Abstract
Transient compression of rat somatosensory cortex has been reported to affect cerebral microvasculature and sensory function simultaneously. However, the effects of long-term cortical compression remain unknown. Here, we investigated whether and to what extent sustained but moderate epidural compression of rat somatosensory cortex impairs somatic sensation and/or cortical microvasculature. Electrophysiological and behavioral tests revealed that sustained compression caused only short-term sensory deficit, particularly at 1 day after injury. Although the diameter of cortical microvessels was coincidentally reduced, no ischemic insult was observed. By measuring Evans Blue and immunoglobulin G extravasation, the blood–brain barrier (BBB) permeability was found to dramatically increase during 1 to 3 days, but this did not lead to brain edema. Furthermore, immunoblotting showed that the BBB component proteins occludin, claudin-5, type IV collagen, and glial fibrillary acidic protein were markedly upregulated in the injured cortex during 1 to 2 weeks when BBB regained integrity. Conversely, treatment of ascorbic acid prevented compression-induced BBB disruption and sensory impairment. Together, these data suggest that sustained compression of the somatosensory cortex compromises BBB integrity and somatic sensation only in the early period. Ascorbic acid may be used therapeutically to modulate cortical compression and/or BBB dysfunction.
Keywords: antioxidants, astrocytes, BBB (blood–brain barrier), electrophysiology, microvasculature, somatosensory cortex
Introduction
Extra mass produced within the closed skull may lead to compression of the brain tissue. In a clinical context, it could be associated with epidural or subdural hematoma, brain tumor, hemangioma, or head trauma (DeAngelis, 2001; Mayer and Rincon, 2005). Although the mechanism contributing to the clinical manifestations of affected patients has not been fully elucidated, it may depend on the mass location and the magnitude to which the mass affects the surrounding tissue, including the brain microvasculature.
A number of studies have indicated that physical compression of the cerebral cortex can interfere with the cerebral microvessels. Compression of the rat sensorimotor cortex for 30 mins can induce cerebral ischemia followed by behavioral sensory and motor deficits, such as paresis of the contralateral forelimbs and hindlimbs (Kundrotiene et al, 2002). In addition, transient compression of the rat somatosensory cortex can result in a synchronized reduction of cerebral blood flow and electrophysiological performance (Burnett et al, 2005). More recently, traumatic brain injury has been shown to cause loss of tight junction proteins in the endothelium, disruption of the blood–brain barrier (BBB), and eventually brain edema (Zhao et al, 2007). These findings suggest that transient compression of the cerebral cortex can elicit pathophysiological alterations of cerebral microvessels and brain parenchyma. However, the effects of long-term cortical compression on the underlying tissue remain unknown.
The BBB is primarily composed of the brain microvascular endothelial cells interconnected with transmembrane tight junction proteins, claudins and occludin, and therefore can restrict paracellular permeability (Furuse et al, 1993; Morita et al, 1999). The claudin family comprises more than 20 members in mammals, among which claudin-5 predominantly expresses in the BBB. The cytoplasmic domains of both claudins and occludin are linked to the actin cytoskeleton by the anchoring proteins zonula occludens (ZO) (Itoh et al, 1999; Morita et al, 1999). Apart from the endothelium, the structural constituents of the BBB also include the perivascular astrocytes, the pericytes, and the basal lamina, which consists of the extracellular matrix such as type IV collagen and laminin (Abbott et al, 2006; Muellner et al, 2003). Under physiological conditions, the BBB permits a constant supply of oxygen and nutrients for brain cells, but precisely limits molecules that may be harmful to neurons from passing into the brain parenchyma.
The aim of this study was to directly investigate the effect of sustained epidural compression of the primary somatosensory cortex on the underlying cortical microvessels and its correlation to the somatic sensation of rats. To evaluate BBB permeability, extravasations of exogenous Evans Blue dye and endogenous serum immunoglobulin G (IgG) were assayed. The morphological alterations of cortical microvessels, the brain water content, and the expressions of BBB components were examined. The astrocytic reaction and its spatial relation to the microvessels were also analyzed. Conversely, reactive oxygen species (ROS; e.g., superoxide, hydrogen peroxide, and hydroxyl radical) and reactive nitrogen species (e.g., nitric oxide (NO) and peroxynitrite) have been shown to affect endothelial cell permeability (Parathath et al, 2006; Schreibelt et al, 2007). Thus, we examined the expressions of the biomarkers 3-nitrotyrosine (3-NT) for protein nitration and 4-hydroxynonenal (4-HNE) for lipid peroxidation, both of which have been extensively used to evaluate the free radical-induced cellular damage (Choi et al, 2007). In addition, a short-term regimen of the antioxidants ascorbic acid (vitamin C), α-tocopherol (vitamin E), nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitor apocynin, xanthine oxidase inhibitor allopurinol, or NO synthase inhibitor N-nitro--arginine methyl ester (-NAME) administration was used on animals to determine the effects of these drugs on the pathophysiological alterations as a result of cortical compression.
Materials and methods
The experiments were conducted under authorization of the Institutional Animal Care and Use Committee of Tzu Chi University. All efforts were made to minimize the number of animals used and to prevent their suffering. Animals were maintained under veterinarian assistance, kept in a temperature-controlled room on a 12/12-h light/dark cycle, and provided with a standard rodent diet and water ad libitum. Adult male Sprague–Dawley rats (n=120, 200 to 250 g; Charles River, BioLASCO, Taipei, Taiwan) subjected to cortical compression were divided and allowed to survive for 1 day, 3 days, 1 week, 2 weeks, and 3 months, respectively. Thirty normal rats were also included. An additional 72 rats received different drug or vehicle treatment (see below).
Epidural Compression of Primary Somatosensory Cortex
Hemispherical plastic beads, 5 mm in diameter and 1.5 mm in thickness, were used for compression of the cerebral cortex. The surgical procedure has been previously detailed (Chen et al, 2003). Briefly, each animal was anesthetized with chloral hydrate (315 mg/kg intraperitoneally; Merck, Darmstadt, Germany) and secured to a stereotaxic device (51600, Stoelting, Wood Dale, IL, USA). An elliptical hole with short diameter 4 mm and long diameter 6 mm was drilled in the skull over the right primary somatosensory cortex under a Zeiss surgical microscope (OPMI pico, Oberkochen, Germany). The plastic bead with the flat surface facing up was slowly implanted in the epidural space, with care not to damage the dura. The coordinates of bead center was 0.84 mm posterior and 4.5 mm lateral to the bregma. In this way, the flat surface of the bead sealed the hole in the skull. The contralateral control side was sham-operated without bead implantation. The wound of the skin was closed with 6-0 sutures (Silkam, B/BRAUN, Tuttlingen, Germany). In normal animals, the dura on both sides were not exposed.
Drug Treatment
On the basis of the preliminary analyses, the efficacious doses of drugs were determined as previously described (Wang et al, 2009). The rats were injected intraperitoneally with ascorbic acid (500 mg/kg) in phosphate-buffered saline (PBS), α-tocopherol (200 mg/kg) in soybean oil, -NAME (100 mg/kg) in PBS, apocynin (50 mg/kg) in PBS, or allopurinol (50 mg/kg) in PBS. These drugs were all purchased from Sigma (St Louis, MO, USA) and applied to animals (n=60) starting immediately after surgery. If animals were to survive for more than 1 day, they received one injection every 24 h. Control animals (n=12) received the same surgery and the corresponding vehicle injections.
Behavioral Test
The von Frey behavioral test was carried out on rats in a quiet room (temperature, 25±2°C; humidity, 55±5%). A series of monofilaments (Touch-Test Sensory Evaluators, North Coast Medical, Wood Dale, IL, USA) were used to determine the touch sensitivity of animals. Individual rats were placed in a Plexiglas chamber with wire mesh floor. After 30 mins for accommodation, filaments (bending force: 0.008, 0.02, 0.04, 0.07, 0.16, 0.4, 0.6, 1.0, 1.4, 2.0, 4.0, 6.0, 8.0, 10.0, 15.0, 26.0, 60.0, and 100.0 g) were delivered to the center of the whisker pad, and the plantar surfaces of the forepaw and hindpaw. Each filament was applied five times to the same region at 5-secs intervals. Head, forepaw, or hindpaw withdrawal on filament application was considered as a positive response. The threshold was defined as the lowest force of the filaments that induced at least three positive responses in five trials. A recovery (5 mins) was allowed to rats after each threshold had been reached. To reduce errors attributed to individual variability, we calculated the threshold ratio as the threshold of the injured (left) side over that of the contralateral side in each animal and the mean threshold ratio as the average of threshold ratios of the same animal group.
