Abstract
The pathogenesis of stroke is multifactorial, and inflammation is thought to have a critical function in lesion progression at early time points. Detection of inflammatory processes associated with cerebral ischemia would be greatly beneficial in both designing individual therapeutic strategies and monitoring outcome. We have recently developed a new approach to imaging components of the inflammatory response, namely endovascular adhesion molecule expression on the brain endothelium. In this study, we show specific imaging of vascular cell adhesion molecule (VCAM)-1 expression in a mouse model of middle cerebral artery occlusion (MCAO), and a reduction in this inflammatory response, associated with improved behavioral outcome, as a result of preconditioning. The spatial extent of VCAM-1 expression is considerably greater than the detectable lesion using diffusion-weighted imaging (25% versus 3% total brain volume), which is generally taken to reflect the core of the lesion at early time points. Thus, VCAM-1 imaging seems to reveal both core and penumbral regions, and our data implicate VCAM-1 upregulation and associated inflammatory processes in the progression of penumbral tissue to infarction. Our findings indicate that such molecular magnetic resonance imaging (MRI) approaches could be important clinical tools for patient evaluation, acute monitoring of therapy, and design of specific treatment strategies.
Keywords: adhesion molecules, focal ischemia, inflammation, molecular imaging, MRI
Introduction
Stroke is the second leading cause of death worldwide and the leading cause of disability in western society. The pathogenesis of stroke is multifactorial, but inflammation has been shown to be detrimental from an early time point (Chamorro and Hallenbeck, 2006; Kim, 1996). Indeed, antiinflammatory therapy has been shown to reduce infarct size and improve outcome after permanent cerebral ischemia (Spera et al, 1998). In the case of transient ischemia and reperfusion, there is a complicated inflammatory response beginning with increased expression of cytokines followed by neutrophil and macrophage recruitment to the cerebral parenchyma (Barone et al, 1992; Feuerstein et al, 1998). Neutrophil infiltration occurs within the first 4 to 6 h of reperfusion and is mediated by increased expression of local inflammatory cytokines and cellular adhesion molecules, including inducible cell adhesion molecule (ICAM), vascular cell adhesion molecule (VCAM), and the selectins (E and P) (Stanimirovic and Satoh, 2000; Wang and Feuerstein, 1995; Zhang et al, 1998). Inhibition of ICAM has been shown to reduce the inflammatory response and improve outcome after ischemia and reperfusion (Kitagawa et al, 1998; Zhang et al, 1995), whereas both ICAM-1 and P-selectin knockout mice show resistance to ischemic injury and improved behavioral outcomes (Connolly et al, 1997; Connolly et al, 1996). Similarly, anti-E-selectin therapy has been shown to reduce infarct volumes, mortality, and neurologic deficits in a murine ischemia–reperfusion model (Huang et al, 2000).
Ischemic preconditioning (IPC) is a phenomenon in which sublethal transient ischemia induces tolerance to a more severe ischemic episode than would normally cause neuronal cell death (Kitagawa et al, 1991). The mechanisms for the protection afforded by IPC has been attributed to a number of different factors, including alterations of metabolism (Stenzel-Poore et al, 2003), differential regulation of the alpha-amino-3-hydroxy-5-methyl-4 isoxazolepropionic acid receptor GluR1 and GluR2 subunits (Sommer and Kiessling, 2002), inhibition of anoxic long-term potentiation (Kawai et al, 1998) and, importantly, a reduction of the inflammatory response (Bowen et al, 2006; Yin et al, 2007).
Despite a wealth of studies in this area, the balance between the beneficial and detrimental effects of inflammation in ischemia remains unclear, as does the contribution of suppressing the inflammatory response in preconditioning. New methods that enable in vivo visualization of inflammatory processes after ischemia are urgently needed to evaluate the role of these processes in stroke. Such technologies would also aid both detection and assessment of disease progression, potentially stratifying patients for therapy, and monitoring response to therapeutic intervention. Magnetic resonance imaging (MRI) is used extensively in the clinic to aid in the evaluation of stroke due to its excellent soft tissue resolution, but currently lacks the ability to image specific elements of pathology, such as inflammatory processes. However, we have recently developed a targeted MRI contrast agent based on microparticles of iron oxide (MPIO) that binds to, and enables visualization of, VCAM-1 on the cerebrovascular endothelium when expression is upregulated in pathology (McAteer et al, 2007).
The aims of this study, therefore, were to determine (1) whether our new VCAM-1-targeted contrast agent (VCAM-MPIO) enables acute detection of inflammatory processes after transient ischemia in a mouse middle cerebral artery occlusion (MCAO) model and (2) whether this VCAM-targeted MRI approach is sufficiently sensitive to allow assessment of therapeutic modulation.
Materials and methods
Animal Preparation
Six-week-old C57Bl/6 mice, weighing 20 to 25 g, were obtained from the Biomedical Services of the University of Oxford (Oxford, UK). All procedures were in accordance with the UK Home Office animals (Scientific Procedures) act 1986. Transient focal ischemia was performed as described elsewhere (Barber et al, 2004b) Briefly, isoflurane anesthesia was induced (2% initial, 1% to 1.5% maintenance) in 30% O2 and 70% N2O. Under the operating microscope, the left common carotid artery, the left external carotid artery (ECA), and the left internal carotid artery (ICA) were isolated and a 6-0 suture was tied at the origin of the ECA and at the distal end of the ECA. The left common carotid artery and ICA were temporarily occluded. The silicon-coated nylon suture was introduced into the ECA and advanced in the ICA until resistance was felt; the filament was inserted ∼9 to 10 mm from the carotid bifurcation, effectively blocking the origin of the MCA. The diameter of the tip of coated suture was considered acceptable between 180 and 220 μm (Hata et al, 1998). The suture remained inserted for 30 mins, after which it was removed and the ECA was permanently occluded.
