Abstract
We hypothesized that pretreatment magnetic resonance imaging (MRI) diffusion-weighted imaging (DWI) and perfusion-weighted imaging (PWI) lesion volumes may have influenced clinical response to thrombolysis in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET). In 98 patients randomized to intravenous (IV) tissue plasminogen activator (tPA) or placebo 3 to 6 h after stroke onset, we examined increasing acute DWI and PWI lesion volumes (Tmax—with 2-sec delay increments), and increasing PWI/DWI mismatch ratios, on the odds of both excellent (modified Rankin Scale (mRS): 0 to 1) and poor (mRS: 5 to 6) clinical outcome. Patients with very large PWI lesions (most had internal carotid artery occlusion) had increased odds ratio (OR) of poor outcome with IV-tPA (58% versus 25% placebo; OR=4.13, P=0.032 for Tmax +2-sec volume >190 mL). Excellent outcome from tPA treatment was substantially increased in patients with DWI lesions <18 mL (77% versus 18% placebo, OR=15.0, P<0.001). Benefit from tPA was also seen with DWI lesions up to 25 mL (69% versus 29% placebo, OR=5.5, P=0.03), but not for DWI lesions >25 mL. In contrast, increasing mismatch ratios did not influence the odds of excellent outcome with tPA. Clinical responsiveness to IV-tPA, and stroke outcome, depends more on baseline DWI and PWI lesion volumes than the extent of perfusion–diffusion mismatch.
Keywords: ischemic stroke, diffusion MRI, perfusion MRI, thrombolysis
Introduction
Tissue plasminogen activator (tPA) significantly improves the chance of independence after ischemic stroke (Hacke et al, 2004; Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group 1995). However, the odds of benefit decrease with time, likely reflecting disappearance of the ischemic penumbra (Darby et al, 1999; Davis et al, 2005). Rescuing hypoperfused but still viable brain tissue is the central premise of stroke thrombolysis, and penumbral imaging may ultimately supplant time-based selection for thrombolysis in individual patients (Davis et al, 2005). However, randomized-controlled trial evidence that penumbral-based selection improves clinical outcomes after thrombolysis is still lacking (Albers et al, 2006; Davis et al, 2008; Hacke et al, 2005; Schellinger et al, 2007).
Perfusion–diffusion magnetic resonance imaging (MRI) is the most established technique to estimate ischemic penumbra in routine clinical practice, where perfusion deficits give an indication of hypoperfused tissue at risk, and diffusion lesions an indication of the irreversibly damaged tissue of the infarct core (Hjort et al, 2005). Perfusion–diffusion ‘mismatch' is thought to be representative of the ischemic penumbra, although it is becoming clear that this may be too simplistic an approach to imaging-based penumbral selection (Hacke et al, 2009; Kakuda et al, 2008; Kidwell et al, 2003). An empirical perfusion/diffusion lesion mismatch ratio of >1.2 used in some studies does not take into account the baseline volume of the PWI and diffusion-weighted imaging (DWI) lesions. Using a greater degree of mismatch to select potential treatment responders may partially offset this problem, although there is also the difficult issue of which perfusion threshold should be used to define critically hypoperfused tissue (Butcher et al, 2003; Butcher et al, 2005; Olivot et al, 2009). Regardless of what perfusion threshold and what degree of perfusion–diffusion mismatch is used to try and better identify penumbral tissue, there remains a complex inter-relationship between tissue at risk and ultimate clinical outcome, even with successful thrombolysis. Some of the missing pieces in this puzzle are likely to include the site of vascular occlusion, particularly the possible adverse effect of internal carotid artery (ICA) occlusion, extent of collateral blood flow, and baseline volume of the diffusion and perfusion lesions (Albers et al, 2006; Miteff et al, 2009; Yoo et al, 2009).
In the primary analysis of the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET), we were intrigued by the finding that in patients with perfusion/diffusion mismatch, intravenous (IV) thrombolysis (nonsignificantly) increased excellent clinical outcome by 15%, but also increased poor outcome by 17% (Davis et al, 2008). We hypothesized that variability in baseline DWI and PWI lesion size within mismatch patients may have been the explanation for this apparently contradictory finding. This study was specifically focused on clinical outcomes in EPITHET, and tested the hypothesis that the size of the baseline DWI lesion and the size and severity of the baseline PWI lesion may have had an influence on the likelihood of both excellent and poor clinical outcomes, independently of the extent of perfusion–diffusion mismatch.
Materials and methods
The EPITHET was a phase II prospective, randomized, double-blinded, placebo-controlled trial of acute ischemic stroke patients imaged with serial MRI and randomized to IV-tPA or placebo at 3 to 6 h after symptom onset (Davis et al, 2008). The EPITHET was conducted between 2001 and 2007 in 15 centers in Australia, New Zealand, Belgium, and Scotland. The study protocol and informed consent procedures were approved by Human Research and Ethics Committees at each site.
The full methodology of the EPITHET trial has been described earlier (Davis et al, 2008). In brief, inclusion criteria included patients with acute hemispheric ischemic stroke who presented 3 to 6 h after symptom onset, were 18 years of age or older, had a National Institutes of Health Stroke Scale score of >4, and had a premorbid modified Rankin score (mRS) of ≤1. Patients were imaged with 1.5-Tesla echoplanar-equipped MRI scanners. The DWI, perfusion-weighted imaging (PWI), and intracranial magnetic resonance angiography (MRA) sequences were obtained before treatment, and at day 3 to 5. T2-weighted imaging, to measure final infarct volume, was obtained at day 90. The MRA was not standardized across sites, 13 of the 15 recruiting sites performed time of flight MRA and 2 sites (20 patients recruited) performed phase contrast MRA. Extracranial MRA was not performed.
All MRI scans were read at the coordinating center by investigators blinded to treatment assignment and clinical outcomes. The DWI lesions were assessed by two independent raters who used standard planimetric software (Analyze 7.0; Biomedical Imaging Resource, Mayo Clinic, Rochester, MN, USA). Postprocessing of perfusion data was performed centrally with deconvolution algorithms to create maps of Tmax, defined as the time to peak of the impulse response (De Silva et al, 2009). The arterial input function was selected from the contralateral middle cerebral artery, with no correction for associated stenosis of the ICA. The PWI lesion definition was varied according to the severity of the threshold for delay. Thus, Tmax thresholds were varied from 2 to 12 secs in 2 secs increments (e.g., Tmax+2 secs, Tmax+4 secs, Tmax+6 secs). The MRA images were analyzed by a neurologist and neuroradiologist to determine the presence and site of occlusion. Reperfusion was defined as >90% reduction in the size of the Tmax+2 secs lesion at day 3 to 5 (Davis et al, 2008). The presence of parenchymal hematoma (PH) was classified by consensus among three raters, using the European Cooperative Acute Stroke Study algorithm (Hacke et al, 1998). Neurological impairment was measured with the National Institutes of Health Stroke Scale by a certified investigator before therapy. Clinical outcome was assessed at day 90 with the mRS by an investigator blinded to MRI findings and treatment assignment. Excellent outcome was defined as mRS 0 to 1, good outcome as mRS 0 to 2, and poor outcome mRS 5 to 6 at day 90.
