Abstract
Traumatic brain injury (TBI) results in reduced cerebral blood flow (CBF) and low levels of the vasodilator nitric oxide (NO) may be involved. Arginase II negatively regulates NO production through competition for the substrate -arginine. We determined whether arginase II-deficient (ArgII−/−) mice would show improved CBF after TBI through arterial spin-labeling magnetic resonance imaging (MRI). The ArgII−/− mice exhibit a significantly improved CBF recovery after trauma in the perilesional brain (P=0.0015) and in various other brain regions. In conclusion, arginase II deficiency leads to a better CBF recovery after TBI and implicates arginase II in hemodynamic processes.
Keywords: arginase II, CBF, MRI, NO, TBI
Introduction
Traumatic brain injury (TBI) results in vascular changes that reduce cerebral blood flow (CBF). Although the exact mechanisms involved in the CBF reduction are still under investigation, alterations of endothelial nitric oxide (NO) metabolism have been implicated (Golding et al, 1999).
Nitric oxide is a vasodilator produced by NO synthases (NOSs) from -arginine. Nitric oxide derived from endothelial and neuronal NOS (eNOS and nNOS) contributes to the regulation of vascular tone. Nitric oxide is known to be involved in the pathophysiology of TBI and has a distinct temporal expression after TBI. Within minutes of trauma, NO levels in injured brain transiently increase above baseline, then decrease and remain below baseline values for hours to days. Constitutive NOS activity and CBF follow a similar trend (Cherian et al, 2004). Administration of -arginine, an NOS substrate, in rodent TBI models results in the restoration of CBF and NO levels to near baseline and decreased neurologic injury (DeWitt et al, 1997; Cherian and Robertson, 2003). -arginine treatment of wild-type (WT) mice shows a recovery of post-TBI CBF and reduced contusion volume, which is absent in eNOS-deficient transgenic mice (Hlatky et al, 2003). This suggests a critical role for eNOS-derived NO in the -arginine-induced recovery of CBF after trauma.
Recently, the two isoforms of arginase, I and II, have been implicated in the development of vascular disease and endothelial dysfunction. Arginase I is an important urea cycle component in the liver, and mitochondria-associated arginase II is found in many different cell types. Both eNOS and arginase II are found in the endothelial cells of the vascular system. The use of -arginine puts arginase II in direct competition with eNOS. Inhibition of arginase in endothelial cells stimulates NO production, whereas overexpression of arginase suppresses NO synthesis and decreases intracellular -arginine levels. Arginase II has been found to regulate vascular endothelial function and vascular tone through eNOS (reviewed by Durante et al, 2007). Shi et al (2001) have reported that arginase II-deficient mice (ArgII−/−) do not have overt abnormalities under normal physiologic conditions; however, Lim et al (2007) have reported a dampened vasocontrictory profile, whereas Huynh et al (2009) have reported a hypertensive phenotype in mice that are 8 to 15 weeks of age. The effects of arginase deficiency might be more prominent in trauma cases in which compensatory mechanisms fail. For example, eNOS-deficient mice show significantly larger CBF deficits after trauma versus WT mice, despite having normal CBF before the trauma and higher mean arterial pressure before and after TBI (Hlatky et al, 2003). The specific effects of arginase II on CBF after TBI are unknown; however, rats pretreated with the arginase-specific inhibitor Nω-hydroxy-nor-arginine showed significantly reduced contusion volume. Arginase II levels are also increased in the brain acutely after TBI in rats (Cherian et al, 2009).
Arterial spin labeling (ASL) is a magnetic resonance imaging (MRI) method, which can be used to quantify CBF noninvasively (Williams et al, 1992) and allows for repeated blood flow measurements in the same animal.
We hypothesize that competition of arginase II with eNOS for the substrate -arginine reduces NO levels and thus CBF after TBI. To determine whether arginase deficiency would increase CBF after TBI, we measured CBF using ASL in ArgII−/− and WT mice before and after TBI.
Materials and methods
All experiments were approved by the institutional Animal Protocol Review Committee of Baylor College of Medicine. Adult male arginase II knockout (ArgII−/−; kindly provided by Dr Brendon Lee, n=10) and C57Bl6/J mice (Harlan Laboratories, Indianapolis, IN, USA; n=11) weighing between 26 to 32 g were subjected to severe TBI and MRI.