In the case of IPC, the suture was inserted as described above for 15 mins. The suture was then removed completely and the wound was sutured. At 72 h reperfusion, the animal was re-anesthetized and the MCAO surgery performed again, but this time a 30-min occlusion was induced.
All mice were regulated to maintain core body temperature of 36.5°C during surgery, occlusion, and the immediate reperfusion period using a homeostatic heating blanket and a rectal probe during surgery (Harvard Apparatus, Edenbridge, Kent, UK). Although they recovered, mice were warmed using a far infrared blanket (Kent Scientific, Torrington, CT, USA); keeping their core body temperature above 34°C throughout.
Laser Doppler Flowmetry
Although the mouse was still under general anesthetic, regional cerebral blood flow (rCBF) was monitored within the parietal cortex. Blood flow velocity was measured before ischemia, during MCAO, and reperfusion. The method used did not require craniotomy. The skull was exposed by a midline scalp incision and a miniature probe holder (MH-05, Oxford Optronix, Milton, Abingdon, Oxfordshire, UK) was glued 3 to 4 mm lateral and 1 mm posterior to Bregma, and the probe MNP100XP (Oxford Optronix) was attached. This allowed CBF measurements to be made during the procedure. The rCBF during occlusion represents the average laser Doppler flowmetry reading normalized to the baseline reading for that animal; baseline readings are recorded at the start of the surgery. The occlusion rCBF is, therefore, given as a percentage of the baseline value. Animals that did not show a reduction in rCBF to ⩽30% of baseline during ischemia were excluded from further analysis (∼30% of all MCAO animals). The number of animals specified in each group hereafter reflect the number that achieved the required decrease in CBF and, hence, went through the full experimental protocol.
Assessment of Behavioral Deficits
A neurologic deficit score was assigned to each mouse on recovery from the MCAO surgery and before imaging. The scoring relates to forelimb flexion, leaning towards paretic side, resistance to lateral push, gait towards paretic side and rotational behavior. The scoring was rated as follows: 0 is normal, 1 is a forelimb flexion to the paretic side that is evident when the animal is lifted by the tail; 2 shows 1 plus a decreased resistance to lateral push in the paretic direction; 3 shows 1, 2 and a tendency for gait in the direction of the paretic side; 4 shows 1 to 3 and a loose rotational gait in the direction of the paretic side; and 5 shows 1 to 4 plus tight circling in the paretic direction.
Vascular Cell Adhesion Molecule-Microparticles of Iron Oxide Synthesis
Purified monoclonal rat antibodies specific to mouse VCAM-1 (clone M/K2, Cambridge Bioscience, Cambridge, Cambridgeshire, UK) or IgG-1 (clone Lo-DNP-1, Serotec, Oxford, Oxfordshire, UK) were conjugated to myOne tosylactivated MPIO (1 μm diameter; iron content 26%) with ρ-toluensulphonyl (tosyl)-reactive surface groups (Invitrogen, Paisley, Renfrewshire, UK) as described in detail earlier (McAteer et al, 2007).
Experimental Protocol
Mice were subjected to MCAO as described above, or were not subjected to MCAO and served as control animals. The mice recovered from the 30-min MCAO for 3 h, at which time the MPIO conjugated to either VCAM-1 or IgG-1 were administered via a tail vein (4 × 108 microparticles; ∼4.5 mg iron per kg body weight). The mice were divided into four different groups: (1) 30 mins MCAO with VCAM-MPIO (n=6), (2) 30 mins MCAO with control IgG-MPIO (n=6), (3) control naive animals with VCAM-MPIO (n=2), and (4) preconditioned +30 mins MCAO with VCAM-MPIO (n=6). The MRI was initiated ∼1 h after intravenous MPIO injection. We have shown earlier that this length of time is sufficient to allow specific binding as well as clearance of unbound particles from the blood pool (McAteer et al, 2007).
A further group of mice (n=4) received a 30-min MCAO but were imaged at 4 h reperfusion using diffusion-weighted imaging (DWI) to assess the extent of the ischemic lesion at the early time point used for the VCAM-1 study. This study was performed in a separate group of animals to enable both the VCAM-1 and diffusion-weighted data to be acquired at the same time point post-ischemia and reperfusion.
Magnetic Resonance Imaging
Anesthesia was induced with 2% isoflurane in 30%O2:70%N2O, and subsequently maintained at 1.5% isoflurane. Animals were placed in an Alderman-Grant resonator coil (i.d. 25 mm) with an in-built stereotaxic frame for imaging. Body temperature was monitored and maintained at 37°C using a rectal probe and circulating warm water system, and heart rate was monitored via subcutaneous electrodes throughout. The MRI was performed on a 7-Tesla horizontal bore magnet with a Varian Inova spectrometer (Varian, Palo Alto, CA, USA). In animals injected with targeted contrast agent a T2*-weighted 3D gradient-echo data set was acquired; flip angle 35°, TR=50 ms, TE=5 ms, field of view 22.5 × 22.5 × 31.6 mm, matrix size 192 × 192 × 360, 2 averages, total acquisition time ∼1 h. The mid-point of the acquisition was ∼1.5 h after MPIO injection. The data were zero-filled to 256 × 256 × 360 and was reconstructed off-line to give a final isotropic resolution of 88 μm. In animals undergoing DWI, diffusion-weighted images were acquired using a navigated pulsed-gradient spin-echo sequence (TR 1.5 s; TE 36.5 ms; field of view 2.5 × 2.5 cm; matrix size 128 × 128; 1 average; total acquisition time 9 mins), with diffusion weighting b values of 125, 500 and 1000 s/mm2, a diffusion time (Δ) of 17.5 ms and a diffusion-gradient duration (δ) of 12.5 ms. Diffusion gradients were applied separately along three orthogonal axes and apparent diffusion coefficient (ADC) ‘trace' maps were calculated (Basser et al, 1994). Navigator echoes were used for motion correction (Ordidge et al, 1994).
