Abstract
Stroke is the third leading cause of death in the USA. Antithrombotic therapy targeting platelet activation is one of the treatments for ischemic stroke. Here we investigate the role of one of the thrombin receptors, protease-activated receptor 4 (PAR4), in a mouse transient middle cerebral artery occlusion (MCAO) model. After a 60 min MCAO and 23 h reperfusion, leukocyte and platelet rolling and adhesion on cerebral venules, blood–brain barrier (BBB) permeability, and cerebral edema were compared in PAR4-deficient mice and wild-type mice. Cerebral infarction volume and neuronal death were also measured. PAR4−/− mice had more than an 80% reduction of infarct volume and significantly improved neurologic and motor function compared with wild-type mice after MCAO. Furthermore, deficiency of PAR4 significantly inhibits the rolling and adhesion of both platelets and leukocytes after MCAO. BBB disruption and cerebral edema were also attenuated in PAR4−/− mice compared with wild-type animals. The results of this investigation indicate that deficiency of PAR4 protects mice from cerebral ischemia/reperfusion (I/R) injury, partially through inhibition of platelet activation and attenuation of microvascular inflammation.
Keywords: platelet/endothelial interactions, protease-activated receptor 4, brain ischemia, inflammation, blood–brain barrier
Introduction
Protease-activated receptors (PARs) belong to the superfamily of seven-transmembrane domain G-protein-coupled receptors. Four subtypes of PAR members (PAR1 to PAR4) are known to date. PAR1, PAR3, and PAR4 can be activated by thrombin, and therefore are also called thrombin receptors (Coughlin, 2000), whereas PAR2 is activated by trypsin (Nystedt et al, 1995). PARs can be activated by the cleavage of the receptor at the extracellular N terminus with the newly exposed N terminus acting as a ligand to bind to the receptor to initiate intracellular signaling (Rasmussen et al, 1991; Vu et al, 1991). The activation of PAR1, PAR3, and PAR4 has been intensively investigated in platelets (Coughlin, 2000; Ishihara et al, 1997; Kahn et al, 1998; Molino et al, 1997). In addition to platelets, PARs are also found to be widely expressed in the brain (Striggow et al, 2001; Wang et al, 2002). PAR4 protein is found on dendrites of the hippocampus and all cortical layers (Striggow et al, 2001). Studies involving thrombin and PARs in the nervous system showed that these receptors have a critical part in maintaining a delicate balance between neuroprotection and neurodegeneration during inflammation, injury, and disease states (Striggow et al, 2000; Vaughan et al, 1995).
Thrombin and its receptors, PARs, have been shown to modulate ischemic, hemorrhagic, and traumatic brain injury. It was shown that after transient focal ischemia in the rat brain, PAR1 mRNA was found to be downregulated. However, PAR3 and PAR4 mRNA levels were increased (Rohatgi et al, 2004) and there was enhanced PAR4 labeling in the penumbra (Henrich-Noack et al, 2006; Striggow et al, 2001). The effects of thrombin were also shown in other experimental ischemic models. Studies with PAR1 null mice and a PAR1 antagonist showed that PAR1 increases infarct volume and causes neuronal damage after both transient focal cerebral ischemia and combined cerebral hypoxia/ischemia (Junge et al, 2003; Olson et al, 2004; Sokolova and Reiser, 2008).
Antithrombotic therapy is the primary therapeutic method used today to prevent stroke-induced injury. However, reagents currently used can cause unexpected bleeding and other unwanted side effects (Smyth et al, 2009). Targeting thrombin signaling has become a novel therapeutic direction to avoiding the risk of bleeding. PAR1 acts as a major PAR in human platelets and its antagonists, SCH530348 and E5555, have been evaluated in clinical investigations (TRACER; ClinicalTrials.gov identifier: NCT00527943 and TRA-2P-TIMI 50; ClinicalTrials.gov identifier: NCT00526474; ClinicalTrials.gov identifiers: NCT00619164, NCT00548587, and NCT00312052). However, because PAR1 does not exist in murine platelets, it is difficult to investigate the antithrombotic role of PAR1 antagonists in mouse models of cerebral ischemic injury. In murine platelets, PAR3, instead of PAR1, and PAR4 are expressed. PAR4 is the major thrombin receptor in murine platelets and is the only PAR that is expressed in both human and murine platelets. Although both PAR4 and PAR1 are activated by thrombin, they respond differently. PAR4 requires a higher concentration of thrombin and causes a prolonged and sustained response, whereas PAR1 can be activated by a low concentration of thrombin, which induces a rapid and transient calcium mobilization (Shapiro et al, 2000). To fully understand the effect of thrombin in brain injury, it is important for us to investigate the role of PAR4 in ischemic injury. It has also been clearly shown that microvascular inflammation, including leukocyte infiltration and blood–brain barrier (BBB) disruption, has an important role in secondary injury after cerebral ischemia. To the best of our knowledge, we are the first to examine the effects of PAR4 in cerebral ischemia/reperfusion (I/R) injury and our data suggest that PAR4 is a critical contributor to the inflammatory responses in the cerebral microvasculature.
