Abstract
Therapeutic hypothermia is a means of neuroprotection well established in the management of acute ischemic brain injuries such as anoxic encephalopathy after cardiac arrest and perinatal asphyxia. As such, it is the only neuroprotective strategy for which there is robust evidence for efficacy. Although there is overwhelming evidence from animal studies that cooling also improves outcome after focal cerebral ischemia, this has not been adequately tested in patients with acute ischemic stroke. There are still some uncertainties about crucial factors relating to the delivery of hypothermia, and the resolution of these would allow improvements in the design of phase III studies in these patients and improvements in the prospects for successful translation. In this study, we discuss critical issues relating first to the targets for therapy including the optimal depth and duration of cooling, second to practical issues including the methods of cooling and the management of shivering, and finally, of factors relating to the design of clinical trials. Consideration of these factors should inform the development of strategies to establish beyond doubt the place of hypothermia in the management of acute ischemic stroke.
Keywords: acute stroke, animal models, clinical trials, hypothermia, shivering MR spectroscopy
Introduction
Therapeutic hypothermia (TH) is one of the most promising treatment strategies for acute ischemic stroke for several reasons. It is the only neuroprotective treatment proven effective in large randomized trials in patients with acute brain injury (HACA, 2002; Bernard et al, 2002; Jacobs et al, 2007). Animal studies indicate a robust benefit of hypothermia after focal cerebral ischemia (van der Worp et al, 2007), without indicating critical publication bias (Sena et al, 2010). Moreover, in contrast to most other neuroprotective treatment strategies, multimodal pathophysiological effects on the ischemic cascade have been described. In contrast, TH has only been rudimentarily studied in stroke patients (Kollmar and Schwab, 2009). So far, no large randomized multicenter trial has been started. The reasons for the lack of appropriate studies may be multifactorial. First of all, no device or pharmacological company has provided sufficient sponsoring for such trials, nor did the government. In accordance, hypothermia in investigator-initiated trials has to compete with trials of the pharmacological industry. In addition, there are more questions than answers about the appropriate elements for TH as a treatment for stroke. Both animal and clinical data on hypothermia in focal cerebral ischemia are reviewed in this study, addressing research questions for future large trials.
Supporting animal data
After ischemia, neurons may die as a result of necrosis and apoptosis (Dirnagl et al, 1999). Important intermediate events include energy depletion, excitotoxicity, calcium influx, free radical formation, and inflammation. Immediately after vessel occlusion, a central core of very low perfusion is surrounded by an area with dysfunction from metabolic and ionic disturbances, but in which structural integrity is still preserved, the so-called ischemic penumbra (Astrup et al, 1981). This is why immediate clinical deficits do not necessarily reflect irreversible damage, and for this reason, the penumbra is the major treatment target in acute ischemic stroke. Depending on residual blood flow and duration of ischemia, the penumbra will eventually be incorporated into the infarct if reperfusion is not achieved. Most of the irreversible damage is inflicted in the first few hours after the onset of ischemia, but pathologic processes may continue for several days (Dirnagl et al, 1999), and in patients, the penumbral tissue has been found for up to 48 hours of ischemic stroke onset (Donnan et al, 2009).
On the basis of the concept of a salvageable ischemic penumbra, ∼500 ‘neuroprotective' treatment strategies have shown to improve outcome in animal models of acute ischemic stroke (O'Collins et al, 2006). In contrast, only very early intravenous thrombolysis with recombinant tissue plasminogen activator (rt-PA) and aspirin have proven to be efficacious in patients, despite numerous clinical trials of other treatment strategies in acute ischemic stroke (van der Worp et al, 2007; Donnan et al, 2008). One of the reasons for the failure of allegedly neuroprotective compounds in patients may be that most of them inhibit only a single step in the chain of events leading to cell death (STAIR, 1999; Gladstone et al, 2002).
Animal models of focal or global cerebral ischemia have suggested that hypothermia affects a wide range of cell death mechanisms, including energy depletion, disruption of the blood–brain barrier, free radical formation, excitotoxicity, and inflammation (Zhao et al, 2007; Yenari et al, 2008). Possibly mediated through these multiple and synergistic effects, the benefit of hypothermia in animal models is more consistent and robust than that of any other treatment strategy. In a systematic review and meta-analysis of animal studies of focal cerebral ischemia, including data obtained from a total of 3,353 animals, hypothermia reduced the infarct size by 44% (95% confidence interval, 40% to 47%) (van der Worp et al, 2007).
Reports on the efficacy of potential treatment strategies in animal models of stroke seem to be profoundly biased by the methodological quality of the studies, which may have contributed to the failure of the translation of these studies to the clinic (Sena et al, 2007a). Hypothermia is no exception. For example, in the systematic review, efficacy was significantly lower compared with random treatment allocation and blinded assessment of outcome than in those without. However, hypothermia still reduced the infarct size by ∼40% in studies of adequate methodological quality, lending support to a true benefit of this therapy (van der Worp et al, 2007).
There is empirical evidence that animal studies of ischemic stroke that report positive or significant results are more likely to be published. Such publication bias will lead to overestimation of treatment effects and can render the readily available evidence unreliable for decision making. It has been estimated that because of publication bias, the above-mentioned meta-analysis overstated the effect of hypothermia by ∼8% (Sena et al, 2010). Even after adjustment for this effect of publication bias, the benefit of hypothermia remains substantial.
The success of translation of animal studies to the clinic may in part depend on the diversity of species in which a particular treatment strategy was tested, and on the testing in animals with comorbidities relevant to the disease under study (STAIR, 1999; Gladstone et al, 2002). Unfortunately, of the 222 experimental comparisons in the systematic review, 214 (96%) involved rats, making any conclusion on differences in efficacy between species futile. The same applies to the effect of sex: 196 comparisons (89%) concerned male animals and 3 (1%) female animals, whereas in 21 comparisons (9%), the sex was unknown. In contrast to patients with stroke, animals in studies of focal cerebral ischemia are generally young (van der Worp et al, 2005). The effect of hypothermia has only recently been tested in 17-month-old rats, and has proven to be consistent with the results of the meta-analysis (Florian et al, 2008). In the systematic review, hypothermia was slightly more effective in hypertensive than in normotensive animals, but to date, just a single study has tested the effect in diabetic rats, preventing a robust conclusion on a potential interaction between hypothermia and diabetes mellitus (van der Worp et al, 2007).
Despite the above-mentioned limitations, we believe that hypothermia has been studied in sufficient detail and under a sufficiently broad variety of experimental conditions in animal models of ischemic stroke to support the translation of this treatment strategy to clinical trials.
What should the aims of therapy be?
Optimal Target Temperature
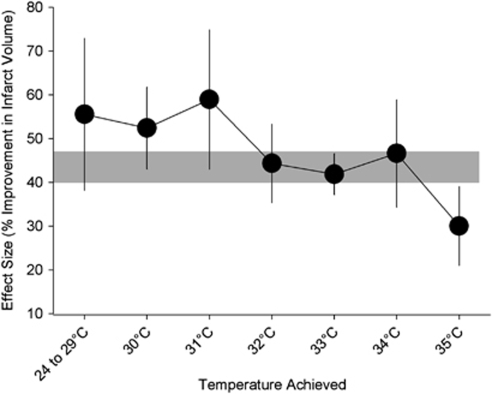
In the above-mentioned systematic review of animal studies, the benefit of hypothermia was inversely related to the temperature achieved (Figure 1). Hypothermia reduced the infarct size by >40% with temperatures of 34°C or below, but the infarct size was still reduced by 30% (95% confidence interval, 21% to 39%) with cooling to 35°C (van der Worp et al, 2007), suggesting that even very modest cooling has considerable potential as a neuroprotective strategy. A later study not included in this review compared cooling with 36, 35, 34, 33, and 32°C for 4 hours, starting 90 minutes after middle cerebral artery (MCA) occlusion in rats, with normothermia (37°C). Only cooling to 34 and 33°C improved outcome, with the largest benefit obtained at 34°C, suggesting that this is the optimal target temperature for hypothermia in acute ischemic stroke (Kollmar et al, 2007). However, because this is only a single study with data not entirely consistent with those of the meta-analysis, these results should be interpreted with some caution.
Figure 1.
Effect of target temperature on the efficacy of hypothermia in improving infarct volume in animal models of stroke. Vertical error bars represent 95% confidence intervals at each point, and the gray bar represents the 95% confidence interval for the overall estimate of efficacy. Adapted from van der Worp et al (2007).
Most early controlled and uncontrolled studies of hypothermia in acute ischemic stroke (Schwab et al, 1998, 2001; Georgiadis et al, 2001; Krieger et al, 2001; De Georgia et al, 2004), as well as most randomized trials of hypothermia in postanoxic encephalopathy after cardiac arrest (Bernard et al, 2002; HACA, 2002), perinatal hypoxic-ischemic encephalopathy (Jacobs et al, 2007), and traumatic brain injury (Hutchison et al, 2008; Peterson et al, 2008) have tested cooling to levels of 32°C to 34°C. Unfortunately, discomfort and shivering increase with lower temperatures, and cooling to these levels therefore generally requires sedation, mechanical ventilation, and admission to an intensive care unit (ICU) (Polderman and Herold, 2009). Owing to the limited availability of intensive care beds in most countries, treatment of even a minority of acute stroke patients is therefore precluded by substantial practical and logistical problems. The fact that intensive care treatment has long been a common clinical practice for patients with severe traumatic brain injury or after cardiac arrest may explain why large randomized clinical trials of moderate hypothermia have been accomplished in these patient populations, whereas such trials have not even been started in patients with acute ischemic stroke.
In addition to the logistical barriers imposed by decreasing temperatures to 34°C or below, side effects of hypothermia also appear to occur more frequently with each degree Celsius reduction in temperature. For example, cardiac arrhythmias become more frequent with temperatures below 30°C, platelet dysfunction occurs when temperatures decrease below 35°C, and coagulation is reduced with temperatures below 33°C (Polderman and Herold, 2009).
Temperature reductions to 35.5 or 35°C have been shown to be feasible and safe in awake patients with acute ischemic stroke by surface cooling, in combination with pethidine (meperidine) to treat shivering (Kammersgaard et al, 2000). Using a similar strategy, body temperature could be reduced to 34.5°C in awake patients with acute myocardial infarction (Ly et al, 2005). Therefore, cooling to 35°C seems to be a feasible strategy to be tested in large randomized trials. The NOCSS (Nordic Cooling Stroke Study) was a randomized trial which tested the effect of temperature reduction to 35°C in awake patients with surface cooling for 9 hours, started within 6 hours of the onset of ischemic stroke. Unfortunately, the trial was terminated because of slow recruitment after the inclusion of 44 patients against a target of 1,000 patients, and results have not yet been published (http://www.strokecenter.org, assessed on 2 November 2009). The ICTuS-L (Intravascular Cooling for the Treatment of Stroke—Longer window) trial is a phase II trial designed to investigate the feasibility and safety of a combination of intravenous thrombolysis and intravascular cooling to 33°C in awake patients with ischemic stroke (Guluma et al, 2006). The trial has recently been terminated and publication of the results is expected shortly.
