Abstract
Inhibition of plasminogen activator inhibitor (PAI)-1 is useful to treat several disorders including thrombosis. An inhibitor of PAI-1 (TM5275) was newly identified by an extensive study of structure-activity relationship based on a lead compound (TM5007) which was obtained through virtual screening by docking simulations. Its antithrombotic efficacy and adverse effects were tested in vivo in rats and nonhuman primates (cynomolgus monkey). TM5275, administered orally in rats (1 to 10 mg/kg), has an antithrombotic effect equivalent to that of ticlopidine (500 mg/kg) in an arterialvenous shunt thrombosis model and to that of clopidogrel (3 mg/kg) in a ferric chloride-treated carotid artery thrombosis model. TM5275 does not modify activated partial thromboplastin time and prothrombin time or platelet activity and does not prolong bleeding time. Combined with tissue plasminogen activator, TM5275 improves the latter's therapeutic efficacy and reduces its adverse effect. Administered to a monkey model of photochemical induced arterial thrombosis, TM5275 (10 mg/kg) has the same antithrombotic effect as clopidogrel (10 mg/kg), without enhanced bleeding. This study documents the antithrombotic benefits of a novel, more powerful, PAI-1 inhibitor in rats and, for the first time, in nonhuman primates. These effects are obtained without adverse effect on bleeding time.
Keywords: bleeding, cynomolgus monkey, PAI-1 inhibitor, thrombosis, tissue plasminogen activator
Introduction
Plasminogen activator inhibitor (PAI)-1, a serine protease inhibitor, is involved in numerous processes including thrombosis and fibrosis, as shown by the fact that disruption of the PAI-1 gene in mice markedly attenuates these processes (Carmeliet et al, 1993; Eitzman et al, 1996, 2000; Weisberg et al, 2005; Nicholas et al, 2005). Its inhibition may thus yield important cardio- and reno-protective benefits (Ha et al, 2009). Recent studies in mice overexpressing human PAI-1 also implicate its involvement in broader biological abnormalities, including alopecia, amyloidosis, and polycystic ovarian syndrome (Eren et al, 2007; Devin et al, 2007). The availability of specific PAI-1 antagonists may thus open new therapeutic avenues (Vaughan et al, 2007).
We have recently developed an original approach to synthesize such orally active inhibitors (Izuhara et al, 2008). Compounds selected virtually by structure based drug design underwent a docking simulation to select those which fitted within the cleft of the PAI-1 three-dimensional structure. They inhibit coagulation in two different rodent models of thrombosis and prevent the fibrotic process initiated by bleomycin in mouse lung (Izuhara et al, 2008).
This study has been undertaken on a newly synthesized PAI-1 inhibitor (TM5275), to test in vivo its antithrombotic efficacy as well as its possible adverse effects. We further assess the clinical benefits of a combination therapy of TM5275 with tissue plasminogen activator (tPA).
The therapeutic efficacy of TM5275 has been evaluated not only in rodents but also in nonhuman primates as recommended by recent guidelines (STAIR, 1999) to reduce discrepancies between preclinical animal trials and clinical studies. Indeed, the pathophysiological mechanisms involved in the development of thrombosis in preclinical rodent models may differ from those implicated in primates, including man (STAIR, 1999). TM5275 proves an effective antithrombotic agent which spares activated partial thromboplastin time/prothrombin time and platelet activity and does not prolong bleeding time. Combined with tPA, it improves the latter's therapeutic efficacy and reduces its adverse effects.
This study is the first to demonstrate in nonhuman primates that PAI-1 inhibition reduces markedly vascular thrombosis without modification of bleeding time, which is the most critical adverse effect of various antithrombotic agents.
Materials and methods
Synthesis of Test Compounds
More than ninety novel compounds (2-acylamino-3-thiophenecarboxylic acid and 2-acylaminobenzoic acid derivatives with comparatively low molecular weights (400 to 550) and without symmetrical structure, designed on the basis of the original PAI-1 inhibitor TM5007 (Izuhara et al, 2008)) as well as PAI-749 (Gardell et al, 2007), a previously reported PAI-1 inhibitor, were synthesized by Hamari Chemicals Ltd (Osaka, Japan). They were screened in vitro for PAI-1 inhibitory activity.
Docking Simulations
Docking simulations were undertaken by the program ASEDock (Goto et al, 2008). The crystal structure of a complex between PAI-1 and the inhibitory reactive-center loop peptide (1A7C) was obtained from the Protein Data Bank (Bernstein et al, 1997) and used as the target structure for the docking simulations.
In Vitro PAI-1 Activity Assay
PAI-1 inhibitory activity was assessed by a previously described chromogenic assay (Izuhara et al, 2008). The composition of the incubation medium was adapted to increase the assay's sensitivity: 0.15 mol/L NaCl, 50 mmol/L Tris-HCl pH8, 0.2 mmol/L CHAPS, 0.1% PEG-6000, 1% dimethylsulfoxide, 5 nmol/L human active PAI-1, 2 nmol/L human 2-chain tPA and 0.2 mmol/L Spectrozyme tPA at final concentration. Tested compounds were added at various concentrations and the IC50 was calculated by logit-log analysis.