Materials and methods
Animals
The cerebral I/R studies were performed on 8- to 10-week-old male PAR4−/− C57BL/6 or C57BL/6 wild-type mice. PAR4−/− mice breeding pairs were a gift from Dr Shaun Coughlin, University of California, San Francisco and wild-type mice were purchase from Jackson Laboratories (Bar Harbor, ME, USA). All experiments were conducted in accordance with the guidelines approved by Institutional Animal Care and Use Committee at Temple University.
Middle Cerebral Artery Occlusion and Reperfusion
Middle cerebral artery occlusion and reperfusion (MCAO/R) was performed as previously described (Zhang et al, 2007). The animals were anesthetized with an intraperitoneal injection of a ketamine (100 mg/mL)/xylazine (20 mg/kg) mixture (1:1) at a dose of 1 mL/kg. The intraluminal filament method (Hata et al, 1998) was used for MCAO. The middle cerebral artery was occluded for 60 mins and reperfusion was confirmed when pulsations were again observed in the internal carotid artery. The same surgical procedures were performed on sham animals without occlusion of the middle cerebral artery. Body temperature was maintained at 37±5°C and regional cerebral blood flow was monitored by laserPro Blood Perfusion Monitor (TSI, Shoreview, MN, USA). The MCAO was considered adequate if regional cerebral blood flow showed a sharp drop to no greater than 25% of the baseline (preischemia) level.
Cranial Windows
Two days before the MCAO/R experiments, cranial windows were implanted in those animals used for observing platelet as well as leukocyte rolling and adhesion as previously described (Zhang et al, 2007). A 4-mm-diameter circular craniotomy was performed using a high-speed drill (Champ-Air Dental Drill; Benco Dental, Horsham, PA, USA) over the right parietal cortex and a 5-mm-diameter cover glass (Fisher Scientific, Pittsburgh, PA, USA) was placed over the exposed brain and sealed with Nexaband Quick Gel. The cover glass provided adequate mechanical protection from infection or contamination.
Platelet Preparation
Approximately 0.9 mL blood was harvested and collected in syringe with 0.1 mL acid citrate-dextrose buffer (38 mmol/L citric acid, 75 mmol/L trisodium citrate, 100 mmol/L dextrose) and centrifuged at 120g for 10 mins. Platelet-rich plasma (PRP) was transferred to a polypropylene tube. Carboxyfluorescein diacetate succinimidyl ester (Molecular Probe, Eugene, OR, USA) was dissolved in dimethyl sulfoxide to a concentration of 14.9 μmol/L. PRP was diluted with Dulbecco's phosphate-buffered saline to 1500 μL and incubated with 9 μL carboxyfluorescein diacetate succinimidyl ester solution for 5 mins at room temperature in the presence of prostaglandin E1 (PGE1) (1 μmol/L). After centrifugation for 10 mins at 450g, the platelet pellet was resuspended with 300μL of Dulbecco's phosphate-buffered saline, stored on ice, and protected from light. Platelet number was manually counted and leukocytes were controlled to be less than 0.05% in the platelet suspension. In each mouse, 50 × 106 platelets were injected before performing MCAO/R. Platelet function was evaluated to make sure that the isolation procedure did not activate the platelets.
Intravital Fluorescence Microscopy and Measurement of Platelet, Leukocyte/Endothelial Interactions
An Olympus epiluminescence intravital microscope (BX10; Olympus, Tokyo, Japan) with a digital Camera (Cooke 1600; Cooke, Romulus, MI, USA) was used to observe the cerebral microcirculation at 24 h after MCAO. Leukocytes were stained in vivo by injection of 0.05 mL of a 0.01% Rhodamine 6G (Sigma, St Louis, MO, USA) through the facial vein (Levene et al, 2007). Platelets were stained in vitro by carboxyfluorescein diacetate succinimidyl ester. The image from the camera was displayed on a computer monitor, captured and recorded by Camware software (The Cooke Corporation, Romulus, MI, USA) at a video frame rate of 25 frames/sec.
Three venules (with diameter 30 to 40 μm) in each animal were assessed. Rolling was considered to be the total number of platelets or leukocytes moving along the endothelial cells at a substantially slower velocity than the midstream blood cell velocity. They were counted when they passed an arbitrary line perpendicular to the longitudinal axis of the vessel during a 30 secs observation period. Adhering platelets and leukocytes were defined as the total number of the platelets and leukocytes firmly attached to the microvascular endothelium for more than 2 and 30 secs, respectively. Adhering platelets and leukocytes were scored as the number of cells per mm2 of the vascular surface area, calculated from the diameter and standardized length (100 μm) of the vessel segment under investigation.
