Abstract
Brain glucose exposure may complicate diabetes and obesity. We used positron emission tomography with 18F-fluorodeoxyglucose in Zucker obese, diabetic, and control rats to determine the contributions of blood glucose mass action versus local mechanisms in regulating central glucose disposal in fasted and acutely glucose-stimulated states, and their adaptations in obesity and diabetes. Our study data indicate that brain glucose uptake is dependent on both local and mass action components, and is stimulated by acute glucose intake in healthy rats. In diseased animals, the organ was chronically overexposed to glucose, due to high fasting glucose uptake, almost abolishing the physiologic response to glucose loading.
Keywords: brain glucose uptake, glucose toxicity, metabolic syndrome, positron emission tomography, diabetes
Introduction
Brain glucose exposure has been implicated in the pathogenesis of the cognitive dysfunction and of the energy unbalance associated with type 2 diabetes and the metabolic syndrome (Reaven et al, 1990; Levin et al, 1999; Gatto et al, 2008). Glucose is the main fuel of the brain, and participates in the control of peripheral processes and central substrate delivery (Levin et al, 1999).
Fundamental, though still elusive notions are whether the body-to-brain glucose flux is a passive process determined by the blood glucose concentration or if local brain mechanisms have an additional role (Peters and Langemann, 2009), and whether a dysregulation at this level may alter the organ exposure to glucose in obesity and type 2 diabetes. Positron emission tomography (PET) with 18F-fluorodeoxyglucose ([18F]FDG) allows discriminate substrate fractional uptake due to local mechanisms from the passive influence of circulating glucose mass action. The fractional uptake rate (FUR) (Ishizu et al, 1994; Thie, 1995) is given by the ratio of brain tissue-to-blood [18F]FDG concentrations, it includes the effects of cerebral extraction and blood flow, but not glycemia, whereas the estimation of glucose uptake as product of FUR and glycemia accounts for the additional influence of circulating glucose concentrations.
This study was undertaken to measure the relative contributions of blood glucose mass action versus brain tissue fractional uptake in regulating central glucose disposal in the fasting and acutely glucose-stimulated state, and their adaptations in obesity and type 2 diabetes. We used a small animal PET device in Zucker obese, ZDF diabetic, and control rats.
Methods
Study Design
Male obese and lean Zucker, and diabetic and nondiabetic ZDF rats were purchased from Charles Rivers Laboratories (Wilmington, MA, USA). The animals were received at 5 weeks of age, after ZDF had developed diabetes; they received a normal chow diet until measurements at 7 to 8 weeks. Half of the animals were studied during fasting and the remaining after i.v. glucose injection. The experimental protocol was conducted in accordance with the D.L. 116/92, implementation of the directive EEC 609/86, regarding the protection of animals used for experimental and other scientific purposes.
PET Scanning Protocol
The study session is summarized in Figure 1A. After an overnight fast, anesthesia was induced/maintained by tiletamine and zolazepam (40 mg/kg, i.p., Zoletil; Virbac Laboratories, France). Femoral veins were catheterized for tracer and glucose administration. A YAP(S)PET (ISE s.r.l., Vecchiano, PI, Italy) was used for μPET imaging. [18F]FDG (67±4 MBq) was injected i.v. In the glucose-stimulated studies, tracer injection was preceded by the i.v. administration of 500 μl of a 50% dextrose solution. For information on arterial blood tracer concentrations, representing the tissue input function, a dynamic scan of the cardiac region was first obtained, followed by a 20 min static acquisition over the brain region (45 to 65 min) to evaluate brain glucose uptake. Blood was frequently sampled through the tail tip for monitoring of circulating glucose (in all animals) or radioactivity levels (in a subgroup of 20 animals) throughout the whole study. Blood glucose levels were determined by strip measurements. Blood radioactivity levels were measured in a cross-calibrated well-counter.