PAI-1/tPA Complex on Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis
The effect of the tested compound on the formation of a PAI-1/tPA complex was estimated in an incubation medium mixing PAI-1, tPA, and the compound. The eventual composition included 100 mmol/L HEPES, pH 7.4, 150 mmol/L NaCl, 0.05% Tween 20, 0.8% dimethylsulfoxide, 0.875 μmol/L PAI-1, 0.7 μmol/L tPA, and compound (160 μmol/L). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and visualized by Coomassie staining.
Pharmacokinetic Studies
Animal experiments were performed in accordance with the Animal Experimentation Guidelines of Tokai University School of Medicine. TM5275, suspended in 0.5% carboxymethyl cellulose sodium salt (CMC) solution, was administered orally by gavage to male ICR mice (50 mg/kg) (CLEA Japan Inc., Tokyo, Japan), male Wistar rats (50 mg/kg) (CLEA Japan Inc.), and male cynomolgus monkeys (1 mg/kg) (Macaca Fascicularis) (Japan SLC, Shizuoka, Japan). Heparinized blood samples were collected from the vein before (0 h) and 1, 2, 6, and 24 h after oral drug administration. Plasma drug concentration was determined on a reverse-phase high-performance liquid chromatography. Maximum drug concentration time (Tmax), maximum drug concentration (Cmax), and drug half-life (T½) were then calculated. For the bioavailability (BA) study in monkeys, heparinized blood samples were collected from the vein before (0 h) and 0.5, 1, 2, 4, 6, 8, 24, 48, 72, 120, and 168 h after oral drug administration, and before (0 h) and 0.08, 0.25, 0.5, 1, 2, 4, 6, 8, 24, 48, 72, 120, and 168 h after intravenous drug injection. BA was calculated by noncompartment model analysis using WinNonlin Professional Software, version 5.01 (Pharsight Co., NC, USA).
Toxicity
All toxicity studies followed the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Harmonised Tripartite Guidelines at the non-GLP conditions.
For the evaluation of acute toxicity, TM5275 (1000 mg/kg for mice and 2000 mg/kg for rats and monkeys), suspended in 0.5% CMC solution, was administered orally by gavage to male (n=5) and female (n=5) ICR mice (CLEA Japan Inc.), male (n=5) and female (n=5) Sprague–Dawley rats (Charles River Japan Inc., Kanagawa, Japan), and male cynomolgus monkeys (n=2) (Japan SLC). The animal's body weight was monitored once a week. Various organs underwent histological studies 2 weeks (mice) and 1 week (rats) after drug administration.
For the evaluation of the subacute toxicity, three different doses of TM5275 (200, 600, and 2000 mg/kg/day) were administered for 2 weeks by gavage to male (n=5) and female (n=5) Sprague–Dawley rats (Charles River Japan) and male cynomolgus monkeys (n=2) (Japan SLC). At the end of the study, blood glucose, total cholesterol, triglyceride, aspartate aminotransferase, alanine aminotransferase, creatinine, urea nitrogen, total protein, albumin, hemoglobin, red blood cells, and hematocrit levels as well as activated partial thromboplastin time and prothrombin time were assessed. Body weight was measured and urinary analysis performed.
The following safety pharmacology core battery was used: a modified Irwin's test for the central nervous system in Sprague–Dawley rats dosing TM5275 up to 2 g/kg per os, and three cardiovascular tests. (1) QT interval in telemetry electrocardiogram recording in beagle dogs administered an oral dose of 2 g/kg of TM5275; (2) action potentials of guinea-pig right ventricular papillary muscles at a dose of 5 μmol/L of TM5275; and (3) hERGIkr current measured in stably transfected human embryonic kidney (HEK) 293 cells at a dose of 5 μmol/L of TM5275.
Arteriovenous Shunt Thrombosis Rat Model
Thrombus formation in arteriovenous shunts was achieved in male CD rats (Charles River Japan) by a previously described method (Morishima et al, 1997). Either TM5275 (10 and 50 mg/kg, n=9) or ticlopidine (500 mg/kg, n=6: Wako Pure Industries Ltd, Osaka, Japan), suspended in 0.5% CMC solution, was administered orally by gavage 90 mins before the study. Control rats were administered only a 0.5% CMC solution (n=10). Blood was allowed to circulate through the shunt for 30 mins. The wet weight of the thrombus covering the silk thread was eventually measured.
Ferric Chloride-Treated Carotid Artery Thrombosis Rat Model
Male Sprague–Dawley rats weighing 280 to 310 g (Japan SLC) were anesthetized with pentobarbital sodium (50 mg/kg, intraperitoneally) and fixed on a heating pad. During the experiment, rectal temperature was maintained at 38°C. The left common carotid artery was exposed, and a piece of filter paper (2.5 × 4.2 mm) was folded around it. The probe of a pulsed Doppler flowmeter (Model PDV-20, Crystal Biotech America, Hopkinton, MA, USA) was placed to measure the arterial blood flow. After obtention of a steady baseline flow, 2 μL of ferric chloride (FeCl3) saline solution (35% (w/w)) was added to the filter paper. Five minutes later, the filter paper was removed and the artery washed with saline. Blood flow in the common carotid artery was continuously monitored for 30 mins after FeCl3 saline exposure. Time to primary occlusion was calculated.