Electrophysiological Measurement
To confirm the results of behavioral tests, we measured the somatosensory evoked potentials (SSEPs) of the same group of animals, which were not processed further for other experiments. The animals were anesthetized and secured to a stereotaxic device. The core temperature of animals was maintained at 37±0.5°C and electrophysiological recordings were carried out with a 4-channel EMG/NCV/EP system (Nicolet VikingQuest, Madison, WI, USA). A silver electrode was placed on the dura over the primary somatosensory cortex. The contralateral whisker pad (1.5 mA), forepaw (4.5 mA), and hindpaw (6 mA) were transcutaneously stimulated by electric pulses (0.2 msecs, 4 Hz). Analysis time of 60 msecs and filters of 30–3000 Hz were applied. Each measurement was repeated 20 times and averaged. Amplitudes between the first negative (N1) and the first positive (P1) peaks of the waveforms (as shown in Figure 2B) were analyzed by VikingQuest 7.3.1 software (Madison, WI, USA). The amplitude ratio was derived from the average of amplitudes of the injured side over that of the contralateral side in each animal and the mean amplitude ratio was the average of amplitude ratios of the same animal group.
Tissue Preparation
Animals were deeply anesthetized and perfused transcardially with normal saline followed by a fixative containing 4% paraformaldehyde in phosphate buffer (PB) at room temperature for 30 mins. After perfusion, brains were dissected out, post-fixed in the same fixative for 4 h, and then immersed in 30% sucrose in PB at 4°C until they sank. Frozen brains were cut into 20-μm-thick sections on a LEICA cryostat (CM1850, Nussloch, Germany). These sections were processed for histochemistry or immunohistochemistry (see below). All sections except those for immunofluorescence were mounted on gelatin-coated slides and coverslipped with Permount (Fisher, Fair Lawn, NJ, USA). Mounted sections were inspected using a Zeiss Axioplan epifluorescence microscope and images were captured by a SPOT cooled CCD camera system (Diagnostic, Sterling Heights, MI, USA) combined with SPOT software (VR 4.6.3.8).
Cytochrome Oxidase Reaction
To ensure that our sections contain the primary somatosensory cortex and to demarcate cortical layers I to IV and V to VI, selective sections were incubated in a solution, 0.05% 3,3′-diaminobenzidine tetrahydrochloride (Sigma), 4% sucrose (Sigma), and 0.02% cytochrome c (Sigma) in 0.1 mol/L PBS (pH 7.4), at 37°C for 3 h in the dark and then washed with cold PBS. In addition, to verify the boundaries between cortical layers, selective sections were stained with cresyl violet or antiserum to protein gene product 9.5 (data not shown) as previously described (Wang et al, 2009).
Alkaline Phosphatase Histochemistry
To reveal cortical microvessels, selective sections containing the primary somatosensory cortex were reacted with a solution, 0.1 mol/L NaCl (Sigma), 20 mmol/L MgCl2 (Sigma), 0.0333% nitro-blue tetrazolium (Sigma), 0.0165% 5-bromo-4-chloro-3-indolyl phosphate p-toluidine salt (Sigma), 0.5% Triton X-100 (Sigma), and 0.89% dimethyl formamide (Sigma) in 0.1 mol/L Tris buffer (pH 9.0), for 30 mins at room temperature. Sections were then washed with 10 mmol/L Tris buffer (pH 7.5). In each section, the total area occupied by cortical microvessels in the right somatosensory cortex was divided by the total area of the right somatosensory cortex. The obtained value of the right (lesion) side was further divided by that of the left (contralateral) side, acquiring the area ratio. The diameter ratio was derived from the average diameter of all cortical microvessels of the injured cortex over that of the contralateral cortex in each section.
Immunohistochemistry
Selective sections were processed for free-floating immunohistochemistry. First, sections were quenched in 1% H2O2 for 1 h and then immersed in 10% normal goat serum (Vector, Burlingame, CA, USA) for 1 h at room temperature. Subsequently, sections were incubated in mouse anti-glial fibrillary acidic protein (GFAP, 1:400; Sigma), mouse anti-3-NT (1:500; Chemicon, Temecula, CA, USA), or rabbit anti-4-HNE (1:2000; Calbiochem, La Jolla, CA, USA) at 4°C overnight and then in biotinylated goat anti-mouse IgG (1:200; Vector) or goat anti-rabbit IgG (1:200; Vector) for 1 h at room temperature. To label endogenous IgG, sections were incubated in biotinylated rabbit anti-rat IgG (1:200; Vector). After rinses in PBS, all sections were treated in avidin–biotin–horseradish peroxidase reagent (1:100; Vector) and reacted with 0.05% 3,3′-diaminobenzidine tetrahydrochloride and 0.01% H2O2 in Tris buffer, pH 7.4. For 3-NT, 4-HNE, and IgG analysis, the optical density (OD) ratio was derived from the OD of the whole area of the injured somatosensory cortex over that of the contralateral cortex in each section and the mean OD ratio was the average of OD ratios of the same animal group. For immunofluorescence, selective sections were treated with GFAP labeling, but the secondary antibody was conjugated with FITC (Vector).
Cerebral Infarct Measurement
To determine whether cortical compression causes ischemic damage, slices containing the primary somatosensory cortex were stained with 2,3,5-triphenyltetrazolium chloride. Animals were decapitated under deep anesthesia, and their brains were removed and immersed in ice-cold PBS for 20 mins to facilitate cutting. Brains were cut into 2-mm thick slices in the coronal plane using an RBM-4000C rodent brain matrix (ASI, Warren, MI, USA). The brain slices were then stained with 2% 2,3,5-triphenyltetrazolium chloride (Sigma) in PBS at 37°C for 6 mins, and after surface reversal of the slices, for another 6 mins.
Evans Blue Extravasation
Evans Blue was used to assess the BBB permeability as this dye has a very high affinity for serum albumin. Three percent Evans Blue (Sigma) in saline was injected slowly through the femoral vein (4 mL/kg) and allowed to circulate for 90 mins. Animals were then transcardially perfused with lukewarm saline, followed by 4% paraformaldehyde. The brains were harvested and photographed. The selected cortical region was cut and incubated in 500 μL formamide (Sigma) at 55°C for 24 h. After incubation, the solution was centrifuged at 20,000 g for 20 mins. The supernatant was collected, and the OD at 620 nm was measured using a Beckman Coulter spectrophotometer (DU-7400, Fullerton, CA, USA) to determine the relative amount of Evans Blue in each sample. The OD ratio was derived from the OD of the injured (right) side over that of the contralateral side in each animal.
Brain Edema Measurement
To measure the brain water content, we used the wet-dry method. After decapitation, brains of animals were quickly dissected out. The cerebellums and brainstems were discarded, the remaining right and left hemispheres were separated, and the wet weight of each hemisphere was measured using a high-precision analytical balance (AE 50, Mettler, Greifensee, Switzerland). The tissues were then completely dried in a desiccating oven at 100°C for 48 h and then the dry weight of each hemisphere was measured. The percentage of brain water content is calculated for each hemisphere using the following formula: ((wet weight−dry weight)/wet weight) × 100.