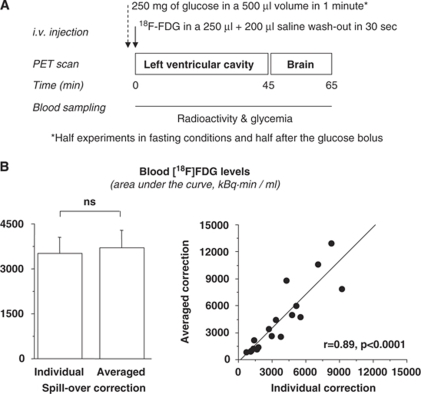
Figure 1.
(A) Design of a positron emission tomography (PET) study session. (B) Areas under the blood tracer concentration curve (input function) after correcting for individually measured or averaged spillover fractions in 20 animals undergoing frequent blood sampling, showing no difference (left) and a tight correlation (right) between the two methods.
Image Analysis
Images were reconstructed using an iterative OSEM algorithm, in a matrix of 68 × 68 pixels, with voxels of 0.75 × 0.75 × 1.5 mm3.
Small regions of interest were drawn on dynamic images corresponding to the left ventricular cavity of the heart to extract the arterial tracer concentration over time. In 20 animals, the true blood radioactivity was measured and used to calculate the spillover fraction, by direct comparison with the image-derived curve (Laforest et al, 2005). Areas under the curves were estimated using blood concentrations corrected with the measured and with the averaged fraction to show their equivalence before implementation of the latter in all animals. Thus, the input function used was composed of the time–activity curve measured in the cardiac cavity, discounted for the average fraction of activity associated with spillover.
Large regions of interest were drawn on the brain image. Fractional uptake rate (min−1) was calculated as ratio of the time-averaged integral of brain tracer concentration at 45 to 65 min (kBq/ml) and the integral of the blood concentration from time 0 to 45 min (kBq × min/ml) (Ishizu et al, 1994; Thie, 1995). It was multiplied by glycemia to obtain brain glucose uptake rates in μmol/min per ml of tissue. To account for the substrate competition caused by higher glucose concentrations, we adapted the method suggested in Dunn et al (2009).
Statistical Analysis
Data are presented as mean±s.e.m. Analysis of variance was used in group comparisons. Statistical significance was defined as P<0.05.
Results
A total of 34 rats were studied (n=10 in the diabetic, n=8 in the other groups). By selection, obese rats were 20% heavier (353±11 g) than the other groups (P<0.05). During scanning, glycemia was progressively higher from lean Zucker (fasting 6.6±0.8 versus hyperglycemic studies 21.1±2.4 mmol/l, P<0.05) and nondiabetic ZDF (6.3±0.5 versus 20.7±2.0, P<0.05) control groups, to obese (19.5±3.1 versus 27.7±3.1, NS) and diabetic animals (26.6±2.9 versus 30.5±0.8, NS), and higher in diseased than control rats (P<0.05).
Images were of sufficient quality to enable drawing of whole brain regions of interest. The spillover correction of the input function with individually measured versus averaged fractions provided similar and tightly correlated results (Figure 1B). Thus, the latter average could be extended to all animals. In two animals, the input function could not be determined due to technical problems, and it was replaced by the average value obtained within the same group and within the same metabolic study condition. Statistical analysis of brain glucose uptake was not affected by these two cases.
The areas under the input function curves (Table 1) were smaller during acute hyperglycemia in control animals, and during both fasting and acute glucose loading in the diseased compared with control groups, indicative of greater whole-body clearance. Brain FUR was significantly greater in ZDF rats than in the other three groups, and in obese Zucker as compared with control animals. Brain glucose uptake showed even larger differences, due to the contribution of progressively higher glucose levels in the diseased groups. These differences were most pronounced in fasting than glucose loading studies, in which the increment was confined to diabetic, but not obese rats.