Several concentrations of TM5275 (0.3, 1, 5 mg/kg) and clopidogrel (1, 3, 10 mg/kg) (Sanofi Aventis, Tokyo, Japan), suspended in 0.5% CMC solution, were administered orally by gavage (n=8, each group) 2 h before FeCl3 exposure. After a 30 mins blood flow monitoring, a sphygmomanometer cuff was placed on the tail and inflated to 40 mm Hg. An incision was made with an animal lancet (Goldenrod, Medipoint Inc., Mineola, NY, USA) and, every 30 secs, a wick of filtration paper was inserted on the wound until no further staining was observed. Bleeding time was determined to the nearest 30 secs. If it lasted more than 10 mins, the experiment was discontinued.
The benefits of combining TM5275 with tPA were further ascertained. Either TM5275 (5 mg/kg, n=10), tPA (0.3 or 3 mg/kg, n=10 each: Kyowa Hakko Kirin Co. Ltd, Tokyo, Japan), or TM5275 (5 mg/kg) plus tPA (0.3 mg/kg) (n=10) were administered orally (TM5275) or intravenously (tPA). The experiments were performed along the conditions described above.
Photochemically Induced Arterial Thrombosis Monkey Model
Three- to 4-year-old male cynomolgus monkeys (Japan SLC) weighing 2.8 to 3.5 kg underwent anesthesia by an intramuscular injection of 10 mg/kg ketamine hydrochloride followed by the intravenous injection of 25 mg/kg pentobarbital sodium. Animals were fixed on a heating pad, and rectal temperature was maintained at 36.5 to 37.5°C. The saphenous artery was exposed by a 2 cm incision and thrombosis was induced by a photochemical reaction according to the modified method of Umemura et al (1993). Briefly, the saphenous artery was irradiated with green light (wave length 540 nm, 900,000 lx) generated by a xenon lamp (L4887, Hamamatsu Photonics, Shizuoka, Japan) with a heat-absorbing filter and a green filter. Irradiation was directed by a 3-mm diameter optic fiber mounted on a micromanipulator. The probe of a pulsed Doppler flowmeter (Model PDV-20, Crystal Biotech America) was placed on the saphenous artery to measure arterial blood flow. Once the baseline flow was steady, a 20 mins photo-irradiation was undertaken and a 6 mins intravenous rose bengal (20 mg/kg) injection initiated. TM5275 (10 mg/kg) or clopidogrel (10 mg/kg) suspended in 0.5% CMC solution were administered by gavage (n=6, each group) 2 h before photochemical thrombosis. Blood flow of the saphenous artery was monitored for 3 h after the start of photo-irradiation. In monkeys, in contrast with rodents, photochemically induced arterial thrombosis progressively reduced cerebral blood flow, and was followed by recanalization and eventually by rethrombosis, a sequence observed in stroke patients and called cyclical flow reduction (Maeda et al, 2005a, 2005b). Therefore, total occlusion time was calculated during the experiment.
Bleeding time was measured in monkeys previously acclimated to chair restraint during repeated training sessions several times before the experiment. A sphygmomanometer cuff, placed on the upper leg of conscious monkeys fixed to a monkey chair, was inflated to 40 mm Hg. TM5275 (50 mg/kg) or clopidogrel (10 mg/kg) suspended in 0.5% CMC solution were administered orally by gavage (n=3, each group) 2 h before the study. Bleeding was produced inside the lower leg by a Micro Lancet (Abbot Japan, Tokyo, Japan) with a 21-gauge needle. Every 10 sec, a wick of filtration paper was placed on the wound until no further staining was observed. Bleeding time was determined to the nearest 10 secs. Maximum observation period was 10 mins.
Statistics
All data are expressed as the mean±s.e. Comparisons between two groups were performed using an unpaired t-test. For multiple comparisons, one-way analysis of variance and Dunnett's post hoc test were performed. The effect of TM5275 combined with tPA in the FeCl3 model was performed by one-way analysis of variance followed by Bonferroni's multiple comparisons. Values are considered significant at P<0.05. All statistical analyses were performed on the statistical package SPSS for Windows (Version 15.0, SPSS, Chicago, IL, USA).
Results
TM5275
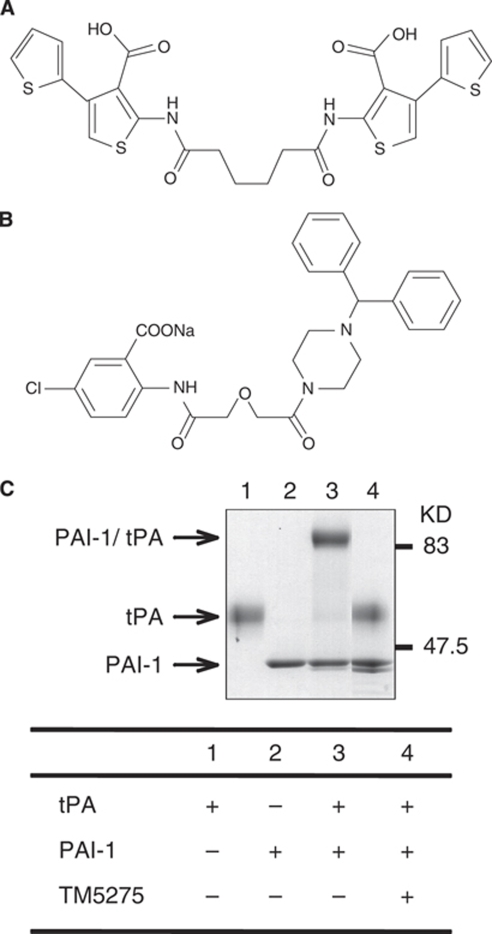
TM5275, 5-chloro-2-[({2-[4-(diphenylmethyl) piperazin-1-yl]-2-oxoethoxy}acetyl)amino]benzoate (Figure 1B), was discovered through an extensive structure-activity relationship study with more than 90 compounds designed and synthesized on the basis of the structure of TM5007 (Figure 1A). TM5275 was eventually selected as the test compound after taking into consideration the in vitro PAI-1 inhibitory activity and pharmacokinetic studies (Tmax, Cmax, T½,) (see below).