Mice were killed immediately after the MRI scan by transcardial perfusion with phosphate-buffered saline. The fresh brains were quickly removed from the skull, rinsed with saline, and sectioned in a brain mould to obtain a 2-mm thick striatal slice. This slice was cut in half and each hemisphere was quickly frozen and analyzed for VCAM mRNA levels.
Magnetic Resonance Imaging Analysis
The MRI data were assigned a numerical code at the time of data acquisition and subsequently analyzed in batches to minimize bias. For each image, the brain was manually masked to exclude extracerebral structures, and the signal intensity in the ventricles was set at a constant level across all data sets to maintain consistency in thresholding. The low-signal areas were segmented in 40 continuous images, spanning the territory of the MCA. Low signal areas were calibrated on 10 evenly spaced slices per brain, to control for minor variations in absolute signal intensity between the individual scans. The median signal intensity value was applied to the fully automated, histogram-based batch analysis of the 40-slice sequence. The data were extracted for the left and right sides of the brain simultaneously with identical parameters. The voxel volumes were summated and expressed as raw volumes in cubic micrometers without surface rendering or smoothing effects. ImagePro Plus (Media Cybernetics, Bethesda, MD, USA) was used to segment and quantify contrast volume. The data are presented as volumes of VCAM-MPIO-induced hypointensity in the ischemic hemisphere, defined as ‘left' minus ‘right' contrast volume.
Real-Time Polymerase Chain Reaction
Total RNA extraction was performed using TRIzol (Invitrogen) following the manufacturer's instructions, with one modification. Briefly, 1 mL of TRIzol was added to the eppendorf tubes containing the tissue, and the samples were homogenized on ice. Once all the samples were homogenized, they were incubated at room temperature for 10 mins, and then 200 μL of chloroform was added to each tube, and the tubes were vortex mixed and centrifuged at 13 000 g for 20 mins at 4C. The clear phase contained the RNA, and this was transferred to a new tube. An equal volume of ethanol was added to the RNA phase and this was applied to the column from the RNeasy kit (Qiagen, Crawley, West Sussex, UK), and the RNA was bound within the column. This was then eluted with 30 μL of DEPC water and the RNA was stored at −80°C until needed.
The extracted RNA was converted to complementary DNA using the Quantitech SYBR green RT kit (Qiagen). Quantitative real-time polymerase chain reaction was performed to determine the levels of the VCAM-1 transcript using the forward primer TCTTACCTGTGCGCTGTGAC and the reverse primer ACAGGTCTCCCATGCACAA generated by primer express (Applied Biosystems, Foster City, CA, USA), and the polymerase chain reaction was normalized to the housekeeping gene cyclophilin.
Immunohistochemistry
As brain tissue from all animals undergoing MRI had been used for polymerase chain reaction analysis, additional animals underwent either protocol 1 (30 mins MCAO with VCAM-MPIO) or protocol 4 (preconditioned +30 mins MCAO with VCAM-MPIO) for immunohistochemical analysis. At the time point corresponding to the midpoint of the 3D T2*-weighted data acquisition, animals were deeply anesthetized with sodium pentobarbital, and transcardially perfused with 10 mL 0.9% heparinized saline followed by 10 mL 4% paraformaldehyde in phosphate-buffered saline. After dissection, the brains were post-fixed, cryoprotected, embedded, and frozen in isopentane at −40°. Immunostaining was performed on 10 μm-thick coronal brain sections through the MCA territory using the M/K2 hybridoma for mouse VCAM-1 (2.8 μg/mL) and standard ABC amplification procedures. Sections were counterstained with cresyl violet, and examined for VCAM-1 expression (stained brown) and the presence of MPIO within VCAM-1-positive vessels. Within each individual immunohistochemistry protocol, a positive control (interleukin-1β-activated brain endothelium) and a negative control, in which the primary antibody was omitted, were included. Sections were then treated with chloroform for 10 mins to disrupt the polythene coating on the MPIO and were stained with Perl's Prussian blue for ferric ions to reveal the presence of MPIO beads within vessels (stained blue).
Statistics
One-sided ANOVA was used with the Bonferroni statistic post hoc for all statistics of multiple comparisons. Results were considered significant when P<0.05.
Results
Diffusion-Weighted Imaging
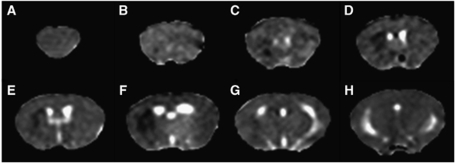
In accord with earlier studies (Barber et al, 2005), DWI revealed a small area of reduced ADC in the territory of the MCA at 4 h after reperfusion (Figure 1). The diffusion-weighted lesion occupied 3±0.5% of the total brain volume.
Figure 1.
Representative ADC maps from mouse brain after 30 mins MCAO and 4 h reperfusion. ADC maps are shown for the eight coronal slices acquired spanning the forebrain (A–H). A small hypointense region (reduced ADC) can be seen in the striatum of the left hemisphere within the core region of the MCA territory (B–G).