Table 1. Glucose fractional extraction and uptake during PET scanning.
| Controls | Obese rats | Controls | Diabetic rats | |
|---|---|---|---|---|
| Fasting state | ||||
| Blood [18F]FDG (AUC) | 9278±1997 | 2369±715* | 7822±1137 | 1216±81* |
| Brain FUR (min−1) | 0.063±0.009 | 0.135±0.020* | 0.076±0.018 | 0.192±0.014*# |
| Brain GU (μmol/min per 100 ml) | 40±1 | 285±76* | 47±11 | 517±76* |
| Glucose load | ||||
| Blood [18F]FDG (AUC) | 4050±847¶ | 1724±235* | 2811±659¶ | 1194±154* |
| Brain FUR (min−1) | 0.098±0.004¶ | 0.129±0.022 | 0.156±0.048§ | 0.262±0.076* |
| Brain GU (μmol/min per 100 ml) | 211±33¶ | 359±73 | 327±101¶ | 792±225* |
AUC, area under the time–activity concentration curve (kBq × min/ml); FUR, fractional uptake rate of glucose; GU, glucose uptake rate (computed as product of FUR and glycemia).
¶P<0.05 versus fasting state, §P=0.17 versus fasting state, *P<0.05 versus lean or both control groups, #P<0.05 versus obese group. The lower level of significance (P=0.17) is likely explained by higher variability of data in this subgroup, as shown by the standard error.
Discussion
Brain glucose uptake and its acute glucose regulation were compared in models of progressive metabolic disease severity and in respective controls, showing a pronounced increase of fasting glucose uptake in the brain of obese and diabetic rats associated with a blunted response to acute glucose loading. The uninterrupted overexposure of the organ to glucose(-toxicity) may lead to damage, and the lack of metabolic flexibility may neutralize the body-to-brain feedback response to acute glucose intake.
Type 2 diabetes and the metabolic syndrome are associated with cognitive deficits, and an inadequate glucose control is considered the main mediator of this complication (Reaven et al, 1990; Munshi et al, 2006). Hyperglycemia can induce functional and structural abnormalities through the accumulation of advanced glycosylation end products and reactive oxygen species, and vascular damage (Brownlee, 2001; Aragno et al, 2005; Mastrocola et al, 2005). Notwithstanding its important implications, the direct demonstration and mechanisms of an enhanced brain glucose exposure in the above disorders are lacking. Studies addressing this matter, either directly or indirectly by measuring glucose transporters, have mostly focused on insulin-deficient animals or patients (Matthaei et al, 1986; Duckrow, 1988; Choi et al, 1989; Pardridge et al, 1990; Simpson et al, 1999), in which the effects of hyperglycemia may be partly offset by the lack of insulin. The animal models used in this study, covering a progressive spectrum of metabolic disease severity from obesity to type 2 diabetes, well reproduce all features of the metabolic syndrome, together with the cognitive decline observed in corresponding human disorders (Winocur et al, 2005).
The current results show that brain glucose uptake is upregulated on average by 7-fold in fasting obese, and by 11- and 2.4-fold in fasting or glucose-loaded diabetic animals, respectively, thus suggesting that glucose toxicity is already pronounced in the former, becoming extremely severe in the latter phenotype. Furthermore, this study indicates that the healthy brain participates in the regulation of its glucose uptake, showing a 5- to 6-fold stimulation induced by glucose intake. Brain glucose uptake and glycolysis are tightly coupled in the physiologic situation, but the [18F]FDG-PET methodology does not permit measuring beyond the uptake. It is possible that glucose uptake was increased to a greater extent than glucose consumption under the conditions of this study, amplifying the formation of glycosylation products and glucotoxicity over vessels and tissue, and better explaining the several-fold elevation observed.
According to the original theory involving the brain in the control of peripheral metabolism, the body-to-brain energy flux was a passive process, only determined by glycemia. More recent data suggest that the brain has an active role in this process (Peters and Langemann, 2009). In this study, PET was used to distinguish the mass action of blood glucose from the local regulatory mechanisms, including brain substrate extraction and cerebral blood flow. These two local processes are lumped in the FUR, which does not depend on glucose mass action. The latter effect appears when the fractional uptake is multiplied by glycemia to estimate brain glucose uptake. Thus, brain FUR and glucose uptake rates represented our target measurements for local compared with mass action outcomes.
