments in the number of newborn neurons.
In conclusion, the result of our study shows that the administration of VEGF after TBI leads to proneurogenic, proangiogenic, and neuroprotective effects that are mediated through phospho-Akt signaling and that result in better functional outcome.
All the authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Beauchamp K, Mutlak H, Smith WR, Shohami E, Stahel PF. Pharmacology of traumatic brain injury: where is the ‘golden bullet'. Mol Med. 2008;14:731–740. doi: 10.2119/2008-00050.Beauchamp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hur T. Immunomodulation by neural stem cells. J Neurol Sci. 2007;265:102–104. doi: 10.1016/j.jns.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Beni-Adani L, Gozes I, Cohen Y, Assaf Y, Steingart RA, Brenneman DE, Eizenberg O, Trembolver V, Shohami E. A peptide derived from activity-dependent neuroprotective protein (ADNP) ameliorates injury response in closed head injury in mice. J Pharmacol Exp Ther. 2001;296:57–63. [PubMed] [Google Scholar]
- Chirumamilla S, Sun D, Bullock MR, Colello RJ. Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J Neurotrauma. 2002;19:693–703. doi: 10.1089/08977150260139084. [DOI] [PubMed] [Google Scholar]
- Cirulli V, Yebra M. Netrins: beyond the brain. Nat Rev Mol Cell Biol. 2007;8:296–306. doi: 10.1038/nrm2142. [DOI] [PubMed] [Google Scholar]
- Dash PK, Mach SA, Moore AN. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J Neurosci Res. 2001;63:313–319. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Wang X, Mehedi A, Kreipke CW, Rafols JA. Differential expression of capillary VEGF isoforms following traumatic brain injury. Neurol Res. 2007;29:395–403. doi: 10.1179/016164107X204729. [DOI] [PubMed] [Google Scholar]
- Forsberg-Nilsson K, Behar TN, Afrakhte M, Barker JL, McKay RD. Platelet-derived growth factor induces chemotaxis of neuroepithelial stem cells. J Neurosci Res. 1998;53:521–530. doi: 10.1002/(SICI)1097-4547(19980901)53:5<521::AID-JNR2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J. Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Gotts JE, Chesselet MF. Vascular changes in the subventricular zone after distal cortical lesions. Exp Neurol. 2005;194:139–150. doi: 10.1016/j.expneurol.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Abe K, Suzuki H, Itoyama Y. Rapid induction of vascular endothelial growth factor gene expression after transient middle cerebral artery occlusion in rats. Stroke. 1997;28:2039–2044. doi: 10.1161/01.str.28.10.2039. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Palmer TD. New roles for astrocytes: the nightlife of an ‘astrocyte'. La vida loca! Trends Neurosci. 2003;26:597–603. doi: 10.1016/j.tins.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Mao XO, Greenberg DA. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J Neurobiol. 2006;66:236–242. doi: 10.1002/neu.20215. [DOI] [PubMed] [Google Scholar]
- Kilic E, Kilic U, Wang Y, Bassetti CL, Marti HH, Hermann DM. The phosphatidylinositol-3 kinase/Akt pathway mediates VEGF's neuroprotective activity and induces blood brain barrier permeability after focal cerebral ischemia. FASEB J. 2006;20:1185–1187. doi: 10.1096/fj.05-4829fje. [DOI] [PubMed] [Google Scholar]
- Langlois J, Ruthland-Brown W, THOMAS K. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA; 2004. [Google Scholar]
- Leker RR, Shohami E. Cerebral ischemia and trauma-different etiologies yet similar mechanisms: neuroprotective opportunities. Brain Res Brain Res Rev. 2002;39:55–73. doi: 10.1016/s0165-0173(02)00157-1. [DOI] [PubMed] [Google Scholar]
- Leker RR, Soldner F, Velasco I, Gavin DK, Androutsellis-Theotokis A, McKay RD. Long-lasting regeneration after ischemia in the cerebral cortex. Stroke. 2007;38:153–161. doi: 10.1161/01.STR.0000252156.65953.a9. [DOI] [PubMed] [Google Scholar]
- Morgan R, Kreipke CW, Roberts G, Bagchi M, Rafols JA. Neovascularization following traumatic brain injury: possible evidence for both angiogenesis and vasculogenesis. Neurol Res. 2007;29:375–381. doi: 10.1179/016164107X204693. [DOI] [PubMed] [Google Scholar]
- Nag S, Takahashi JL, Kilty DW. Role of vascular endothelial growth factor in blood–brain barrier breakdown and angiogenesis in brain trauma. J Neuropathol Exp Neurol. 1997;56:912–921. doi: 10.1097/00005072-199708000-00009. [DOI] [PubMed] [Google Scholar]