Infarct Volume and Cerebral Edema Assessment
The animals were killed with an overdose of pentobarbital (200 mg/kg intraperitoneally) 24 h after MCAO and the brains were removed. After chilling on ice for 10 mins, five 2 mm coronal sections were cut using a mouse brain matrix (Zivic Lab, Pittsburgh, PA, USA). The sections were immersed in a 2% triphenyltetrazolium chloride (TTC) (Sigma, St Louis, MO, USA) saline solution for 20 mins at 37°C in the dark. The brain sections were then fixed in 4% paraformaldehyde at 4°C for 24 h and the anterior and caudal face of each section were scanned by a flatbed color scanner (Microtek, Carson, CA, USA). The resulting images were analyzed with ImageJ software (ImageJ 1.410, National Institutes of Health, Bethesda, MD, USA). The infarct volumes were corrected for brain edema/swelling. For this purpose, the hemispheric infarct volume in each section was calculated by subtracting the area of normal TTC-stained tissue in the hemisphere ipsilateral to the ligation from the contralateral nonischemic area to generate the infarct fraction (%) as described byLin et al (1993) and Swanson et al (1990). Cerebral edema was determined by the percent increase of the ipsilateral/contralateral hemisphere area (Vannucci et al, 2001).
Neurologic Deficit Score and Rotarod Test
The severity of neurologic deficits was evaluated 24 h after ischemic injury using a five-point deficit score (0=normal motor function, 1=flexion of torso and of contralateral forelimb on lifting of the animal by tail, 2=circling to the contralateral side but normal posture at rest, 3=leaning to contralateral side at rest, and 4=no spontaneous motor activity) (Hata et al, 1998). A rotarod was used to evaluate the motor function 24 h after MCAO (Gupta et al, 2002). Animals were trained on the rotarod at a speed of 10 rpm before MCAO and tested 24 h after MCAO at the same speed. The running time on the rotarod was recorded automatically.
Quantitative Evaluation of Evans Blue Extravasation
Blood–brain barrier disruption was assessed by quantitatively measuring Evans blue (EB) extravasation as previously described with some modifications (Belayev et al, 1996). Mice were intravenously injected with 0.1 mL 2% EB 24 h after MCAO. After 60 mins the animals were perfused transcardially with 0.9% saline to remove intravascular EB dye. Ischemic and nonischemic hemispheres were dissected and weighed. The hemispheres were homogenized in 500 μL 0.1 mol/L phosphate-buffered saline and 500 μL of trichloroacetic acid. The samples were incubated at 4°C for at least 1 h and centrifuged at 10,000g for 30 min. The resulting supernatants were measured for absorbance of EB at 610 nm using a spectrophotometer.
Histology
Triphenyltetrazolium chloride-stained brain sections were postfixed in 4% paraformaldehyde and dehydrated in 30% sucrose. After freezing, the 2 mm sections were cut into 40 μm slices on a cryostat and mounted on gelatin-coated slides. Slides were stained with cresyl violet and microscopic images were collected.
Statistical Analysis
Students' t-test was used to evaluate the difference of cerebral infarction and neurologic score, rotarod running time, and leukocyte/endothelial or platelet/endothelial interactions in PAR4−/− and wild-type mice. One-way analysis of variance was used to analyze EB extravasation in PAR4−/− and wild-type mice. Data were presented as the mean±s.e.m. A statistically significant difference was assumed at P<0.05.
Results
Evaluation of Tissue Damage after Cerebral I/R Injury in WT and PAR4−/− Mice
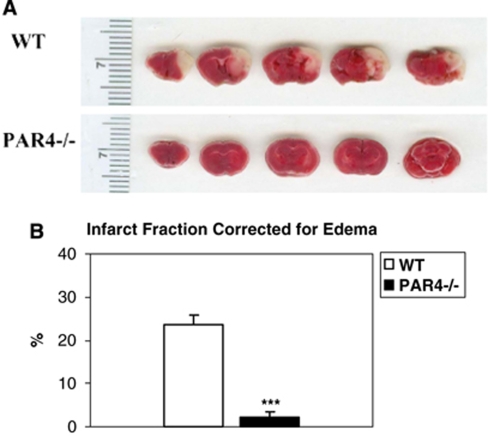
PAR4−/− and wild-type mice were subjected to MCAO for 1 h followed by 23 h of reperfusion. Cerebral infarction lesion area was determined by using TTC staining. Figure 1 shows that compared with wild-type mice, deficiency of PAR4 caused a more than 10-fold reduction of cerebral infarct fraction (23.86%±1.86 versus 2.16%±1.23, respectively) (P<0.001). There was no cerebral infarct volume in sham animals.
Figure 1.
Infarct volume is significantly reduced in PAR4−/− mice. (A) Representative TTC-stained coronal sections of wild-type and PAR4−/− mice. The white area represents the lesion area. (B) Average infarct volume was measured and adjusted for brain edema/swelling as infarct fraction: infarct fraction=(contralateral area−(ipsilateral area−infarct area))/contralateral area. Wild-type infarct size (23.725%±2.156, n=8; mean±s.e.m.) was significantly larger (***P<0.001) than PAR4−/− infarct size (2.156%±1.22, n=10; mean±s.e.m.).
