Abstract
In addition to the neuronal and behavioral consequences of excess glucocorticoid exposure, the cerebrovascular system can also be adversely affected by stressors. This study determined that chronic stress in adulthood decreased the vascular area fraction of the hippocampus and increased the vascular area fraction of the amygdala. In addition, the data indicated that prenatal exposure to synthetic glucocorticoids modulated the effects of adult stress on vascular area fraction of the hippocampus and amygdala. These data indicate that in addition to the well-documented stress-induced changes in neurons and glia, cerebral vasculature is also altered by exposure to stressors.
Keywords: angiogenesis, anhedonia, dexamethasone, prenatal stress, vascular
Introduction
Stressor exposure in early life or in adulthood has been reported to have detrimental consequences, and the combination of early life stress and stress in adulthood is particularly damaging. Both early life stress and exposure to stressors in adulthood can alter brain morphology, the hypothalamic–pituitary–adrenal axis, and behavior (Neigh et al, 2009). Adult exposure to exogenous glucocorticoids inhibits cerebral angiogenesis (Ekstrand et al, 2008), induces apoptosis in cerebral microvascular pericytes (Katychev et al, 2003), and causes cerebral microvascular endothelial cell rarefaction (Vogt and Schmid-Schonbein, 2001). Furthermore, exposure to chronic social stress in adulthood reduces the vascularization by 30% in the hippocampus (Czeh et al, 2010). An understanding of the effects of prenatal glucocorticoid and adult stress exposure on cerebral vasculature is particularly important given the role of vascular function in the maintenance of neuronal integrity (Zauner et al, 2002). This study tested the hypothesis that prenatal exposure to glucocorticoids sensitizes offspring to the behavioral and cerebral vascular effects of chronic stress in adulthood.
Materials and methods
Prenatal Treatment
Long Evans dams (Harlan, Indianapolis, IN, USA) arrived on gestational day 12 and were housed individually on a 12:12 light:dark cycle at 20°C±4°C and at a relative humidity of 50%±5%. Tap water and food (Harlan Teklad, Madison, WI, USA) were available ad libitum. On gestational days 14 to 21, pregnant dams were given subcutaneous injections of dexamethasone (DEX; 100 μg/kg in 0.1 mL sterile saline) or saline (SAL; 0.1 mL). Dexamethasone is a synthetic glucocorticoid that is reported to mimic maternal stress exposure (O'Regan et al, 2004). Pups were weaned on PND 22 and group housed until experimental protocols commenced on PND 90.
Adult Stress
Adult male rats derived from the described prenatal treatments were divided into two cohorts, one for behavioral analysis and one for vascular area fraction. Each cohort contained: (1) prenatal SAL+adult control (SAL+CON) (n=6), (2) prenatal SAL+adult stress (SAL+STRESS) (n=6), (3) prenatal DEX+adult control (DEX+CON) (n=7), and (4) prenatal DEX+adult stress (DEX+STRESS) (n=6). Each experimental group for each cohort contained only one rat from each litter. The control condition consisted of handling for ∼30 seconds each day during the light cycle. Restraint stress was administered for 2 hours/day for 14 days using immobilization bags (Braintree Scientific, Braintree, MA, USA) during the light cycle. This duration of restraint was chosen because 10 days of exposure is sufficient to alter neuronal architecture (Vyas et al, 2002) and changes in vasculature often require up to 2 weeks of stress to be detectable (LaManna et al, 2004).
Behavioral Testing
Saccharin preference
Preference for a saccharin solution (0.015% in filtered tap water) versus regular drinking water was used as an indirect measure of hedonic state (Pijlman et al, 2003). The ratio of the total amount of saccharin and nonsaccharin drinking water consumed was calculated after a 24-hour exposure.
Open field
Dark-cycle activity measurements were evaluated by placing the rats in an open field (40 × 40 × 39 cm3) under red light. Activity was recorded at 5-minute intervals for 25 minutes and quantified by a computer-operated tracking system of 16 photo beams per side (TruScan System; Coulbourn Instruments, Allentown, PA, USA). The distance traveled in each 5-minute interval was measured as the total of all vectored x–y-coordinate changes.
Histology
At the conclusion of the 14th day of treatment, rats were deeply anesthetized and transcardially perfused. Brains were paraffin embedded, cut on a microtome (8 μm), and mounted on polylysine slides. Sections were stained for von Willebrand factor (Chemicon, Billerica, MA, USA), an endothelial cell marker, visualized, counterstained with hemotoxylin, and coverslipped (full details of the methods are available in Supplementary Methods).