Figure 1.
Chemical structures of TM5007 (A) and TM5275 (B). Molecular weights for TM5007 and TM5275 are 560.02 and 543.97, respectively. On SDS-PAGE (C), PAI-1 formed a covalent complex with tPA, whereas no PAI-1/tPA complex formation was observed when PAI-1 was preincubated with TM5275.
The PAI-1 inhibitory activity of TM5275, measured by tPA-dependent hydrolysis of peptide substrate, is comparable to that of TM5007 and PAI-749: Half-maximal inhibition (IC50) values of TM5275, TM5007, and PAI-749 are 6.95, 5.60, and 8.37 μmol/L, respectively.
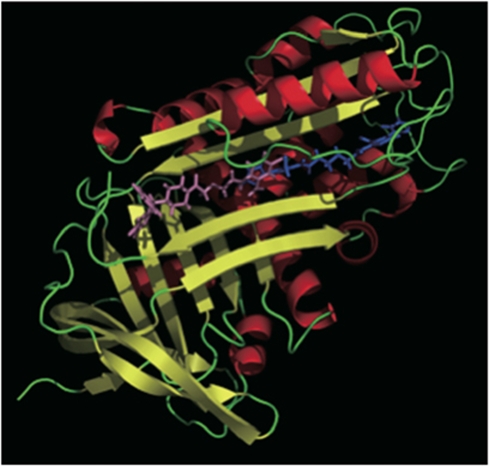
Docking simulation of the PAI-1 moiety and TM5275 was undertaken to understand the mechanism of TM5275 action. TM5275 binds to strand 4 of the A β-sheet (s4A) position of PAI-1. Although both TM5275 and TM5007 bind within the cleft to the s4A segment of PAI-1, closer inspection reveals that their binding sites differ, as illustrated in Figure 2: the bulky diphenylmethyl group of TM5275 cannot be accommodated to the binding site of TM5007, so that TM5275 is markedly shifted in the s4A cleft.
Figure 2.
The binding modes of TM5007 (cyan) and TM5275 (pink) obtained by docking simulations are shown. Figures were drawn by a software PyMOL version 0.97 (DeLano Scientific LLC, San Carlo, CA, USA).
In vitro, TM5275 (up to 100 μmol/L) does not interfere with other serpin/serine protease systems (that is, alpha1-antitrypsin/trypsin and alpha2-antiplasmin/plasmin). Its PAI-1 inhibitory activity thus appears specific. On sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the PAI-1 covalent complex formed with tPA is not observed when PAI-1 is preincubated with TM5275 (data not shown).
Pharmacokinetics
The pharmacokinetics of TM5275 improved significantly when compared with that of TM5007. An oral dose of 50 mg/kg of TM5275, administered in rats, yields calculated plasma Tmax, Cmax, and T½ of 2 h, 34 μmol/L, and 2.5 h, respectively, versus 18 h, 8.8 μmol/L, and 124 h, respectively, in rats administered the same dose of TM5007. TM5275 thus increases Cmax fourfold, and markedly shortens both Tmax and T½. In mice, an oral dose of 50 mg/kg of TM5275 yields the following values for these parameters: 1 h, 6.9 μmol/L, and 6.5 h, respectively. In monkeys, an oral dose of 1 mg/kg of TM5275 yields Tmax, Cmax, and T½ values of 6 h, 10.5 μmol/L, and 114.7 h, respectively. Bioavailability of TM5275 reaches 96% in monkeys.
Toxicity
Acute toxicity has been evaluated in vivo. A single dose of TM5275 of 1000 mg/kg in mice and 2000 mg/kg in rats and monkeys elicited no symptoms after 2 weeks in the former group and after 1 week in the latter two groups. Body weight and the histology of various organs are not modified.
Subacute toxicity has been assessed in rats and monkeys administered daily three different doses of TM5275 (200, 600, and 2000 mg/kg/day) for 2 weeks. Body weight and the histology of various organs are not modified. No abnormality is noted in the biochemistry of plasma and urine, including activated partial thromboplastin time, prothrombin time, and red blood cell count.
In safety pharmacology studies, TM5275 does not modify tests of the central nervous system (a modified Irwin's test in rats) or of the cardiovascular system: (1) QT interval in electrocardiogram recording in dogs; (2) action potentials of guinea-pig right ventricular papillary muscles; and (3) hERGIkr current in HEK293 cells.
Rat Thrombosis Models
The antithrombotic effectiveness of TM5275 in a rat arteriovenous shunt model is described in Table 1. Blood clot weights are significantly lower in rats administered 10 and 50 mg/kg of TM5275 (60.9±3.0 and 56.8±2.8 mg, respectively) than in vehicle-treated rats (72.5±2.0 mg). Up to 300 mg/kg of TM5007 are needed to reach the efficacy of 50 mg/kg of TM5275 in the same model. The antithrombotic effectiveness of TM5275 (50 mg/kg) is equivalent to that of ticlopidine (500 mg/kg), a reference antithrombotic compound. Plasma concentration of TM5275 reaches 17.5±5.2 μmol/L after a dose of 10 mg/kg.