It is noteworthy that even the maintenance of normothermia in the first few days after stroke onset may improve outcome. In the randomized PAIS (Paracetamol (Acetaminophen) In Stroke) trial, treatment of patients with a baseline body temperature of 37°C or above with high-dose paracetamol, started within 12 hours of stroke onset and continued for 3 days, resulted in a temperature reduction of just 0.3°C at 24 hours, and also in an improved outcome at 3 months (Den Hertog et al, 2009b). Although biologically plausible, this finding requires confirmation in a second randomized trial as this was observed in a post hoc subgroup analysis.
As cooling to 33 or 34°C may be more effective than to 35°C, there is a strong need to compare the feasibility and safety of cooling with these levels in patients with acute ischemic stroke. Two of such small phase II trials have just started in Germany (http://www.strokecenter.org/Trials/TrialDetail.aspx?tid=883) and Finland (http://www.strokecenter.org/Trials/TrialDetail.aspx?tid=949).
Duration of Cooling
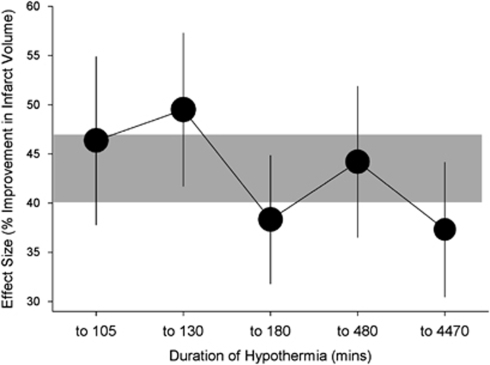
In animal models of focal cerebral ischemia, the diverse pathophysiological processes that are invoked exert their deleterious effects over different time courses extending from the first hours to several days after vessel occlusion (Dirnagl et al, 1999). Such observations would suggest that hypothermia should in fact be more efficacious if prolonged. In the above-mentioned meta-analysis, there was a small and unexpected inverse relationship between the duration of hypothermia and effect size (Figure 2). However, 193 of the 277 (70%) individual experiments tested hypothermia for ≤3 hours, and just 32 (12%) the effect of hypothermia for >12 hours, precluding firm conclusions about the relative benefit of longer cooling. In addition, the two individual studies that compared the effect of cooling for ≥12 hours with cooling for ≤3 hours found a considerably larger benefit with these longer treatment durations (Yanamoto et al, 1996, 1999). In a recent study of cooling to 33°C initiated 1 hour after permanent MCA occlusion in rats, hypothermia for 48 hours seemed to provide better functional and morphologic outcomes than hypothermia for 24 or 12 hours, with the smallest effects observed with the shortest duration of cooling (Clark et al, 2008).
Figure 2.
Effect of duration of cooling on the efficacy of hypothermia in improving infarct volume in animal models of stroke. Vertical error bars represent 95% confidence intervals at each point, and the gray bar represents the 95% confidence interval for the overall estimate of efficacy. Adapted from van der Worp et al (2007).
In clinical trials of cardiac arrest, hypothermia has been proven efficacious if maintained for 12 (Bernard et al, 2002) or 24 hours (HACA, 2002). In none of these two trials, a well-founded reason for the selection of these treatment durations was provided, and reports of pilot studies suggest that this was not much more than an educated guess (Zeiner et al, 2000).
In short, there is no adequate information on the optimal duration of hypothermia in patients with acute ischemic stroke, and this may even depend on the severity of ischemia and on the occurrence of reperfusion. On theoretical grounds, longer durations may be more effective, but these may also be less well tolerated by awake patients and may be associated with a higher risk of complications. One small phase II trial is currently comparing the feasibility of cooling for 12 and 24 hours in patients with acute ischemic stroke.
Time Window for Starting Hypothermia
In preclinical studies, time is the most critical factor in the treatment of cerebral ischemia (Dirnagl et al, 1999). A pooled analysis of six randomized trials of intravenous thrombolysis with rt-PA has also strongly suggested a greater benefit the sooner treatment was started, with potential efficacy up to 6 hours after stroke onset (Hacke et al, 2004). Expediting the delivery of care should therefore be a crucial factor in any acute stroke trial.
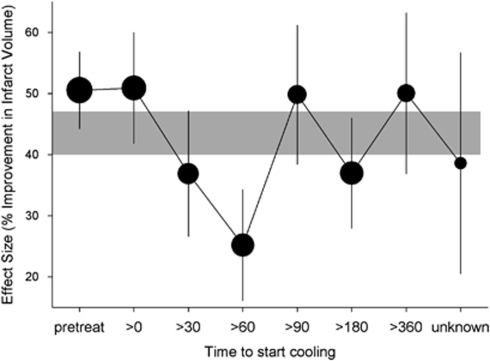
In animal studies, the effect of hypothermia was most consistent when treatment was started before or at the onset of focal cerebral ischemia, but the benefit remained substantial with treatment delays of up to 6 hours, without a clear time dependency (Figure 3). Both the number of experiments with treatment delays of >3 hours and the number of animals included in these experiments were too small to draw firm conclusions (van der Worp et al, 2007). In individual studies testing the effect of delaying the start of cooling, the benefit of hypothermia subsided more strongly over time than suggested by the results of the meta-analysis. In these studies, a statistically significant benefit was not observed anymore at 45 minutes (Markarian et al, 1996), or at 2 (Baker et al, 1992), 3 (Maier et al, 2001), or 6 hours (Ren et al, 2004; Ohta et al, 2007). This discrepancy may reflect pitfalls of the meta-analysis, the individual studies, or both, or may reflect true differences in windows of therapeutic opportunity between species or strains, different durations of ischemia, or different temperatures.
Figure 3.
Effect of delay to initiation of treatment on the efficacy of hypothermia in improving infarct volume in animal models of stroke. Vertical error bars represent 95% confidence intervals at each point, and the gray bar represents the 95% confidence interval for the overall estimate of efficacy. Adapted from van der Worp et al (2007).
In the European Hypothermia After Cardiac Arrest Study, cooling improved functional outcome despite a median interval of 8 hours between the restoration of spontaneous circulation and the attainment of the target temperature (HACA, 2002). There are no data on the maximum time to start efficacious cooling in patients with acute ischemic stroke. However, it is expected that a potential benefit will be greater the earlier cooling is started.
On the first day after stroke onset, spontaneous reperfusion occurs in only a minority of the patients (Jorgensen et al, 1994; Bowler et al, 1998). Even after intravenous thrombolysis, complete recanalization of an occluded MCA 2 hours after start of treatment is found only in up to one-third of patients (Alexandrov et al, 2004; Molina et al, 2006). In the first few hours after stroke onset, most infarcts will therefore still lack adequate perfusion. For a novel treatment strategy to be successful, this strategy should either lead to a higher rate of recanalization or be effective independent of the occurrence of reperfusion. In animal models, the benefit of hypothermia was larger in temporary than in permanent occlusion models, but the effect in the latter was still substantial (37% 95% confidence interval, 30% to 43%). In temporary occlusion models, the benefit was largest when cooling was started before or during ischemia, but also substantial when started during reperfusion (van der Worp et al, 2007). Hypothermia is therefore a promising treatment strategy with or without the achievement of reperfusion.
Practicalities of the delivery of hypothermia
The Method of Cooling
Two major methods for inducing systemic hypothermia in stroke patients can be distinguished: surface cooling and endovascular cooling (Polderman and Herold, 2009). In general, surface cooling methods have the advantage of being noninvasive, and are therefore probably applicable on a larger scale, as experience with introducing catheters in large veins is not required. In addition, their noninvasive nature probably allows the combination of hypothermia with concurrent thrombolysis. However, as the body's natural response to heat loss consists of an increase in sympathetic tone and vasoconstriction of vessels in the skin, maintenance of hypothermia at the target temperature may be more difficult with surface cooling than with endovascular cooling, and patient discomfort and shivering may be more difficult to prevent (Polderman and Herold, 2009; Sessler, 2009a). Simple and relatively inexpensive surface cooling techniques include the use of fans, convective air blankets, water mattresses, and alcohol bathing. However, these methods have a low-to-intermediate speed of cooling and are generally labor intensive. A more rapid induction of hypothermia can be obtained using ice packs, but temperature cannot be controlled sufficiently during the maintenance and rewarming phases. Automated surface cooling systems have relatively high cooling rates and temperature can be controlled throughout all phases.
Endovascular cooling is generally faster and patient comfort may be greater if warming blankets are applied. Disadvantages are its invasive nature, leading to time loss and preventing hypothermia concurrent with thrombolysis, and the required experience with endovascular techniques, preventing an easy wide-spread application (Polderman and Herold, 2009).
For a more rapid induction of hypothermia, chilled intravenous fluids (e.g., normal saline at 4°C, 20 to 30 mL/kg body weight in 30 minutes) may be infused at the onset of surface or endovascular cooling. In patients with postanoxic encephalopathy, subarachnoid hemorrhage, or traumatic brain injury, the infusion of large volumes of fluids is generally tolerated well (Baumgardner et al, 1999; Bernard et al, 2003; Polderman et al, 2005), and a recent small study has suggested that this is also safe in patients with acute stroke (Baumgardner et al, 1999; Bernard et al, 2003; Polderman et al, 2005; Kollmar et al, 2009b).
Selective head (and neck) cooling using helmets, head caps, and neck bands may have the advantage of avoiding systemic complications and has been tested in sedated patients with traumatic brain injury (Wang et al, 2004; Qiu et al, 2006) or after cardiac arrest (Wandaller et al, 2009). However, these methods appear less feasible in awake patients with acute stroke and have not been tested in this patient population. In addition, the target temperature is hard to assess and control during all phases of the cooling process, as this requires invasive monitoring. In a modeling study, temperatures below 36°C could not be reached in the deep brain tissue with head cooling alone, whereas additional application of neckbands covering the carotid bifurcation did lead to a considerable temperature reduction in the deep brain tissue. Temperature sensors had to be applied at least 2 cm below the cortical surface to yield values representative for deep brain tissue (Keller et al, 2009). Selective brain cooling with cold saline circulating in balloon catheters introduced into the nasal cavity has been shown to be feasible in pigs (Covaciu et al, 2008), but is still awaiting clinical studies.