Vasculature was assessed using the stereological method of area fraction (Mouton, 2002) by counting the area occupied by blood vessels. The CA3 region of the hippocampus and the basolateral nucleus of the amygdala were targeted for assessment, owing to the established involvement of these regions in stress-induced neuronal remodeling (Vyas et al, 2002). Eighteen randomly selected fields per region were counted for each rat. An average was calculated for each brain region for each rat and used for statistical analyses.
Data Analysis and Statistics
SigmaStat 3.0 (SPSS, Chicago, IL, USA) was used to conduct all statistical analyses. Data sets were assessed for equal variance and normality before parametric testing. The effects of the prenatal and adult conditions on saccharin preference and area fraction of capillaries in the amygdala and hippocampus were assessed using two-way analysis of variance. If there was a main effect or interaction, then specific group differences were assessed using the Tukey post hoc test. Differences were considered statistically significant if P<0.05.
Results
Saccharin Preference
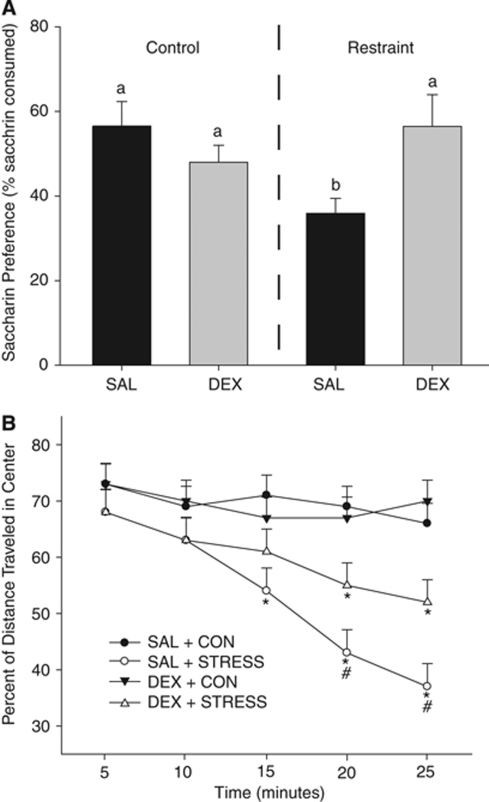
Chronic stress in adulthood reduced saccharin preference (F1,19=7.4; P<0.05; SAL+CON=56.6±5.1% and SAL+STRESS=35.9±3.5% Figure 1A). In addition, among the rats exposed to chronic stress as adults, those that received DEX in utero had a greater preference for saccharin than the rats exposed to SAL in utero (P<0.05); the rats exposed to DEX+STRESS had a preference for saccharin, similar to both the SAL and DEX rats that were in the adult control conditions (P>0.05).
Figure 1.
(A) Exposure to chronic stress in adulthood reduced saccharin preference (P<0.05 as compared with all groups designated with ‘a'). Prenatal DEX prevented the stress-induced reduction in saccharin preference (P>0.05 as compared with all groups designated with ‘a' and P<0.05 as compared with ‘b'). (B) Chronic stress in adulthood reduced central tendency in an open field (*P<0.05 compared with SAL+CON or DEX+CON). Rats exposed to DEX in utero followed by STRESS in adulthood showed a reduction in central tendency (*P<0.05 compared with SAL+CON or DEX+CON), but to a lesser degree than those rats exposed to SAL+STRESS (#P<0.05 compared with SAL+STRESS). Data are presented as mean±s.e.m.; n=6 for SAL+CON, SAL+STRESS, DEX+STRESS; n=7 for DEX+CON. DEX+CON, dexamethasone+adult control; SAL+CON, saline+adult control.
Open Field
Exposure to DEX in utero reduced total activity in the open field as compared with SAL-treated offspring (F1,49=5.6, P<0.01; data not shown). Central tendency, or the percent of distance traveled in the center of the arena over the duration of the test, was reduced in rats exposed to chronic stress as adults, regardless of prenatal treatment (F3,47=3.6, P<0.05; SAL+CON=69.5%±4.6%, DEX+CON=67.9%±4.6%, SAL+STRESS=49.7%±5.2%, and DEX+STRESS=57.5%±4.9% Figure 1B illustrates this effect over the 5-minute blocks of the test).