Table 1. Effect of TM5275 on thrombus weight in a rat arteriovenous shunt model.
| Treatment | N | Thrombus weight subtracting thread (mg) |
|---|---|---|
| Vehicle | ||
| 0.5% Carboxymethyl cellulose sodium salt (per os) | 10 | 72.5±2.0 |
| TM5275 | ||
| 10 mg/kg (per os) | 9 | 60.9±3.0* |
| 50 mg/kg (per os) | 9 | 56.8±2.8† |
| Ticlopidine | ||
| 500 mg/kg (per os) | 6 | 51.8±2.2† |
Data are expressed as mean±s.e. *P<0.01, †P<0.001 versus vehicle.
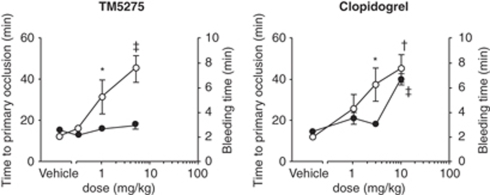
The antithrombotic effectiveness of TM5275 in a rat FeCl3 carotid artery thrombosis model is illustrated in Figure 3. TM5275 and clopidogrel, another standard antithrombotic drug, prove antithrombotic in a dose-dependent manner (Figure 3). The minimum effective doses of TM5275 and clopidogrel are 1 and 3 mg/kg, respectively. It corresponds to a TM5275 plasma concentration of 4.9±3.6 μmol/L. As expected, clopidogrel prolongs bleeding time in a dose-dependent manner (Figure 3). By contrast, TM5275 does not affect bleeding time, a potential benefit as an antithrombotic agent. Two hours after oral administration, TM5275 (10 mg/kg) does not affect platelet aggregation induced by ADP and collagen (data not shown). Its antithrombotic effect is thus independent of any effect on platelets.
Figure 3.
Effects of TM5275 and clopidogrel on primary occlusion time (open circles) and bleeding time (closed circles) in the rat FeCl3-induced thrombosis model. *P<0.05, †P<0.01, ‡P<0.001 versus vehicle. n=8, each group.
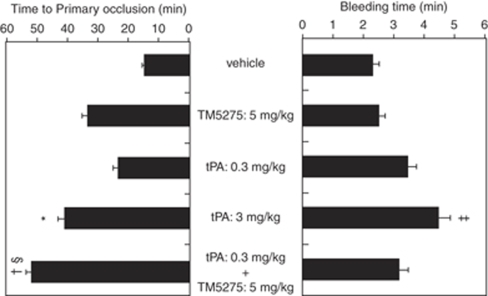
TM5275 has been further combined with tPA in the same model, as illustrated in Figure 4. tPA (0.3 mg/kg) alone does not provide a significant antithrombotic effect. However, TM5275 (5 mg/kg) combined with tPA (0.3 mg/kg) significantly enhances the antithrombotic effect of tPA (0.3 mg/kg) alone and provides a benefit similar to that of a high tPA dose (3 mg/kg). The bleeding time of the combination therapy is similar to that of a low tPA dose (0.3 mg/kg) alone.
Figure 4.
Effect of combination of TM5275 and tPA on primary occlusion time (left panel) and bleeding time (right panel) in the rat FeCl3-induced thrombosis model. *P<0.05, †P<0.01, ‡P<0.001 versus vehicle, §P<0.05 versus tPA (0.3 mg/kg) alone. n=10, each group.
Monkey Thrombosis Model
The antithrombotic effect of TM5275 has been evaluated in a cynomolgus monkey model of photochemically induced thrombosis (Table 2). Total occlusion time is significantly reduced in both the TM5275 (53.9±19.9 mins) and clopidogrel (39.4±25.8 mins) groups (10 mg/kg, each) in comparison with the vehicle group (119.0±17.4 mins). Plasma concentration of TM5275 reaches 18.9±3.7 μmol/L in the TM5275 group.
Table 2. Effect of TM5275 on total occlusion time in the monkey photochemically induced thrombosis model.
| Treatment | N | Total occlusion time (min) |
|---|---|---|
| Vehicle | ||
| 0.5% Carboxymethyl cellulose sodium salt (per os) | 6 | 119.0±17.4 |
| Clopidogrel | ||
| 10 mg/kg (per os) | 6 | 39.4±25.8* |
| TM5275 | ||
| 10 mg/kg (per os) | 6 | 53.9±19.9* |
Total occlusion time was calculated during the experiment. Data are expressed as mean±s.e. *P<0.05 versus vehicle.
The benefits of TM5275, as an antithrombotic agent devoid of effects on bleeding time, have been confirmed in nonhuman primates (Table 3). Two hours after administration of 50 mg/kg (five times higher than the effective dose) of TM5275, the bleeding time is only slightly longer (146.7±3.3 secs) than before administration (83.3±6.7 secs), whereas it is markedly extended in the 10 mg/kg clopidogrel group (>600 secs) above that observed before administration (113.3±8.8 secs).
Table 3. Effect of TM5275 on bleeding time in the monkey photochemically induced thrombosis model.
| Bleeding time (secs) | |||
|---|---|---|---|
| Treatment | N | Before administration | After administration |
| Clopidogrel | |||
| 10 mg/kg (per os) | 3 | 113.3±8.8 | >600 |
| TM5275 | |||
| 50 mg/kg (per os) | 3 | 83.3±6.7 | 146.7±3.3 |
Data are expressed as mean±s.e.