Statistical Analysis
Statistical analysis was performed using STATA (Version 10, 2001; College Station, TX, USA). First, logistic regression was performed to examine the effects of acute DWI lesion volume, acute Tmax lesion volumes, and mismatch ratio on both excellent (mRS: 0 to 1) and poor (mRS: 5 to 6) outcome. A regression model was generated for each Tmax threshold (e.g., Tmax+2 secs, Tmax+4 secs, up to Tmax+12 secs). This also dictated how the mismatch ratio was defined (e.g., Tmax+2 secs lesion volume/DWI lesion volume). All three independent variables were used as continuous variables.
On the basis of the results of the regression modeling, recursive-partitioning analysis was used to determine the optimal cut points for acute DWI lesion volume and acute Tmax lesion volumes in predicting both excellent and poor clinical outcome. Recursive partitioning is a form of decision-tree analysis that automatically generates cut points that best separate the data in terms of a dichotomous outcome, using nominated variables (Sedrakyan et al, 2006). Using the lesion volume cut points generated by recursive partitioning, logistic regression was then used to determine whether treatment group (tPA or placebo) changed the odds of an excellent or poor outcome in patients fulfilling the acute Tmax and DWI lesion cut point inclusion/exclusion criteria. In a similar manner, the influence of increasing Tmax threshold and mismatch ratio (in place of the Tmax and DWI lesion cut point criteria) on the effect of treatment group on clinical outcome was examined. We also explored the influence of other baseline factors such as stroke severity, age, time to treatment, and a diagnosis of diabetes on the Tmax and DWI lesion volume cut point regression models. An additional variable, volume of hypoperfused tissue with Tmax+12 secs, was added to the regression models for poor outcome given recent evidence that poor collateral status may worsen outcome after thrombolysis (Bang et al, 2008; Miteff et al, 2009). Thus, the volume of tissue with the greatest delay in Tmax measured in our study (>12 secs), was used as a marker of poor collateral flow. We also examined the influence of Tmax+12 secs delay on subsequent reperfusion and infarct growth (follow-up infarct volume—acute DWI volume). A further exploratory analysis of poor outcome where the presence of ICA occlusion was substituted in place of large Tmax lesions was performed.
Finally, after identifying that an acute DWI lesion cut point was a strong predictor of response to IV-tPA, we performed an analysis to explore the mechanisms behind this. We performed logistic regression of excellent and poor outcomes using a DWI lesion cut point as an independent variable, as well as other potential baseline imaging predictors of outcome; Tmax+2 secs lesion volume, extent of Tmax+12 secs lesion delay (as a marker of poor collateral flow), and occlusion site on MRA. We also examined the influence of subsequent reperfusion and infarct growth (follow-up infarct volume—acute DWI volume) on the ability of the DWI lesion cut point to predict outcome of this analysis. These variables were also compared between patients above and below the DWI lesion cut point.
Results
There were 99 of 100 patients enrolled in EPITHET with functional outcome determined at day 90, and 98 patients had both baseline PWI and DWI, of whom 49 were randomized to tPA and 49 to placebo.
Regression Modeling
Acute Tmax+2 secs and DWI lesion volumes were independent predictors of excellent (smaller volumes) and poor (larger volumes) outcome. As Tmax threshold increased, the predictive ability of Tmax lesion volume increased until the Tmax+8 secs threshold was reached, with Tmax+8 secs volume the best univariate predictor of clinical outcome (Table 1). Tmax+8 secs combined with DWI volume was the best overall predictive model. Perfusion–diffusion mismatch ratio was not a predictor of outcome at any Tmax threshold, in either univariate or multivariate analyses.
Table 1. Regression models of excellent outcome.
| Univariate | Multivariate (Tmax vol or MM with DWI) | ||||
|---|---|---|---|---|---|
| Variable | P value | R2 | DWI vol P value in equation | Tmax vol or MM ratio P value in equation | Combined R2 |
| DWI vol | 0.003 | 0.086 | |||
| Tmax2 vol | 0.003 | 0.090 | 0.037 | 0.023 | 0.131 |
| Tmax4 vol | 0.001 | 0.112 | 0.079 | 0.013 | 0.141 |
| Tmax6 vol | 0.001 | 0.119 | 0.111 | 0.011 | 0.143 |
| Tmax8 vol | 0.0001 | 0.121 | 0.152 | 0.011 | 0.153 |
| Tmax10 vol | 0.001 | 0.112 | 0.195 | 0.028 | 0.128 |
| Tmax12 vol | 0.001 | 0.110 | 0.208 | 0.040 | 0.121 |
| Tmax2MM | 0.509 | 0.005 | 0.003 | 0.345 | 0.091 |
| Tmax4MM | 0.507 | 0.005 | 0.003 | 0.342 | 0.091 |
| Tmax6MM | 0.501 | 0.005 | 0.003 | 0.337 | 0.091 |
| Tmax8MM | 0.488 | 0.005 | 0.003 | 0.315 | 0.092 |
| Tmax10MM | 0.455 | 0.006 | 0.003 | 0.284 | 0.093 |
Abbreviations: MM, mismatch ratio; Vol, volume.
Recursive Partitioning
Given that perfusion–diffusion mismatch ratio did not add to prediction of clinical outcome, the recursive partitioning analysis was restricted to DWI and Tmax lesion volumes. Two Tmax thresholds were used separately in combination with DWI lesion volume: (1) Tmax+2 secs volume, as this was the primary perfusion lesion definition in EPITHET and (2) Tmax+8 secs volume—as this was the best predictor of clinical outcome in the regression modeling.
Recursive Partitioning—Excellent Outcome (Modified Rankin Score: 0 to 1)
Baseline DWI lesion volume (Supplementary Figure 1a) was the first discriminator of excellent outcome in decision-tree analysis using the Tmax+2 secs model. Twenty-two of 46 (48%) patients with baseline DWI lesions <18 mL had an excellent outcome compared with only 8/53 (15%) with DWI lesions >18 mL (λ2=12.5, P<0.001). In patients above and below the DWI 18 mL cut point, the next discriminator of excellent outcome was a very large Tmax+2 secs lesion (>190 mL). In patients with DWI above 18 mL, only 1/28 (4%) patients with Tmax+2 secs lesions >190 mL had an excellent outcome, compared with 7/24 (29%) with Tmax+2 secs lesions <190 mL. In the DWI above 18 mL group with Tmax+2 secs lesions <190 mL, an additional DWI cut point was discriminatory. Three of six (50%) patients with DWI lesions between 18 and 25 mL had an excellent outcome, compared with only four of 18 (22%) patients with DWI lesions >25 mL. Overall, the group with baseline Tmax+2 secs lesions <190 mL and DWI <18 mL had high rates of excellent outcome (17/31, 55%). However within this group, a small Tmax+2 secs lesion (<20 mL) was also discriminatory; 5 of the 7 patients with small Tmax+2 secs lesions had an excellent outcome (71%), compared with 12/24 (50%) with Tmax+2 secs lesions >20 mL.