Vascular Area Fraction
Amygdala
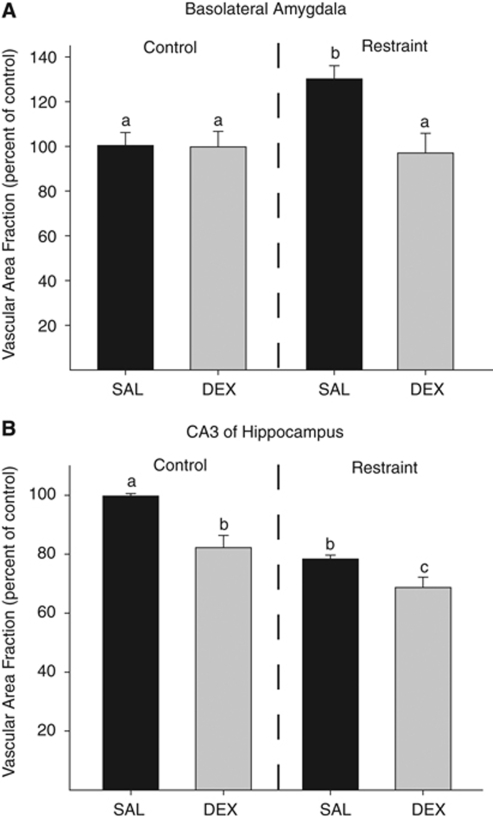
The area fraction of capillaries in the basolateral amygdala was altered by prenatal treatment and adult stress exposure (F1,21=4.7, P<0.05; Figure 2A). Post hoc analysis showed that those rats that were exposed to SAL+STRESS had a 30% higher area fraction of capillaries in the basolateral amygdala than those in the SAL+CON group (P<0.05). Furthermore, those rats in the DEX+STRESS group had an area fraction of capillaries in the amygdala similar to that of the rats in the SAL+CON group (P>0.05) and lower than that of the rats in the SAL+STRESS group (P<0.05).
Figure 2.
(A) Chronic stress in adulthood increased vascularization in the basolateral amygdala (P<0.05 as compared with all conditions labeled with ‘a'). Dexamethasone in utero buffered against the chronic stress-induced change in vascularization in the amygdala such that there was no difference among DEX+STRESS and the control conditions (P>0.05), but the SAL+STRESS group had more area occupied by vasculature in the amygdala than those rats in the DEX+STRESS group (P<0.05). (B) Rats exposed to prenatal DEX have a reduced vascularization of the hippocampus as compared with rats prenatally exposed to saline (SAL; P<0.05). Chronic stress in adulthood also reduced hippocampal vascularization as compared with handled rats (SAL+CON; P<0.05). The combination of prenatal DEX+STRESS was additive such that vascularization was reduced as compared with SAL+CON, DEX+CON, and SAL+STRESS (P<0.05). Data are presented as mean±s.e.m.; n=6 for SAL+CON, SAL+STRESS, DEX+STRESS; n=7 for DEX+CON. DEX+CON, dexamethasone+adult control; SAL+CON, saline+adult control.
Hippocampus
The area fraction of capillaries in the CA3 region of the hippocampus was reduced by prenatal treatment with DEX (F1,21=14.7, P<0.05; Figure 2B) and by adult stress exposure (F1,21=25.0, P<0.05). Post hoc analysis showed that exposure to DEX in utero reduced the area fraction occupied by capillaries in the hippocampus by 16% when compared with SAL-treated offspring (P<0.05). In addition, exposure to chronic stress in adulthood reduced the area fraction occupied by capillaries by 20% as compared with control-treated adults (P<0.05). An a priori t-test showed that rats given DEX in utero and exposed to chronic stress as adults had a lower area fraction occupied by capillaries in the CA3 region of the hippocampus than those rats that received SAL in utero and chronic stress as adults (t12=2.6, P<0.05), and the DEX+STRESS group showed a 29% reduction in vascularization in the hippocampus as compared with the SAL+CON group.
Discussion
Chronic stress increased vascular density in the amygdala and decreased vascular density in the hippocampus. Our hippocampal finding is consistent with a recent report that documented a reduction in hippocampal vascularization after 5 weeks of social defeat stress (Czeh et al, 2010) and mirrors the well-established chronic stress-induced retraction of dendritic branching in the hippocampus (Vyas et al, 2002). To the best of our knowledge, the data reported here that show a vascular hypertrophy in the amygdala are the first report on the effects of chronic stress on vasculature in the amygdala. The effect of chronic stress on amygdalar vasculature again reflected the established effects of chronic stress on neuronal cytoarchitecture, as chronic stress has been shown to cause hypertrophy of dendrites in the amygdala (Vyas et al, 2003). This data set does not allow us to establish whether the changes in vasculature precede or follow the changes in neuronal cytoarchitecture. Future work will ascertain the temporal relationship between chronic stress-induced changes in neuronal cytoarchitecture and vascularization. Given the crucial role of vascular support in the cerebrum (Zauner et al, 2002), a relationship between these two types of stress-induced remodeling merits further consideration.