Discussion
In contrast with a previous rather inefficient high-throughput random screening approach of a large chemical library, we recently reported a novel method to identify small molecular PAI-1 inhibitors, that is, structure-based drug design relying on the virtual screening of small compounds based on the PAI-1 three-dimensional structure (Izuhara et al, 2008). Two small molecular PAI-1 inhibitors, TM5001 and TM5007, were thus synthesized and their antithrombotic activity documented in rats (Izuhara et al, 2008). In this study, we use the same approach and synthesize a new, more effective PAI-1 inhibitor, TM5275. We show its antithrombotic efficacy not only in rodents but also in monkeys, a more human relevant model.
In limited toxicology studies performed so far, TM5275 appears to be nontoxic. Single doses of 1.0 to 2.0 g/kg elicit no abnormality in rodents or monkeys. Daily doses of 200, 600, or 2000 mg/kg administered for 2 weeks to rats or monkeys also fail to produce symptoms, biochemical disorders, or histological abnormalities in tested organs. More pointed safety pharmacologic tests disclose no neural or cardiovascular toxicity.
Of major interest, TM5275 is 6 times more effective than TM5007: at a dosage of 50 mg/kg, its antithrombotic activity, in a rat arteriovenous shunt model, is equivalent to that obtained by 300 mg/kg of TM5007. This difference is best accounted for by a higher plasma drug concentration of TM5275 reached within 2 h after a 50 mg/kg oral dose, that is, 22.4 μmol/L versus only 5.2 μmol/L after a 300 mg/kg oral dose of TM5007. It fits with the different pharmacokinetic profiles of both drugs: a higher Tmax reached within a shorter delay after TM5275 (34 μmol/L within 2 h) than after TM5007 (8.8 μmol/L within 18 h). T½ is more rapid for TM5275 (2.5 h) than for TM5007 (124 h). Interestingly, a lower oral dose of 10 mg/kg administered to nonhuman primates results in intermediary values: Tmax of 10.5 μmol/L reached within 6 h with a T½ of 114.7 h. These characteristics argue in favor of oral TM5275 to prevent or dissolve clots in humans, a conclusion further supported by the very high bioavailability of oral TM5275 in monkeys (96%).
Clearly, TM5275 is effective in the prevention of thrombosis in rat models: it dose-dependently reduces blood clot weight and, at the higher 50 mg/kg doses, is equivalent to a single dose of 500 mg/kg of ticlopidine. In the FeCl3 carotid artery thrombosis model, it (1 mg/kg) also prolongs occlusion time to an extent similar to that of clopidogrel (3 mg/kg) but, unlike the latter, without increasing the bleeding time. In monkeys, the benefits of TM5275 (10 mg/kg) are also apparent as total occlusion time is markedly attenuated to a degree similar to that achieved by clopidogrel (10 mg/kg), but, in contrast to the latter, with only a minor increase in bleeding time.
The differences observed between rodents and monkeys, in TM5275 pharmacokinetics and antithrombotic effects, fully support the recent guidelines (STAIR, 1999) advocating tests in nonhuman primates to reduce discrepancies between preclinical models (usually rodents) and human clinical data. In contrast to rodent thrombosis models, the photochemically induced arterial thrombosis developed in monkeys shows a cyclical flow reduction, that is, a progressive decrease in blood flow with rethrombosis followed by recanalization closely resembling human cerebral thrombosis (Maeda et al, 2005a, 2005b). This difference might reflect specific platelet and coagulation systems. In this nonhuman primate model, TM5275 provides a powerful antithrombotic effect without adverse effects on bleeding time, suggesting its potential benefit, for example, in combination therapy with tPA, post-tPA therapy to avoid arterial reocclusion (Alexandrov and Grotta, 2002), treatment for patients with cerebral microbleeds (Nighoghossian et al, 2002; Wong et al, 2003), or intracranial branch atheromatous disease (Caplan, 1989).
Although tPA has recently become the most effective therapeutic agent for acute ischemic stroke (The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group, 1995), symptomatic intracranial hemorrhage remains the most serious, concurrent complication associated with tPA treatment (Derex and Nighoghossian, 2008). In The National Institute of Neurological Disorders and Stroke tPA trial (1995), 6.4% of patients showed a symptomatic intracranial hemorrhage with deterioration of the clinical status in the tPA group, compared with 0.6% in the placebo group. The mortality rate in cases with symptomatic intracranial hemorrhage reached up to 47% (The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group, 1997). Recently, an increased risk of intracranial hemorrhage in patients receiving tPA, assessed by concomitant cerebral microbleeding on T2*-weighted magnetic resonance imaging, has been pointed out (Nighoghossian et al, 2002), and an alternative thrombolytic therapy devoid of worrisome bleeding disorders often encountered with tPA therapy is requested. We show that, in rats, TM5275 augments its antithrombotic efficacy: added to a standard intravenous dose of tPA (0.3 mg/kg), it more than doubles (P<0.05 versus 0.3 mg/kg tPA alone) its time to primary occlusion without increasing bleeding time. Conversely, tPA (3 mg/kg) significantly prolongs the TM5275-induced time to primary occlusion with a small but significant increase in bleeding time. It is noted that the TM5275-tPA (0.3 mg/kg) combination, compared with a 10 times higher dose of injected tPA (3 mg/kg), achieves a longer time to primary occlusion (P<0.01 versus vehicle) with a decreased bleeding time. These data obtained in rats suggest that an appropriate combination of oral TM5275 with intravenous tPA might prove valuable in clinical emergencies without inducing worrisome bleeding disorders.