As mentioned above, surface cooling has been shown to be feasible in awake patients with ischemic stroke with target temperatures of ∼35°C (Kammersgaard et al, 2000). Cooling awake stroke patients to 33°C for a total of 24 hours has been shown to be possible with an endovascular device in the inferior vena cava, and a combination of buspirone, meperidine, and cutaneous warming with a heating blanket to suppress shivering (Guluma et al, 2006). There are no randomized trials in which the safety, feasibility, and speed to target temperature of surface and endovascular cooling have been compared in awake patients.
Where Should Cooling be Performed?
As mentioned above, all successful cooling trials in patients with ischemic brain injury were performed in an ICU. However, the number of beds attributed to neurology in ICUs is relatively small worldwide, and even in countries with a highly organized stroke management structure such as Germany, the number of beds in special neurologic ICUs is only 500, whereas the annual incidence of stroke in Germany is ∼250,000 individuals (Kolominsky-Rabas and Heuschmann, 2002). The number of stroke units (SUs) is increasing and 161 SUs with a capacity of up to 20 beds have been identified in Germany (Deutsche Schlaganfall Hilfe). As a consequence, treatment strategies for stroke should be safe and feasible for use in regular SUs. As mentioned above, cooling to 35.5°C has been shown to be feasible in an SU (Kammersgaard et al, 2000), but whether this also applies to lower temperatures remains to be seen.
Preventing Shivering and Discomfort
Humans have an effective thermoregulatory system which opposes the induction and maintenance of hypothermia (Sessler, 2009b). In particular, shivering and peripheral vasoconstriction counteract cooling (Delaunay et al, 1993; Cheng et al, 1995), and are accompanied by sympathetic activation, tachycardia, and hypertension (Frank et al, 1997; Frank et al, 1999). These may lead to discomfort (Mokhtarani et al, 2001), and a stressful autonomic response may also be dangerous in critically ill patients.
Human thermoregulatory mechanisms comprise sensory inputs, a central control system, and efferent systems which act to increase or decrease temperature to keep this in the normal range (Lopez et al, 1994; Cheng et al, 1995; Sessler, 2009b). Body temperature is controlled by the hypothalamus by autonomic reactions, such as sweating and cutaneous vasodilation to decrease temperature, and cutaneous arterio-venous shunt constriction and shivering to increase temperature. Heat distribution is not uniform (Sessler, 2009b), with the trunk and head forming a core compartment with homogenous temperature because of high perfusion within the body and the arms and legs being 2°C to 4°C colder than the core and having substantial internal temperature gradients (Rajek et al, 2000). The contribution of the skin temperature to thermoregulatory control depends on the defense mechanism. Skin temperature contributes ∼10% to control of sweating, and ∼20% to control of vasoconstriction and shivering. Autonomic responses are therefore largely based on alterations in core temperature (Sessler, 2009b).
General anesthesia fundamentally impairs thermoregulatory defense (Schwab et al, 1998; Sessler, 2009b), but the induction and maintenance of hypothermia is a major challenge in awake individuals for whom such mechanisms are intact. In a small study in stroke patients, vasoconstriction and shivering thresholds were 37.1°C±0.4°C and 36.6°C±0.4°C, respectively (Zweifler and Sessler, 1996). Peripheral vasoconstriction due in part to hypothermia-induced increases in serum catecholamines (Frank et al, 1997) leads to isolation of the body core and thereby preserves metabolic heat (Matsukawa et al, 1995). Unfortunately, this impaired perfusion may limit the efficiency of surface cooling.
Shivering is another autonomic response to hypothermia and leads to metabolic heat production (Horvath et al, 1956). The shivering threshold is normally set at ∼35.5°C, which is ∼1°C below the vasoconstriction threshold (Lopez et al, 1994). Physical and pharmacological approaches to prevent and/or treat shivering and vasoconstriction have been reviewed in detail elsewhere (Sessler, 2009a) and are discussed briefly below.
Pharmacological Strategies Against Shivering
The opioid meperidine (pethidine) is frequently used (Zweifler and Sessler, 1996; Mokhtarani et al, 2001; Lyden et al, 2005; Kollmar et al, 2009b), and at high doses, reduced the shivering threshold in healthy volunteers to 33.4°C (Mokhtarani et al, 2001). Unfortunately, it has a wide range of side effects, including nausea and sedation. Other drugs which might be useful alone or in combination include magnesium (Zweifler et al, 2004; Meloni et al, 2009), clonidine, and dexetomidine (Delaunay et al, 1993; Talke et al, 1997; De Witte et al, 1998). For example, low-dose buspirone and low-dose meperidine act synergistically to reduce the shivering threshold, while causing little sedation or respiratory insufficiency (Mokhtarani et al, 2001), and this combination has recently been used in patients with acute stroke (Lyden et al, 2005; Kollmar et al, 2009b).
Physical Methods Against Shivering
As skin temperature contributes ∼20% to the autonomic control of shivering (Lenhardt et al, 1999), surface cooling might lead to greater discomfort and more severe shivering and vasoconstriction than endovascular cooling. Local skin counter warming may blunt this effect (Mekjavic and Eiken, 1985; Iaizzo et al, 1999; Kimberger et al, 2007), with each 1°C increase in mean skin temperature decreasing the shivering threshold by 0.25°C (Cheng et al, 1995). Effects of counter warming have been reported in nonsedated volunteers (Iaizzo et al, 1999; Sweney et al, 2001) as well as in sedated and ventilated brain injury patients (Badjatia et al, 2009), but are not consistent (Doufas et al, 2003). A combination of pharmacological and physical techniques is attractive, and in healthy volunteers, meperidine plus skin counter warming reduced the shivering threshold to below 34°C without significant side effects (Kimberger et al, 2007).
Temperature Measurement
Any cooling strategy will involve measurement of temperature, even if only to show that cooling has been achieved. Often, however, the cooling strategy will require that temperature feedback is provided to the cooling device to allow adjustment of the intensity of cooling and thereby stability at target temperature and control of rewarming. It is presumed that any benefit of hypothermia is manifest through direct effects on ischemic brain tissue (and in particular in the ischemic penumbra), rather than through indirect effects on, for instance, the systemic inflammatory response. The primary temperature of interest is therefore the temperature in the region of the infarct. At equilibrium, any systemic measure of temperature would be appropriate, but in the context of active cooling and controlled rewarming, there may be differences between brain and systemic temperatures, which must be taken into account in planning the best peripheral temperature measurement to be used. A further consideration relates to the delivery of cooling to the affected brain; in patients with ischemic stroke in whom no reperfusion has occurred, cerebral blood flow (CBF), and thereby delivery of cooling to the affected brain tissue, is by definition compromised.
How can we demonstrate that cooling the body cools the infracted brain? Measurement of brain temperature in vivo has until recently required direct instrumentation of brain substance, for instance, using intracranial probes. These are seldom used routinely in patients with stroke, and given the potential associated morbidity, their use in a research context is limited. However, in a small group of patients with large space-occupying MCA stroke treated with moderate hypothermia wherein brain temperature was measured directly, this decreased to 33.3°C when body temperature was cooled to 33°C (Schwab et al, 1998). Magnetic resonance spectroscopic thermometry is a technique that exploits temperature-dependent changes in the separation of peaks in magnetic resonance spectroscopy to report changes in temperature in a volume of brain (Karaszewski et al, 2006). Although the ability to measure absolute temperature with precision is still in some doubt, it is certainly possible to measure differences in temperature (e.g., between the noninfarcted hemisphere, ischemic core, and penumbra) with some precision. Temperature can be reported in voxels of 8 mm3 across two different slices with an acquisition time of only 8 minutes, making this practicable in the emergency situation in patients undergoing active cooling. The Edinburgh HAIST (Hypothermia in Acute Ischemic Stroke Trial) will measure ischemic core and penumbral temperatures 2 to 3 hours after the initiation of cooling in patients with acute ischemic stroke, seeking to show that cooling the body does indeed cool the brain.
What is the best peripheral temperature measurement to monitor cooling? The optimal peripheral temperature measurement will provide continuous feedback to the cooling device to allow adjustment of cooling intensity, and hence candidate measures include esophageal, rectal, or bladder temperature probes. Bladder probes are probably least invasive (many patients will already have a urinary catheter in situ), but have the potential disadvantage that ice-cold saline rapidly infused may be rapidly cleared by the kidneys, therefore giving inappropriate temperature readings in the early stages of cooling and potentially leading to an overshoot of hypothermia. Rectal probes have the disadvantage that there may be a substantial delay between changes in core temperature and changes in rectal temperature. Although esophageal temperature probes probably give the most precise and dynamic indication of core temperature, they are also the most invasive approach. In the same manner that bladder temperature probes also function as urinary catheters, it may be that esophageal probes that also function as nasogastric tubes might be developed for this purpose.
Acid–Base Management: The Role of CO2
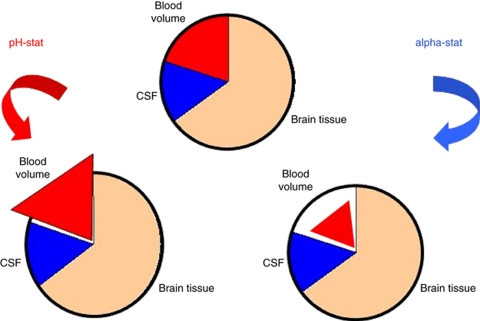
Mechanoregulatory mechanisms maintain near-constant CBF over a wide range of cerebral perfusion pressures (Lavi et al, 2003). The vascular tone is also regulated through chemoregulation by endothelial nitric oxide. Levels of endothelial nitric oxide are regulated by local metabolic activity, pH, and pCO2, with PaCO2 being the most potent known vasodilator; global changes in PaCO2 can easily overcome local mechanoregulatory mechanisms and therefore influence CBF (Murkin et al, 1987). Both PaCO2 and PaO2 are strongly influenced by temperature (Murkin, 2007), and their manipulation by changing proportions of inspired ventilatory gases or by adjusting ventilation parameters further complicates the situation. It is therefore somewhat surprising how little attention has been given to the interaction between temperature and blood gases in ischemic stroke (Kollmar et al, 2009a). In ventilated patients, the standard (α-stat) approach is to maintain PaCO2 at 40 mm Hg when measured ex vivo at 37°C (Murkin, 2007). In contrast, during pH-stat management, the measured PaCO2 is adjusted to take into account patient temperature (du Plessis et al, 1997; Kollmar et al, 2002, 2009a). The α-stat strategy results in a higher measured PaCO2 leading to increased minute volume settings, reduced PaCO2, and decreased CBF; this is avoided with pH-stat management (Murkin, 2007; Figure 4).
Figure 4.
Effects of α- and pH-stat on different intracranial compartments. CSF, cerebrospinal fluid.
These strategies have been compared in a rodent model of transient focal cerebral ischemia (Kollmar et al, 2002), in which cooling to 33°C was established during the 5-h reperfusion period. Consistent with the above hypothesis, CBF was higher and infarct size was smaller in pH-stat-ventilated animals compared with α-stat-ventilated animals.