The study results indicate that both the stimulation of brain glucose uptake in healthy animals and the chronic overexposure of the brain to glucose in diabetes and obesity are higher than expected from the contribution of circulating glucose levels alone, and the participation of local brain responses was shown. Our blood tracer data suggest higher whole-body clearance in hyperglycemic conditions, resulting in a lower input function. This change was not accompanied by a proportional decline in brain tracer concentrations, indicating that the organ was more avidly retaining the tracer, leading to a higher brain-to-blood activity ratio (FUR). Cerebral blood flow is unlikely to account for this upregulation, because it was reported to be reduced due to hyperglycemia (Duckrow et al, 1987; Wang et al, 2008). Instead, it may have significantly contributed to the blunted response to acute hyperglycemia in diseased animals, the data of which are compatible with the increase in glucose transporters at the blood–brain barrier described in other models of chronic hyperglycemia, accompanied by higher passive diffusion, which is nonsaturable and proportional to plasma glucose levels (Pelligrino et al, 1992).
Hyperglycemia was induced by i.v. glucose administration to avoid between-subject differences in glucose absorption through the visceral peritoneum and passage through the liver before entering the circulation. The potential influence of the procedure on cerebral blood flow was kept as systematic as possible by reproducible injection volumes, but we cannot exclude a bolus-hypertonic effect on the blood–brain barrier, and our findings are circumscribed to an abrupt, severe hyperglycemia. We used strip glucose measurements, allowing frequent determinations, minimal invasiveness, and immediate readings. Though laboratory assays have higher accuracy, we found them to provide similar and correlated results versus strip measurements in another study (r=0.99, P<0.01). We did not measure glucose levels in the cerebrospinal fluid, which may have reinforced our findings; however, one advantage of PET studies is their noninvasiveness, and glycemia is normally used in the estimation of brain glucose uptake.
In conclusion, the current study shows that brain glucose uptake depends on the dual contribution of blood glucose mass action and local glucose uptake mechanisms, resulting in a remarkable response to acute glucose administration in healthy animals. In obese and type 2 diabetic animals, the brain was chronically overexposed to glucose, due to very high fasting glucose uptake, almost abolishing the physiologic flexibility in response to glucose loading.
The authors declare no conflict of interest.
References
- Aragno M, Mastrocola R, Medana C, Restivo F, Catalano MG, Pons N, Danni O, Boccuzzi G. Up-regulation of advanced glycated products receptors in the brain of diabetic rats is prevented by antioxidant treatment. Endocrinology. 2005;146:5561–5567. doi: 10.1210/en.2005-0712. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Choi TB, Boado RJ, Pardridge WM. Blood–brain barrier glucose transporter mRNA is increased in experimental diabetes mellitus. Biochem Biophys Res Commun. 1989;164:375–380. doi: 10.1016/0006-291x(89)91729-4. [DOI] [PubMed] [Google Scholar]
- Duckrow RB. Glucose transfer into rat brain during acute and chronic hyperglycemia. Metab Brain Dis. 1988;3:201–209. doi: 10.1007/BF00999236. [DOI] [PubMed] [Google Scholar]
- Duckrow RB, Beard DC, Brennan RW. Regional cerebral blood flow decreases during chronic and acute hyperglycemia. Stroke. 1987;18:52–58. doi: 10.1161/01.str.18.1.52. [DOI] [PubMed] [Google Scholar]