Clinical deterioration after tPA therapy was observed in the National Institute of Neurological Disorders and Stroke rt-PA Stroke Study (Grotta et al, 2001), and early cerebral arterial reocclusion occurred in 34% of tPA-treated patients with any initial recanalization (Alexandrov and Grotta, 2002). Oral administration of TM5275, followed by intravenous tPA treatment, may be beneficial. Also, the presence of cerebral microbleeds on T2*-weighted magnetic resonance imaging has been pointed out as a marker of increased risk of intracranial hemorrhage in patients receiving tPA (Nighoghossian et al, 2002) or taking aspirin (Wong et al, 2003). An alternative thrombolytic and/or antiplatelet therapy devoid of worrisome bleeding disorders, such as TM5275, may therefore provide benefits.
Intracranial branch atheromatous disease is another candidate for TM5275 treatment. Patients with atheromatous disease of an arterial branch present a gradual or stepwise progression of clinical signs, because platelet-fibrin plugs may form, break off, and embolize distally (Caplan, 1989), a mechanism mimicking the cyclical flow reduction by the photochemically induced arterial thrombosis (Maeda et al, 2005a, 2005b) shown in our study using cynomolgus monkeys.
Several characteristics of TM5275 might prove helpful in the treatment of thrombotic disorders. First of all, unlike other types of antithrombotic agents, for example, anticoagulation or antiplatelet agents it does not prolong bleeding time and has virtually no influence on activated partial thromboplastin time/prothrombin time and platelet activity. Second, its impressive bioavailability warrants an administration per os, a substantial advantage over tPA. The demonstration that, combined with the latter, it enhances its activity supports a joint administration.
It is noteworthy that inhibition of PAI-1 in humans may not induce serious adverse effects. Currently, two genetic defects in the PAI-1 gene have been documented in humans (Mehta and Shapiro, 2008). Affected individuals do have a mild bleeding tendency but rarely exhibit severe bleeding events commonly seen in other procoagulant deficiencies: most episodes are post-traumatic or post-surgical. The majority of bleeding events are controlled with antifibrinolytic agents, such as tranexamic acid and ɛ-aminocaproic acid. In PAI-1-deficient patients, a normal lifespan is achieved. Further clinical studies are needed to support these conclusions.
Acknowledgments
This study was supported by a grant from the New Energy and Industrial Technology Development Organization in Japan (to TM and NH).
The authors declare no conflict of interest.
References
- Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology. 2002;59:862–867. doi: 10.1212/wnl.59.6.862. [DOI] [PubMed] [Google Scholar]
- Bernstein FC, Koetzle TF, Williams GJ, Meyer EF, Jr, Brice MD, Rodgers JR, Kennard O, Shimanouchi T, Tasumi M. The protein data bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1997;112:535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Caplan LR. Intracranial branch atheromatous disease: a neglected, understudied, and underused concept. Neurology. 1989;39:1246–1250. doi: 10.1212/wnl.39.9.1246. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Stassen JM, Schoonjans L, Ream B, van den Oord JJ, De Mol M, Mulligan RC, Collen D. Plasminogen activator inhibitor-1 gene-deficient mice: II Effects on hemostasis, thrombosis, and thrombolysis. J Clin Invest. 1993;92:2756–2760. doi: 10.1172/JCI116893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derex L, Nighoghossian N. Intracerebral haemorrhage after thrombolysis for acute ischaemic stroke: an update. J Neurol Neurosurg Psychiatry. 2008;79:1093–1099. doi: 10.1136/jnnp.2007.133371. [DOI] [PubMed] [Google Scholar]
- Devin JK, Johnson JE, Eren M, Gleaves LA, Bradham WS, Bloodworth JR, Jr, Vaughan DE. Transgenic overexpression of plasminogen activator inhibitor-1 promotes the development of polycystic ovarian changes in female mice. J Mol Endocrinol. 2007;39:9–16. doi: 10.1677/JME-06-0057. [DOI] [PubMed] [Google Scholar]
- Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D, Simon RH. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J Clin Invest. 1996;97:232–237. doi: 10.1172/JCI118396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitzman DT, Westrick RJ, Xu Z, Tyson J, Ginsburg D. Plasminogen activator inhibitor-1 deficiency protects against atherosclerosis progression in the mouse carotid artery. Blood. 2000;96:4212–4215. [PubMed] [Google Scholar]