In a nonrandomized study of patients with large MCA territory ischemic stroke, CBF was substantially higher with pH-stat than with α-stat ventilation (Kollmar et al, 2009a). However, pH-stat ventilation was associated with critical increases in intracranial pressure after 3 days, possibly because of effects on cerebral blood volume, and pH-stat should therefore be used with caution in patients with space-occupying hemispheric infarction. Furthermore, the increased PaCO2 occurring with pH-stat may lead to a steal phenomenon, diverting blood away from the injured to the healthy brain tissue (Oshima et al, 2000). The clinical importance of these issues needs to be better understood to guide the use of hypothermia in mechanically ventilated patients.
Other considerations for clinical trial design
Cooling Patients With Intracerebral Hemorrhage
In animal models of focal cerebral ischemia (van der Worp et al, 2007; Sena et al, 2007b) and in clinical trials in stroke (Hacke et al, 2004), efficacy declines with delay to treatment, and expediting the delivery of care should therefore be a crucial factor in any acute stroke trial (Macleod et al, 2003; Thrift et al, 2006). For this reason, cooling may have a larger effect if started in the field instead of in the emergency room or in the ward. However, imaging (computed tomography or magnetic resonance imaging) is required to reliably distinguish ischemic stroke from intracerebral hemorrhage (ICH); therefore, hypothermia can only be initiated in the field if it does not harm patients with ICH.
However, even mild hypothermia may have effects on platelet function and coagulation, which could preclude its use in patients with ICH. In animals, moderate hypothermia has been associated with reversible platelet dysfunction, prolonged bleeding, prothrombin, and partial prothrombin times, and reduced thromboxane B2 (Valeri et al, 1987; Staab et al, 1994). In humans, conflicting evidence suggests that hypothermia may either increase (Zhang et al, 2004; Xavier et al, 2007; Scharbert et al, 2010) or decrease (Hessel et al, 1980; Yoshihara et al, 1985; Romlin et al, 2007) platelet function, and further data are required. These effects may be temperature dependent, with lower temperatures leading to larger aberrations (Polderman and Herold, 2009). Rapid rewarming has been reported to be associated with lethal disseminated intravascular coagulation (Mahajan et al, 1981).
Prolonged hypothermia has been reported to reduce inflammation, edema, and blood–brain barrier in animal models of ICH (Kawai et al, 2001; Fingas et al, 2007). However, the reported beneficial effects of mild hypothermia are inconsistent and depend on the model, severity of the hemorrhage, and the time of treatment (MacLellan et al, 2009); in one study, hypothermia increased bleeding and worsened outcome (MacLellan et al, 2004). There are limited clinical data on cooling in ICH, but one small nonrandomized study suggested that mild hypothermia (35°C), started more than 12 hours from ICH onset, and when maintained for 10 days, appeared to reduce case fatality and was not associated with recurrent bleeding (Kollmar et al, unpublished communication).
Inflammation and Infections During Hypothermia
Infections have been reported in up to a third of patients with acute stroke and to be related to poor functional outcome (Emsley and Hopkins, 2008). Experimental and clinical studies have shown that brain injury can lead to a ‘central nervous system injury-induced immune deficiency syndrome' (Meisel et al, 2005). In the mouse, focal cerebral ischemia has been reported to lead to a reduction in the peripheral blood lymphocytes count and impaired T and natural killer activity, leading to increased pneumonia and mortality rates (Prass et al, 2003; Meisel et al, 2004). Decreased peripheral blood lymphocyte count has also been reported in acute stroke patients, along with increased expression of proinflammatory, proapoptotic, or adhesion-relevant genes in the majority of peripheral blood mononuclear cells subpopulations (Kassner et al, 2009).
Cooling may accentuate this immunosuppression (Russwurm et al, 2002), perhaps through a reduction of cytokine response and the induction of an antiinflammatory T-cell cytokine profile (Lee et al, 2001). Experimental data suggest that hypothermia is associated with reduced inflammation in ischemic brain regions (Zhao et al, 2007), and this may be one reason for its protective effects. However, if this immunosuppression is more general, it may predispose to infectious complications and may thereby be associated with an increased length of hospital stay, poor outcome, and increased mortality. In patients with space-occupying hemispheric infarction treated with induced hypothermia, pneumonia rates of up to 83% have been reported (Schwab et al, 1998, 2001; Georgiadis et al, 2001, 2002), but these may be explained in large part by sedation, mechanical ventilation, and the use of muscle relaxants. In patients with acute stroke, the use of mechanical ventilation should probably be restricted because of the risk of ventilator-associated pneumonia (Porzecanski and Bowton, 2006). In all, 10% to 20% of patients who were mechanically ventilated for >48 hours develop ventilator-associated pneumonia, with mortality rates of 15% to 50%, and ICU length of stay in patients with ventilator-associated pneumonia is increased by a mean of 6 days and leads to an increase in treatment costs (Safdar et al, 2005).
Data for infection rates in stroke patients treated with hypothermia without mechanical ventilation are limited. Although many studies report a trend toward more pneumonia in the hypothermia group (Kammersgaard et al, 2002; Knoll et al, 2002; De Georgia et al, 2004), these results should be interpreted with caution because of the small numbers of patients included and the lack of a standard definition of pneumonia.
Each of stroke, cooling itself, the sedative medication used to prevent shivering, and mechanical ventilation may therefore increase the risk of infection (Polderman and Herold, 2009). This might be reduced by limiting the duration of cooling. Kammersgaard et al (2002) showed that with 6 hours of cooling, infection rates were only slightly higher than in the normothermia group, and in patients with myocardial infarction treated with mild hypothermia for 4 hours using a standard antishivering approach, there was no increased pneumonia (Dixon et al, 2002; Kandzari et al, 2004; Table 1). Similarly, limiting the depth of hypothermia to 35°C might reduce the risk of infection both directly and through lower requirements for sedation (Mokhtarani et al, 2001), but both of these approaches are likely to lead to reduced neuroprotection. Clinical trials of hypothermia in stroke patients should not only carefully assess the incidence of pneumonia using a standard definition but should also seek to minimize this risk by careful use of sedative antishivering medication, identifying biomarkers of developing pneumonia, and instituting immediate treatment when pneumonia is identified. The depth and duration of cooling tested in a clinical trial should seek the optimum balance between efficacy and adverse effects, such as the development of infections.
Combination With Other Treatments for Acute Stroke
Any novel treatment for stroke should not affect the delivery of other treatments known to be effective, or alternatively should deliver greater efficacy than those treatments they displace. For acute ischemic stroke, there is good evidence for the efficacy of SU care, aspirin, thrombolysis, and decompressive hemicraniectomy (van der Worp and van Gijn, 2007; Donnan et al, 2008).
Stroke Unit Care: Transfer from an intensive care setting to that of rehabilitation in an SU may be slightly delayed by TH, depending on the modality and duration of cooling. This is unlikely to be of significance, particularly if cooling is delivered in an environment experienced in the management of stroke. Of greater potential significance is the delay to the initiation of rehabilitation, wherein there is some evidence to suggest that very early mobilization (within 24 hours of stroke onset) may convey additional benefits (Bernhardt et al, 2006; Sorbello et al, 2009), and this may be one of the mechanisms through which the beneficial effects of SU care are made manifest. Delays to the initiation of rehabilitation may be attributed to active cooling (i.e., cooling for 24 hours of longer), controlled rewarming (particularly in case of cooling to lower temperatures, in which the rewarming phase may last up to 12 hours), or may be a consequence of the groin puncture required for endovascular cooling. The benefits of very early mobilization are currently being tested in a randomized controlled trial (Bernhardt et al, 2006), and these will have to be taken into account when considering the potential benefits of hypothermia.
Aspirin: Both hypothermia and aspirin may be associated with increased risk of gastric ulceration (Krieger et al, 2001; Baigent et al, 2009) and of coagulopathy, but the extent to which this might be clinically significant is not known. The benefits of immediate aspirin are limited, and in patients receiving thrombolysis, aspirin is not administered in the first 24 hours; hence, if there is evidence of increased risk in patients receiving aspirin and hypothermia, then it would be reasonable to omit aspirin for the duration of cooling. Unfortunately, the randomized controlled trials of hypothermia in cardiac arrest did not report whether those studies included taking aspirin, and if so whether this had any effect on outcome (HACA, 2002; Bernard et al, 2002).
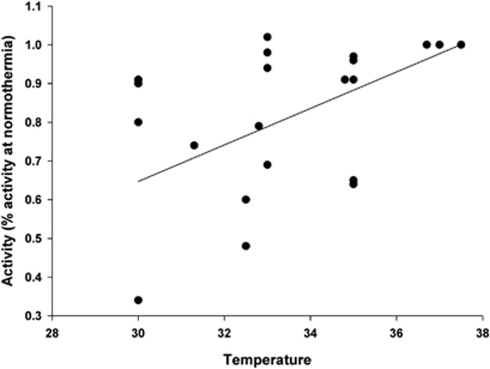
Thrombolysis: Therapeutic thrombolysis depends on the activity of a series of enzymes including rt-PA itself and plasmin, and enzyme activity may be exquisitely sensitive to temperature. The effect of temperature on the efficacy of rt-PA has been examined both in vitro and in vivo. Taking all the in vitro data together (Figure 5), there seems to be a relative decrease in lytic activity of ∼5% per degree of hypothermia, equivalent to a 20% reduction in activity with cooling to 33°C (Yenari et al, 1995; Schwarzenberg et al, 1998; Shaw et al, 2006, 2007). However, given that rt-PA is likely to be administered to eligible patients as soon as is practicable (and at or before the initiation of cooling), it is unlikely that such target temperatures would be achieved when most lysis is occurring. Furthermore, in vivo studies have suggested no effect of hypothermia on mortality or reperfusion, and probably improved infarct volume compared with thrombolysis of hypothermia alone (Meden et al, 1994a 1994b; Kollmar et al, 2004).
Figure 5.
Effect of temperature on the in vitro lytic activity of tPA. Linear regression gives r2=0.348, suggesting a 4.7% decline in thrombolytic activity per degree of hypothermia. Data were obtained from the studies by Yenari et al (1995), Schwarzenberg et al (1998), Shaw et al (2006), and Shaw et al (2007).
Endovascular cooling involves femoral puncture and instrumentation of the inferior vena cava; this might present problems if the puncture was to be performed when lytic activity was at its height, or if rt-PA administration was delayed until a femoral catheter was established. However, such considerations are not seen as an impediment to intraarterial lysis and clot retrieval in patients who have received intravenous rt-PA (Khatri et al, 2008); therefore, although such considerations may favor the adoption of a surface rather than endovascular approach to cooling, they cannot be seen as an absolute contraindication to endovascular induction of hypothermia in patients receiving intravenous rt-PA.