Western Blotting
To analyze the expressions of the BBB components, Western blotting was performed. The whole brains of animals were removed and the dissected right or left somatosensory cortices were homogenized in an ice-cold RIPA buffer (Sigma). The homogenates were then centrifuged at 15,000 g at 4°C for 10 mins and the supernatant fraction was collected. The protein contents were determined by the Bio-Rad Protein Assay (Hercules, CA, USA). After boiling in Laemli's buffer (Bio-Rad) for 10 mins, 5 μg of protein samples per lane were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto polyvinylidene difluoride membranes (Bio-Rad). The membranes were then incubated with blocking buffer (0.1 mol/L PBS, 0.05% Tween-20, 5% non-fat dry milk) at room temperature for 1 h and then probed with primary antibodies, mouse anti-β-actin (1:400,000, Sigma), mouse anti-GFAP (1:400,000, Sigma), mouse anti-type IV collagen (1:50,000, Chemicon), rabbit anti-ZO-1 (1:20,000, Zymed, Carlsbad, CA, USA), mouse anti-occludin (1:50,000, Zymed), or mouse anti-claudin-5 (1:50,000, Zymed), at 4°C overnight. After rinses with wash buffer (0.1 mol/L PBS, 0.05% Tween-20), the blots were probed with horseradish peroxidase-conjugated secondary antibodies at room temperature for 1 h and then revealed by Amersham ECL Advance Western Blotting Detection Kit (RPN2135, GE Healthcare, Buckinghamshire, UK). The immunoblots were quantitated using a BioSpectrum AC imaging system (UVP, Upland, CA, USA) combined with VisionWorksLS software (UVP, Upland, CA, USA).
Image and Data Analyses
All quantifications were performed by an individual masked to the treatment groups. To enable equal comparison, care was taken to ensure that both images of injured and contralateral somatosensory cortices were acquired using the same brightness and exposure settings. Image-Pro Plus software (Media Cybernetics, Silver Spring, MD, USA) was used to analyze digital photographs of histochemical and immunohistochemical sections. All data were presented as mean±standard error of the mean (s.e.m.). Statistical significance was determined by one-way analysis of variance followed by Tukey's post hoc test, using Prism 4.03 software (GraphPad, San Diego, CA, USA).
Results
Primary Somatosensory Cortex Revealed by Cytochrome Oxidase Reaction
As this study was focused on the primary somatosensory cortex, the compressed region needed to be verified. One principal hallmark of the primary somatosensory cortex is the cellular organization of layer IV. Through cytochrome oxidase reaction, stellate cells in layer IV of the barrel field were observed to form a series of barrels (Figures 1A and 1B, black arrowheads), which corresponds to the topographic distribution of the contralateral whiskers. In forelimb and hindlimb regions, although stellate cells did not form barrels, layer IV was still discernible (white arrowheads).
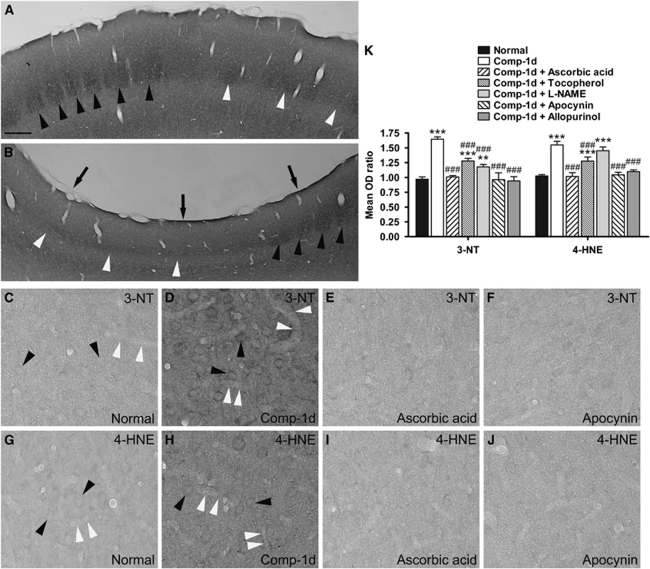
Figure 1.
Photomicrographs from coronal sections of the primary somatosensory cortices processed with cytochrome oxidase reaction, 3-nitrotyrosine (3-NT), or 4-hydroxynonenal (4-HNE) immunolabeling. (A and B) Show the contralateral (left) and compressed (right) sides, respectively, of the same section from one representative rat at 1 day after surgery. The staining technique revealed the barrels (black arrowheads) in layer IV of the somatosensory cortex, which is the topographic area of the contralateral whiskers. Layer IV, contiguous to the barrel field, represents the forelimb and hindlimb area (white arrowheads), which did not form barrels but is still evident. Physical compression caused a concave (arrows in B) on the somatosensory cortex and decreased the cortical thickness (compare A with B). (C–J) Show the immunoreactivities of 3-NT (C–F) and 4-HNE (G–J) in the right somatosensory cortex of a representative normal rat (C and G) and rats that received cortical compressin (D and H), cortical compression and ascorbic acid injection (E and I), or cortical compression and apocynin injection (F and J), and survived for 1 day after surgery. Compared with the normal rat (C and G), immunoreactivities of 3-NT and 4-HNE in stellate cells (black arrowheads) and microvessel walls (white arrowheads) were apparently increased after compression (D and H). (K) Shows the mean OD ratios of 3-NT and 4-HNE immunoreactivity in the total somatosensory cortical areas. Mean±s.e.m., n=4 per group, d=day. **P<0.01, ***P<0.001, compared with the normal group; ###P<0.001, compared with the animal group surviving for 1 day after compression (Comp-1d). Scale bar=300 μm (A and B) or 25 μm (C–J).
Oxidative Stress Induced by Cortical Compression
We first investigated whether compression of the primary somatosensory cortex elicits oxidative stress. We found that compared with the normal animals (Figures 1C and 1G), the expressions of 3-NT and 4-HNE were markedly elevated in layer IV of compressed somatosensory cortices (P<0.001) at 1 day after compression (Figures 1D, 1H, and 1K). The increase in both biomarkers was seen in stellate cells (black arrowheads), microvessel walls (white arrowheads), and neuropils. The expressions of both biomarkers in the contralateral (left) somatosensory cortices of compressed animals were not significantly different from those of normal animals (data not shown). These results indicate that epidural compression rapidly enhanced oxidative stress in the underlying somatosensory cortex. We next examined whether antioxidants could block the oxidative stress caused by cortical compression. We found that treatment of ascorbic acid or α-tocopherol significantly prevented increases in 3-NT and 4-HNE at 1 day after compression (P<0.001), although ascorbic acid seems to be more effective than α-tocopherol (Figures 1E, 1I, and 1K). Treatment of -NAME significantly prevented the increase in 3-NT (P<0.001) but not in 4-HNE (Figure 1K).
Behavioral and Electrophysiological Tests
The von Frey behavioral test was used to assess the sensory function of animals. For quantification, the mean threshold ratios were calculated as mentioned in ‘Materials and methods' section. In normal rats, the mean threshold ratios obtained by stimulation on whisker pads, forepaws, and hindpaws were all 1 (Figure 2A), indicating that touch sensitivity was not different between both sides of normal animals. In contrast, the mean threshold ratios all abruptly increased at 1 day after compression, no matter which body region was tested (Figure 2A). However, as the survival time of rats prolonged, the ratios were apparently reduced. In addition, we found that treatment of ascorbic acid, α-tocopherol, or -NAME significantly prevented whisker pad sensory impairment at 1 day after compression (P<0.001), but it is noteworthy that ascorbic acid or α-tocopherol was more effective than -NAME. Similar results were also seen in the forepaw and hindpaw.
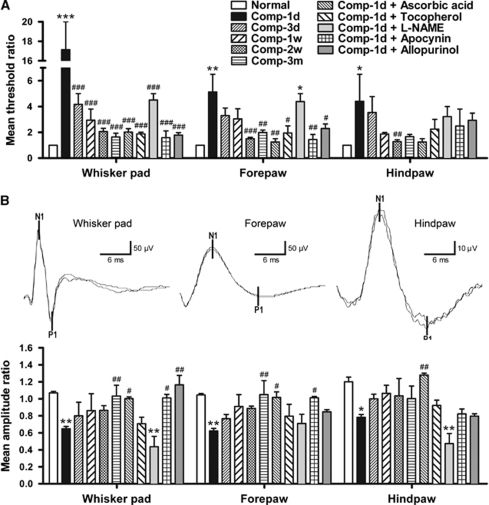
Figure 2.