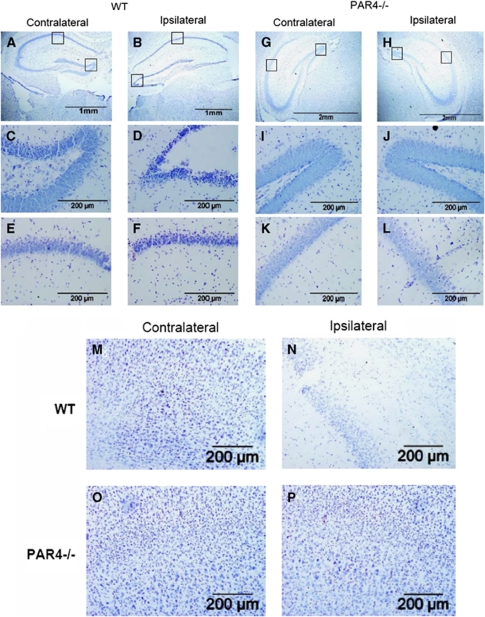
To further characterize the cellular response in wild-type and PAR4−/− mice after I/R injury, we used cresyl violet staining to evaluate neuronal death after stroke. More cell loss was observed in the ipsilateral hippocampus (Figure 2) of wild-type mice than PAR4−/− mice. Neurodegeneration was noticed in both CA1 pyramidal cells and in the dentate gyrus of the wild-type ipsilateral hippocampus (Figures 2A and B). The loss of the hilar neurons in the ipsilateral dentate gyrus of wild-type animals was greater than on the contralateral side (Figures 2C and D). Similarly, more cell loss in the hippocampal CA1 was observed in the wild-type ipsilateral side compared with the contralateral side (Figures 2E and F). Ischemia and reperfusion did not cause significant cell loss in PAR4−/− mice (Figures 2G-L). There was no detectable cell loss in either the hippocampal CA1 or the dentate gyrus of the PAR4−/− mice. Similar results were observed in the cortex (Figures 2M-P). These results indicate that deficiency of PAR4 decreased neuronal death after ischemic injury.
Figure 2.
Neurodegeneration in the ipsilateral, but not contralateral hippocampus and cortex of wild-type but not PAR4 null mice after I/R injury. Representative photographs with cresyl violet staining at low (A, B, G, H) and high (C to F and I to P) magnification of the wild-type (n=5) ipsilateral hippocampus (B, D, and F) and cortex (N); wild-type (n=5) contralateral hippocampus (A, C, and E) and cortex (M); PAR4−/− ipsilateral hippocampus (H, J, and L) and cortex (P); PAR4−/− contralateral hippocampus (G, I, and K) and cortex (O).
Effects of PAR4 on Cerebral Edema after I/R Injury
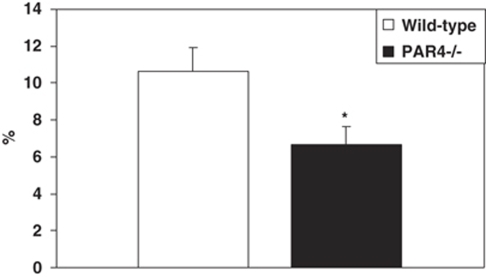
As determined from the percent increase of the ipsilateral/contralateral volume, cerebral I/R injury caused brain edema. There was a significant reduction in the magnitude of edema in PAR4−/− mice (6.67%±0.99) compared with wild-type mice (10.66%±1.23) (Figure 3). No cerebral edema was detected in sham animals.
Figure 3.
Deficiency of PAR4 reduced edema after I/R injury. Edema was assessed after 1 h MCAO and 23 h reperfusion in both wild-type (n=9) and PAR4−/−mice (n=10). Cerebral edema was determined by the percent increase of the ipsilateral/contralateral hemisphere area. (*P<0.05 is considered as significantly different compared with wild type; mean±s.e.m.).
Deficiency of PAR4 Attenuated Neurologic Deficits after Cerebral I/R Injury
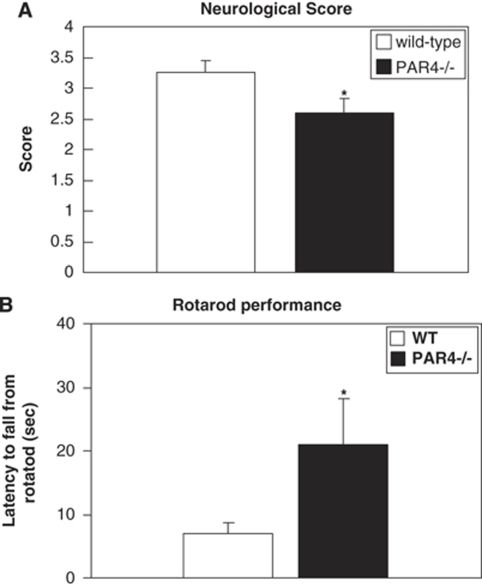
The neurologic deficit score and rotarod test were used to evaluate the neurologic function after MCAO. PAR4−/− mice showed improved neurologic function (2.6±0.233) and prolonged running time on the rotarod (21.02±7.21) compare with wild-type mice (3.33±0.19) and (7±1.59) (Figure 4). As a control, all of the sham animals displayed normal behavior (neurologic score is 0) and motor function.
Figure 4.