For decision-tree analysis using Tmax+8 secs, perfusion lesion volume >150 mL was the strongest discriminator of outcome (Supplementary Figure 1b). None of the 23 patients with a Tmax+8 secs lesion >150 mL had an excellent outcome, compared with 30 of 76 (39%) with lesions below the 150 mL cut point (P<0.001). Next, patients with small (<10 mL) Tmax+8 secs lesions had higher rates of excellent outcome (9/15, 57%) than those with lesions between 10 and 150 mL (22/62, 35%). The final discriminatory cut point in those with Tmax+8 secs lesions between 10 and 150 mL was an acute DWI lesion of 25 mL; 18 of 40 (45%) patients with DWI <25 mL an excellent outcome compared with only 4/22 (18%) patients with DWI >25 mL.
Recursive Partitioning—Poor Outcome (Modified Rankin Score: 5 to 6)
In the Tmax+2 secs model, acute DWI lesion volume (Supplementary Figure 2a) was the strongest discriminator of poor outcome; 12 of 17 (71%) patients with baseline DWI lesions >65 mL had a poor outcome compared with only 16/82 (20%) with DWI <65 mL (λ2=18.1, P<0.001). In patients with acute DWI below 65 mL, very large (>190 mL) Tmax+2 secs volume was the next discriminator; with 9/31 (29%) patients having a poor outcome compared with 7/51 (14%) patients with Tmax+2 secs lesions below 190 mL. Notably, none of the eight patients with Tmax+2 secs lesions below 20 mL had a poor outcome, compared with 7 of 43 (16%) with Tmax+2 secs lesions between 20 and 190 mL. In this group (DWI <65 mL and Tmax+2 secs lesion between 20 and 190 mL), an additional DWI cut point further discriminated the chance of poor outcome. Four of 13 (31%) patients with a DWI lesion >25 mL had a poor outcome compared with only 3/30 (10%) with acute DWI below 25 mL.
The acute DWI lesion cut point of 65 mL was also the strongest discriminator of poor outcome with the Tmax+8 secs model (Supplementary Figure 2b). In patients with DWI lesions below 65 mL, a very large (>150 mL) Tmax+8 secs lesion was a discriminator of poor outcome, with 4/11 (36%) patients having a poor outcome compared with 12/71 (17%) with Tmax+8 secs lesions below 150 mL.
Effect of the Application of Tmax and Diffusion-Weighted Imaging Volume Selection Criteria by Treatment Group—Excellent Outcome (Modified Rankin Score: 0 to 1)
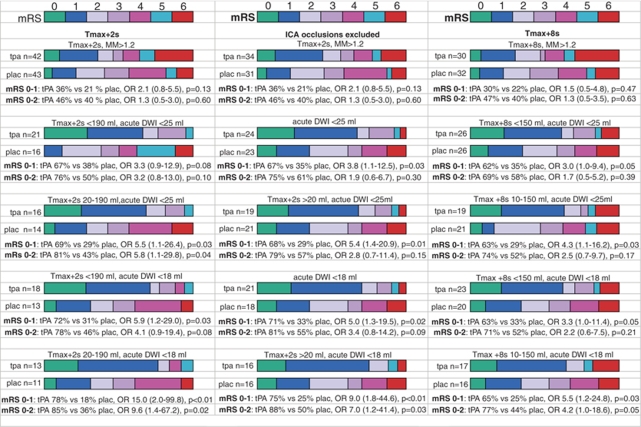
With the primary EPITHET perfusion–diffusion mismatch definition (Tmax+2 secs mismatch ratio >1.2) tPA-treated patients had a nonsignificant 15% increase in excellent outcome compared with the placebo group (Figure 1). Applying the first two cut points from the Tmax+2 secs recursive partitioning model (DWI lesion <18 mL and Tmax+2 secs lesion <190 mL), tPA-treated patients had a 41% increase in excellent outcomes (odds ratio (OR)=5.9, P=0.027). If small Tmax+2 secs lesions (<20 mL) were also excluded, then excellent outcomes in the placebo group dropped even further, making the odds in favor of tPA very high (OR=15.0, P=0.008), albeit with less than a quarter of the original EPITHET cohort of patients. There was a similar benefit in favor of tPA for good outcome using these selection criteria (Figure 1). In regression analysis of the patients fulfilling these cut points, age was the only other univariate baseline predictor of excellent outcome in these patients, but was not significant in multivariate analysis, whereas tPA treatment was (Table 2).
Figure 1.
Graphic comparison of the modified Rankin Scale for tPA versus placebo patients fulfilling various PWI, DWI, and ICA occlusion criteria for excellent and good outcomes. Below the graphs for each cut point are the odds ratio (OR) for tPA versus placebo for excellent (mRS: 0 to 1) and good (mRS: 0 to 2) outcomes. MM, mismatch ratio. 95% confidence intervals are displayed in brackets beside the odds ratio.
Table 2. Baseline predictors of excellent outcome in patients fulfilling the various selection criteria.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | P value | OR (95% CI) | P value |
| Tmax +2 secs 20–190 mL, acute DWI <18 mL | ||||
| Age | 0.81 (0.67–0.98) | 0.032 | 0.71 (0.48–1.05) | 0.089 |
| tPA | 15.0 (2.0–111.2) | 0.008 | 29.14 (1.37–617.6) | 0.030 |
| Acute NIHSS | 1.0 (0.81–1.23) | 1.000 | ||
| Diabetes | 0.60 (0.81–4.45) | 0.617 | ||
| Tmax+2 secs MM | 1.04 (0.96–1.12) | 0.324 | ||
| Time to Rx | 1.01 (0.99–1.03) | 0.411 | ||
| Tmax +8 secs 10–150 mL, acute DWI <18 mL | ||||
| Age | 0.95 (0.89–1.01) | 0.095 | 0.94 (0.88–1.01) | 0.091 |
| tPA | 5.5 (1.22–24.81) | 0.027 | 6.5 (1.25–33.27) | 0.026 |
| Acute NIHSS | 0.88 (0.72–1.07) | 0.194 | ||
| Diabetes | 0.54 (0.08–3.45) | 0.514 | ||
| Tmax+8 secs MM | 0.97 (0.91–1.04) | 0.424 | ||
| Time to Rx | 1.01 (0.99–1.02) | 0.269 | ||
| ICA occlusion excluded, Tmax +2 secs >20 mL, acute DWI <18 mL | ||||
| Age | 0.92 (0.84–1.01) | 0.095 | 0.92 (0.83–1.02) | 0.111 |
| tPA | 9.0 (1.8–44.6) | 0.007 | 9.48 (1.68–53.36) | 0.011 |
| Acute NIHSS | 0.93 (0.77–1.12) | 0.449 | ||
| Diabetes | 0.43 (0.66–2.76) | 0.373 | ||
| Tmax+2 secs MM | 1.04 (0.96–1.12) | 0.324 | ||
| Time to Rx | 1.00 (0.99–1.02) | 0.713 | ||
Abbreviations: CI, confidence interval; MM, mismatch ratio; NIHSS, National Institutes of Health Stroke Scale.