Anesthesia, Ventilation, and Surgical Preparation
Preparation for TBI was performed as described by Liu et al (2002). Briefly, mice were initially anesthetized with 5% isoflurane in 100% oxygen and maintained on 2% to 3% isoflurane. Transoral intubation was carried out by exposing the trachea through a midline neck incision and observing the entry of the endotracheal tube into the trachea. The neck incision was sutured close. A volume-controlled ventilator (MiniVent Type 845, Hugo Sachs Elektronik, March-Hugstetten, Germany) was used to mechanically ventilate the mice on 2% to 3% isoflurane in oxygen for the remainder of the experiment and adjusted to obtain an end-tidal CO2 level (EtCO2) of 35 mm Hg (7 μL/g body weight—stroke volume with respiratory rate was adjusted to achieve the 35 mm Hg EtCO2 level). EtCO2 levels were monitored with a microcapnograph (Columbus Instruments, Columbus, OH, USA) during the surgery. Rectal temperature was maintained at 36°C to 37°C using a heat pad and a rectal thermistor.
Production of Brain Injury
Traumatic brain injury was induced as described by Liu et al (2002). Briefly, mice were placed in a stereotaxic frame and secured with ear and incisor bars. After hair removal, a midline cranial incision was made. A 3-mm craniectomy was carried out over the right parietal cortex with the anterior edge −0.82 mm from the bregma and 1 mm lateral of the sagittal suture. The impacter rod was positioned perpendicular to the exposed brain surface. Controlled cortical impact injury (3 m/second; 1.5 mm deformation, 100 milliseconds) was performed using a voltage-driven impactor (Benchmark Stereotaxic Impactor, myNeuroLab, St Louis, MO, USA). The wound was sutured and mice were prepared for MRI. Mice were excluded from analysis if death occurred before or during imaging. Four mice (WT n=2, ArgII−/− n=2) were excluded because of this criterion. Three sham TBI WT mice acted as controls.
Magnetic Resonance Imaging
Mice were imaged on a 9.4 Bruker Avance Biospec Spectrometer, 21-cm bore horizontal scanner with a 35-mm volume resonator (Bruker BioSpin, Billerica, MA, USA). Mice were anesthetized with 5% isoflurane and 100% oxygen and then maintained on 1% to 2% isoflurane during imaging. After TBI, mice were maintained on mechanical ventilation to avoid respiratory distress. Respiration and temperature were monitored using a respiratory pad and rectal probe, respectively (Small Animal Instruments, Stony Brook, NY, USA). The temperature was maintained at 37°C with an air-heating system. Set-up scans aligned a slice to the anterior edge of the hippocampus (Figure 1). A series of flow-sensitive alternating inversion recovery ASL scans were performed to assess the relative CBF (rCBF) at the following time points: before TBI, 15 minutes after TBI, and 1 hour after TBI. The ASL parameters were as follows: repetition time=7555.4; echo time=16.73; field of view=1.50 cm; slice thickness=1.5 mm; matrix=64 × 64; NEX=2. Selective and nonselective ASL images were acquired. Blood samples were collected after imaging for arterial blood gas measurements.
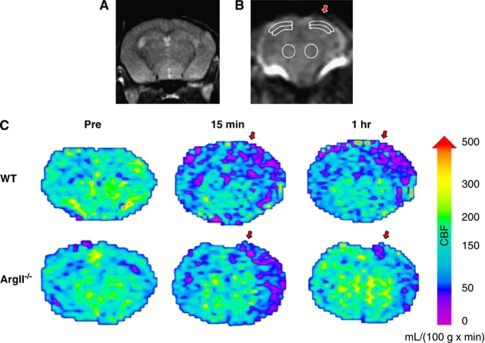
Figure 1.
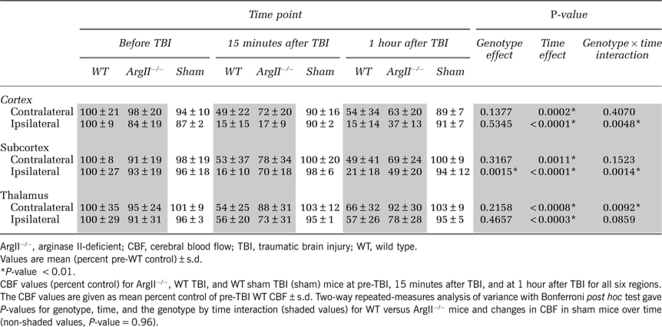
Regions of interest (ROIs) for cerebral blood flow (CBF) calculations. (A–C) Regions of interest and CBF maps for arterial spin labeling (ASL) MRI mouse brain scans. (A) Coronal brain slice used for CBF measurements. (B) Six ROIs were used to determine CBF, three from each hemisphere. Regions contralateral and ipsilateral to the traumatic brain injury (TBI) (indicated by the red arrow) were chosen in the cortex, subcortex, and striatum. (C) Cerebral blood flow map of an arginase II-deficient (ArgII−/−) mouse and a wild-type (WT) mouse before TBI, 15 minutes after TBI, and 1 hour after TBI. The ArgII−/− mice show less CBF blunting and/or better CBF recovery. Maps were created by applying the relative CBF (rCBF) equation (rCBF = λ × 60,000 × ((1/T1(selective))−(1/T1(nonselective))) to T1 maps of the brain created from the ASL scans. A color map was applied. Relative CBF units: mL/100 g/minute. Red arrows indicate the site of TBI impact.