- Dunn JT, Anthony K, Amiel SA, Marsden PK. Correction for the effect of rising plasma glucose levels on quantification of MR(glc) with FDG-PET. J Cereb Blood Flow Metab. 2009;29:1059–1067. doi: 10.1038/jcbfm.2009.21. [DOI] [PubMed] [Google Scholar]
- Gatto NM, Henderson VW, St John JA, McCleary C, Hodis HN, Mack WJ. Metabolic syndrome and cognitive function in healthy middle-aged and older adults without diabetes. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2008;15:627–641. doi: 10.1080/13825580802036936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizu K, Nishizawa S, Yonekura Y, Sadato N, Magata Y, Tamaki N, Tsuchida T, Okazawa H, Miyatake S, Ishikawa M. Effects of hyperglycemia on FDG uptake in human brain and glioma. J Nucl Med. 1994;35:1104–1109. [PubMed] [Google Scholar]
- Laforest R, Sharp TL, Engelbach JA, Fettig NM, Herrero P, Kim J, Lewis JS, Rowland DJ, Tai YC, Welch MJ. Measurement of input functions in rodents: challenges and solutions. Nucl Med Biol. 2005;32:679–685. doi: 10.1016/j.nucmedbio.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Routh VH. Brain glucose sensing and body energy homeostasis: role in obesity and diabetes. Am J Physiol. 1999;276:R1223–R1231. doi: 10.1152/ajpregu.1999.276.5.R1223. [DOI] [PubMed] [Google Scholar]
- Mastrocola R, Restivo F, Vercellinatto I, Danni O, Brignardello E, Aragno M, Boccuzzi G. Oxidative and nitrosative stress in brain mitochondria of diabetic rats. J Endocrinol. 2005;187:37–44. doi: 10.1677/joe.1.06269. [DOI] [PubMed] [Google Scholar]
- Matthaei S, Horuk R, Olefsky JM. Blood–brain glucose transfer in diabetes mellitus. Decreased number of glucose transporters at blood–brain barrier. Diabetes. 1986;35:1181–1184. doi: 10.2337/diab.35.10.1181. [DOI] [PubMed] [Google Scholar]
- Munshi M, Grande L, Hayes M, Ayres D, Suhl E, Capelson R, Lin S, Milberg W, Weinger K. Cognitive dysfunction is associated with poor diabetes control in older adults. Diabetes Care. 2006;29:1794–1799. doi: 10.2337/dc06-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM, Triguero D, Farrell CR. Downregulation of blood–brain barrier glucose transporter in experimental diabetes. Diabetes. 1990;39:1040–1044. doi: 10.2337/diab.39.9.1040. [DOI] [PubMed] [Google Scholar]
- Pelligrino DA, LaManna JC, Duckrow RB, Bryan RM, Jr, Harik SI. Hyperglycemia and blood–brain barrier glucose transport. J Cereb Blood Flow Metab. 1992;12:887–899. doi: 10.1038/jcbfm.1992.126. [DOI] [PubMed] [Google Scholar]
- Peters A, Langemann D. Build-ups in the supply chain of the brain: on the neuroenergetic cause of obesity and type 2 diabetes mellitus. Front Neuroenergetics. 2009;1:2. doi: 10.3389/neuro.14.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven GM, Thompson LW, Nahum D, Haskins E. Relationship between hyperglycemia and cognitive function in older NIDDM patients. Diabetes Care. 1990;13:16–21. doi: 10.2337/diacare.13.1.16. [DOI] [PubMed] [Google Scholar]
- Simpson IA, Appel NM, Hokari M, Oki J, Holman GD, Maher F, Koehler-Stec EM, Vannucci SJ, Smith QR. Blood–brain barrier glucose transporter: effects of hypo- and hyperglycemia revisited. J Neurochem. 1999;72:238–247. doi: 10.1046/j.1471-4159.1999.0720238.x. [DOI] [PubMed] [Google Scholar]
- Thie JA. Clarification of a fractional uptake concept. J Nucl Med. 1995;36:711–712. [PubMed] [Google Scholar]
- Wang Z, Luo W, Li P, Qiu J, Luo Q. Acute hyperglycemia compromises cerebral blood flow following cortical spreading depression in rats monitored by laser speckle imaging. J Biomed Opt. 2008;13:064023. doi: 10.1117/1.3041710. [DOI] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE, Piroli GG, Grillo CA, Reznikov LR, Reagan LP, McEwen BS. Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behav Neurosci. 2005;119:1389–1395. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]