If the more lenient DWI lesion >25 mL exclusion cut point from decision-tree analysis was applied, in combination with the exclusion of both very large Tmax+2 secs (>190 mL) and small Tmax+2 secs (<20 mL) lesions, there was still a 40% absolute increase in excellent outcome with tPA (OR=5.5, P=0.033). Similar benefit for tPA treatment was seen for mRS 0 to 2 (OR=5.8, P=0.04), and, the proportion of eligible cases also increased slightly to 30% of the EPITHET cohort (Figure 1). Further increasing the DWI lesion exclusion cut point to >30 mL raised the sample to 38% of the original EPITHET sample, but the odds of excellent outcome from tPA dropped and were not significant (14/22, 64% versus 6/16, 38% OR=2.9, P=0.116). Increasing Tmax+2 secs perfusion–diffusion mismatch ratio did not increase the odds of excellent outcome with tPA (Supplementary Figure 3).
Using the cut points from decision-tree analysis from the Tmax+8 secs model; tPA-treated patients with Tmax+8 secs lesions between 10 and 150 mL and DWI <25 mL had a 34% absolute increase in excellent outcome compared with the placebo group (OR=4.3, P=0.032), although the 22% increase in good outcome was not significant (Figure 1). These cut points included 40% of the original EPITHET sample. If the more stringent DWI cut point (<18 mL) was applied, there were significant increases in excellent outcome (40%) and good outcome (30%) with tPA treatment (Figure 1). There were no other significant univariate baseline predictors of excellent outcome in these patients. Further increasing the DWI exclusion cut point to >30 mL raised the sample to 53% of the original EPITHET population, but the odds of tPA increasing excellent outcomes dropped and were not significant (16/27, 59% versus 9/26, 35% OR=2.8, P=0.076). In regression analysis of the patients fulfilling the Tmax+8 secs lesion volume 10 to 150 mL and DWI<18 mL criteria, age was the only other univariate baseline predictor of excellent outcome, but was not significant in multivariate analysis, whereas tPA treatment was (Table 2). Increasing Tmax+8 secs perfusion–diffusion mismatch ratio did not increase the odds of excellent outcome with tPA (Supplementary Figure 3).
Effect of the Application of Tmax and Diffusion-Weighted Imaging Volume Selection Criteria by Treatment Group—Poor Outcome (Modified Rankin Score: 5 to 6)
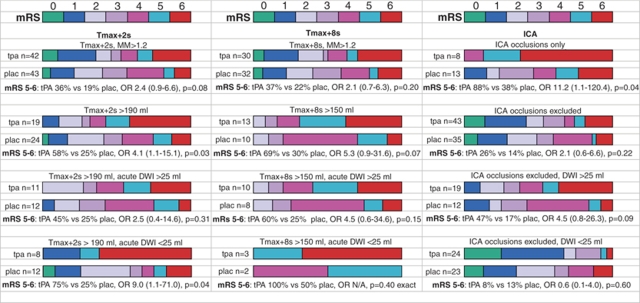
Patients with baseline DWI lesion >65 mL had a very high rate of poor outcome, regardless of treatment group (Supplementary Figure 4). However, in patients with Tmax+2 secs above 190 mL (regardless of DWI lesion volume), the odds of a poor outcome were increased in tPA treated compared with placebo patients (Figure 2). Age, and the extent of Tmax+12 secs delay were also univariate predictors of poor outcome in this group (Table 3). Notably, the volume of the PWI lesion with Tmax+12 secs delay tended to be higher in the tPA group (median 94 mL versus 55 mL, P=0.09), as was the extent of acute-day 90 infarct expansion (median 80 mL in tPA group versus 55 mL in placebo group, P=0.14). This was despite a similar, if not higher, rate of reperfusion in the tPA group (7/16 versus 4/24 placebo, P=0.08). However, the odds of poor outcome remained higher with tPA in multivariate analysis, even after correcting for Tmax+12 secs delay (Table 3).
Figure 2.
Graphic comparison of the modified Rankin Scale for tPA versus placebo patients fulfilling various PWI, DWI, and ICA occlusion criteria for poor outcomes. Below the graphs for each cut point are the odds ratio (OR) for tPA versus placebo for poor (mRS: 5 to 6) outcomes. MM, mismatch ratio. 95% confidence intervals are displayed in brackets beside the odds ratio.
Table 3. Baseline predictors of poor outcome—large Tmax lesions or ICA occlusion.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | P value | OR (95% CI) | P value |
| Tmax +2 secs>190 mL | ||||
| Age | 1.12 (1.01–1.22) | 0.003 | 1.17 (1.05–1.31) | 0.004 |
| tPA | 4.13 (1.11–15.1) | 0.032 | 7.99 (1.12–57.14) | 0.028 |
| Acute NIHSS | 1.11 (0.98–1.27) | 0.108 | ||
| Diabetes | 2.29 (0.52–10.18) | 0.276 | ||
| Acute DWI vol | 1.00 (0.99–1.02) | 0.437 | ||
| Tmax +12 secs vol | 1.02 (1.01–1.03) | 0.023 | 1.02 (1.0–1.04) | 0.061 |
| Tmax +8 secs>150 mL | ||||
| Age | 1.14 (1.02–1.27) | 0.017 | 1.12 (1.02–1.29) | 0.045 |
| tPA | 5.25 (0.87–31.55) | 0.070 | 9.31 (0.9–340.9) | 0.065 |
| Acute NIHSS | 1.06 (0.87–1.28) | 0.578 | ||
| Diabetes | 0.89 (0.14–5.72) | 0.901 | ||
| Acute DWI vol | 1.00 (0.99–1.02) | 0.876 | ||
| Tmax +12 secs vol | 1.03 (1.01–1.05) | 0.043 | 1.02 (1.0–1.05) | 0.083 |
| Internal carotid artery occlusion | ||||
| Age | 1.15 (1.03–1.29) | 0.017 | 1.16 (1.01–1.33) | 0.031 |
| tPA | 11.2 (1.06–120.4) | 0.046 | 11.66 (0.9–177.5) | 0.075 |
| Acute NIHSS | 1.05 (0.9–1.23) | 0.505 | ||
| Diabetes | 1.75 (0.24–12.64) | 0.579 | ||
| Acute DWI vol | 1.01 (0.99–1.03) | 0.212 | ||
| Tmax +12 secs vol | 1.02 (1.00–1.04) | 0.081 | 1.01 (1.0–1.04) | 0.098 |
Abbreviations: CI, confidence interval; NIHSS, National Institutes of Health Stroke Scale; vol, volume.