Magnetic Resonance Imaging of Vascular Cell Adhesion Molecule-1 Expression
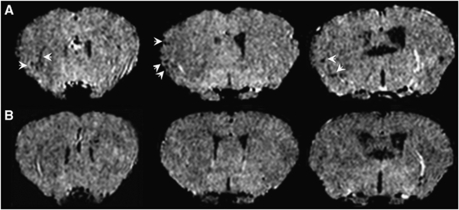
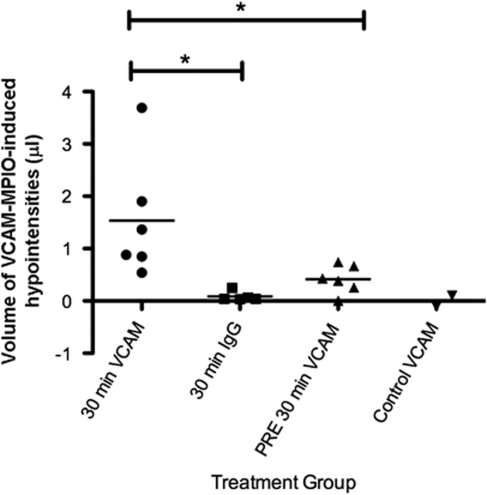
The VCAM-MPIO binding was evident in the lesioned hemisphere when animals were imaged ∼4.5 h after 30 mins transient ischemia, detected as areas of hypointensity (Figure 2A). In contrast, very little MPIO binding was observed in animals undergoing the same ischemia protocol, but IgG-MPIO injection (Figure 2B). The VCAM-MPIO binding in the ischemic hemisphere was significantly (P<0.05) greater than IgG-MPIO retention after 30 mins MCAO (Figure 3).
Figure 2.
Representative images showing VCAM-MPIO binding in the ischemic hemisphere. Sets of T2*-weighted images taken from full 3D data sets spanning the brain. (A) Binding of VCAM-MPIO is evident as focal hypointensities (arrows) in the 30-min MCAO animal, whereas negligible binding is seen in MCAO animals injected with IgG-MPIO (B).
Figure 3.
Volumes of VCAM-MPIO-induced hypointensities. A 30-mins MCAO-induced binding of VCAM-MPIO in the ischemic hemisphere, whereas negligible binding was seen in IgG-MPIO-injected animals. Significantly reduced VCAM-MPIO binding was measured in preconditioned MCAO animals compared with nonpreconditioned animals. *P<0.05. Average neurologic deficit score for each group are as follows: 30 mins VCAM=2.5; 30 mins IgG=2.5; PRE 30 mins MCAO=1.2; control=0.
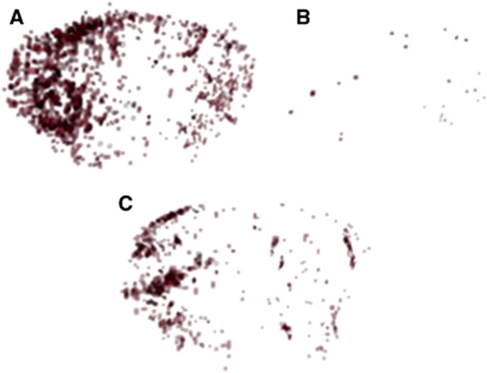
The volume of hypointensity reflecting VCAM-MPIO binding, 25±6% of total brain volume, was considerably greater than the lesion volume identified on diffusion-weighted images (∼3%). Three-dimensional reconstructions of the hypointensities on the T2*-weighted images illustrate the spatial extent of VCAM-MPIO binding within the brain (Figure 4). It is notable that binding seems denser in the striatal areas of the lesion that correspond to areas showing reduced diffusion in animals imaged using DWI (Figure 1), whereas the radial vessels of the cortex are also particularly apparent. In contrast, naive control animals injected with VCAM-MPIO showed minimal binding and resultant hypointensities.
Figure 4.
Representative 3D reconstructions of thresholded T2*-weighted images from MCAO animals showing extent of VCAM-MPIO binding. (A) VCAM-MPIO binding is substantially greater in the ischemic (left) hemisphere than the control side of MCAO animals. (B) Negligible VCAM-MPIO binding is evident in animals injected with IgG-MPIO, whereas significantly reduced VCAM-MPIO binding was found in preconditioned compared with nonpreconditioned MCAO animals (C).
Ischemic Preconditioning
The VCAM-MPIO binding was apparent throughout the ischemic territory in preconditioned MCAO animals. However, a clear reduction in the intensity of binding was evident across the ischemic region with the exception of a small focal area of dense binding in the lateral striatum (Figure 4). The VCAM-MPIO binding in the lesioned hemisphere was significantly (P<0.05) reduced in preconditioned ischemic animals compared with nonpreconditioned ischemic animals (Figure 3).
Behavioral Scores
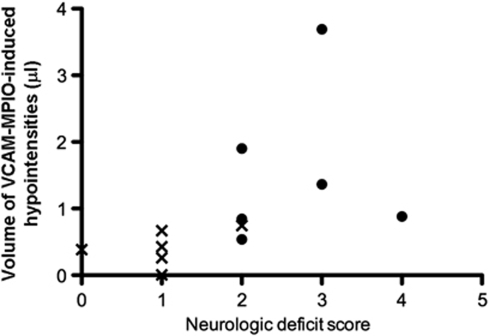
Mice that had more severe neurologic symptoms, as determined by the neurologic deficit score, tended to exhibit greater VCAM-MPIO binding in the ischemic hemisphere (Figure 5). Moreover, preconditioned MCAO mice had a better behavioral outcome before imaging than nonpreconditioned MCAO animals, correlating with lower VCAM-MPIO binding in these animals. The variation in behavioral outcome of the nonpreconditioned animals likely reflects in part the variation in CBF reduction achieved during MCAO (minimum CBF=19±8%, mean±s.d.), although the most severe reductions did not always correlate with the worst neurologic scores.