Effect of PAR4 on neurologic function in mice subjected to I/R injury. (A) Neurologic score was evaluated after 1 h MCAO and 23 h reperfusion. (B) Rotarod testing of sensorimotor deficits in wild-type (n=8) and PAR4−/−(n=10) mice after 1 h MCAO and 23 h reperfusion was tested. Deficiency of PAR4 causes significantly improved both neurologic score and sensorimotor function compare to wild type. (*P<0.05; mean±s.e.m.).
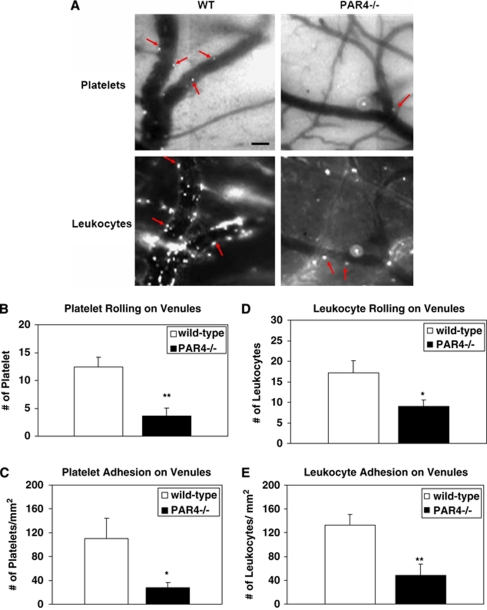
Effects of PAR4 on Platelet/Endothelial and Leukocyte/Endothelial Interactions During MCAO/R
The level of platelet and leukocyte rolling and adhesion was observed in cerebral venules of both wild-type and PAR4−/− mice 24 h after cerebral I/R injury. PAR4−/− mice subjected to MCAO/R showed a lower level of leukocyte rolling (10.25±1.25/30 secs) and adhesion (60±20 per mm2) on venules as compared with wild-type mice (19.75±1.75/30 secs) and (118±13.61 per mm2) (Figure 5). In addition, the number of platelets rolling (3.65±1.43/30 secs) and adhering (28.22±7.95 per mm2) in PAR4−/− mice was significantly lower compared with wild-type mice (12.38±1.84/30 secs) and (110.4±33.98 per mm2) (Figure 5).
Figure 5.
Effect of PAR4 on the rolling and adhesion of platelets and leukocytes. (A) Video images of capturing platelets labeled with CFDASE or leukocytes labeled with Rhodamine 6G in the ischemic area 24 h after MCAO in both wild-type and PAR4−/− mice. Arrows indicate labeled platelets or leukocytes flowing through venules. Scale bar=40 μm. The rolling and adhesion of platelets (B and C) and leukocytes (D and A) in murine brain microvascular venules after 1 h MCAO and 23 h reperfusion were evaluated. Five animals were evaluated in each group. *P<0.05; **P<0.01 relative to the corresponding wild-type group values.
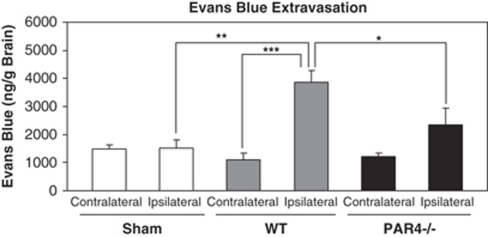
Effects of PAR4 on BBB Disruption after MCAO/R
Focal cerebral ischemia significantly increased EB extravasation in the ischemic hemisphere of wild-type mice (3876±393.7 ng/g) compared with sham animals (1522±280.4 ng/g). PAR4−/− mice showed significantly attenuated EB extravasation in the ischemic hemisphere (2352±586.1 ng/g) compared with wild-type mice (3876±393.7 ng/g) after 1 h of ischemia and 24 h reperfusion. The results also indicated that the BBB was not altered in the nonischemic hemisphere in either wild-type mice (1113±215.6 ng/g) or PAR4−/− mice (1218±129.2 ng/g) when compared with sham mice (1475±168 ng/g) (Figure 6).
Figure 6.
Effect of PAR4 on the changes in the BBB permeability to Evans blue. The leakage of Evans blue dye after 1 h MCAO and 23 h reperfusion in PAR4 null mice was significantly decreased than wild-type mice (*P<0.05, n=10; mean±s.e.m.).
Discussion
In this study, we investigated the role of PAR4 in a mouse model of cerebral I/R injury. The most important finding is that deficiency of PAR4 protected the mouse brain from I/R-induced injury. We observed that the infarct volume of PAR4-deficient mice decreased more than 10-fold compared with wild-type mice and as expected, motor function in PAR4 null mice was significantly improved. In addition, cresyl violet staining showed significant neuronal loss in ipsilateral cortex and hippocampus of wild-type but not PAR4−/− mice after MCAO/R. We found that PAR4 null mice showed less cerebral microvascular inflammation, including attenuated platelet and leukocyte/endothelial cell interactions, BBB disruption and cerebral edema after I/R injury.