Similarly, there was a strong trend for poor outcome to be increased with tPA in patients with Tmax +8 secs lesions >150 mL (Table 3), and again, poor outcomes were high in the tPA group regardless of DWI lesion volume (Figure 2). Thus, patients with very large Tmax+2 secs (>190 mL) or Tmax+8 secs (>150 mL) lesions showed no benefit from IV-tPA.
Additional Exploratory Analysis—Role of Internal Carotid Artery Occlusion
Given the high rate of poor outcomes seen in patients with very large Tmax+2 secs and +8 secs lesions, we hypothesized that ICA occlusion may have a similar association with poor outcome (Tables 3 and 4). There were nine patients with baseline ICA occlusion in the tPA group (one did not have 3-month outcome studies), and 13 in the placebo group. Only one of these patients had a clear terminal ICA occlusion on MRA, the remainder had no flow observed in the entire intracranial ICA. Reperfusion was considerably more frequent in the patients without ICA occlusion (31/63 versus 1/19, P<0.001). We therefore repeated the original regression modeling of acute DWI volume, Tmax volumes, and mismatch ratios on clinical outcome in the 79 patients without ICA occlusion (Supplementary Table 1). As large mismatch ratios may be seen with ICA occlusion, combined with the lack of reperfusion seen in these patients, we wished to exclude this as an explanation as to why mismatch ratio did not predict outcome in the original analysis. However, repeat regression modeling with ICA occlusions excluded did not alter the original regression results. Smaller Tmax and DWI lesion volumes were still independent predictors of excellent outcome, with Tmax+8 secs volume the best individual predictor of clinical outcome. Again, perfusion–diffusion mismatch ratio was not a predictor of outcome at any Tmax threshold, in either univariate or multivariate (combined with DWI volume) analyses.
Table 4. Role of acute DWI lesion cut point (<>25 mL) in predicting excellent and poor outcome (patients without ICA occlusion).
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | P value | OR (95% CI) | P value |
| Excellent outcome—placebo group (n=35) | ||||
| DWI <25 mL | 2.7 (0.5–15.3) | 0.270 | ||
| Excellent outcome—tPA (n=43) | ||||
| DWI <25 mL | 17.0 (3.1–92.4) | 0.001 | 15.8 (2.03–99.8) | 0.008 |
| Infarct growth | 0.99 (0.97–1.00) | 0.132 | ||
| Occlusion site | 0.99 (0.81–1.19) | 0.886 | ||
| Tmax +2 secs vol | 1.0 (0.99–1.00) | 0.256 | ||
| Tmax +12 secs vol | 0.98 (0.96–1.00) | 0.079 | 0.99 (0.98–1.02) | 0.983 |
| Reperfusion | 1.0 (0.21–4.56) | 1.0 | ||
| Poor outcome—placebo group (n=35) | ||||
| DWI >25 mL | 1.3 (0.2–9.3) | 0.772 | ||
| Poor outcome—tPA (n=43) | ||||
| DWI >25 mL | 9.9 (1.8–54.4) | 0.008 | 1.78 (0.19–16.5) | 0.313 |
| Infarct growth | 1.02 (1.01–1.04) | 0.017 | 1.03 (1.00–1.07) | 0.040 |
| Occlusion site | 0.96 (0.74–1.24) | 0.742 | ||
| Tmax +2 secs vol | 1.0 (0.99–1.00) | 0.150 | ||
| Tmax +12 secs vol | 1.02 (1.00–1.03) | 0.055 | 1.02 (0.99–1.05) | 0.068 |
| Reperfusion | 0.26 (0.04–1.90) | 0.184 | ||
| tPA patients (n=43) | ||||
| Variable | DWI <25 mL | DWI >25 mL | P value | |
| Infarct growth | 0 (0–12.1) | 7.9 (0–93.1) | 0.118 | |
| Occl site (M1) | 7/22 (32%) | 6/15 (40%) | 0.609 | |
| Occl site (M2) | 4/22 (18%) | 2/15 (13%) | 1.0 | |
| Occl site (nil) | 11/22 (50%) | 7/15 (47%) | 0.842 | |
| Tmax +2 secs vol | 122 (41–168) | 156 (99–220) | 0.178 | |
| Tmax +12 secs vol | 8 (0–16) | 49 (17–121) | 0.002 | |
| Reperfusion | 13/18 (72%) | 8/13 (62%) | 0.701 | |
Abbreviations: CI, confidence interval; Occl, occlusion, lesion volumes are median and inter-quartile range in brackets.
Perfusion lesions were very large in the ICA occlusion group. Median Tmax+2 secs volume in the tPA group was 288 mL versus 265 mL in the placebo group (P=0.87). Indeed, 19 of the 43 patients with very large Tmax+2 secs (>190 mL) lesions had ICA occlusion, compared with only 3/55 patients with Tmax+2 secs <190 mL (P<0.001). In the ICA occlusion group, more severe Tmax delay lesion volumes were also large, and clinical severity was high; suggesting that isolated extracranial ICA occlusion/high-grade stenosis was unlikely. Median Tmax+8 secs volume in the tPA group was 163 mL versus 155 mL in the placebo group (P=0.48), and median baseline National Institutes of Health Stroke Scale was 15 in the tPA group versus 17 in the placebo group (P=0.82).
For patients with ICA occlusion, the odds of a poor outcome were increased in the tPA group (tPA 7/8 versus placebo 5/13, OR=11.2, P=0.046). This was not due to PH, as only 1 of the 11 PH in the tPA group was in a patient with ICA occlusion, and none of the placebo patients with ICA occlusion had PH. Age was a significant predictor of poor outcome in univariate and multivariate analysis (Table 3). There was also a trend for the extent of Tmax+12 secs delay to be a univariate and multivariate predictor of poor outcome. Notably, Tmax+12 secs delay tended to be higher in the tPA group (median 91 mL versus 64 mL, P=0.11) and the extent of acute-day 90 infarct expansion also tended to be higher in tPA patients (median 137 mL versus 88 mL, P=0.19). After correcting for age and extent of Tmax+12 secs delay, the odds of poor outcome with tPA remained high but were not significant (Table 3).