Figure 5.
Comparison of neurologic deficit score (NDS) with volume of VCAM-MPIO-induced hypointensities. • represents 30 mins MCAO animals, whereas X represents preconditioned +30 mins MCAO animals, showing that preconditioned animals had an improved NDS and lower levels of specific contrast in the ischemic hemisphere.
Vascular Cell Adhesion Molecule-1 Transcript Levels
A 30-min MCAO seemed to slightly increase the VCAM transcript levels in the lesioned hemisphere as compared with the nonlesioned hemisphere in both MCAO and preconditioned MCAO mice when determined at 5.5 h after reperfusion (sample obtained after MRI), but this was not significant. No significant difference in the VCAM-1 transcript levels in the lesioned hemisphere was found between MCAO and preconditioned MCAO animals (P=0.7), indicating that the preconditioning ischemia did not alter the transcription of this adhesion molecule (Figure 6).
Figure 6.
Relative expression of the VCAM-1 transcript after ischemia or control. The ipsilateral (left; L) hemisphere showed increased expression of the VCAM-1 transcript in both MCAO and preconditioned MCAO animals compared with the contralateral (right; R) hemisphere, although this did not reach significance compared with either the nonlesioned hemispheres or control animals. The increase in VCAM-1 transcript was not significantly different between the two groups MCAO groups irrespective of preconditioning (P=0.7).
Immunohistochemistry
Marked upregulation of VCAM-1 was apparent throughout the ischemic territory in MCAO animals (Figures 7A, 7B, and 7D), whereas little VCAM-1 expression was found in the contralateral hemisphere (Figure 7C). Moreover, bound VCAM-MPIO were clearly evident in numerous VCAM-1-positive vessels within the ischemic lesion (Figures 7E–7G), and this was verified using Perl's Prussian Blue stain for ferric iron after chloroform treatment (H–J). In preconditioned MCAO animals, the level of VCAM-1 staining seemed to be less intense than in nonpreconditioned animals, but nevertheless was still present throughout the ischemic region in accord with the MRI data. Thus, it seems that the VCAM-MPIO MRI findings provide a fair representation of the extent of VCAM-1 expression within the ischemic lesion.
Figure 7.
Expression of VCAM-1 protein after MCAO. (A) Increased expression of VCAM-1 was clearly evident in the ischemic hemisphere (arrow) of MCAO animals, and was most highly expressed on vessels (arrows) in the striatum (B). Very little VCAM-1 was detectable in the contralateral hemisphere (C), but VCAM-1 expression in the ipsilateral hemisphere was found throughout the ischemic territory including cortical regions (D). High-power photomicrographs (E–G) revealed the presence of VCAM-MPIO (arrows) in VCAM-1-positive vessels within the ischemic lesion. (H) Perl's Prussian Blue stain for ferric iron after chloroform treatment on a film of VCAM-MPIO. The same approach confirmed the presence of MPIO (blue) in VCAM-1 positive (brown) vessels in both the core (I) and outer edge (J) of the ischemic lesion. Bars=20 μm.
Discussion
In this study, we have shown that a relatively short ischemic insult followed by a few hours of reperfusion was sufficient to induce an inflammatory response in the ipsilateral hemisphere, which was detectable using VCAM-MPIO and MRI. The deleterious inflammatory response due to cell adhesion molecules is visualized at a relatively early time point in the pathogenesis of ischemic stroke (Amantea et al, 2009; Danton and Dietrich, 2003; Fisher, 2008). This transient ischemic insult followed by reperfusion also resulted in a focal region of reduced ADC, as has been reported earlier (Barber et al, 2005). However, this region of reduced ADC was very restricted, compared with the total volume of the MCA territory, corresponding to only 3% of the total brain volume, whereas the area of VCAM-MPIO binding was much more extensive, involving 25% of the total brain volume. Importantly, negligible binding of the nonspecific IgG-MPIO was evident in MCAO animals, indicating that the binding of the VCAM-MPIO was specific to increased VCAM-1 expression on the luminal endothelium of the ischemic hemisphere. This conclusion is supported by immunohistochemical analysis showing both VCAM-1 expression throughout the ischemic territory and binding of VCAM-MPIO to VCAM-1 positive vessels at the same time point. Animals that had been preconditioned with a sub-lethal duration of ischemia showed improved behavioral outcomes after 30-mins MCAO and reperfusion, and also had significantly reduced VCAM-MPIO binding in the lesioned hemisphere. This finding suggests that the preconditioning ischemia attenuated the inflammatory response in the brain, and may be an important mechanism underlying the protection afforded by IPC.
A small number of recent studies using ligand-targeted contrast agents (either MRI or fluorescence based) have reported efficacy of this approach for identifying inflammatory markers in ischemic lesions in vivo (Barber et al, 2004a; Klohs et al, 2008). However, both studies report only late-stage time points in lesion evolution (24 to 80 h post-reperfusion) when inflammatory processes are known to be markedly upregulated and damage is well progressed. In contrast, here we show that the VCAM-MPIO enable detection of inflammatory processes in ischemic lesions in vivo in the acute stages of lesion evolution, at times when this information could drive therapy on an individual basis and when such therapy may be of considerably greater benefit than at later stages.