Image Analysis
Relative CBF was calculated from changes in T1 relaxation times between selective and nonselective scans using the following equation:
where λ is the brain partition coefficient, equal to 90 mL per 100 g (Herscovitch and Raichle, 1985). Here, 60,000 is the conversion factor for T1 relaxation times from milliseconds to minutes.
Relative CBFs from six regions of interest in the cortex and thalamus ipsilateral and contralateral to the injury site were determined from a coronal brain slice (slice 8 of 18 from an anatomy scan, with the first slice at the anterior edge of the olfactory bulb) (Figure 1A). The regions of interest were copied between animals and rCBF values were converted to percent control rCBF with WT preinjury mice as the control.
T1 maps were also created. These maps were then subjected to the rCBF equation pixel by pixel to create CBF maps of the WT and ArgII−/− brain slice.
Statistics
Values for CBF are given as percent control and shown as mean±s.d. A two-factor repeated-measure analysis of variance followed by Bonferroni post hoc tests was applied to CBF values to determine the between-genotype differences and differences between genotypes over time. A P-value <0.05 was considered significant.
Results
Improved rCBF in ArgII−/− Mice Versus WT Mice After Severe TBI as Investigated by ASL Sequences
ArgII−/− and WT mice underwent TBI and MRI. Relative CBF was quantified from ASL MRI scans. The rCBF changes over time, differences between genotypes, and the interaction between time and genotype (temporal CBF profile) were analyzed. Preinjury baseline CBF for all regions was not significantly different between WT and ArgII−/− mice (Table 1, P-value=0.9882). As expected after severe TBI, CBF significantly decreased in both genotypes but not in the sham TBI group (time effect, Table 1).
Table 1. CBF values by region.
In WT animals, CBF in the ipsilateral cortex decreased to 15% of the pre-TBI levels within 15 minutes after TBI and remained low (15%) even at 1 hour after TBI (Table 1). The CBF in the WT ipsilateral subcortex was also drastically reduced at 15 minutes and 1 hour after TBI (Table 1, 16% and 21% of the pre-TBI CBF, respectively). Considerable CBF reductions were also seen in the WT contralateral cortex (49%) and subcortex (53%) by 15 minutes after TBI. These regions showed little or no recovery by 1 hour (Table 1, 54% and 49%, respectively). Overall, the WT mice showed substantial CBF deficits after TBI.
At 15 minutes after TBI in the ipsilateral cortex, ArgII−/− mice had a CBF loss similar to WT mice (17%), which increased to 37% by 1 hour after TBI. The CBF temporal profile also improved significantly over WT mice (Table 1, P=0.0048). The CBF in the ArgII−/− ipsilateral subcortex was significantly higher than that in the WT mice after TBI (70% at 15 minutes and 49% at 1 hour after TBI, P=0.0015). The CBF temporal profile in the ipsilateral subcortex of ArgII−/− mice was significantly different from that of WT (Table 1, P=0.0014). In the contralateral thalamus, the temporal CBF profile was significantly improved in ArgII−/− mice compared with WT mice (Table 1, temporal profile P=0.0092) and approached significance in the ipsilateral thalamus of ArgII−/− mice (Table 1, temporal profile, P=0.0859). The CBF maps also indicate that the ArgII−/− mice showed improved CBF after TBI compared with WT, particularly in the ipsilateral hemisphere (Figure 1B).
Sham TBI WT mice did not show significant changes in CBF (Table 1, P-value=0.96). There was no significant difference in terminal arterial blood gases (WT: pH=7.32±0.1, pCO2=32.1±4.8; ArgII−/−: pH=7.35±.07, pCO2=35.6±5.1) between genotypes.
Discussion
Traumatic brain injury can result in NO-mediated cerebral hypoperfusion, which predisposes the brain to further injury. This study shows that the absence of arginase II significantly improves CBF recovery after trauma in the perilesional brain region (ipsilateral subcortex; P=0.0015). Significant improvements in CBF over time were also observed in the ipsilateral cortex (P=0.0048), ipsilateral subcortex (P=0.0014), and the contralateral thalamus (P=0.0092).
The exact mechanism(s) behind the effect of arginase II on CBF is unknown; however, one potential explanation is the direct competition of arginase II with eNOS for the substrate -arginine. This hypothesis aligns with reports that -arginine increases eNOS-mediated CBF (DeWitt et al, 1997; Hlatky et al, 2003).