Only 2 of 21 patients with ICA occlusion, both in the placebo group, had an excellent outcome. If ICA occlusion was substituted as an exclusion criterion instead of a very large PWI lesion, along with the exclusion of small Tmax +2 secs lesions (<20 mL) and larger DWI lesions (>18 mL), the odds of excellent outcome with tPA were very high (OR=9.0, P=0.005), as were the odds of mRS 0 to 2 (Figure 1). There were no other significant univariate baseline predictors of excellent outcome in this group (Table 2). There was also a higher proportion (32%) of the original EPITHET cohort than the 24% selected using the very large Tmax +2 secs lesion (>190 mL) exclusion. If the higher DWI exclusion cut point (>25 mL) was applied in combination with the exclusion of ICA occlusions and small Tmax+2 secs (<20 mL) lesions, the odds of tPA benefit dropped somewhat but were still significant for excellent outcome (OR=5.42, P=0.01). Furthermore, the proportion of the original EPITHET sample increased to 40% (Figure 1).
Similar increased odds of excellent outcome with tPA (OR=6.24, P=0.014) were seen if ICA occlusions, small Tmax+8 secs lesions (<10 mL), and larger acute DWI lesions (>25 mL) were excluded (Supplementary Figure 3). Higher odds of tPA benefit were again seen with exclusion of DWI lesions above 18 mL for excellent (OR=9.2 P=0.01), and good outcomes (OR=7.6, P=0.03). Thus, using ICA occlusion as an exclusion rather than either of the very large Tmax (+2 secs or +8 secs) lesion cut points increased odds of excellent outcome with tPA, with equivalent or less removal of subjects from the original EPITHET population (Figure 1).
Why was Baseline Diffusion-Weighted Imaging Lesion Volume Cut Point such a Strong Predictor of tPA Response?
In patients with ICA occlusion, poor outcomes were seen above and below the DWI lesion cut point of 25 mL (Figure 2). In this group, reperfusion was minimal and the infarct expansion was considerable no matter what baseline DWI lesion volume was. Conversely, in patients without ICA occlusion, a DWI lesion below 25 mL was a very strong predictor of excellent outcome, and, substantially reduced the odds of a poor outcome (Table 4). However, this effect seemed predominantly to be in tPA patients; in that the odds of excellent outcome below a DWI cut point of 25 mL in the tPA group were extremely high (and odds of poor outcome extremely low), but the DWI 25 mL cut point did not significantly affect outcomes in the placebo group.
Considering the tPA patients in isolation: for excellent outcome, only the DWI 25 mL cut point and Tmax+12 secs volume were univariate predictors (infarct expansion was not). Only the DWI cut point remained significant in multivariate analysis (Table 4). For poor outcome in the tPA group: infarct expansion, the DWI 25 mL cut point, and Tmax+12 secs volume were univariate predictors. Only infarct expansion remained significant in multivariate analysis. With respect to comparison of baseline and follow-up imaging variables between tPA patients, arterial occlusion site did not differ between those with acute DWI <25 and >25 mL. However, despite similar Tmax+2 secs volumes, there was a clear difference in extent of Tmax+12 secs delay between the DWI <25 and >25 mL groups (Table 4). As well as worse collateral status in the larger DWI group, there appeared to be a much larger range of infarct expansion (95% centile for infarct expansion was 176 mL in the large DWI group versus only 18 mL in the small DWI group), but this was not significant in the overall comparison between the groups. Reperfusion rates were similar in the DWI <25 and >25 mL groups (Table 4). Thus, a baseline DWI lesion <25 mL was the most important determinant of an excellent outcome from tPA, and the extent of subsequent infarct expansion was the most important determinant of poor outcome from tPA, and was independent of the DWI 25 mL cut point.
Discussion
This study has identified a subgroup of ischemic stroke patients who benefit dramatically from IV thrombolytic therapy 3 to 6 h after symptom onset. These are patients with a baseline DWI lesion of <18 mL, who have a substantially increased chance of good or excellent outcomes if treated with tPA. However, the odds of benefit from tPA dropped rapidly with increasing baseline DWI lesion volume, with little treatment benefit with a DWI lesion above 25 mL. Thrombolytic treatment also resulted in no benefit in patients with very large PWI lesions (Tmax+2 secs >190 mL or Tmax+8 secs >150 mL). Conversely, both tPA- and placebo-treated patients with small perfusion lesions (Tmax+2 secs <20 mL or Tmax+8 secs <10 mL) had high rates of excellent outcome. These tPA-responder or nonresponsive groups could not be identified by perfusion–diffusion mismatch alone, implying that the mismatch concept in isolation is probably too simplistic as an imaging-based selection approach for thrombolysis.
Large baseline DWI lesions (>65 mL) were strongly associated with poor outcome. A similar DWI lesion volume cut point (70 mL) for poor outcome has recently been identified with intra-arterial thrombolysis (Yoo et al, 2009). Interestingly, these volumes are significantly lower than the previously suggested >100 mL DWI lesion volume exclusion derived from observational studies (Albers et al, 2006; Schellinger et al, 2007). Moreover, patients with DWI lesions between 25 and 65 mL were not necessarily harmed by tPA, but there did not appear to be a positive treatment effect in these patients.
This study is the first placebo-controlled trial to show that patients with very large PWI lesions fail to benefit from tPA although others have suggested very large perfusion lesions may be a marker of poor response to tPA (Albers et al, 2006; Schellinger et al, 2007). Although larger baseline PWI deficits correlate with larger DWI lesions, this was not the mechanism of the adverse effect of treatment, as tPA-treated patients with small DWI lesions (but large PWI lesions) also had greater odds of poor outcome (Figure 2). The high rate of poor outcomes seen overall in the patients with large perfusion lesions is probably explained by the close relationship with ICA occlusion, as both spontaneous and IV-tPA recanalization rates are low in patients with ICA occlusion (Rha and Saver, 2007). However, it is more difficult to explain why worse outcomes were seen in the tPA group compared with placebo. Potential confounders include the relatively small number of cases in this analysis, and we could not be certain whether these patients had extensive intracranial ICA thrombus, tandem ICA/middle cerebral artery occlusion, or isolated extracranial ICA occlusion, which should carry a better prognosis (Gupta et al, 2006). However, the patients in the ICA group did have very severe clinical deficits and very large lesions with severe Tmax delay, making isolated extracranial ICA occlusion/stenosis very unlikely.
A possible explanation for the worse outcomes seen in the tPA patients with large PWI lesions/ICA occlusion may relate to differences in collateral flow observed between the tPA and placebo groups. We used volume of tissue with very severe Tmax delay (+12 secs) as a marker of poor collateral flow, as collateral flow is poorly visualized directly on conventional MRA sequences (Bang et al, 2008; Miteff et al, 2009). Extent of Tmax+12 secs delay was a predictor of poor outcome in the large PWI/ICA occlusion groups. Furthermore, extent of Tmax+12 secs delay tended to be higher in the tPA than the placebo patients in this group, as did infarct expansion. Thus, it is possible that the higher rate of poor outcome seen in tPA patients with large PWI/ICA occlusion may have been at least partially related to poorer collateral flow, contributing to greater infarct expansion. This occurred despite similar low rates of reperfusion in both tPA and placebo groups. Regardless of mechanism, our data suggest that strong consideration should be given to excluding patients with ICA occlusion (or very large PWI lesions) from IV-tPA therapy in the 3 to 6 h time window. Fewer patients will be excluded from thrombolytic therapy using the ICA occlusion approach, and the odds of tPA benefit are similar or higher. Our findings certainly emphasize the need to investigate alternative treatment approaches in patients with ICA occlusion.