- Eren M, Gleaves LA, Atkinson JB, King LE, Declerck PJ, Vaughan DE. Reactive site-dependent phenotypic alterations in plasminogen activator inhibitor-1 transgenic mice. J Thromb Haemost. 2007;5:1500–1508. doi: 10.1111/j.1538-7836.2007.02587.x. [DOI] [PubMed] [Google Scholar]
- Gardell SJ, Krueger JA, Antrilli TA, Elokdah H, Mayer S, Orcutt SJ, Crandall DL, Vlasuk GP. Neutralization of plasminogen activator inhibitor 1 (PAI-1) by the synthetic antagonist PAI-749 via a dual mechanism of action. Mol Pharmacol. 2007;72:897–906. doi: 10.1124/mol.107.037010. [DOI] [PubMed] [Google Scholar]
- Goto J, Kataoka R, Muta H, Hirayama N. ASEDock-docking based on alpha spheres and excluded volumes. J Chem Inf Model. 2008;48:583–590. doi: 10.1021/ci700352q. [DOI] [PubMed] [Google Scholar]
- Grotta JC, Welch KM, Fagan SC, Lu M, Frankel MR, Brott T, Levine SR, Lyden PD. Clinical deterioration following improvement in the NINDS rt-PA Stroke Trial. Stroke. 2001;32:661–668. doi: 10.1161/01.str.32.3.661. [DOI] [PubMed] [Google Scholar]
- Ha H, Oh EY, Lee HB. The role of plasminogen activator inhibitor 1 in renal and cardiovascular diseases. Nat Rev Nephrol. 2009;5:203–211. doi: 10.1038/nrneph.2009.15. [DOI] [PubMed] [Google Scholar]
- Izuhara Y, Takahashi S, Nangaku M, Takizawa S, Ishida H, Kurokawa K, van Ypersele de Strihou C, Hirayama N, Miyata T. Inhibition of plasminogen activator inhibitor-1: its mechanism and effectiveness on coagulation and fibrosis. Arterioscler Thromb Vasc Biol. 2008;28:672–677. doi: 10.1161/ATVBAHA.107.157479. [DOI] [PubMed] [Google Scholar]
- Maeda M, Moriguchi A, Mihara K, Aoki T, Takamatsu H, Matsuoka N, Mutoh S, Goto T. FK419, a nonpeptide platelet glycoprotein IIb/IIIa antagonist, ameliorates brain infarction associated with thrombotic focal cerebral ischemia in monkeys: comparison with tissue plasminogen activator. J Cereb Blood Flow Metab. 2005a;25:108–118. doi: 10.1038/sj.jcbfm.9600013. [DOI] [PubMed] [Google Scholar]
- Maeda M, Takamatsu H, Furuichi Y, Noda A, Awaga Y, Tatsumi M, Yamamoto M, Ichise R, Nishimura S, Matsuoka N. Characterization of a novel thrombotic middle cerebral artery occlusion model in monkeys that exhibits progressive hypoperfusion and robust cortical infarction. J Neurosci Methods. 2005b;146:106–115. doi: 10.1016/j.jneumeth.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Mehta R, Shapiro AD. Plasminogen activator inhibitor type 1 deficiency. Haemophilia. 2008;14:1255–1260. doi: 10.1111/j.1365-2516.2008.01834.x. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Tanabe K, Terada Y, Hara T, Kunitada S. Antithrombotic and hemorrhagic effects of DX-9065a, a direct and selective factor Xa inhibitor: comparison with a direct thrombin inhibitor and antithombin III-dependent anticoagulants. Thromb Haemost. 1997;78:1366–1371. [PubMed] [Google Scholar]
- Nicholas SB, Aguiniga E, Ren Y, Kim J, Wong J, Govindarajan N, Noda M, Wang W, Kawano Y, Collins A, Hsueh WA. Plasminogen activator inhibitor-1 deficiency retards diabetic nephropathy. Kidney Int. 2005;67:1297–1307. doi: 10.1111/j.1523-1755.2005.00207.x. [DOI] [PubMed] [Google Scholar]
- Nighoghossian N, Hermier M, Adeleine P, Blanc-Lasserre K, Derex L, Honnorat J, Philippeau F, Dugor JF, Froment JC, Trouillas P. Old microbleeds are a potential risk factor for cerebral bleeding after ischemic stroke: a gradient–echo T2*-weighted brain MRI study. Stroke. 2002;33:735–742. doi: 10.1161/hs0302.104615. [DOI] [PubMed] [Google Scholar]
- Stroke Therapy Academic Industry Roundtable (STAIR) Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. The NINDS t-PA Stroke Study Group. Stroke. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- Umemura K, Wada K, Uematsu T, Nakashima M. Evaluation of the combination of a tissue-type plasminogen activator, SUN9216, and a thromboxane A2 receptor antagonist, vapiprost, in a rat middle cerebral artery thrombosis model. Stroke. 1993;24:1077–1082. doi: 10.1161/01.str.24.7.1077. [DOI] [PubMed] [Google Scholar]
- Vaughan DE, De Taeye BM, Eren M. PAI-1 antagonists: predictable indications and unconventional applications. Curr Drug Targets. 2007;8:962–970. doi: 10.2174/138945007781662364. [DOI] [PubMed] [Google Scholar]
- Weisberg AD, Albornoz F, Griffin JP, Crandall DL, Elokdah H, Fogo AB, Vaughan DE, Brown NJ. Pharmacological inhibition and genetic deficiency of plasminogen activator inhibitor-1 attenuates angiotensin II/salt-induced aortic remodeling. Arterioscler Thromb Vasc Biol. 2005;25:365–371. doi: 10.1161/01.ATV.0000152356.85791.52. [DOI] [PubMed] [Google Scholar]
- Wong KS, Chan YL, Liu JY, Gao S, Lam WW. Asymptomatic microbleeds as a risk factor for aspirin-associated intracerebral hemorrhages. Neurology. 2003;60:511–513. doi: 10.1212/01.wnl.0000046583.40125.20. [DOI] [PubMed] [Google Scholar]