Time-dependent changes in the mean threshold ratios of the von Frey behavioral test (A) and the mean amplitude ratios of the somatosensory evoked potentials (SSEPs) (B) from normal animals and animals subjected to compression of the right somatosensory cortices. The upper panels in B were example SSEP waveforms recorded from a normal animal. The N1-P1 component was used to analyze the peak-to-peak amplitude. Mean±s.e.m., n=4 per group, d=day, w=week, m=month. *P<0.05, **P<0.01, ***P<0.001, compared with each corresponding normal; #P<0.05, ##P<0.01, ###P<0.001, compared with each corresponding animal group surviving for 1 day after compression (Comp-1d).
We also used electrophysiological techniques to confirm the behavioral results. In normal animals, the mean amplitude ratios of SSEP were 1.07±0.02 for the whisker pad, 1.05±0.02 for the forepaw, and 1.20±0.06 for the hindpaw (Figure 2B). At 1 day after cortical compression, the mean amplitude ratios were dramatically reduced to 0.65±0.03 for the whisker pad (P<0.01), 0.62±0.03 for the forepaw (P<0.01), and 0.78±0.03 for the hindpaw (P<0.05). In contrast, starting at 3 days after compression, the mean amplitude ratios for all three stimulation sites gradually recovered. We also examined the effect of the drugs we used on the SSEP performance. We found that in contrast to the mean amplitude ratios 0.62 to 0.78 at 1 day after compression, treatment of ascorbic acid maintained the ratios at 1.00 to 1.28 (Figure 2B, compare Comp-1d with Comp-1d+ascorbic acid). In contrast, the effect of α-tocopherol was not prominent and -NAME failed to prevent the compression-induced decrease in SSEP amplitudes. Treatment of normal animals with antioxidants or vehicles and treatment of compressed animals with vehicles had no significant effect on the behavioral and electrophysiological performance (data not shown).
Morphological Alterations of Cortical Microvasculature
We next used alkaline phosphatase histochemistry to illustrate the cortical microvessels in the primary somatosensory cortices to evaluate their morphological alterations in response to cortical compression (Figure 3). Microvessels in layers I to IV and in layers V to VI were analyzed separately as implanted beads might exert pressure of varying degrees on these two compartments. To quantify this, high-power images were analyzed with an Image-Pro Plus analysis system. We found that in normal animals, the average diameter of microvessels in layers I to IV of the somatosensory cortex was 7.70 μm, ranging from 3.57 to 23.04 μm. In layers V to VI, the average was 7.61 μm, ranging from 3.85 to 25.33 μm. We also measured the area occupied by individual microvessels in cortical sections. In layers I to IV of normal animals, the average area was 267.78 μm2, ranging from 5.10 to 2,225.51 μm2. In layers V to VI, the average was 252.10 μm2, ranging from 13.78 to 2,578.57 μm2. We further calculated the mean diameter ratios and the mean area ratios (Figures 3K and 3L) as mentioned in ‘Materials and methods' section. At 1 day after compression, the mean diameter ratio and the mean area ratio in layers I to IV were significantly reduced to 0.71 (P<0.05) and 0.57 (P<0.05), respectively. Those in layers V to VI were also reduced to 0.79 (P<0.05) and 0.65 (P>0.05), respectively. During the 3 days to 3 months post-compression, both ratios were markedly restored in both layers I to IV and V to VI. In addition, treatment of ascorbic acid or α-tocopherol, rather than -NAME, prohibited the decrease of both ratios.
Figure 3.
The cortical microvasculature, revealed by alkaline phosphatase histochemistry, in the coronal sections of the normal and the compressed somatosensory cortices. Micrographs were taken from the left (contralateral) and the right (lesion) sides of the same sections of representative normal rats (A and B) and rats surviving for 1 day (C and D), 3 days (E and F), 1 week (G and H), and 2 weeks (I and J) after surgery. The vertical lines on the top of the figure depict the boundaries between cortical layers IV and V in all panels. (K and L) Show the time-dependent changes in the mean diameter ratios and the mean area ratios of cortical microvessels. Mean±s.e.m., n=4 per group, d=day, w=week, m=month. *P<0.05, compared with each corresponding normal; #P<0.05, ##P<0.01, ###P<0.001, compared with each corresponding animal group surviving for 1 day after compression (Comp-1d). Scale bar=300 μm.
Furthermore, we examined whether the observed morphological alterations of cortical microvessels could lead to cerebral infarct. Through 2,3,5-triphenyltetrazolium chloride staining, no ischemic insult was observed in response to cortical compression at 1 day, 3 days (Figures 4A–4D), and any other time point we examined (data not shown).
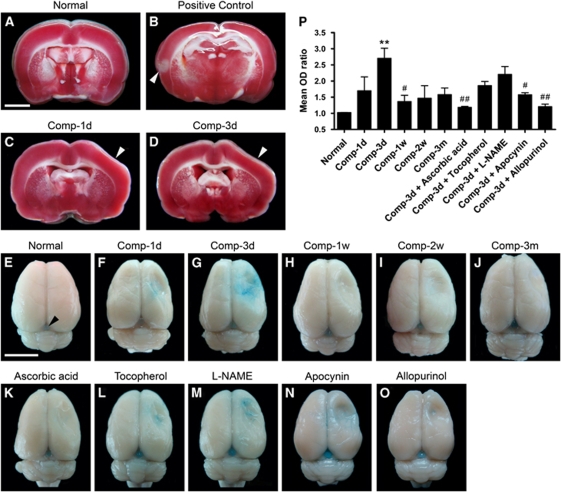
Figure 4.
Effects of sustained cortical compression on the 2,3,5-triphenyltetrazolium chloride staining and the Evans Blue extravasation. (A) Shows a representative 2,3,5-triphenyltetrazolium chloride-stained coronal slice from a normal rat. (B) Shows a 2,3,5-triphenyltetrazolium chloride-stained slice from a rat that received a permanent ligation of only a branch of the middle cerebral artery (to reduce the suffering of the rat) and survived for 1 day after surgery. Note that the ischemic sign (loss of red stain) was detected in this slice (arrowhead). (C and D) Show representative 2,3,5-triphenyltetrazolium chloride-stained slices from the rats surviving for 1 day and 3 days after compression. (E–O) Are representative whole brains from different animal groups receiving Evans Blue perfusion. In the normal animal (E), no Evans Blue was seen in the tissue except in the pineal gland (arrowhead). (P) Shows the time-dependent changes in the mean OD ratios of extravasated Evans Blue. Mean±s.e.m., n=4 per group, d=day, w=week, m=month. **P<0.01, compared with the normal animal group; #P<0.05, ##P<0.01, compared with the animal group surviving for 3 days after compression (Comp-3d). Scale bars=3 mm (A–D) and 10 mm (E–O).
BBB Permeability
The rationale for these experiments was to determine whether and to what extent cortical compression affects the BBB permeability of cortical microvessels. In normal animals, no Evans Blue extravasation was detected in the somatosensory cortices, as shown in Figure 4E. In contrast, the pineal gland, which is located between the cerebrum and cerebellum, was stained and could serve as a positive control (Figure 4E, arrowhead), as it is devoid of the BBB. In the compressed somatosensory cortices, Evans Blue extravasation was observed starting at 1 day, peaking at 3 days, but apparently decreasing at the remaining time points (Figures 4F–4J and 4P). In addition, the Evans Blue extravasation as a result of injury was attenuated in rats treated with vitamins or -NAME, but the statistical significance was shown only in the case of ascorbic acid (P<0.01) (Figures 4K–4M and 4P).