The mechanism of ischemia-induced brain injury is complicated and still under investigation. Lack of blood-derived oxygen causes direct neuronal death whereas the secondary injury, mainly because of the inflammatory responses after reperfusion, exacerbates tissue damage and neurologic deficits. Inflammatory responses in cerebral microvessels lead to leukocyte and platelet accumulation on the surface of endothelial cells where they physically occlude microvessels, release inflammatory cytokines, and break down the BBB. There is subsequent extravasation of inflammatory cells and fluid into the brain parenchyma contributing to increased tissue damage and cerebral edema (Sandoval and Witt, 2008). This study was the first demonstration that deficiency of PAR4 attenuates BBB breakdown after ischemia injury. BBB function was significantly improved when PAR4 receptor was deleted.
Protease-activated receptors are widely expressed. Although PAR4 is mainly expressed in platelets, it is also expressed on endothelial cells. On endothelial cells, PAR1 is the major PAR and it initiates the downstream signaling when it is activated (Kataoka et al, 2003). Previous studies have shown that PAR1 has important influence on pulmonary and intestinal microvascular permeability (Chin et al, 2003; Vogel et al, 2000). However, a recent study also showed that PAR4 and its agonist cathepsin G are involved in alterations of the colonic epithelial barrier in ulcerative colitis (Dabek et al, 2009). In addition, PAR1 deletion did not influence BBB disruption in the central nervous system after cerebral ischemic injury (Junge et al, 2003). Our study shows that deficiency of PAR4 prevents BBB disruption after stroke, which indicates that in the central nervous system, PAR4, instead of PAR1, has a role in the regulation of BBB function after ischemic injury.
The neuroprotective effects in PAR4-deficient mice could involve at least two possible mechanisms. First, as indicated by diminished platelet and white cell interaction with endothelial cells and improved preservation of BBB function, PAR4 deficiency may reduce microvascular inflammation and diminish inflammatory cell infiltration. Because PAR4 is also expressed on neurons, it is also possible that inhibiting expression of PAR4 in the brain has a direct neuroprotective effect after brain ischemic injury. Other studies also showed that PAR4 mRNA levels were found to be significantly increased 12 h after ischemia in the rat injured hemisphere and this increased level was reduced only after 7 days (Rohatgi et al, 2004). Because PAR4 receptors are widely distributed and deficiency of PAR4 showed multiple effects in cerebral I/R injury, it is still difficult to determine the exact mechanisms through which PAR4 acts.
PAR4 is the only PAR member that is expressed on both human and murine platelets and has an important role in thrombin-induced platelet activation. Platelet activation has a key role in vascular occlusion during ischemic stroke and is the target of antithrombotic therapy (Smyth et al, 2009). Platelets are regarded as classic inflammatory cells, which contain and release adhesion proteins, interact with other inflammatory cells, produce numerous chemokines, and express chemokine receptors (Gear and Camerini, 2003). Platelet/leukocyte/endothelial cell interactions have been implicated in the pathogenesis of I/R injury in several vascular beds (Ishikawa et al, 2004). P-selectin secreted from platelet-α granules and Weibel–Palade bodies of endothelial cells is critical for leukocyte rolling. P-selectin from platelets is important for the formation of platelet-leukocyte aggregation and recruitment of leukocytes to the endothelial surface (Ishikawa et al, 2004). Thrombin activates the PAR4 receptor and causes P-selectin secretion in platelets. In rat mesenteric venules, thrombin-induced leukocyte rolling and adhesion is influenced by PAR4 (Vergnolle et al, 2002). Recently, the activation of PAR4, but not other PARs on platelets, was shown to have a major role in soluble tissue factor-induced inflammation (Busso et al, 2008). In our study, we found that both leukocyte and platelet rolling and adhesion after ischemic injury in PAR4 null mice were decreased compared with wild-type animals, which may contribute to inhibition of the inflammatory effects in PAR4 null mice. Decreased P-selectin secretion through thrombin-induced PAR4 activation may have a role.
Furthermore, because PAR4 is the major thrombin receptor in murine platelets, deficiency of PAR4 will abolish the thrombin-induced murine platelet response. This will mimic a double inhibition of both PAR1 and PAR4 response in human platelets. Studies on guinea pigs already showed a prolonged time before occlusion when blocking both PAR1 and PAR4 compared with blocking only PAR1 in a ferric chloride-induced injury model (Derian et al, 2003). Our data further show that complete inhibition of the thrombin-induced platelet response will also dramatically prevent brain ischemic injury.
In conclusion, this study showed that deficiency of PAR4 is neuroprotective in cerebral I/R injury, partially through the attenuation of cerebral microvascular inflammation. Hence, blockade of PAR4 might indicate a new therapeutic strategy for the treatment of stroke.
Acknowledgments
We thank Mr Zachary Reichenbach and Ms Varshana Gurvshmy, Temple University School of Medicine, for proofreading the paper. This work was supported by HL81322, HL80444 and HL60683; DA P30 13429, DA 03672 and DA 05488 from National Institutes of Health and under a grant with the Pennsylvania Department of Health.
The authors declare no conflict of interest.