The baseline DWI and PWI lesion volume selection criteria identify a subpopulation of the EPITHET cohort who have greater odds of benefit from tPA than recently seen in a similar time window, or earlier time windows, using standard clinical selection criteria, lending support to the notion that tissue-based selection may ultimately replace time-based criteria (Hacke et al, 2008). Although we have identified a subgroup of patients with the greatest odds of clinical benefit from thrombolysis, it is possible that patients with small PWI lesions may still occasionally benefit from tPA given that relatively small treatment effects may have been missed in our study. It is also important not to equate our findings as being incompatible with the mismatch hypothesis.
Treatment with tPA still attenuates infarct growth in patients with larger baseline DWI lesions, but our data support a DWI lesion volume ‘threshold' for clinical benefit from IV-tPA, in that the size of the acute DWI lesion has a critical effect on ultimate clinical outcome, as it reflects the amount of tissue already damaged. In patients without ICA occlusion, there was a dramatic difference in outcomes seen in tPA patients above and below the 25 mL DWI cut point. Baseline DWI lesion <25 mL was the only independent predictor of an excellent outcome from tPA (Table 4). This did not appear to reflect more distal arterial occlusion or greater subsequent reperfusion, which were similar in those above and below the DWI cut point. The poorer treatment response seen with larger acute DWI lesions also cannot be explained by more proximal arterial occlusion and lack of reperfusion. Indeed, extent of subsequent infarct expansion was the only multivariate determinant of poor outcome from tPA, and this was independent of the baseline DWI lesion cut point. Despite similar arterial occlusion sites and subsequent reperfusion, the DWI >25 mL group still had a trend toward more infarct expansion. Notably, collateral status was clearly poorer in the DWI>25 mL group, and this may well have contributed to both larger baseline DWI lesions and greater subsequent infarct growth, as recently demonstrated with CT (Miteff et al, 2009).
Excluding subgroups with small PWI and larger DWI lesions from a phase III imaging-based selection thrombolytic trial highlights the challenges involved in the design of such a trial. A sample size calculation indicates that only 17 patients in each arm would have 90% power of a significant result using the selection criteria that give the greatest absolute difference between excellent outcomes in tPA versus placebo groups (Tmax+2 secs lesion between 20 and 190 mL, and DWI lesion <18 mL). However, these criteria would have included only 24% of the EPITHET patients. Such strict entry criteria would not only hinder a phase III study's recruitment rate, but also limit the generalizability of any result from such a study. In terms of the best trade-off between study feasibility/generalizability and targeting treatment responders, we hypothesize that patients with an acute DWI lesion of >25 mL and/or ICA occlusion could be excluded from thrombolytic therapy. The ICA occlusion is probably preferable to a very large PWI lesion as an exclusion criterion because it is easy to identify and less patients will be excluded from thrombolytic therapy. Excluding patients with small PWI lesions is also associated with increased odds of benefit from tPA therapy, although this will also limit the study population. Nonetheless, it is important to include PWI in the selection algorithm, as it ensures that a biologically plausible imaging ‘target' is present for thrombolytic therapy. Further, the exclusion of small PWI lesions, in combination with a DWI lesion cut point identifies the tPA-responder group, whereas using a perfusion–diffusion mismatch ratio in isolation does not.
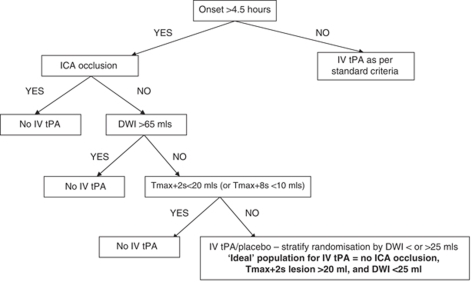
Limitations of our study include the testing of post hoc hypotheses on a single trial population, multiple statistical analyses, and potential risks of both type 1 and 2 errors. Furthermore, the patient numbers are relatively small. For these reasons, one must be particularly cautious about interpreting the size of treatment effects at the multiple cut points we have examined. We recognize that our conclusions should be considered hypothesis generating and require further testing. Despite these limitations, this study reignites the promise of imaging-based selection for thrombolysis and strongly supports the need for a phase III trial of IV-tPA using this approach in an extended time window. The group who have much higher odds of treatment benefit than seen with standard time-based clinical selection involves exclusion of patients with: (1) larger baseline DWI lesions (>25 mL), (2) ICA occlusion (or very large Tmax deficits), and (3) small PWI deficits (Tmax+2 secs <20 mL or Tmax+8 secs <10 mL). However, exclusion of patients with baseline DWI lesions above 25 mL from a phase III randomized placebo-controlled trial of IV-tPA may seem too radical a step. A more conservative approach might be to stratify randomization by baseline DWI lesion volume (e.g., <25 mL and 25 to 65 mL), and/or to have a prespecified interim analysis for both safety and futility for the larger baseline DWI lesion group. We propose a patient selection and treatment stratification algorithm (Figure 3) that pending further validation on an independent data set, could be used in future trials, such as the EXtension of Thrombolysis for Emergent Neurological Deficits trial. This is a phase III study aimed at testing the benefits and risks of IV-tPA in patients with a favorable PWI/DWI profile randomized 4.5 to 9 h after stroke onset (ClinicalTrials.gov number NCT00887328. http://clinicaltrials.gov/ct2/show/NCT00887328).
Figure 3.
Proposed imaging-based selection algorithm for a phase III IV-tPA/placebo-controlled trial in an extended time window.
Acknowledgments
The study was funded by grants from the Australian National Health and Medical Research Foundation, and National Stroke Foundation and received no pharmaceutical funding.