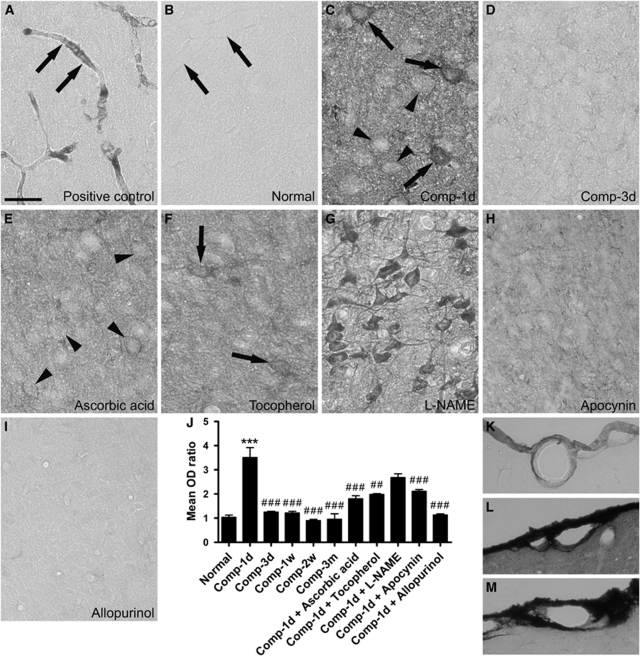
Evans Blue is an exogenous tracer and the possibility that it might directly act on the BBB could not be entirely ruled out. To further substantiate the notion that the BBB breakdown is attributable to cortical compression, we also measured the endogenous IgG extravasation as IgG is normally confined within cortical microvessels and virtually absent from brain parenchyma (Figures 5A and 5B). At 1 day after compression, IgG leakage into the brain parenchyma soon reached a maximum (P<0.001), but declined rapidly at the remaining time points (P<0.001) (Figures 5C, 5D, and 5J). Treatment of ascorbic acid or α-tocopherol significantly prevented IgG extravasation at 1 day after compression (P<0.01) (Figures 5E, 5F, and 5J). Interestingly, after treatment with -NAME, the leaked IgG was observed to concentrate on the cortical neurons (Figure 5G). In addition, we also observed IgG immunoreactivity in the pial vessels superficial to the somatosensory cortices (Figures 5K and 5L). We found that contrary to the normal rats (Figure 5K), intensive immunolabeling appeared in the vessel wall and the neighboring meninges at 1 day after compression (Figure 5L). Moreover, the IgG extravasation from the pial vessels was much more drastic than that from the parenchymal vessels within the cortical tissue (Figure 5L). This suggests that physical compression differentially modulated the permeability of pial and parenchymal vessels.
Figure 5.
Endogenous IgG extravasation after cortical compression. (A) Shows a representative image of the somatosensory cortex from a normal rat. The rat was not perfused with saline, which can clear the content of vasculature and thus the serum IgG remains in the microvessels (arrows). (B) In contrast, a normal rat received saline perfusion, and therefore the IgG immunostaining is negative in the microvessels (arrows). (C) Cortical compression led to drastic IgG leakage into the brain parenchyma at 1 day after surgery. The immunoreactivity was also present within the cytoplasm of layer IV neurons (arrowheads) and some of them were intensely stained (arrows). (D) At 3 days after compression, the IgG immunoreactivity was abruptly decreased in the brain parynchyma. (E, F, H, and I) Treatment of ascorbic acid, α-tocopherol, apocynin, or allopurinol markedly blocked the IgG leakage at 1 day after compression, although some neurons were still immunostained (arrowheads in E and arrows in F). (G) In contrast, treatment of -NAME caused the extravasated IgG to concentrate on the cortical neurons. (J) Shows the time-dependent changes in the mean OD ratios of IgG immunoreactivity in the total somatosensory cortical areas. Mean±s.e.m., n=4 per group, d=day, w=week, m=month. ***P<0.001, compared with the normal group; ##P<0.01, ###P<0.001, compared with the animal group surviving for 1 day after compression (Comp-1d). (K–M) Show the IgG immunoreactivity in the pial vessels dorsal to the somatosensory cortices of a normal rat (K), a rat surviving for 1 day after compression (L), and a rat that received cortical compression and allopurinol injection and survived for 1 day after compression (M). Scale bar=25 μm (A–I) or 50 μm (K–M).
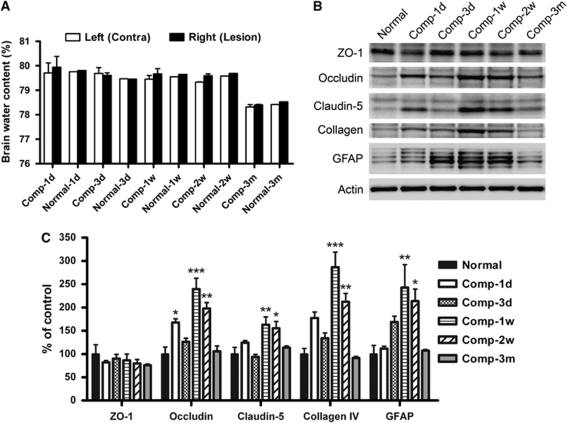
We further investigated whether the BBB breakdown observed can cause brain edema. We found that the brain water content in normal adult male Sprague–Dawley rats was 78% to 80% (Figure 6A). Sustained compression of the primary somatosensory cortex has no significant effect on percentage in all of the animals that we examined.
Figure 6.
Analyses of the brain water content and the expressions of the BBB components after cortical compression. (A) To exclude the aging factor, each group of compressed (Comp) animals (n=4) was accompanied by a group of normal animals (n=2). The brain water content in both the left and right cerebral hemispheres of all normal rats was maintained within 78% to 80%, although a slight decrease was seen in the older rats that had survived for 3 months (Normal-3m). Sustained compression of the somatosensory cortex did not elicit significant alteration of the percentage at all time points, relative to each corresponding normal. (B and C) Representative Western blots and summary data from the normal and the compressed somatosensory cortices. β-Actin served as the internal control. Mean±s.e.m., n=4 per group, d=day, w=week, m=month. *P<0.05, **P<0.01, ***P<0.001, compared with each corresponding normal.
Expression of BBB Components
To investigate the molecular basis for the BBB breakdown and restoration observed, the cortical tissue was extracted and immunoblotted. We found that the expression of ZO-1 in the compressed somatosensory cortex showed no or negligible alteration at any given time point (Figures 6B and 6C). At 1 day, sustained cortical compression gave rise to the upregulation of occludin, claudin-5, type IV collagen, and GFAP in the affected cortical region, but the statistical significance was shown only in occludin (P<0.05) (Figures 6B and 6C). In contrast, the immunoreactivities of these BBB components were drastically and significantly enhanced at 1 and 2 weeks after compression but returned to the normal level at 3 months.
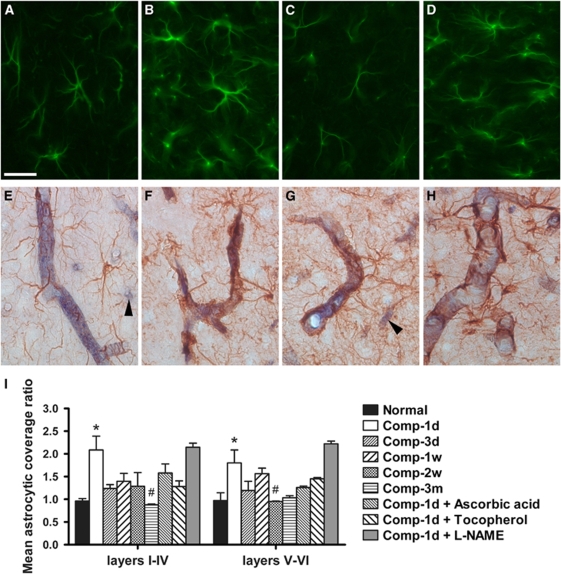
Astrocytic Reaction
To elucidate the specific role of astrocytes in BBB modulation, we used morphological methods to examine the astrocytic reaction after cortical compression. Consistent with the GFAP immunoblotting results, the astrocytic reaction revealed by immunofluorescence was obviously increased both in layers I to IV and V to VI of the compressed somatosensory cortex at 1 week after compression, relative to the corresponding contralateral cortical region (Figures 7A–7D). In contrast, the astrocytic coverage on cortical microvessels was significantly increased only at 1 day after compression (P<0.05) (Figures 7E–7I). Treatment of ascorbic acid or α-tocopherol apparently prevented the increase in astrocytic coverage at 1 day after compression, but this was not statistically significant (Figure 7I). The astrocytic coverage was unchanged after treatment of -NAME (compare Comp-1d with Comp-1d+-NAME).