Decompressive Hemicraniectomy: In patients with space-occupying MCA infarction, decompressive hemicrainectomy leads to substantial improvements in survival and functional outcome (Vahedi et al, 2007). Although surgery might be considered to be associated with greater risks, particularly of hemorrhage or infection, in the context of hypothermia, this does not seem to be the case, and indeed the induction of hypothermia is considered by some to be a useful adjunct to decompressive hemicraniectomy, and is the subject of ongoing studies (Poca et al, 2010).
Problems With Sample Size Calculation for Studies
In any clinical trial program, there are competing ethical imperatives to ensure first that sufficient patients are included to obtain a robust and precise estimate of treatment effects and second that trials are not unnecessarily large, continuing to allocate patients to a treatment when the presence or absence of efficacy is already known. These competing demands can be resolved first through the use of sample size calculations which use plausible and realistic estimates of treatment effect sizes, and of likely patient losses to follow-up, withdrawals from active treatment, and crossing over between treatment groups, and second through the staging of preplanned interim analyses by an independent data and safety monitoring committee, with clearly defined procedures for when they would recommend that a study be halted before full recruitment being achieved, on the basis of safety (i.e., hazard), efficacy (i.e., benefit), or futility.
Where trials in hypothermia test a range of interventions, this situation is made more complex by the substantial heterogeneity and lack of consensus regarding the optimal depth and duration of hypothermia, the different technologies that are available to achieve hypothermia, and the different shivering strategies that might be proposed. These factors are likely to influence not only the efficacy of the intervention but also the adverse effects. For instance, endovascular cooling to 32°C for 72 hours might be expected to be more effective than surface cooling to 35°C for 12 hours, but would also be expected to be associated with greater hazard.
There are two broad approaches to clinical trial program design which might be used to address these issues. A number of pilot studies are planned or are in progress, testing different means, depths, and durations of hypothermia. These will give some information about the safety of different approaches, but will allow only indirect comparison between the different approaches and so will need to be interpreted with caution. However, they will provide patient data with which it might be possible to test whether there is any benefit of cooling independent of means, depth, and duration. Such a meta-analysis would be most powerful if the protocols of pilot studies were aligned to minimize unnecessary heterogeneity in trial design and if the definitions used in data collection were uniform. There is a precedent for this type of meta-analysis from the decompressive hemicraniectomy trial lists (Vahedi et al, 2007), and we propose that in the case of planned and ongoing hypothermia studies, it might be possible to go so far as having common trial infrastructures such as electronic Case Report Forms (eCRF) systems and a common data and safety monitoring board.
Second, a major problem in this field of research is the evolution of both cooling technologies and management strategies (for instance, for shivering) over time. One response to this which has been used in other domains is the use of adaptive trial designs, whereby the various treatment groups available, and the planned size of each individual treatment group, can be adjusted during the course of the trial. The hypothesis herein relates not so much to relative efficacy of different depths and durations of hypothermia (although this might be possible) but rather to selecting the intervention which is best tolerated, which has fewest associated adverse events, and which results in a brain temperature closest to the target. If a new cooling strategy becomes available during the course of the trial which seems better against these criteria, than that currently in use, or if a safer, more effective cooling strategy becomes available, it would be possible to adjust the trial protocol according to prespecified rules and prespecified limits.
The Cochrane review of physical cooling methods suggests that hypothermia may be associated with an odds ratio for an improvement in outcome of 0.85 (Den Hertog et al, 2009a); if death and dependency were seen in 50% of the control group, then 700 patients per group would be required to give 80% power to detect such a difference using a dichotomous outcome measure, and assuming that 20% of patients cross over or do not complete treatment, a trial would require 1,750 patients; 90% power would require 2,310 patients. However, the review included data from just two physical cooling trials (of which only one randomized), in a total of 59 patients. In addition, the two studies were feasibility studies, with time windows for start of treatment of up to 12 hours. The benefit of hypothermia may therefore be completely different from that suggested by the Cochrane review.
It has been suggested (Bath et al, 2007) that the use of a sliding dichotomous outcome may reduce the required patient numbers by ∼30%, but this may not be appropriate where treatment may be associated with increased harm in some patients, as is seen with thrombolysis and may also be the case with hypothermia.
Conclusions
Next to reperfusion strategies, TH is the most promising treatment for patients with acute ischemic stroke. At present, clinical data on the use of hypothermia in these patients are very limited and do not provide sufficient information on potential efficacy to support its use outside clinical trials. The effect of hypothermia on functional outcome in stroke is a priority question to be tested in large randomized clinical trials, but given the limited potential for commercial exploitation, it is likely that such trials will be investigator driven.
The translational difficulties previously encountered in testing other potentially neuroprotective interventions will be diminished by a more detailed understanding of the most appropriate target depth and duration of cooling, along with the optimization of the means of cooling, the management of potential complications including shivering and infections, and increased understanding of how hypothermia might interact with existing treatments. The EuroHyp investigators are coordinating a number of small pilot studies addressing these issues to inform the design of a pan European multicenter randomized controlled trial.
Acknowledgments
The EuroHYP investigators are H Bart van der Worp, Malcolm Macleod, Rainer Kollmar, Stephan Schwab, Jesper Petterssen, Derk Kreiger, Gregor Broessner, Francesco Orzi, and Risto Roine. MRM acknowledges the support of Chest Heart and Stroke Scotland and the Edinburgh MRC Trials Methodology Hub.
The authors declare no conflict of interest.
References
- Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, Montaner J, Saqqur M, Demchuk AM, Moye LA, Hill MD, Wojner AW. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia—the ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- Badjatia N, Strongilis E, Prescutti M, Fernandez L, Fernandez A, Buitrago M, Schmidt JM, Mayer SA. Metabolic benefits of surface counter warming during therapeutic temperature modulation. Crit Care Med. 2009;37:1893–1897. doi: 10.1097/CCM.0b013e31819fffd3. [DOI] [PubMed] [Google Scholar]
- Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CJ, Onesti ST, Solomon RA. Reduction by delayed hypothermia of cerebral infarction following middle cerebral artery occlusion in the rat: a time-course study. J Neurosurg. 1992;77:438–444. doi: 10.3171/jns.1992.77.3.0438. [DOI] [PubMed] [Google Scholar]
- Bath PM, Gray LJ, Collier T, Pocock S, Carpenter J. Can we improve the statistical analysis of stroke trials? Statistical reanalysis of functional outcomes in stroke trials. Stroke. 2007;38:1911–1915. doi: 10.1161/STROKEAHA.106.474080. [DOI] [PubMed] [Google Scholar]
- Baumgardner JE, Baranov D, Smith DS, Zager EL. The effectiveness of rapidly infused intravenous fluids for inducing moderate hypothermia in neurosurgical patients. Anesth Analg. 1999;89:163–169. doi: 10.1097/00000539-199907000-00029. [DOI] [PubMed] [Google Scholar]
- Bernard S, Buist M, Monteiro O, Smith K. Induced hypothermia using large volume, ice-cold intravenous fluid in comatose survivors of out-of-hospital cardiac arrest: a preliminary report. Resuscitation. 2003;56:9–13. doi: 10.1016/s0300-9572(02)00276-9. [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Bernhardt J, Dewey H, Collier J, Thrift A, Lindley R, Moodie M, Donnan G. A Very Early Rehabilitation Trial (AVERT) Int J Stroke. 2006;1:169–171. doi: 10.1111/j.1747-4949.2006.00044.x. [DOI] [PubMed] [Google Scholar]
- Bowler JV, Wade JP, Jones BE, Nijran KS, Steiner TJ. Natural history of the spontaneous reperfusion of human cerebral infarcts as assessed by 99mTc HMPAO SPECT. J Neurol Neurosurg Psychiatry. 1998;64:90–97. doi: 10.1136/jnnp.64.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Matsukawa T, Sessler DI, Ozaki M, Kurz A, Merrifield B, Lin H, Olofsson P. Increasing mean skin temperature linearly reduces the core-temperature thresholds for vasoconstriction and shivering in humans. Anesthesiology. 1995;82:1160–1168. doi: 10.1097/00000542-199505000-00011. [DOI] [PubMed] [Google Scholar]