Dr Davis is a member of the Boehringer Ingelheim, and Servier advisory boards. He has received honoraria for lectures from Novo Nordisk, Servier, Sanofi-Aventis, Pfizer, and Boehringer Ingelheim. Dr Donnan is a member of the Boehringer Ingelheim, Servier, and Sanofi-Aventis advisory boards, has accepted honoraria or consultancy payments from, and has had the costs of participating in scientific meetings reimbursed by Boehringer Ingelheim, Sanofi-Aventis, and Servier. Dr Donnan has received grants from Sanofi-Aventis, and grants and consultancy payments from Boehringer Ingelheim. Dr Parsons has received honoraria or consultancy payments from Boehringer Ingelheim. Dr Levi has accepted honoraria or consultancy payments and travel grants from Boehringer Ingelheim and Sanofi-Aventis. Dr Butcher has accepted honoraria or consultancy payments from Boehringer Ingelheim, Sanofi-Aventis, and Roche. Dr Barber has received honoraria or consultancy payments from Pfizer. Drs De Silva, Christensen, Ebinger, and Bladin have no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, Bammer R, Kakuda W, Lansberg MG, Shuaib A, Coplin W, Hamilton S, Moseley M, Marks MP. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B, Kim D, Jahan R, Duckwiler GR, Yoon SR, Vinuela F, Liebeskind DS. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2008;79:625–629. doi: 10.1136/jnnp.2007.132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher K, Parsons M, Baird T, Barber A, Donnan G, Desmond P, Tress B, Davis S. Perfusion thresholds in acute stroke thrombolysis. Stroke. 2003;34:2159–2164. doi: 10.1161/01.STR.0000086529.83878.A2. [DOI] [PubMed] [Google Scholar]
- Butcher KS, Parsons M, MacGregor L, Barber PA, Chalk J, Bladin C, Levi C, Kimber T, Schultz D, Fink J, Tress B, Donnan G, Davis S. Refining the perfusion-diffusion mismatch hypothesis. Stroke. 2005;36:1153–1159. doi: 10.1161/01.str.0000166181.86928.8b. [DOI] [PubMed] [Google Scholar]
- Darby DG, Barber PA, Gerraty RP, Desmond PM, Yang Q, Parsons M, Li T, Tress BM, Davis SM. Pathophysiological topography of acute ischemia by combined diffusion-weighted and perfusion MRI. Stroke. 1999;30:2043–2052. doi: 10.1161/01.str.30.10.2043. [DOI] [PubMed] [Google Scholar]
- Davis SM, Donnan GA, Butcher KS, Parsons M. Selection of thrombolytic therapy beyond 3 h using magnetic resonance imaging. Curr Opin Neurol. 2005;18:47–52. doi: 10.1097/00019052-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, Barber PA, Bladin C, De Silva DA, Byrnes G, Chalk JB, Fink JN, Kimber TE, Schultz D, Hand PJ, Frayne J, Hankey G, Muir K, Gerraty R, Tress BM, Desmond PM. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- De Silva DA, Fink JN, Christensen S, Ebinger M, Bladin C, Levi CR, Parsons M, Butcher K, Barber PA, Donnan GA, Davis SM. Assessing reperfusion and recanalization as markers of clinical outcomes after intravenous thrombolysis in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) Stroke. 2009;40:2872–2874. doi: 10.1161/STROKEAHA.108.543595. [DOI] [PubMed] [Google Scholar]
- Gupta R, Vora NA, Horowitz MB, Tayal AH, Hammer MD, Uchino K, Levy EI, Wechsler LR, Jovin TG. Multimodal reperfusion therapy for acute ischemic stroke: factors predicting vessel recanalization. Stroke. 2006;37:986–990. doi: 10.1161/01.STR.0000209303.02474.27. [DOI] [PubMed] [Google Scholar]
- Hacke W, Albers G, Al-Rawi Y, Bogousslavsky J, Davalos A, Eliasziw M, Fischer M, Furlan A, Kaste M, Lees KR, Soehngen M, Warach S. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36:66–73. doi: 10.1161/01.STR.0000149938.08731.2c. [DOI] [PubMed] [Google Scholar]
- Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, Brott T, Frankel M, Grotta JC, Haley EC, Jr, Kwiatkowski T, Levine SR, Lewandowski C, Lu M, Lyden P, Marler JR, Patel S, Tilley BC, Albers G, Bluhmki E, Wilhelm M, Hamilton S. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- Hacke W, Furlan AJ, Al-Rawi Y, Davalos A, Fiebach JB, Gruber F, Kaste M, Lipka LJ, Pedraza S, Ringleb PA, Rowley HA, Schneider D, Schwamm LH, Leal JS, Sohngen M, Teal PA, Wilhelm-Ogunbiyi K, Wintermark M, Warach S. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2009;8:141–150. doi: 10.1016/S1474-4422(08)70267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D. Thrombolysis with alteplase 3 to 4.5 h after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Fieschi C. Randomised double-blind placebo controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II) Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- Hjort N, Butcher K, Davis SM, Kidwell CS, Koroshetz WJ, Rother J, Schellinger PD, Warach S, Ostergaard L. Magnetic resonance imaging criteria for thrombolysis in acute cerebral infarct. Stroke. 2005;36:388–397. doi: 10.1161/01.STR.0000152268.47919.be. [DOI] [PubMed] [Google Scholar]
- Kakuda W, Lansberg MG, Thijs VN, Kemp SM, Bammer R, Wechsler LR, Moseley ME, Marks MP, Albers GW. Optimal definition for PWI/DWI mismatch in acute ischemic stroke patients. J Cereb Blood Flow Metab. 2008;28:887–891. doi: 10.1038/sj.jcbfm.9600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell CS, Alger JR, Saver JL. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke. 2003;34:2729–2735. doi: 10.1161/01.STR.0000097608.38779.CC. [DOI] [PubMed] [Google Scholar]
- Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009;132 (Part 8:2231–2238. doi: 10.1093/brain/awp155. [DOI] [PubMed] [Google Scholar]
- Olivot JM, Mlynash M, Thijs VN, Kemp S, Lansberg MG, Wechsler L, Bammer R, Marks MP, Albers GW. Optimal Tmax threshold for predicting penumbral tissue in acute stroke. Stroke. 2009;40:469–475. doi: 10.1161/STROKEAHA.108.526954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- Schellinger PD, Thomalla G, Fiehler J, Kohrmann M, Molina CA, Neumann-Haefelin T, Ribo M, Singer OC, Zaro-Weber O, Sobesky J. MRI-based and CT-based thrombolytic therapy in acute stroke within and beyond established time windows: an analysis of 1210 patients. Stroke. 2007;38:2640–2645. doi: 10.1161/STROKEAHA.107.483255. [DOI] [PubMed] [Google Scholar]
- Sedrakyan A, Zhang H, Treasure T, Krumholz HM. Recursive partitioning-based preoperative risk stratification for atrial fibrillation after coronary artery bypass surgery. Am Heart J. 2006;151:720–724. doi: 10.1016/j.ahj.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group N Engl J Med. 1995. pp. 1581–1587. [DOI] [PubMed]
- Yoo AJ, Verduzco LA, Schaefer PW, Hirsch JA, Rabinov JD, Gonzalez RG. MRI-based selection for intra-arterial stroke therapy: value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke. 2009;40:2046–2054. doi: 10.1161/STROKEAHA.108.541656. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.