References
- Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood–brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739:88–96. doi: 10.1016/s0006-8993(96)00815-3. [DOI] [PubMed] [Google Scholar]
- Busso N, Chobaz-Peclat V, Hamilton J, Spee P, Wagtmann N, So A. Essential role of platelet activation via protease activated receptor 4 in tissue factor-initiated inflammation. Arthritis Res Ther. 2008;10:R42. doi: 10.1186/ar2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin AC, Vergnolle N, MacNaughton WK, Wallace JL, Hollenberg MD, Buret AG. Proteinase-activated receptor 1 activation induces epithelial apoptosis and increases intestinal permeability. Proc Natl Acad Sci USA. 2003;100:11104–11109. doi: 10.1073/pnas.1831452100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- Dabek M, Ferrier L, Roka R, Gecse K, Annahazi A, Moreau J, Escourrou J, Cartier C, Chaumaz G, Leveque M, Ait-Belgnaoui A, Wittmann T, Theodorou V, Bueno L. Luminal cathepsin g and protease-activated receptor 4: a duet involved in alterations of the colonic epithelial barrier in ulcerative colitis. Am J Pathol. 2009;175:207–214. doi: 10.2353/ajpath.2009.080986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derian CK, Damiano BP, Addo MF, Darrow AL, D'Andrea MR, Nedelman M, Zhang HC, Maryanoff BE, Andrade-Gordon P. Blockade of the thrombin receptor protease-activated receptor-1 with a small-molecule antagonist prevents thrombus formation and vascular occlusion in nonhuman primates. J Pharmacol Exp Ther. 2003;304:855–861. doi: 10.1124/jpet.102.042663. [DOI] [PubMed] [Google Scholar]
- Gear AR, Camerini D. Platelet chemokines and chemokine receptors: linking hemostasis, inflammation, and host defense. Microcirculation. 2003;10:335–350. doi: 10.1038/sj.mn.7800198. [DOI] [PubMed] [Google Scholar]
- Gupta YK, Sinha K, Chaudhary G. Transient focal ischemia induces motor deficit but does not impair the cognitive function in middle cerebral artery occlusion model of stroke in rats. J Neurol Sci. 2002;203-204:267–271. doi: 10.1016/s0022-510x(02)00303-9. [DOI] [PubMed] [Google Scholar]
- Hata R, Mies G, Wiessner C, Fritze K, Hesselbarth D, Brinker G, Hossmann KA. A reproducible model of middle cerebral artery occlusion in mice: hemodynamic, biochemical, and magnetic resonance imaging. J Cereb Blood Flow Metab. 1998;18:367–375. doi: 10.1097/00004647-199804000-00004. [DOI] [PubMed] [Google Scholar]
- Henrich-Noack P, Riek-Burchardt M, Baldauf K, Reiser G, Reymann KG. Focal ischemia induces expression of protease-activated receptor1 (PAR1) and PAR3 on microglia and enhances PAR4 labeling in the penumbra. Brain Res. 2006;1070:232–241. doi: 10.1016/j.brainres.2005.10.100. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Connolly AJ, Zeng D, Kahn ML, Zheng YW, Timmons C, Tram T, Coughlin SR. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature. 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Cooper D, Arumugam TV, Zhang JH, Nanda A, Granger DN. Platelet-leukocyte-endothelial cell interactions after middle cerebral artery occlusion and reperfusion. J Cereb Blood Flow Metab. 2004;24:907–915. doi: 10.1097/01.WCB.0000132690.96836.7F. [DOI] [PubMed] [Google Scholar]
- Junge CE, Sugawara T, Mannaioni G, Alagarsamy S, Conn PJ, Brat DJ, Chan PH, Traynelis SF. The contribution of protease-activated receptor 1 to neuronal damage caused by transient focal cerebral ischemia. Proc Natl Acad Sci USA. 2003;100:13019–13024. doi: 10.1073/pnas.2235594100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn ML, Zheng YW, Huang W, Bigornia V, Zeng D, Moff S, Farese RV, Jr, Tam C, Coughlin SR. A dual thrombin receptor system for platelet activation. Nature. 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- Kataoka H, Hamilton JR, McKemy DD, Camerer E, Zheng YW, Cheng A, Griffin C, Coughlin SR. Protease-activated receptors 1 and 4 mediate thrombin signaling in endothelial cells. Blood. 2003;102:3224–3231. doi: 10.1182/blood-2003-04-1130. [DOI] [PubMed] [Google Scholar]
- Levene HB, Zhang M, Erb CJ, Jallo JI, Loftus CM, Tuma RF. Method to perform IV injections on mice using the facial vein. J Neurosci Methods. 2007;164:304–307. doi: 10.1016/j.jneumeth.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–121. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- Molino M, Bainton DF, Hoxie JA, Coughlin SR, Brass LF. Thrombin receptors on human platelets. Initial localization and subsequent redistribution during platelet activation. J Biol Chem. 1997;272:6011–6017. doi: 10.1074/jbc.272.9.6011. [DOI] [PubMed] [Google Scholar]