- Clark DL, Penner M, Orellana-Jordan IM, Colbourne F. Comparison of 12, 24 and 48 h of systemic hypothermia on outcome after permanent focal ischemia in rat. Exp Neurol. 2008;212:386–392. doi: 10.1016/j.expneurol.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Covaciu L, Allers M, Enblad P, Lunderquist A, Wieloch T, Rubertsson S. Intranasal selective brain cooling in pigs. Resuscitation. 2008;76:83–88. doi: 10.1016/j.resuscitation.2007.07.002. [DOI] [PubMed] [Google Scholar]
- De Georgia MA, Krieger DW, Abou-Chebl A, Devlin TG, Jauss M, Davis SM, Koroshetz WJ, Rordorf G, Warach S. Cooling for Acute Ischemic Brain Damage (COOL AID): a feasibility trial of endovascular cooling. Neurology. 2004;63:312–317. doi: 10.1212/01.wnl.0000129840.66938.75. [DOI] [PubMed] [Google Scholar]
- De Witte JL, Kim JS, Sessler DI, Bastanmehr H, Bjorksten AR. Tramadol reduces the sweating, vasoconstriction, and shivering thresholds. Anesth Analg. 1998;87:173–179. doi: 10.1097/00000539-199807000-00036. [DOI] [PubMed] [Google Scholar]
- Delaunay L, Bonnet F, Liu N, Beydon L, Catoire P, Sessler DI. Clonidine comparably decreases the thermoregulatory thresholds for vasoconstriction and shivering in humans. Anesthesiology. 1993;79:470–474. doi: 10.1097/00000542-199309000-00009. [DOI] [PubMed] [Google Scholar]
- Den Hertog HM, van der Worp HB, Tseng MC, Dippel DW.2009aCooling therapy for acute stroke Cochrane Database Syst Rev (1Article number CD001247 [DOI] [PMC free article] [PubMed]
- Den Hertog HM, van der Worp HB, van Gemert HM, Algra A, Kappelle LJ, van Gijn J, Koudstaal PJ, Dippel DW. The Paracetamol (Acetaminophen) In Stroke (PAIS) trial: a multicentre, randomised, placebo-controlled, phase III trial. Lancet Neurol. 2009b;8:434–440. doi: 10.1016/S1474-4422(09)70051-1. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Dixon SR, Whitbourn RJ, Dae MW, Grube E, Sherman W, Schaer GL, Jenkins JS, Baim DS, Gibbons RJ, Kuntz RE, Popma JJ, Nguyen TT, O′Neill WW. Induction of mild systemic hypothermia with endovascular cooling during primary percutaneous coronary intervention for acute myocardial infarction. J Am Coll Cardiol. 2002;40:1928–1934. doi: 10.1016/s0735-1097(02)02567-6. [DOI] [PubMed] [Google Scholar]
- Donnan GA, Baron JC, Ma H, Davis SM. Penumbral selection of patients for trials of acute stroke therapy. Lancet Neurol. 2009;8:261–269. doi: 10.1016/S1474-4422(09)70041-9. [DOI] [PubMed] [Google Scholar]
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- Doufas AG, Wadhwa A, Lin CM, Shah YM, Hanni K, Sessler DI. Neither arm nor face warming reduces the shivering threshold in unanesthetized humans. Stroke. 2003;34:1736–1740. doi: 10.1161/01.STR.0000077014.47422.DB. [DOI] [PubMed] [Google Scholar]
- du Plessis AJ, Jonas RA, Wypij D, Hickey PR, Riviello J, Wessel DL, Roth SJ, Burrows FA, Walter G, Farrell DM, Walsh AZ, Plumb CA, del Nido P, Burke RP, Castaneda AR, Mayer JE, Jr, Newburger JW. Perioperative effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 1997;114:991–1000. doi: 10.1016/S0022-5223(97)70013-8. [DOI] [PubMed] [Google Scholar]
- Emsley HC, Hopkins SJ. Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol. 2008;7:341–353. doi: 10.1016/S1474-4422(08)70061-9. [DOI] [PubMed] [Google Scholar]
- Fingas M, Clark DL, Colbourne F. The effects of selective brain hypothermia on intracerebral hemorrhage in rats. Exp Neurol. 2007;208:277–284. doi: 10.1016/j.expneurol.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Florian B, Vintilescu R, Balseanu AT, Buga AM, Grisk O, Walker LC, Kessler C, Popa-Wagner A. Long-term hypothermia reduces infarct volume in aged rats after focal ischemia. Neurosci Lett. 2008;438:180–185. doi: 10.1016/j.neulet.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Frank SM, Higgins MS, Fleisher LA, Sitzmann JV, Raff H, Breslow MJ. Adrenergic, respiratory, and cardiovascular effects of core cooling in humans. Am J Physiol. 1997;272:R557–R562. doi: 10.1152/ajpregu.1997.272.2.R557. [DOI] [PubMed] [Google Scholar]
- Frank SM, Raja SN, Bulcao CF, Goldstein DS. Relative contribution of core and cutaneous temperatures to thermal comfort and autonomic responses in humans. J Appl Physiol. 1999;86:1588–1593. doi: 10.1152/jappl.1999.86.5.1588. [DOI] [PubMed] [Google Scholar]
- Georgiadis D, Schwarz S, Aschoff A, Schwab S. Hemicraniectomy and moderate hypothermia in patients with severe ischemic stroke. Stroke. 2002;33:1584–1588. doi: 10.1161/01.str.0000016970.51004.d9. [DOI] [PubMed] [Google Scholar]
- Georgiadis D, Schwarz S, Kollmar R, Schwab S. Endovascular cooling for moderate hypothermia in patients with acute stroke: first results of a novel approach. Stroke. 2001;32:2550–2553. doi: 10.1161/hs1101.097382. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- Guluma KZ, Hemmen TM, Olsen SE, Rapp KS, Lyden PD. A trial of therapeutic hypothermia via endovascular approach in awake patients with acute ischemic stroke: methodology. Acad Emerg Med. 2006;13:820–827. doi: 10.1197/j.aem.2006.03.559. [DOI] [PubMed] [Google Scholar]
- HACA Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, Brott T, Frankel M, Grotta JC, Haley EC, Jr, Kwiatkowski T, Levine SR, Lewandowski C, Lu M, Lyden P, Marler JR, Patel S, Tilley BC, Albers G, Bluhmki E, Wilhelm M, Hamilton S. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- Hessel EA, Schmer G, Dillard DH. Platelet kinetics during deep hypothermia. J Surg Res. 1980;28:23–34. doi: 10.1016/0022-4804(80)90078-5. [DOI] [PubMed] [Google Scholar]
- Horvath SM, Spurr GB, Hutt BK, Hamilton LH. Metabolic cost of shivering. J Appl Physiol. 1956;8:595–602. doi: 10.1152/jappl.1956.8.6.595. [DOI] [PubMed] [Google Scholar]
- Hutchison JS, Ward RE, Lacroix J, Hebert PC, Barnes MA, Bohn DJ, Dirks PB, Doucette S, Fergusson D, Gottesman R, Joffe AR, Kirpalani HM, Meyer PG, Morris KP, Moher D, Singh RN, Skippen PW. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358:2447–2456. doi: 10.1056/NEJMoa0706930. [DOI] [PubMed] [Google Scholar]
- Iaizzo PA, Jeon YM, Sigg DC. Facial warming increases the threshold for shivering. J Neurosurg Anesthesiol. 1999;11:231–239. doi: 10.1097/00008506-199910000-00002. [DOI] [PubMed] [Google Scholar]
- Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P.2007Cooling for newborns with hypoxic ischaemic encephalopathy Cochrane Database Syst Rev (4Article number CD003311 [DOI] [PubMed]
- Jorgensen HS, Sperling B, Nakayama H, Raaschou HO, Olsen TS. Spontaneous reperfusion of cerebral infarcts in patients with acute stroke. Incidence, time course, and clinical outcome in the Copenhagen Stroke Study. Arch Neurol. 1994;51:865–873. doi: 10.1001/archneur.1994.00540210037011. [DOI] [PubMed] [Google Scholar]
- Kammersgaard LP, Jorgensen HS, Rungby JA, Reith J, Nakayama H, Weber UJ, Houth J, Olsen TS. Admission body temperature predicts long-term mortality after acute stroke: the Copenhagen Stroke Study. Stroke. 2002;33:1759–1762. doi: 10.1161/01.str.0000019910.90280.f1. [DOI] [PubMed] [Google Scholar]
- Kammersgaard LP, Rasmussen BH, Jorgensen HS, Reith J, Weber U, Olsen TS. Feasibility and safety of inducing modest hypothermia in awake patients with acute stroke through surface cooling: a case-control study: the Copenhagen Stroke Study. Stroke. 2000;31:2251–2256. doi: 10.1161/01.str.31.9.2251. [DOI] [PubMed] [Google Scholar]
- Kandzari DE, Chu A, Brodie BR, Stuckey TA, Hermiller JB, Vetrovec GW, Hannan KL, Krucoff MW, Christenson RH, Gibbons RJ, Sigmon KN, Garg J, Hasselblad V, Collins K, Harrington RA, Berger PB, Chronos NA, Hochman JS, Califf RM. Feasibility of endovascular cooling as an adjunct to primary percutaneous coronary intervention (results of the LOWTEMP pilot study) Am J Cardiol. 2004;93:636–639. doi: 10.1016/j.amjcard.2003.11.038. [DOI] [PubMed] [Google Scholar]
- Karaszewski B, Wardlaw JM, Marshall I, Cvoro V, Wartolowska K, Haga K, Armitage PA, Bastin ME, Dennis MS. Measurement of brain temperature with magnetic resonance spectroscopy in acute ischemic stroke. Ann Neurol. 2006;60:438–446. doi: 10.1002/ana.20957. [DOI] [PubMed] [Google Scholar]
- Kassner SS, Kollmar R, Bonaterra GA, Hildebrandt W, Schwab S, Kinscherf R. The early immunological response to acute ischemic stroke: differential gene expression in subpopulations of mononuclear cells. Neuroscience. 2009;160:394–401. doi: 10.1016/j.neuroscience.2009.02.050. [DOI] [PubMed] [Google Scholar]
- Kawai N, Kawanishi M, Okauchi M, Nagao S. Effects of hypothermia on thrombin-induced brain edema formation. Brain Res. 2001;895:50–58. doi: 10.1016/s0006-8993(01)02026-1. [DOI] [PubMed] [Google Scholar]
- Keller E, Mudra R, Gugl C, Seule M, Mink S, Frohlich J. Theoretical evaluations of therapeutic systemic and local cerebral hypothermia. J Neurosci Methods. 2009;178:345–349. doi: 10.1016/j.jneumeth.2008.12.030. [DOI] [PubMed] [Google Scholar]
- Khatri P, Hill MD, Palesch YY, Spilker J, Jauch EC, Carrozzella JA, Demchuk AM, Martin R, Mauldin P, Dillon C, Ryckborst KJ, Janis S, Tomsick TA, Broderick JP. Methodology of the Interventional Management of Stroke III Trial. Int J Stroke. 2008;3:130–137. doi: 10.1111/j.1747-4949.2008.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberger O, Ali SZ, Markstaller M, Zmoos S, Lauber R, Hunkeler C, Kurz A. Meperidine and skin surface warming additively reduce the shivering threshold: a volunteer study. Crit Care. 2007;11:R29. doi: 10.1186/cc5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll T, Wimmer ML, Gumpinger F, Haberl RL. The low normothermia concept—maintaining a core body temperature between 36 and 37 degrees C in acute stroke unit patients. J Neurosurg Anesthesiol. 2002;14:304–308. doi: 10.1097/00008506-200210000-00006. [DOI] [PubMed] [Google Scholar]
- Kollmar R, Blank T, Han JL, Georgiadis D, Schwab S. Different degrees of hypothermia after experimental stroke: short- and long-term outcome. Stroke. 2007;38:1585–1589. doi: 10.1161/STROKEAHA.106.475897. [DOI] [PubMed] [Google Scholar]