Figure 7.
Astrocytic reaction and coverage on the microvasculature after cortical compression. (A–D) Are representative immunofluorescent micrographs showing the astrocytic reaction in layer IV (A and B) and layer V (C and D) of the somatosensory cortices. In contrast to the contralateral cortex (A and C), astrocytic reaction was markedly upregulated in the compressed cortex at 1 week after surgery (B and D). (E–H) Are representative micrographs double-labeled with the GFAP immunohistochemistry to reveal astrocytes and the alkaline phosphatase histochemistry to reveal cortical microvessels. In contrast to the normal rats (E), astrocytic coverage on the cortical microvessels was abruptly increased at 1 day after compression, regardless of the size of the blood vessels (F–H). The tiny microvessels with a diameter less than 5 μm (arrowheads in E and G) were difficult to analyze and not included in the quantification. (I) Shows the time-dependent changes in the mean astrocytic coverage ratios. Mean±s.e.m., n=4 per group, d=day, w=week, m=month. *P<0.05, compared with each corresponding normal; #P<0.05, compared with each corresponding animal group surviving for 1 day after compression (Comp-1d). Scale bar=25 μm.
The Source of Oxidative Stress
On the basis of the above experimental results, the antioxidant vitamin ascorbic acid was a potent drug to prevent BBB disruption and sensory deficit caused by compression of the primary somatosensory cortex. This prompted us to find out the source of the ROS involved in the compression-induced pathophysiological responses. The major ROS superoxide is a natural by-product of mitochondrial respiration that is also generated by xanthine oxidase, NADPH oxidase, and cyclooxygenase (Halliwell, 1994). Thus, we used the NADPH oxidase inhibitor apocynin and the xanthine oxidase inhibitor allopurinol to treat animals. We found that treatment of apocynin or allopurinol significantly prevented increases in 3-NT and 4-HNE in the affected cortical region (Figures 1F, 1J, and 1K) and the sensory deficits detected in the whisker pads and forepaws (Figures 2A and 2B) at 1 day after compression. Although both drugs had no conspicuous effect on the permeability of pial vessels (compare Figures 5L with 5M), they significantly prohibited the BBB disruption after cortical compression, shown by Evans Blue (Figures 4N–4P) and IgG (Figures 5H–5J) quantifications. These results suggest that both xanthine oxidase and NADPH oxidase were implicated in the pathological signs caused by cortical compression.
Discussion
In previous studies, short-term compression (less than 2 h) has been applied on the cerebral cortex through a Plexiglas piston or brass or plastic cylinder to intentionally lower the cortical surface approximately 3 mm, thereby inducing a decrease in cerebral blood flow and, in turn, ischemia (Burnett et al, 2005; Kundrotiene et al, 2002; Watanabe et al, 2001). These studies were focused mainly on ischemia-induced brain damage. In contrast, the plastic bead used herein was an optimal compression tool for the current investigation, which ensured the long-term (up to 3 months) and persistent compression of the somatosensory cortex when the animals were unrestrained in the cage. The thickness of 1.5 mm of the plastic beads was used as, in preliminary experiments, 2-mm thick beads implantation produced a localized cortical infarct, evidenced by coagulated necrosis and cell damage starting at 3 days after compression and 1-mm thick beads did not cause any Evans Blue and IgG extravasation (data not shown). Although cortical compression through 1.5-mm thick beads transiently reduced the size of cortical microvessels (Figures 3K and 3L), no substantial ischemia-induced brain tissue damage or cell death was found on the basis of the results of 2,3,5-triphenyltetrazolium chloride stain, caspase-3 immunoblotting, and H&E stain (data not shown). Therefore, by the present surgery paradigm, the investigation of the effects of long-term cortical compression on the cortical microvessels and the BBB could be achieved.
In this study, we show for the first time that sustained epidural compression on the primary somatosensory cortex induced only transient BBB breakdown, evidenced by IgG and Evans Blue extravasation. As shown in Figure 5, IgG extravasation peaked at 1 day but almost completely vanished at 3 days after compression. In contrast, the BBB showed more prolonged permeability to the exogenous tracer Evans Blue (960 Da), which is a marker for serum albumin and was observed to extravasate maximally at 3 days after compression (Figure 4). These results suggest that the degree of the BBB opening was large enough to permit both the macromolecules albumin and IgG to pass, starting before 24 h post-compression. Nevertheless, the BBB tightness partially recovered to some extent on the third day, as a result of which the larger molecule IgG (150 kDa) extravasation resolved but the smaller-sized albumin (67 kDa) leakage into brain parenchyma was still evident. However, in any case, the BBB integrity was completely restored before 1 week post-compression as shown by the observation that both serum proteins stopped leaking from cortical microvessels. It suggests that the BBB compromise could be rapidly reversed after cortical compression and this may help prevent the brain edema. The repair of the BBB may begin at as early as the third day after compression as IgG extravasated maximally at 1 day but nearly disappeared at 3 days. We recently explored the effect of decompression with preceding 1-week compression of somatosensory cortex as the removal of compressive mass is a common clinical practice (Chen et al, 2004). Interestingly, we found that decompression also elicited short-lasting BBB disruption and sensory deficits in the early stage (unpublished). Together, these findings indicate that the BBB is a dynamic structure that could be rapidly modulated.
Another intriguing finding of this study is that the leaked IgGs accumulated not only in the neuropil but also within the cytoplasm of many cortical neurons at 1 day after compression. The uptake of IgG or other serum proteins by neurons has been implicated in the neuronal dysfunction, which is associated with epilepsy, abnormal cortical development, and neurodegenerative disease (Hallene et al, 2006; Orr et al, 2005; Rigau et al, 2007). Consistent with these reports, we found that persistent compression on the somatosensory cortex also caused the impairment of somatic sensation, which was concurrent with the breakdown of the BBB and the neuronal accumulation of IgG. Collectively, our results suggest that the leaked serum proteins owing to the BBB disruption may directly enter the cortical neurons and thus interfere with the neuronal function after cortical compression. However, to our surprise, the leaked serum proteins could be rapidly cleared from the neuropil and the cortical neurons (compare Figures 5C with 5D). This may help facilitate the recovery of neuronal function. Indeed, the sensory deficit owing to sustained compression of the somatosensory cortex was found to occur only during the initial several days and resolve soon within 2 weeks. This rapid adaptation may involve the regulation of thalamocortical connections. We recently found that thalamocortical fibers did not withdraw from layer IV after cortical compression (unpublished). Furthermore, cortical compression caused a decreased expression of glutamate receptor subunits and an increased expression of γ-aminobutyric acid receptor subunits in layer IV stellate cells only during the initial several days after compression (unpublished). These findings may explain the rapid adaptation of somatic sensation in response to sustained compression of the primary somatosensory cortex.