- Nystedt S, Emilsson K, Larsson AK, Strombeck B, Sundelin J. Molecular cloning and functional expression of the gene encoding the human proteinase-activated receptor 2. Eur J Biochem. 1995;232:84–89. doi: 10.1111/j.1432-1033.1995.tb20784.x. [DOI] [PubMed] [Google Scholar]
- Olson EE, Lyuboslavsky P, Traynelis SF, McKeon RJ. PAR-1 deficiency protects against neuronal damage and neurologic deficits after unilateral cerebral hypoxia/ischemia. J Cereb Blood Flow Metab. 2004;24:964–971. doi: 10.1097/01.WCB.0000128266.87474.BF. [DOI] [PubMed] [Google Scholar]
- Rasmussen UB, Vouret-Craviari V, Jallat S, Schlesinger Y, Pages G, Pavirani A, Lecocq JP, Pouyssegur J, Van Obberghen-Schilling E. cDNA cloning and expression of a hamster alpha-thrombin receptor coupled to Ca2+ mobilization. FEBS Lett. 1991;288:123–128. doi: 10.1016/0014-5793(91)81017-3. [DOI] [PubMed] [Google Scholar]
- Rohatgi T, Henrich-Noack P, Sedehizade F, Goertler M, Wallesch CW, Reymann KG, Reiser G. Transient focal ischemia in rat brain differentially regulates mRNA expression of protease-activated receptors 1 to 4. J Neurosci Res. 2004;75:273–279. doi: 10.1002/jnr.10847. [DOI] [PubMed] [Google Scholar]
- Sandoval KE, Witt KA. Blood–brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Shapiro MJ, Weiss EJ, Faruqi TR, Coughlin SR. Protease-activated receptors 1 and 4 are shut off with distinct kinetics after activation by thrombin. J Biol Chem. 2000;275:25216–25221. doi: 10.1074/jbc.M004589200. [DOI] [PubMed] [Google Scholar]
- Smyth SS, Woulfe DS, Weitz JI, Gachet C, Conley PB, Goodman SG, Roe MT, Kuliopulos A, Moliterno DJ, French PA, Steinhubl SR, Becker RC. G-protein-coupled receptors as signaling targets for antiplatelet therapy. Arterioscler Thromb Vasc Biol. 2009;29:449–457. doi: 10.1161/ATVBAHA.108.176388. [DOI] [PubMed] [Google Scholar]
- Sokolova E, Reiser G. Prothrombin/thrombin and the thrombin receptors PAR-1 and PAR-4 in the brain: localization, expression and participation in neurodegenerative diseases. Thromb Haemost. 2008;100:576–581. [PubMed] [Google Scholar]
- Striggow F, Riek-Burchardt M, Kiesel A, Schmidt W, Henrich-Noack P, Breder J, Krug M, Reymann KG, Reiser G. Four different types of protease-activated receptors are widely expressed in the brain and are up-regulated in hippocampus by severe ischemia. Eur J Neurosci. 2001;14:595–608. doi: 10.1046/j.0953-816x.2001.01676.x. [DOI] [PubMed] [Google Scholar]
- Striggow F, Riek M, Breder J, Henrich-Noack P, Reymann KG, Reiser G. The protease thrombin is an endogenous mediator of hippocampal neuroprotection against ischemia at low concentrations but causes degeneration at high concentrations. Proc Natl Acad Sci USA. 2000;97:2264–2269. doi: 10.1073/pnas.040552897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Willing LB, Goto S, Alkayed NJ, Brucklacher RM, Wood TL, Towfighi J, Hurn PD, Simpson IA. Experimental stroke in the female diabetic, db/db, mouse. J Cereb Blood Flow Metab. 2001;21:52–60. doi: 10.1097/00004647-200101000-00007. [DOI] [PubMed] [Google Scholar]
- Vaughan PJ, Pike CJ, Cotman CW, Cunningham DD. Thrombin receptor activation protects neurons and astrocytes from cell death produced by environmental insults. J Neurosci. 1995;15:5389–5401. doi: 10.1523/JNEUROSCI.15-07-05389.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnolle N, Derian CK, D'Andrea MR, Steinhoff M, Andrade-Gordon P. Characterization of thrombin-induced leukocyte rolling and adherence: a potential proinflammatory role for proteinase-activated receptor-4. J Immunol. 2002;169:1467–1473. doi: 10.4049/jimmunol.169.3.1467. [DOI] [PubMed] [Google Scholar]
- Vogel SM, Gao X, Mehta D, Ye RD, John TA, Andrade-Gordon P, Tiruppathi C, Malik AB. Abrogation of thrombin-induced increase in pulmonary microvascular permeability in PAR-1 knockout mice. Physiol Genomics. 2000;4:137–145. doi: 10.1152/physiolgenomics.2000.4.2.137. [DOI] [PubMed] [Google Scholar]
- Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Wang H, Ubl JJ, Reiser G. Four subtypes of protease-activated receptors, co-expressed in rat astrocytes, evoke different physiological signaling. Glia. 2002;37:53–63. doi: 10.1002/glia.10012. [DOI] [PubMed] [Google Scholar]
- Zhang M, Martin BR, Adler MW, Razdan RK, Jallo JI, Tuma RF. Cannabinoid CB(2) receptor activation decreases cerebral infarction in a mouse focal ischemia/reperfusion model. J Cereb Blood Flow Metab. 2007;27:1387–1396. doi: 10.1038/sj.jcbfm.9600447. [DOI] [PMC free article] [PubMed] [Google Scholar]