- Kollmar R, Frietsch T, Georgiadis D, Schabitz WR, Waschke KF, Kuschinsky W, Schwab S. Early effects of acid-base management during hypothermia on cerebral infarct volume, edema, and cerebral blood flow in acute focal cerebral ischemia in rats. Anesthesiology. 2002;97:868–874. doi: 10.1097/00000542-200210000-00018. [DOI] [PubMed] [Google Scholar]
- Kollmar R, Georgiadis D, Schwab S. Alpha-stat versus pH-stat guided ventilation in patients with large ischemic stroke treated by hypothermia. Neurocrit Care. 2009a;10:173–180. doi: 10.1007/s12028-008-9162-z. [DOI] [PubMed] [Google Scholar]
- Kollmar R, Henninger N, Bardutzky J, Schellinger PD, Schabitz WR, Schwab S. Combination therapy of moderate hypothermia and thrombolysis in experimental thromboembolic stroke—an MRI study. Exp Neurol. 2004;190:204–212. doi: 10.1016/j.expneurol.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kollmar R, Schellinger PD, Steigleder T, Kohrmann M, Schwab S. Ice-cold saline for the induction of mild hypothermia in patients with acute ischemic stroke: a pilot study. Stroke. 2009b;40:1907–1909. doi: 10.1161/STROKEAHA.108.530410. [DOI] [PubMed] [Google Scholar]
- Kollmar R, Schwab S. Hypothermia in focal ischemia: implications of experiments and experience. J Neurotrauma. 2009;26:377–386. doi: 10.1089/neu.2008.0564. [DOI] [PubMed] [Google Scholar]
- Kolominsky-Rabas PL, Heuschmann PU. [Incidence, etiology and long-term prognosis of stroke] Fortschr Neurol Psychiatr. 2002;70:657–662. doi: 10.1055/s-2002-35857. [DOI] [PubMed] [Google Scholar]
- Krieger DW, De Georgia MA, Abou-Chebl A, Andrefsky JC, Sila CA, Katzan IL, Mayberg MR, Furlan AJ. Cooling for acute ischemic brain damage (cool aid): an open pilot study of induced hypothermia in acute ischemic stroke. Stroke. 2001;32:1847–1854. doi: 10.1161/01.str.32.8.1847. [DOI] [PubMed] [Google Scholar]
- Lavi S, Egbarya R, Lavi R, Jacob G. Role of nitric oxide in the regulation of cerebral blood flow in humans: chemoregulation versus mechanoregulation. Circulation. 2003;107:1901–1905. doi: 10.1161/01.CIR.0000057973.99140.5A. [DOI] [PubMed] [Google Scholar]
- Lee SL, Battistella FD, Go K. Hypothermia induces T-cell production of immunosuppressive cytokines. J Surg Res. 2001;100:150–153. doi: 10.1006/jsre.2001.6230. [DOI] [PubMed] [Google Scholar]
- Lenhardt R, Greif R, Sessler DI, Laciny S, Rajek A, Bastanmehr H. Relative contribution of skin and core temperatures to vasoconstriction and shivering thresholds during isoflurane anesthesia. Anesthesiology. 1999;91:422–429. doi: 10.1097/00000542-199908000-00016. [DOI] [PubMed] [Google Scholar]
- Lopez M, Sessler DI, Walter K, Emerick T, Ozaki M. Rate and gender dependence of the sweating, vasoconstriction, and shivering thresholds in humans. Anesthesiology. 1994;80:780–788. doi: 10.1097/00000542-199404000-00009. [DOI] [PubMed] [Google Scholar]
- Ly HQ, Denault A, Dupuis J, Vadeboncoeur A, Harel F, Arsenault A, Gibson CM, Bonan R. A pilot study: the Noninvasive Surface Cooling Thermoregulatory System for Mild Hypothermia Induction in Acute Myocardial Infarction (the NICAMI Study) Am Heart J. 2005;150:933. doi: 10.1016/j.ahj.2005.02.049. [DOI] [PubMed] [Google Scholar]
- Lyden PD, Allgren RL, Ng K, Akins P, Meyer B, Al Sanani F, Lutsep H, Dobak J, Matsubara BS, Zivin J. Intravascular Cooling in the Treatment of Stroke (ICTuS): early clinical experience. J Stroke Cerebrovasc Dis. 2005;14:107–114. doi: 10.1016/j.jstrokecerebrovasdis.2005.01.001. [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Clark DL, Silasi G, Colbourne F. Use of prolonged hypothermia to treat ischemic and hemorrhagic stroke. J Neurotrauma. 2009;26:313–323. doi: 10.1089/neu.2008.0580. [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Girgis J, Colbourne F. Delayed onset of prolonged hypothermia improves outcome after intracerebral hemorrhage in rats. J Cereb Blood Flow Metab. 2004;24:432–440. doi: 10.1097/00004647-200404000-00008. [DOI] [PubMed] [Google Scholar]
- Macleod MR, Lewis SC, Dennis MS. Effect of deprivation on time to hospital in acute stroke. J Neurol Neurosurg Psychiatry. 2003;74:545–546. doi: 10.1136/jnnp.74.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan SL, Myers TJ, Baldini MG. Disseminated intravascular coagulation during rewarming following hypothermia. JAMA. 1981;245:2517–2518. [PubMed] [Google Scholar]
- Maier CM, Sun GH, Kunis D, Yenari MA, Steinberg GK. Delayed induction and long-term effects of mild hypothermia in a focal model of transient cerebral ischemia: neurological outcome and infarct size. J Neurosurg. 2001;94:90–96. doi: 10.3171/jns.2001.94.1.0090. [DOI] [PubMed] [Google Scholar]
- Markarian GZ, Lee JH, Stein DJ, Hong SC. Mild hypothermia: therapeutic window after experimental cerebral ischemia. Neurosurgery. 1996;38:542–550. doi: 10.1097/00006123-199603000-00024. [DOI] [PubMed] [Google Scholar]
- Matsukawa T, Sessler DI, Sessler AM, Schroeder M, Ozaki M, Kurz A, Cheng C. Heat flow and distribution during induction of general anesthesia. Anesthesiology. 1995;82:662–673. doi: 10.1097/00000542-199503000-00008. [DOI] [PubMed] [Google Scholar]
- Meden P, Overgaard K, Pedersen H, Boysen G. Effect of hypothermia and delayed thrombolysis in a rat embolic stroke model. Acta Neurol Scand. 1994a;90:91–98. doi: 10.1111/j.1600-0404.1994.tb02686.x. [DOI] [PubMed] [Google Scholar]
- Meden P, Overgaard K, Pedersen H, Boysen G. The influence of body temperature on infarct volume and thrombolytic therapy in a rat embolic stroke model. Brain Res. 1994b;647:131–138. doi: 10.1016/0006-8993(94)91407-9. [DOI] [PubMed] [Google Scholar]
- Meisel C, Prass K, Braun J, Victorov I, Wolf T, Megow D, Halle E, Volk HD, Dirnagl U, Meisel A. Preventive antibacterial treatment improves the general medical and neurological outcome in a mouse model of stroke. Stroke. 2004;35:2–6. doi: 10.1161/01.STR.0000109041.89959.4C. [DOI] [PubMed] [Google Scholar]
- Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6:775–786. doi: 10.1038/nrn1765. [DOI] [PubMed] [Google Scholar]
- Mekjavic IB, Eiken O. Inhibition of shivering in man by thermal stimulation of the facial area. Acta Physiol Scand. 1985;125:633–637. doi: 10.1111/j.1748-1716.1985.tb07765.x. [DOI] [PubMed] [Google Scholar]
- Meloni BP, Campbell K, Zhu H, Knuckey NW. In search of clinical neuroprotection after brain ischemia: the case for mild hypothermia (35 degrees C) and magnesium. Stroke. 2009;40:2236–2240. doi: 10.1161/STROKEAHA.108.542381. [DOI] [PubMed] [Google Scholar]
- Mokhtarani M, Mahgoub AN, Morioka N, Doufas AG, Dae M, Shaughnessy TE, Bjorksten AR, Sessler DI. Buspirone and meperidine synergistically reduce the shivering threshold. Anesth Analg. 2001;93:1233–1239. doi: 10.1097/00000539-200111000-00038. [DOI] [PubMed] [Google Scholar]
- Molina CA, Ribo M, Rubiera M, Montaner J, Santamarina E, Delgado-Mederos R, Arenillas JF, Huertas R, Purroy F, Delgado P, Alvarez-Sabin J. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke. 2006;37:425–429. doi: 10.1161/01.STR.0000199064.94588.39. [DOI] [PubMed] [Google Scholar]
- Murkin JM. Cerebral autoregulation: the role of CO2 in metabolic homeostasis. Semin Cardiothorac Vasc Anesth. 2007;11:269–273. doi: 10.1177/1089253207311159. [DOI] [PubMed] [Google Scholar]
- Murkin JM, Farrar JK, Tweed WA, McKenzie FN, Guiraudon G. Cerebral autoregulation and flow/metabolism coupling during cardiopulmonary bypass: the influence of PaCO2. Anesth Analg. 1987;66:825–832. [PubMed] [Google Scholar]
- O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- Ohta H, Terao Y, Shintani Y, Kiyota Y. Therapeutic time window of post-ischemic mild hypothermia and the gene expression associated with the neuroprotection in rat focal cerebral ischemia. Neurosci Res. 2007;57:424–433. doi: 10.1016/j.neures.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Oshima H, Katayama Y, Hirayama T. Intracerebral steal phenomenon associated with global hyperemia in moyamoya disease during revascularization surgery. J Neurosurg. 2000;92:949–954. doi: 10.3171/jns.2000.92.6.0949. [DOI] [PubMed] [Google Scholar]
- Peterson K, Carson S, Carney N. Hypothermia treatment for traumatic brain injury: a systematic review and meta-analysis. J Neurotrauma. 2008;25:62–71. doi: 10.1089/neu.2007.0424. [DOI] [PubMed] [Google Scholar]
- Poca MA, Benejam B, Sahuquillo J, Riveiro M, Frascheri L, Merino MA, Delgado P, Alvarez-Sabin J. Monitoring intracranial pressure in patients with malignant middle cerebral artery infarction: is it useful. J Neurosurg. 2010;112:648–57. doi: 10.3171/2009.7.JNS081677. [DOI] [PubMed] [Google Scholar]
- Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med. 2009;37:1101–1120. doi: 10.1097/CCM.0b013e3181962ad5. [DOI] [PubMed] [Google Scholar]
- Polderman KH, Rijnsburger ER, Peerdeman SM, Girbes AR. Induction of hypothermia in patients with various types of neurologic injury with use of large volumes of ice-cold intravenous fluid. Crit Care Med. 2005;33:2744–2751. doi: 10.1097/01.ccm.0000190427.88735.19. [DOI] [PubMed] [Google Scholar]
- Porzecanski I, Bowton DL. Diagnosis and treatment of ventilator-associated pneumonia. Chest. 2006;130:597–604. doi: 10.1378/chest.130.2.597. [DOI] [PubMed] [Google Scholar]
- Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U, Volk HD, Meisel A. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W, Shen H, Zhang Y, Wang W, Liu W, Jiang Q, Luo M, Manou M. Noninvasive selective brain cooling by head and neck cooling is protective in severe traumatic brain injury. J Clin Neurosci. 2006;13:995–1000. doi: 10.1016/j.jocn.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Rajek A, Greif R, Sessler DI, Baumgardner J, Laciny S, Bastanmehr H. Core cooling by central venous infusion of ice-cold (4 degrees C and 20 degrees C) fluid: isolation of core and peripheral thermal compartments. Anesthesiology. 2000;93:629–637. doi: 10.1097/00000542-200009000-00010. [DOI] [PubMed] [Google Scholar]