Interestingly, conflicting vasoconstrictory profiles for arginase II have been reported. Lim et al (2007) observed dampened vasoconstriction in the ArgII−/− mice. Conversely, one group has reported development of a hypertensive phenotype in ArgII−/− mice (Huynh et al, 2009). A diminished vasoconstriction profile or a hypertensive phenotype may help explain why ArgII−/− mice show improved CBF recovery after TBI. The cause of increased CBF in the thalamus after injury in ArgII−/− mice is not currently known, but may be a compensatory mechanism for decreased blood flow in the cortex.
In summary, although the mechanisms behind low post-trauma CBF are not yet fully elucidated, arginase II is emerging as an important regulator of eNOS activity. In our study, ArgII−/− mice show a better recovery of CBF in the injured brain after TBI than WT mice. From these data and others, it may be inferred that arginase II inhibition presents an interesting new target for in vivo manipulation of NO-mediated CBF after TBI.
Acknowledgments
This project was funded by a grant from Mission Connect (LCM) and the NIH grant POI-N538660 (CSR). The interdepartmental Program in Translational Biology and Molecular Medicine is funded in part by the Howard Hughes Medical Institutes Med into Grad Initiative.
The authors declare no conflict of interest.
References
- Cherian L, Hlatky R, Robertson CS. Nitric oxide in traumatic brain injury. Brain Pathol. 2004;14:195–201. doi: 10.1111/j.1750-3639.2004.tb00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian L, Robertson CS. L-arginine and free radical scavengers increase cerebral blood flow and brain tissue nitric oxide concentrations after controlled cortical impact injury in rats. J Neurotrauma. 2003;20:77–85. doi: 10.1089/08977150360517209. [DOI] [PubMed] [Google Scholar]
- Cherian L, Sheiko T, Bryan R, Craigen W, Goodman C, Robertson CS. Inhibition of arginase with N-hydroxy-nor-L-arginine (NOHA) reduces histological damage after cortical impact injury. J Neurotrauma. 2009;26:A-96. [Google Scholar]
- DeWitt DS, Smith TG, Deyo DJ, Miller KR, Uchida T, Prough DS. L-arginine and superoxide dismutase prevent or reverse cerebral hypoperfusion after fluid-percussion traumatic brain injury. J Neurotrauma. 1997;14:223–233. doi: 10.1089/neu.1997.14.223. [DOI] [PubMed] [Google Scholar]
- Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol. 2007;34:906–911. doi: 10.1111/j.1440-1681.2007.04638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding EM, Robertson CS, Bryan RM. The consequences of traumatic brain injury on cerebral blood flow and autoregulation: a review. Clin Exp Hypertens. 1999;21:299–332. doi: 10.3109/10641969909068668. [DOI] [PubMed] [Google Scholar]
- Herscovitch P, Raichle ME. What is the correct value for the brain–blood partition coefficient for water. J Cereb Blood Flow Metab. 1985;5:65–69. doi: 10.1038/jcbfm.1985.9. [DOI] [PubMed] [Google Scholar]
- Hlatky R, Lui H, Cherian L, Goodman JC, O'Brien WE, Contant CF, Robertson CS. The role of endothelial nitric oxide synthase in the cerebral hemodynamics after controlled cortical impact injury in mice. J Neurotrauma. 2003;20:995–1006. doi: 10.1089/089771503770195849. [DOI] [PubMed] [Google Scholar]
- Huynh NN, Andrews KL, Head GA, Khong SML, Mayorov DN, Murphy AJ, Lambert G, Kiriazis H, Xu Q, Du X, Chin-Dusting JPF. Arginase II knockout mouse displays a hypertensive phenotype despite a decreased vasoconstrictory profile. Hypertension. 2009;54:294–301. doi: 10.1161/HYPERTENSIONAHA.108.121731. [DOI] [PubMed] [Google Scholar]
- Lim HK, Lim HK, Ryoo S, Benjo A, Shuleri K, Miriel V, Baraban E, Camara A, Soucy K, Nyhan D, Shoukas A, Berkowitz DE. Mitochondrial arginase II constrains endothelial NOS-3 activity. Am J Physiol Heart Circ Physiol. 2007;293:H3317–H3324. doi: 10.1152/ajpheart.00700.2007. [DOI] [PubMed] [Google Scholar]
- Liu H, Goodman JC, Robertson CS. The effects of L-arginine on cerebral hemodynamics after controlled cortical impact injury in the mouse. J Neurotrauma. 2002;19:327–334. doi: 10.1089/089771502753594891. [DOI] [PubMed] [Google Scholar]
- Shi O, Morris SM, Zoghbi H, Porter CW, O′Brien WE. Generation of a mouse model for arginase II deficiency by targeted disruption of the arginase II gene. Mol Cell Biol. 2001;21:811–813. doi: 10.1128/MCB.21.3.811-813.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci USA. 1992;89:212–216. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]