The early inflammatory response (within 4 to 6 h) seems to be detrimental for the brain after focal cerebral ischemia and is mediated by cytokines, chemokines, and cell adhesion molecules (Amantea et al, 2009). Importantly, increased VCAM-1 expression has been found in infarcted areas of autopsy specimens from patients with recent stroke, suggesting that enhanced expression of this cell adhesion molecule may be important for the pathogenesis of human ischemic stroke (Krupinski et al, 1994). The recently published MITICO study investigated risk factors for recurrent stroke in a population of stroke patients, and identified increased levels of circulating VCAM-1 in the plasma as one of the main predictors of future ischemic stroke (Castillo et al, 2009). The VCAM-1 is shed from activated blood vessels and has been proposed as a marker of the extent of neuroinflammatory disease. We have shown earlier the technique of imaging inflammation using our novel VCAM-MPIO in an animal model of interleukin-1β-induced CNS inflammation, and in a mouse model of atherosclerosis (McAteer et al, 2008; McAteer et al, 2007). We now show the utility of this approach for identifying acute endothelial activation after transient MCAO, and hence the inflammatory component of the ischemic pathology. Our data indicate that VCAM-1 expression is markedly upregulated within the 3 to 4 h reperfusion window after a short ischemic episode. These data suggest that this approach will be efficacious in identifying inflammatory processes associated with ischemic lesions and their time course of onset.
Diffusion-weighted MRI has also been used to assess infarct progression in the mouse MCAO model earlier (Hesselbarth et al, 1998), and lesions can be visualized as early as 60 mins after induction of ischemia. It is well established that in the MCAO model, a core region of tissue at the center of the ischemic territory rapidly undergoes irreversible damage and will progress to infarction, whereas a penumbral region surrounding the core contains tissue that is at risk but potentially salvageable (Barone, 2009; Ferrer et al, 2003). At the acute time points after an ischemic challenge, the lesion identified by DWI is generally considered to reflect the core of the lesion only. Over time, the size of the DWI lesion continues to increase, and by ∼24 h encompasses the final extent of infarcted tissue. In this study, the DWI lesion observed 4 h after reperfusion encompassed a relatively small percentage of the total brain volume (∼3%) located mainly within the striatum, which we take to reflect predominantly the core of the ischemic lesion based on earlier studies. In contrast, the area of VCAM-1 expression at the same time point was considerably greater (∼25% of total brain volume) than that identified by DWI and in spatial extent resembled maps of perfusion deficit reported earlier in MCAO animals. The VCAM-MPIO binding was notably denser in the striatal region that exhibited reduced diffusion. As has been found for DWI/perfusion MRI mismatches, the difference in lesion extent obtained from the VCAM and diffusion-imaging modalities may provide a measure of penumbral, and hence potentially salvageable, tissue.
Ischemic preconditioning has previously been shown to significantly reduce infarct size in various animal models of stroke (Barone et al, 1998; Hoyte et al, 2006; Stenzel-Poore et al, 2003) and has been suggested to induce a number of survival mechanisms that confer protection from a future ischemic insult. It has been proposed that a key mechanism may be a reduction in the early, deleterious, inflammatory cascade (Bowen et al, 2006; Yin et al, 2007). Reducing inflammation at the early time points of reperfusion has been shown to improve outcome after ischemic stroke (Spera et al, 1998; Yrjanheikki et al, 1999). In this study, VCAM-MPIO binding was significantly reduced in MCAO animals after preconditioning, particularly in cortical areas. These findings support the concept that preconditioning reduces the acute inflammatory response associated with transient ischemia. Earlier studies have shown protective effects of inhibiting adhesion molecules, such as ICAM and E-/P-selectin, in ischemia and our findings indicate that VCAM-1 may also have a function in ischemic injury. Interestingly, one earlier study failed to show improvement with anti-VCAM-1 antibody treatment after focal stroke and reperfusion in both rats and mice (Justicia et al, 2006). However, the duration of stroke was relatively prolonged in those models and the potential efficacy of anti-VCAM therapy may depend on a multitude of factors including duration, severity, and site of lesion.
The reduction in VCAM-MPIO binding after IPC did not seem to be due to decreased total transcription of the adhesion molecule, as preconditioning did not significantly reduce transcript levels, but may rather reflect altered stability of the transcript or translation failures. It is also of note that activated microglia and macrophages are known to express VCAM-1, which may not be susceptible to the preconditioning regime and, thus, whole tissue analysis could mask a reduction in endothelial VCAM-1 transcript levels specifically. In either case, our findings indicate that the overall level of VCAM-1 transcript from the ischemic tissue is not a reliable indicator of protein expression on the cerebrovasculature, as VCAM-MPIO binding was significantly reduced where mean transcript levels were not.
Stroke is a major health issue throughout the world, and the incidence will continue to rise as the population ages. One of the earliest aspects of the pathology is an inflammatory response and the ability to image this pathology could greatly enhance our ability to both tailor therapy and monitor outcome. In this study, we have shown specific imaging of VCAM-1 expression in a mouse model of MCAO, and a reduction in this inflammatory response, associated with improved behavioral outcome, as a result of preconditioning. Our findings indicate that the use of targeted contrast agents with MRI could be an important clinical tool for patient evaluation, acute monitoring of therapy, and the design of specific treatment strategies.
Acknowledgments
LH was funded by the Canadian Institutes of Health Research and an Overseas Research Student Award. This work was also funded by the MRC and the Fondation Leducq (AB), Cancer Research UK (NS) and The Wellcome Trust (RC). The authors thank Dr Alexandr Khrapichev for technical assistance with the MRI.
The authors declare no conflict of interest.