All of the BBB components have been reported to participate in the regulation of the BBB permeability, such as tight junctions of endothelial cells, astrocytes, pericytes, and the basal lamina (Abbott et al, 2006; Colgan et al, 2007; Muellner et al, 2003). Among these, the expression of tight junction proteins is considered to be essential to the BBB integrity as claudin-5-deficiency has been shown to result in BBB compromise (Nitta et al, 2003). However, in this study, although the expressions of ZO-1, occludin, and claudin-5 were maintained or upregulated at 1 day after compression (Figure 6C), the IgG extravasation was most drastic at this time (Figure 5J). It means that the maintained or increased expression of tight junction proteins is not sufficient to guarantee the BBB integrity. In our model, despite no ischemic signs, cortical compression significantly reduced the size of the cortical microvessels. This may cause the alteration of the blood flow-associated hemodynamic forces such as shear stress and cyclic strain on the vascular endothelium, which have been evidenced to activate integrin and Rac1, to decrease the occludin tyrosine phosphorylation and increase the ZO-1 serine/threonine phosphorylation, and to modulate the expression, association, and distribution of occludin and ZO-1 (Colgan et al, 2007; Collins et al, 2006; Tzima et al, 2002). Moreover, increasing evidences have suggested that the BBB permeability is strictly regulated by the redistribution of tight junction proteins between the cell membrane and the cytoplasmic fraction of endothelial cells, and the underlying mechanism involves the phosphorylation of tyrosine, serine, or threonine in the tight junction proteins (Andras et al, 2007; Elias et al, 2009). Interestingly, we recently found that cortical compression elicited the alteration of phosphorylation status of tight junction proteins in the early period (unpublished). Thus, it is likely that the redistribution, rather than the total expression, of ZO-1, occludin, and claudin-5, predominantly affects the BBB permeability within the initial several days in our model. This may also be a reasonable cause for the rapid reverse of the BBB compromise as mentioned above. In contrast, the expressions of occludin, claudin-5, type IV collagen, and GFAP were dramatically increased at 1 and 2 weeks after compression. This may be beneficial to the BBB integrity as the BBB tightness recovered entirely during this period. Taken together, our results are consistent with the idea that the redistribution of tight junction proteins may predominantly underlie the BBB compromise in the early stages after compression. On the contrary, the recovery of the BBB tightness may be contributed and accelerated by both the redistribution and enhanced total expression of the BBB component proteins. However, the deduction needs to be further investigated.
In this study, the astrocytic reaction was also found to be biphased. The early phase was 1 to 3 days after compression when the total expression of GFAP in the somatosensory cortex was only slightly elevated but the astrocytic coverage on the cortical microvessels was largely increased. The late phase was 1 to 2 weeks after compression when the total expression of GFAP was drastically enhanced, but the astrocytic coverage on the cortical microvessels returned to near the normal level. Astrocytes are well known to enwrap brain capillaries by their endfeet and to promote the formation and maintenance of the BBB through intercellular contact, diffusible factors, or GFAP (Abbott et al, 2006; Reuss et al, 2003). It is also well established that water channel aquaporin-4 is highly expressed in the hypertrophied perivascular astrocytic processes, which help clear the vasogenic edema because of the BBB disruption (Tomas-Camardiel et al, 2005). Thus, in our model, the increased astrocytic coverage on cortical microvessels in the early phase may help prevent brain edema. On the contrary, the elevated astrocytic reaction in the late phase may trigger an increase in tight junction protein expressions as astrocytes have been shown to enhance the occludin protein expression and BBB integrity in vitro (Colgan et al, 2008). Together, all of the above results and considerations support the deduction that astrocytes may have distinct roles in the early and late phases in our model.
In addition, we show for the first time that the systemic administration of ascorbic acid or α-tocopherol, rather than -NAME, prevented the BBB disruption caused by cortical compression. Under physiologic conditions, ascorbic acid and α-tocopherol function as a potent ROS scavenger, thereby prohibiting the oxidation of lipid, protein, and DNA. In contrast, -NAME is a competitive NO synthase inhibitor that can retard the production of NO. In this regard, ROS seems to have a more important role than reactive nitrogen species in the BBB disruption in our model. Superoxide, one of the major ROSs, has been shown to regulate the endothelial tight junction proteins and increase the BBB permeability through RhoA, phosphatidylinositol 3 kinase (PI3 kinase), and the protein kinase B (PKB/Akt) signaling pathway (Schreibelt et al, 2007; Sheth et al, 2003). Activation of protein tyrosine kinase by superoxide has also been implicated in dissociation of occluding–ZO-1 and E-cadherin–β-catenin complexes from the cytoskeleton (Rao et al, 2002). In addition, direct treatment of 4-HNE or hydrogen peroxide to brain endothelial cells has been shown to impair the BBB integrity (Lee et al, 2004; Mertsch et al, 2001). In this study, both the NADPH oxidase inhibitor apocynin and the xanthine oxidase inhibitor allopurinol prevented the BBB disruption, which is consistent with the findings from other laboratories that these two oxidases contribute to the BBB dysfunction (Kahles et al, 2007; Schreibelt et al, 2007; Sheth et al, 2003). Collectively, these results indicate that ROSs have a detrimental role in the BBB integrity. In addition, with regard to reactive nitrogen species, we found that the treatment of NO synthase inhibitor -NAME reduced the extravasation of Evans Blue and IgG, despite no statistical significance (Figures 4P and 5J). However, ascorbic acid, which protected the BBB in our model, has been shown to be capable of increasing the NO production and bioactivity without affecting the NO synthase expression (Heller et al, 2001; Huang et al, 2000). Actually, up to now, a number of reports on the role of NO in the BBB modulation are also contradictory (Boje and Lakhman, 2000; Kuhlmann et al, 2006; Wong et al, 2004). Thus, NO and/or its derivatives may be a double-edged sword in the BBB modulation. Conversely, the oxidative stress-mediated effect on sensory deficit caused by compression of somatosensory cortex may not need to occur indirectly by influencing on the BBB but instead directly on the layer IV neurons. Indeed, we found that both 3-NT and 4-HNE were markedly upregulated in stellate cells and neuropils after compression. 4-HNE is a highly reactive aldehyde derived from lipid peroxidation because of superoxide and its derivatives, and 3-NT is produced through tyrosine nitration by peroxynitrite, a combined product of superoxide and NO (Choi et al, 2007). Thus, it is reasonable that both apocynin and allopurinol, which can block superoxide production, prevented the upregulation of 3-NT and 4-HNE in response to cortical compression in our model. Taken together, in this study, the cortical compression may cause sensory deficit through two routes. First, cortical compression elicits high oxidative stress (and/or hemodynamic forces as mentioned above) on microvessels, leading to disruption of the BBB, accumulation of serum proteins in cortical neurons, and eventually dysfunction of neurons. Second, cortical compression directly elicits high oxidative stress in cortical neurons and neuropils and thus impairs their function.
In conclusion, this study provides direct evidence that sustained but moderate compression of the rat somatosensory cortex impairs the BBB integrity and somatic sensation only in the early period. Oxidative stress may be implicated in these pathophysiological changes and ascorbic acid may be used therapeutically to modulate cortical compression complicated by the BBB dysfunction. In addition, the animal model of this study could serve as a convenient and effective tool for further investigation of the molecular mechanisms underlying the relationship between the BBB disruption and neuronal function.
Acknowledgments
This work was supported by a grant from the National Science Council of Taiwan (NSC 97-2320-B-320-005-MY2) and a grant from Tzu Chi University (TCIRP 95003-04) to Pei-Hsin Liu.
The authors declare no conflict of interest.