- Ren Y, Hashimoto M, Pulsinelli WA, Nowak TS., Jr Hypothermic protection in rat focal ischemia models: strain differences and relevance to ‘reperfusion injury'. J Cereb Blood Flow Metab. 2004;24:42–53. doi: 10.1097/01.WCB.0000095802.98378.91. [DOI] [PubMed] [Google Scholar]
- Romlin B, Petruson K, Nilsson K. Moderate superficial hypothermia prolongs bleeding time in humans. Acta Anaesthesiol Scand. 2007;51:198–201. doi: 10.1111/j.1399-6576.2006.01181.x. [DOI] [PubMed] [Google Scholar]
- Russwurm S, Stonans I, Schwerter K, Stonane E, Meissner W, Reinhart K. Direct influence of mild hypothermia on cytokine expression and release in cultures of human peripheral blood mononuclear cells. J Interferon Cytokine Res. 2002;22:215–221. doi: 10.1089/107999002753536185. [DOI] [PubMed] [Google Scholar]
- Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005;33:2184–2193. doi: 10.1097/01.ccm.0000181731.53912.d9. [DOI] [PubMed] [Google Scholar]
- Scharbert G, Kalb ML, Essmeister R, Kozek-Langenecker SA. Mild and moderate hypothermia increases platelet aggregation induced by various agonists: a whole blood in vitro study. Platelets. 2010;21:44–48. doi: 10.3109/09537100903420269. [DOI] [PubMed] [Google Scholar]
- Schwab S, Georgiadis D, Berrouschot J, Schellinger PD, Graffagnino C, Mayer SA. Feasibility and safety of moderate hypothermia after massive hemispheric infarction. Stroke. 2001;32:2033–2035. doi: 10.1161/hs0901.095394. [DOI] [PubMed] [Google Scholar]
- Schwab S, Schwarz S, Spranger M, Keller E, Bertram M, Hacke W. Moderate hypothermia in the treatment of patients with severe middle cerebral artery infarction. Stroke. 1998;29:2461–2466. doi: 10.1161/01.str.29.12.2461. [DOI] [PubMed] [Google Scholar]
- Schwarzenberg H, Muller-Hulsbeck S, Brossman J, Gluer CC, Bruhn HD, Heller M. Hyperthermic fibrinolysis with rt-PA: in vitro results. Cardiovasc Intervent Radiol. 1998;21:142–145. doi: 10.1007/s002709900231. [DOI] [PubMed] [Google Scholar]
- Sena E, van der Worp HB, Howells D, Macleod M. How can we improve the pre-clinical development of drugs for stroke. Trends Neurosci. 2007a;30:433–439. doi: 10.1016/j.tins.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Sena ES, van der Worp HB, Bath PMW, Howells DW, Macleod MR.2010Publication bias in reports of animal stroke studies leads to major overstatement of efficacy PLoS Biol 8e1000344. doi: 10.1371/journal.pbio.1000344(in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena E, Wheble P, Sandercock P, Macleod M. Systematic review and meta-analysis of the efficacy of tirilazad in experimental stroke. Stroke. 2007b;38:391. doi: 10.1161/01.STR.0000254462.75851.22. [DOI] [PubMed] [Google Scholar]
- Sessler DI. Defeating normal thermoregulatory defenses: induction of therapeutic hypothermia. Stroke. 2009a;40:e614–e621. doi: 10.1161/STROKEAHA.108.520858. [DOI] [PubMed] [Google Scholar]
- Sessler DI. Thermoregulatory defense mechanisms. Crit Care Med. 2009b;37:S203–S210. doi: 10.1097/CCM.0b013e3181aa5568. [DOI] [PubMed] [Google Scholar]
- Shaw GJ, Bavani N, Dhamija A, Lindsell CJ. Effect of mild hypothermia on the thrombolytic efficacy of 120 kHz ultrasound enhanced thrombolysis in an in-vitro human clot model. Thromb Res. 2006;117:603–608. doi: 10.1016/j.thromres.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Shaw GJ, Dhamija A, Bavani N, Wagner KR, Holland CK. Arrhenius temperature dependence of in vitro tissue plasminogen activator thrombolysis. Phys Med Biol. 2007;52:2953–2967. doi: 10.1088/0031-9155/52/11/002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorbello D, Dewey HM, Churilov L, Thrift AG, Collier JM, Donnan G, Bernhardt J. Very early mobilisation and complications in the first 3 months after stroke: further results from phase II of A Very Early Rehabilitation Trial (AVERT) Cerebrovasc Dis. 2009;28:378–383. doi: 10.1159/000230712. [DOI] [PubMed] [Google Scholar]
- Staab DB, Sorensen VJ, Fath JJ, Raman SB, Horst HM, Obeid FN. Coagulation defects resulting from ambient temperature-induced hypothermia. J Trauma. 1994;36:634–638. doi: 10.1097/00005373-199405000-00006. [DOI] [PubMed] [Google Scholar]
- STAIR Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Sweney MT, Sigg DC, Tahvildari S, Iaizzo PA. Shiver suppression using focal hand warming in unanesthetized normal subjects. Anesthesiology. 2001;95:1089–1095. doi: 10.1097/00000542-200111000-00011. [DOI] [PubMed] [Google Scholar]
- Talke P, Tayefeh F, Sessler DI, Jeffrey R, Noursalehi M, Richardson C. Dexmedetomidine does not alter the sweating threshold, but comparably and linearly decreases the vasoconstriction and shivering thresholds. Anesthesiology. 1997;87:835–841. doi: 10.1097/00000542-199710000-00017. [DOI] [PubMed] [Google Scholar]
- Thrift AG, Dewey HM, Sturm JW, Paul SL, Gilligan AK, Srikanth VK, Macdonell RA, McNeil JJ, Macleod MR, Donnan GA. Greater incidence of both fatal and nonfatal strokes in disadvantaged areas: the Northeast Melbourne Stroke Incidence Study. Stroke. 2006;37:877–882. doi: 10.1161/01.STR.0000202588.95876.a7. [DOI] [PubMed] [Google Scholar]
- Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, Amelink GJ, Schmiedeck P, Schwab S, Rothwell PM, Bousser MG, van der Worp HB, Hacke W. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215–222. doi: 10.1016/S1474-4422(07)70036-4. [DOI] [PubMed] [Google Scholar]
- Valeri CR, Feingold H, Cassidy G, Ragno G, Khuri S, Altschule MD. Hypothermia-induced reversible platelet dysfunction. Ann Surg. 1987;205:175–181. doi: 10.1097/00000658-198702000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Worp HB, de HP, Morrema E, Kalkman CJ. Methodological quality of animal studies on neuroprotection in focal cerebral ischaemia. J Neurol. 2005;252:1108–1114. doi: 10.1007/s00415-005-0802-3. [DOI] [PubMed] [Google Scholar]
- van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain. 2007;130:3063–3074. doi: 10.1093/brain/awm083. [DOI] [PubMed] [Google Scholar]
- van der Worp HB, van Gijn J. Clinical practice. Acute ischemic stroke. N Engl J Med. 2007;357:572–579. doi: 10.1056/NEJMcp072057. [DOI] [PubMed] [Google Scholar]
- Wandaller C, Holzer M, Sterz F, Wandaller A, Arrich J, Uray T, Laggner AN, Herkner H. Head and neck cooling after cardiac arrest results in lower jugular bulb than esophageal temperature. Am J Emerg Med. 2009;27:460–465. doi: 10.1016/j.ajem.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Wang H, Olivero W, Lanzino G, Elkins W, Rose J, Honings D, Rodde M, Burnham J, Wang D. Rapid and selective cerebral hypothermia achieved using a cooling helmet. J Neurosurg. 2004;100:272–277. doi: 10.3171/jns.2004.100.2.0272. [DOI] [PubMed] [Google Scholar]
- Xavier RG, White AE, Fox SC, Wilcox RG, Heptinstall S. Enhanced platelet aggregation and activation under conditions of hypothermia. Thromb Haemost. 2007;98:1266–1275. doi: 10.1160/th07-03-0189. [DOI] [PubMed] [Google Scholar]
- Yanamoto H, Hong SC, Soleau S, Kassell NF, Lee KS. Mild postischemic hypothermia limits cerebral injury following transient focal ischemia in rat neocortex. Brain Res. 1996;718:207–211. doi: 10.1016/0006-8993(96)00122-9. [DOI] [PubMed] [Google Scholar]
- Yanamoto H, Nagata I, Nakahara I, Tohnai N, Zhang Z, Kikuchi H. Combination of intraischemic and postischemic hypothermia provides potent and persistent neuroprotection against temporary focal ischemia in rats. Stroke. 1999;30:2720–2726. doi: 10.1161/01.str.30.12.2720. [DOI] [PubMed] [Google Scholar]
- Yenari M, Kitagawa K, Lyden P, Perez-Pinzon M. Metabolic downregulation: a key to successful neuroprotection. Stroke. 2008;39:2910–2917. doi: 10.1161/STROKEAHA.108.514471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenari MA, Palmer JT, Bracci PM, Steinberg GK. Thrombolysis with tissue plasminogen activator (tPA) is temperature dependent. Thromb Res. 1995;77:475–481. doi: 10.1016/0049-3848(95)93883-2. [DOI] [PubMed] [Google Scholar]
- Yoshihara H, Yamamoto T, Mihara H. Changes in coagulation and fibrinolysis occurring in dogs during hypothermia. Thromb Res. 1985;37:503–512. doi: 10.1016/0049-3848(85)90096-9. [DOI] [PubMed] [Google Scholar]
- Zeiner A, Holzer M, Sterz F, Behringer W, Schorkhuber W, Mullner M, Frass M, Siostrzonek P, Ratheiser K, Kaff A, Laggner AN. Mild resuscitative hypothermia to improve neurological outcome after cardiac arrest. A clinical feasibility trial. Hypothermia After Cardiac Arrest (HACA) Study Group. Stroke. 2000;31:86–94. doi: 10.1161/01.str.31.1.86. [DOI] [PubMed] [Google Scholar]
- Zhang JN, Wood J, Bergeron AL, McBride L, Ball C, Yu Q, Pusiteri AE, Holcomb JB, Dong JF. Effects of low temperature on shear-induced platelet aggregation and activation. J Trauma. 2004;57:216–223. doi: 10.1097/01.ta.0000093366.98819.fe. [DOI] [PubMed] [Google Scholar]
- Zhao H, Steinberg GK, Sapolsky RM. General versus specific actions of mild-moderate hypothermia in attenuating cerebral ischemic damage. J Cereb Blood Flow Metab. 2007;27:1879–1894. doi: 10.1038/sj.jcbfm.9600540. [DOI] [PubMed] [Google Scholar]
- Zweifler RM, Sessler DI. Thermoregulatory vasococonstriction and shivering impede therapeutic hypothermia in acute ischemic stroke patients. J Stroke Cerebrovasc Dis. 1996;6:100–103. doi: 10.1016/s1052-3057(96)80011-5. [DOI] [PubMed] [Google Scholar]
- Zweifler RM, Voorhees ME, Mahmood MA, Parnell M. Magnesium sulfate increases the rate of hypothermia via surface cooling and improves comfort. Stroke. 2004;35:2331–2334. doi: 10.1161/01.STR.0000141161.63181.f1. [DOI] [PubMed] [Google Scholar]