References
- Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J. 2009;276:13–26. doi: 10.1111/j.1742-4658.2008.06766.x. [DOI] [PubMed] [Google Scholar]
- Barber PA, Foniok T, Kirk D, Buchan AM, Laurent S, Boutry S, Muller RN, Hoyte L, Tomanek B, Tuor UI. MR molecular imaging of early endothelial activation in focal ischemia. Ann Neurol. 2004a;56:116–120. doi: 10.1002/ana.20162. [DOI] [PubMed] [Google Scholar]
- Barber PA, Hoyte L, Colbourne F, Buchan AM. Temperature-regulated model of focal ischemia in the mouse: a study with histopathological and behavioral outcomes. Stroke. 2004b;35:1720–1725. doi: 10.1161/01.STR.0000129653.22241.d7. [DOI] [PubMed] [Google Scholar]
- Barber PA, Hoyte L, Kirk D, Foniok T, Buchan A, Tuor U. Early T1- and T2-weighted MRI signatures of transient and permanent middle cerebral artery occlusion in a murine stroke model studied at 9.4T 1. Neurosci Lett. 2005;388:54–59. doi: 10.1016/j.neulet.2005.06.067. [DOI] [PubMed] [Google Scholar]
- Barone FC. Ischemic stroke intervention requires mixed cellular protection of the penumbra. Curr Opin Investig Drugs. 2009;10:220–223. [PubMed] [Google Scholar]
- Barone FC, Schmidt DB, Hillegass LM, Price WJ, White RF, Feuerstein GZ, Clark RK, Lee EV, Griswold DE, Sarau HM.1992Reperfusion increases neutrophils and leukotriene B4 receptor binding in rat focal ischemia Stroke 231337–1347.discussion 1347–1348 [DOI] [PubMed] [Google Scholar]
- Barone FC, White RF, Spera PA, Ellison J, Currie RW, Wang X, Feuerstein GZ. Ischemic preconditioning and brain tolerance: temporal histological and functional outcomes, protein synthesis requirement, and interleukin-1 receptor antagonist and early gene expression. Stroke. 1998;29:1937–1950. doi: 10.1161/01.str.29.9.1937. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen KK, Naylor M, Vemuganti R. Prevention of inflammation is a mechanism of preconditioning-induced neuroprotection against focal cerebral ischemia. Neurochem Int. 2006;49:127–135. doi: 10.1016/j.neuint.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Castillo J, Alvarez-Sabin J, Martinez-Vila E, Montaner J, Sobrino T, Vivancos J. Inflammation markers and prediction of post-stroke vascular disease recurrence: The MITICO study. J Neurol. 2009;256:217–224. doi: 10.1007/s00415-009-0058-4. [DOI] [PubMed] [Google Scholar]
- Chamorro A, Hallenbeck J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke. 2006;37:291–293. doi: 10.1161/01.STR.0000200561.69611.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly ES, Jr, Winfree CJ, Prestigiacomo CJ, Kim SC, Choudhri TF, Hoh BL, Naka Y, Solomon RA, Pinsky DJ. Exacerbation of cerebral injury in mice that express the P-selectin gene: identification of P-selectin blockade as a new target for the treatment of stroke. Circ Res. 1997;81:304–310. doi: 10.1161/01.res.81.3.304. [DOI] [PubMed] [Google Scholar]
- Connolly ES, Jr, Winfree CJ, Springer TA, Naka Y, Liao H, Yan SD, Stern DM, Solomon RA, Gutierrez-Ramos JC, Pinsky DJ. Cerebral protection in homozygous null ICAM-1 mice after middle cerebral artery occlusion. Role of neutrophil adhesion in the pathogenesis of stroke. J Clin Invest. 1996;97:209–216. doi: 10.1172/JCI118392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 2003;62:127–136. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Friguls B, Dalfo E, Justicia C, Planas AM. Caspase-dependent and caspase-independent signalling of apoptosis in the penumbra following middle cerebral artery occlusion in the adult rat. Neuropathol Appl Neurobiol. 2003;29:472–481. doi: 10.1046/j.1365-2990.2003.00485.x. [DOI] [PubMed] [Google Scholar]
- Feuerstein GZ, Wang X, Barone FC. The role of cytokines in the neuropathology of stroke and neurotrauma. Neuroimmunomodulation. 1998;5:143–159. doi: 10.1159/000026331. [DOI] [PubMed] [Google Scholar]
- Fisher M. Injuries to the vascular endothelium: vascular wall and endothelial dysfunction. Rev Neurol Dis. 2008;5 (Suppl 1:S4–S11. [PubMed] [Google Scholar]
- Hata R, Mies G, Wiessner C, Fritze K, Hesselbarth D, Brinker G, Hossmann KA. A reproducible model of middle cerebral artery occlusion in mice: hemodynamic, biochemical, and magnetic resonance imaging. J Cereb Blood Flow Metab. 1998;18:367–375. doi: 10.1097/00004647-199804000-00004. [DOI] [PubMed] [Google Scholar]
- Hesselbarth D, Franke C, Hata R, Brinker G, Hoehn-Berlage M. High resolution MRI and MRS: a feasibility study for the investigation of focal cerebral ischemia in mice. NMR Biomed. 1998;11:423–429. doi: 10.1002/(sici)1099-1492(199812)11:8<423::aid-nbm538>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Hoyte LC, Papadakis M, Barber PA, Buchan AM. Improved regional cerebral blood flow is important for the protection seen in a mouse model of late phase ischemic preconditioning. Brain Res. 2006;1121:231–237. doi: 10.1016/j.brainres.2006.08.107. [DOI] [PubMed] [Google Scholar]