References
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Andras IE, Deli MA, Veszelka S, Hayashi K, Hennig B, Toborek M. The NMDA and AMPA/KA receptors are involved in glutamate-induced alterations of occludin expression and phosphorylation in brain endothelial cells. J Cereb Blood Flow Metab. 2007;27:1431–1443. doi: 10.1038/sj.jcbfm.9600445. [DOI] [PubMed] [Google Scholar]
- Boje KM, Lakhman SS. Nitric oxide redox species exert differential permeability effects on the blood-brain barrier. J Pharmacol Exp Ther. 2000;293:545–550. [PubMed] [Google Scholar]
- Burnett MG, Detre JA, Greenberg JH. Activation-flow coupling during graded cerebral ischemia. Brain Res. 2005;1047:112–118. doi: 10.1016/j.brainres.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Chen JR, Wang YJ, Tseng GF. The effect of epidural compression on cerebral cortex: a rat model. J Neurotrauma. 2003;20:767–780. doi: 10.1089/089771503767869999. [DOI] [PubMed] [Google Scholar]
- Chen JR, Wang YJ, Tseng GF. The effects of decompression and exogenous NGF on compressed cerebral cortex. J Neurotrauma. 2004;21:1640–1651. doi: 10.1089/neu.2004.21.1640. [DOI] [PubMed] [Google Scholar]
- Choi HB, Ryu JK, Kim SU, McLarnon JG. Modulation of the purinergic P2X7 receptor attenuates lipopolysaccharide-mediated microglial activation and neuronal damage in inflamed brain. J Neurosci. 2007;27:4957–4968. doi: 10.1523/JNEUROSCI.5417-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan OC, Collins NT, Ferguson G, Murphy RP, Birney YA, Cahill PA, Cummins PM. Influence of basolateral condition on the regulation of brain microvascular endothelial tight junction properties and barrier function. Brain Res. 2008;1193:84–92. doi: 10.1016/j.brainres.2007.11.072. [DOI] [PubMed] [Google Scholar]
- Colgan OC, Ferguson G, Collins NT, Murphy RP, Meade G, Cahill PA, Cummins PM. Regulation of bovine brain microvascular endothelial tight junction assembly and barrier function by laminar shear stress. Am J Physiol Heart Circ Physiol. 2007;292:H3190–H3197. doi: 10.1152/ajpheart.01177.2006. [DOI] [PubMed] [Google Scholar]
- Collins NT, Cummins PM, Colgan OC, Ferguson G, Birney YA, Murphy RP, Meade G, Cahill PA. Cyclic strain-mediated regulation of vascular endothelial occludin and ZO-1: influence on intercellular tight junction assembly and function. Arterioscler Thromb Vasc Biol. 2006;26:62–68. doi: 10.1161/01.ATV.0000194097.92824.b3. [DOI] [PubMed] [Google Scholar]
- DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–23. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- Elias BC, Suzuki T, Seth A, Giorgianni F, Kale G, Shen L, Turner JR, Naren A, Desiderio DM, Rao R. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J Biol Chem. 2009;284:1559–1569. doi: 10.1074/jbc.M804783200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallene KL, Oby E, Lee BJ, Santaguida S, Bassanini S, Cipolla M, Marchi N, Hossain M, Battaglia G, Janigro D. Prenatal exposure to thalidomide, altered vasculogenesis, and CNS malformations. Neuroscience. 2006;142:267–283. doi: 10.1016/j.neuroscience.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence. Lancet. 1994;344:721–724. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- Heller R, Unbehaun A, Schellenberg B, Mayer B, Werner-Felmayer G, Werner ER. L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem. 2001;276:40–47. doi: 10.1074/jbc.M004392200. [DOI] [PubMed] [Google Scholar]
- Huang A, Vita JA, Venema RC, Keaney JF., Jr Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J Biol Chem. 2000;275:17399–17406. doi: 10.1074/jbc.M002248200. [DOI] [PubMed] [Google Scholar]
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahles T, Luedike P, Endres M, Galla HJ, Steinmetz H, Busse R, Neumann-Haefelin T, Brandes RP. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke. 2007;38:3000–3006. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- Kuhlmann CR, Lessmann V, Luhmann HJ. Fluvastatin stabilizes the blood-brain barrier in vitro by nitric oxide-dependent dephosphorylation of myosin light chains. Neuropharmacology. 2006;51:907–913. doi: 10.1016/j.neuropharm.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Kundrotiene J, Wagner A, Liljequist S. Extradural compression of sensorimotor cortex: a useful model for studies on ischemic brain damage and neuroprotection. J Neurotrauma. 2002;19:69–84. doi: 10.1089/089771502753460259. [DOI] [PubMed] [Google Scholar]
- Lee HS, Namkoong K, Kim DH, Kim KJ, Cheong YH, Kim SS, Lee WB, Kim KY. Hydrogen peroxide-induced alterations of tight junction proteins in bovine brain microvascular endothelial cells. Microvasc Res. 2004;68:231–238. doi: 10.1016/j.mvr.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Mayer SA, Rincon F. Treatment of intracerebral haemorrhage. Lancet Neurol. 2005;4:662–672. doi: 10.1016/S1474-4422(05)70195-2. [DOI] [PubMed] [Google Scholar]
- Mertsch K, Blasig I, Grune T. 4-Hydroxynonenal impairs the permeability of an in vitro rat blood-brain barrier. Neurosci Lett. 2001;314:135–138. doi: 10.1016/s0304-3940(01)02299-6. [DOI] [PubMed] [Google Scholar]
- Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA. 1999;96:511–516. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellner A, Benz M, Kloss CU, Mautes A, Burggraf D, Hamann GF. Microvascular basal lamina antigen loss after traumatic brain injury in the rat. J Neurotrauma. 2003;20:745–754. doi: 10.1089/089771503767869971. [DOI] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr CF, Rowe DB, Mizuno Y, Mori H, Halliday GM. A possible role for humoral immunity in the pathogenesis of Parkinson's disease. Brain. 2005;128:2665–2674. doi: 10.1093/brain/awh625. [DOI] [PubMed] [Google Scholar]
- Parathath SR, Parathath S, Tsirka SE. Nitric oxide mediates neurodegeneration and breakdown of the blood-brain barrier in tPA-dependent excitotoxic injury in mice. J Cell Sci. 2006;119:339–349. doi: 10.1242/jcs.02734. [DOI] [PubMed] [Google Scholar]
- Rao RK, Basuroy S, Rao VU, Karnaky KJ, Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J. 2002;368:471–481. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss B, Dono R, Unsicker K. Functions of fibroblast growth factor (FGF)-2 and FGF-5 in astroglial differentiation and blood-brain barrier permeability: evidence from mouse mutants. J Neurosci. 2003;23:6404–6412. doi: 10.1523/JNEUROSCI.23-16-06404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigau V, Morin M, Rousset MC, de Bock F, Lebrun A, Coubes P, Picot MC, Baldy-Moulinier M, Bockaert J, Crespel A, Lerner-Natoli M. Angiogenesis is associated with blood-brain barrier permeability in temporal lobe epilepsy. Brain. 2007;130:1942–1956. doi: 10.1093/brain/awm118. [DOI] [PubMed] [Google Scholar]
- Schreibelt G, Kooij G, Reijerkerk A, van Doorn R, Gringhuis SI, van der Pol S, Weksler BB, Romero IA, Couraud PO, Piontek J, Blasig IE, Dijkstra CD, Ronken E, de Vries HE. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 2007;21:3666–3676. doi: 10.1096/fj.07-8329com. [DOI] [PubMed] [Google Scholar]
- Sheth P, Basuroy S, Li C, Naren AP, Rao RK. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J Biol Chem. 2003;278:49239–49245. doi: 10.1074/jbc.M305654200. [DOI] [PubMed] [Google Scholar]
- Tomas-Camardiel M, Venero JL, Herrera AJ, De Pablos RM, Pintor-Toro JA, Machado A, Cano J. Blood-brain barrier disruption highly induces aquaporin-4 mRNA and protein in perivascular and parenchymal astrocytes: protective effect by estradiol treatment in ovariectomized animals. J Neurosci Res. 2005;80:235–246. doi: 10.1002/jnr.20443. [DOI] [PubMed] [Google Scholar]
- Tzima E, Del Pozo MA, Kiosses WB, Mohamed SA, Li S, Chien S, Schwartz MA. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J. 2002;21:6791–6800. doi: 10.1093/emboj/cdf688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SM, Tsai HP, Huang JJ, Huang HC, Lin JL, Liu PH. Inhibition of nitric oxide synthase promotes facial axonal regeneration following neurorrhaphy. Exp Neurol. 2009;216:499–510. doi: 10.1016/j.expneurol.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Hoffman JR, Craik RL, Hand PJ, Croul SE, Reivich M, Greenberg JH. A new model of localized ischemia in rat somatosensory cortex produced by cortical compression. Stroke. 2001;32:2615–2623. doi: 10.1161/hs1101.097384. [DOI] [PubMed] [Google Scholar]
- Wong D, Dorovini-Zis K, Vincent SR. Cytokines, nitric oxide, and cGMP modulate the permeability of an in vitro model of the human blood-brain barrier. Exp Neurol. 2004;190:446–455. doi: 10.1016/j.expneurol.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci. 2007;27:10240–10248. doi: 10.1523/JNEUROSCI.1683-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]