- Nieto M, Schuurmans C, Britz O, Guillemot F. Neural bHLH genes control the neuronal versus glial fate decision in cortical progenitors. Neuron. 2001;29:401–413. doi: 10.1016/s0896-6273(01)00214-8. [DOI] [PubMed] [Google Scholar]
- Ogunshola OO, Stewart WB, Mihalcik V, Solli T, Madri JA, Ment LR. Neuronal VEGF expression correlates with angiogenesis in postnatal developing rat brain. Brain Res Dev Brain Res. 2000;119:139–153. doi: 10.1016/s0165-3806(99)00125-x. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Parcellier A, Tintignac LA, Zhuravleva E, Hemmings BA. PKB and the mitochondria: AKTing on apoptosis. Cell Signal. 2008;20:21–30. doi: 10.1016/j.cellsig.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Shapira Y, Shohami E, Sidi A, Soffer D, Freeman S, Cotev S. Experimental closed head injury in rats: mechanical, pathophysiologic, and neurologic properties. Crit Care Med. 1988;16:258–265. doi: 10.1097/00003246-198803000-00010. [DOI] [PubMed] [Google Scholar]
- Shapira Y, Setton D, Artru AA, Shohami E. Blood–brain barrier permeability, cerebral edema, and neurologic function after closed head injury in rats. Anesth Analg. 1993;77:141–148. doi: 10.1213/00000539-199307000-00028. [DOI] [PubMed] [Google Scholar]
- Shein NA, Tsenter J, Alexandrovich AG, Horowitz M, Shohami E. Akt phosphorylation is required for heat acclimation-induced neuroprotection. J Neurochem. 2007;103:1523–1529. doi: 10.1111/j.1471-4159.2007.04862.x. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Rao MS, Hattiangady B, Zaman V, Shetty GA. Hippocampal neurotrophin levels after injury: relationship to the age of the hippocampus at the time of injury. J Neurosci Res. 2004;78:520–532. doi: 10.1002/jnr.20302. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Skold MK, von Gertten C, Sandberg-Nordqvist AC, Mathiesen T, Holmin S. VEGF and VEGF receptor expression after experimental brain contusion in rat. J Neurotrauma. 2005;22:353–367. doi: 10.1089/neu.2005.22.353. [DOI] [PubMed] [Google Scholar]
- Skold MK, Risling M, Holmin S. Inhibition of vascular endothelial growth factor receptor 2 activity in experimental brain contusions aggravates injury outcome and leads to early increased neuronal and glial degeneration. Eur J Neurosci. 2006;23:21–34. doi: 10.1111/j.1460-9568.2005.04527.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38:3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- Tsenter J, Beni-Adani L, Assaf Y, Alexandrovich AG, Trembovler V, Shohami E. Dynamic changes in the recovery after traumatic brain injury in mice: effect of injury severity on T2-weighted MRI abnormalities, and motor and cognitive functions. J Neurotrauma. 2008;25:324–333. doi: 10.1089/neu.2007.0452. [DOI] [PubMed] [Google Scholar]
- van Bruggen N, Thibodeaux H, Palmer JT, Lee WP, Fu L, Cairns B, Tumas D, Gerlai R, Williams SP, van Lookeren Campagne M, Ferrara N. VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J Clin Invest. 1999;104:1613–1620. doi: 10.1172/JCI8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WY, Dong JH, Liu X, Wang Y, Ying GX, Ni ZM, Zhou CF. Vascular endothelial growth factor and its receptor Flk-1 are expressed in the hippocampus following entorhinal deafferentation. Neuroscience. 2005;134:1167–1178. doi: 10.1016/j.neuroscience.2005.04.064. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jin K, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA. VEGF-overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration. J Neurosci Res. 2007;85:740–747. doi: 10.1002/jnr.21169. [DOI] [PubMed] [Google Scholar]
- Widenfalk J, Lipson A, Jubran M, Hofstetter C, Ebendal T, Cao Y, Olson L. Vascular endothelial growth factor improves functional outcome and decreases secondary degeneration in experimental spinal cord contusion injury. Neuroscience. 2003;120:951–960. doi: 10.1016/s0306-4522(03)00399-3. [DOI] [PubMed] [Google Scholar]
- Zhao XH, Jin WL, Ju G. An in vitro study on the involvement of LINGO-1 and Rho GTPases in Nogo-A regulated differentiation of oligodendrocyte precursor cells. Mol Cell Neurosci. 2007;36:260–269. doi: 10.1016/j.mcn.2007.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.