- Huang J, Choudhri TF, Winfree CJ, McTaggart RA, Kiss S, Mocco J, Kim LJ, Protopsaltis TS, Zhang Y, Pinsky DJ, Connolly ES., Jr Postischemic cerebrovascular E-selectin expression mediates tissue injury in murine stroke. Stroke. 2000;31:3047–3053. [PubMed] [Google Scholar]
- Justicia C, Martin A, Rojas S, Gironella M, Cervera A, Panes J, Chamorro A, Planas AM. Anti-VCAM-1 antibodies did not protect against ischemic damage either in rats or in mice. J Cereb Blood Flow Metab. 2006;26:421–432. doi: 10.1038/sj.jcbfm.9600198. [DOI] [PubMed] [Google Scholar]
- Kawai K, Nakagomi T, Kirino T, Tamura A, Kawai N. Preconditioning in vivo ischemia inhibits anoxic long-term potentiation and functionally protects CA1 neurons in the gerbil. J Cereb Blood Flow Metab. 1998;18:288–296. doi: 10.1097/00004647-199803000-00007. [DOI] [PubMed] [Google Scholar]
- Kim JS. Cytokines and adhesion molecules in stroke and related diseases. J Neurol Sci. 1996;137:69–78. doi: 10.1016/0022-510x(95)00338-3. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Kuwabara K, Tagaya M, Ohtsuki T, Hata R, Ueda H, Handa N, Kimura K, Kamada T. Ischemic tolerance' phenomenon detected in various brain regions. Brain Res. 1991;561:203–211. doi: 10.1016/0006-8993(91)91596-s. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Mabuchi T, Yagita Y, Ohtsuki T, Hori M, Yanagihara T. Deficiency of intercellular adhesion molecule 1 attenuates microcirculatory disturbance and infarction size in focal cerebral ischemia. J Cereb Blood Flow Metab. 1998;18:1336–1345. doi: 10.1097/00004647-199812000-00008. [DOI] [PubMed] [Google Scholar]
- Klohs J, Grafe M, Graf K, Steinbrink J, Dietrich T, Stibenz D, Bahmani P, Kronenberg G, Harms C, Endres M, Lindauer U, Greger K, Stelzer EH, Dirnagl U, Wunder A. In vivo imaging of the inflammatory receptor CD40 after cerebral ischemia using a fluorescent antibody. Stroke. 2008;39:2845–2852. doi: 10.1161/STROKEAHA.107.509844. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- McAteer MA, Schneider JE, Ali ZA, Warrick N, Bursill CA, von zur Muhlen C, Greaves DR, Neubauer S, Channon KM, Choudhury RP. Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide. Arterioscler Thromb Vasc Biol. 2008;28:77–83. doi: 10.1161/ATVBAHA.107.145466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAteer MA, Sibson NR, von Zur Muhlen C, Schneider JE, Lowe AS, Warrick N, Channon KM, Anthony DC, Choudhury RP. In vivo magnetic resonance imaging of acute brain inflammation using microparticles of iron oxide. Nat Med. 2007;13:1253–1258. doi: 10.1038/nm1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordidge RJ, Helpern JA, Qing ZX, Knight RA, Nagesh V. Correction of motional artifacts in diffusion-weighted MR images using navigator echoes. Magn Reson Imaging. 1994;12:455–460. doi: 10.1016/0730-725x(94)92539-9. [DOI] [PubMed] [Google Scholar]
- Sommer C, Kiessling M. Ischemia and ischemic tolerance induction differentially regulate protein expression of GluR1, GluR2, and AMPA receptor binding protein in the gerbil hippocampus: GluR2 (GluR-B) reduction does not predict neuronal death. Stroke. 2002;33:1093–1100. doi: 10.1161/01.str.0000014205.05597.45. [DOI] [PubMed] [Google Scholar]
- Spera PA, Ellison JA, Feuerstein GZ, Barone FC. IL-10 reduces rat brain injury following focal stroke. Neurosci Lett. 1998;251:189–192. doi: 10.1016/s0304-3940(98)00537-0. [DOI] [PubMed] [Google Scholar]
- Stanimirovic D, Satoh K. Inflammatory mediators of cerebral endothelium: a role in ischemic brain inflammation. Brain Pathol. 2000;10:113–126. doi: 10.1111/j.1750-3639.2000.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, Meller R, Rosenzweig HL, Tobar E, Shaw TE, Chu X, Simon RP. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet. 2003;362:1028–1037. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- Wang X, Feuerstein GZ. Induced expression of adhesion molecules following focal brain ischemia. J Neurotrauma. 1995;12:825–832. doi: 10.1089/neu.1995.12.825. [DOI] [PubMed] [Google Scholar]
- Yin W, Signore AP, Iwai M, Cao G, Gao Y, Johnnides MJ, Hickey RW, Chen J. Preconditioning suppresses inflammation in neonatal hypoxic ischemia via Akt activation. Stroke. 2007;38:1017–1024. doi: 10.1161/01.STR.0000258102.18836.ca. [DOI] [PubMed] [Google Scholar]
- Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci USA. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Chopp M, Zhang Z, Jiang N, Powers C. The expression of P- and E-selectins in three models of middle cerebral artery occlusion. Brain Res. 1998;785:207–214. doi: 10.1016/s0006-8993(97)01343-7. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Jiang N, Tang WX, Prostak J, Manning AM, Anderson DC.1995Anti-intercellular adhesion molecule-1 antibody reduces ischemic cell damage after transient but not permanent middle cerebral artery occlusion in the Wistar rat Stroke 261438–1442.discussion 1443 [DOI] [PubMed] [Google Scholar]