Contrary to our hypothesis that prenatal DEX exposure would sensitize rats to the negative effects of chronic stress exposure in adulthood, the developmental exposure to high levels of glucocorticoids seems to have been protective. Although the peripheral cardiovascular effects of prenatal DEX have been reported (O'Regan et al, 2004), effects on the cerebral vasculature have not been investigated earlier. One possible explanation for the seemingly protective effects of prenatal DEX exposure in this study are the theories of stress inoculation, resilience, and hormesis (Edge et al, 2009). In short, these theories postulate that exposure to mild-to-moderate levels of stress, or, in this case, glucocorticoids, during development prepares the offspring for a stressful environment, thereby engendering a buffer against subsequent stress exposure (Edge et al, 2009). Our data suggest that prenatal DEX does in fact lead to a buffering against the effects of chronic stress in adulthood in terms of affective behavior and amygdalar vascularization. However, DEX-mediated buffering of the chronic stress effects on behavior and amygdalar vasculature is at the expense of, or in combination with, an exacerbated effect of chronic stress on decreased vascularization of the hippocampus.
The data presented in this report show that cerebral vascularization can be bidirectionally regulated in a region-specific manner by exposure to adult stress. Furthermore, prenatal exposure to DEX protected against the effects of adult stress on affective behavior and amygdalar vasculature, but exacerbated the retraction of hippocampal vasculature. This suggests that glucocorticoid exposure during development can be either protective or detrimental within the same organism. The implications of these changes in cerebral vasculature are not yet fully defined. Earlier work has shown that after electroconvulsive seizures and enrichment, therapies effective in reducing depressive-like behaviors in rat models, hippocampal vasculature increases by up to 30% (Ekstrand et al, 2008; Hellsten et al, 2005), and the authors suggest a potential role for cerebral vascular changes in affective disorders similar in nature to the established relationship between increased neuronal demand and vascular outgrowth in motor cortex (Black et al, 1990). The specific relationship between stress-induced changes in cerebral vasculature and stress-induced changes in neuronal cytoarchitecture and function cannot be ascertained from this data set; however, these findings show that the effects of chronic stress on the brain are not limited to neurons. Given the crucial role of cerebral vasculature in the maintenance of neuronal health and function and the evidence presented here, stress-induced alterations in cerebral vasculature may contribute to stress-induced neuronal and behavioral dysfunction warranting further consideration.
Gretchen N Neigh, PhD, receives research funding from NARSAD, AHA, NIH, and GlaxoSmithKline. Michael J Owens, PhD, receives research funding from NIH, Eli Lilly, Lundbeck, Cyberonics, Ortho-McNeil Janssen, AstraZeneca, and Dainippon Sumitomo. Dr Owens consults for H Lundbeck A/S. Charles B Nemeroff, MD, PhD, has research funding from NIH, is on the Scientific Advisory Board for AFSP, AstraZeneca, Forest Laboratories, NARSAD, Quintiles, Janssen/Ortho-McNeil, PharmaNeuroboost, and Mt Cook Pharma; is a stockholder in Corcept, Revaax, NovaDel Pharma, CeNeRx, and PharmaNeuroboost; and is on the Board of directors for American Foundation for Suicide Prevention (AFSP), George West Mental Health Foundation, NovaDel Pharma, and Mt Cook Pharma. Dr Nemeroff and Dr Owens have a patent entitled ‘A method to estimate transporter occupancy.' Dr Nemeroff has a patent on the method and devices for transdermal delivery of lithium (US 6,375,990 B1).
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci USA. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Abumaria N, Rygula R, Fuchs E. Quantitative changes in hippocampal microvasculature of chronically stressed rats: no effect of fluoxetine treatment. Hippocampus. 2010;20:174–85. doi: 10.1002/hipo.20599. [DOI] [PubMed] [Google Scholar]
- Edge MD, Ramel W, Drabant EM, Kuo JR, Parker KJ, Gross JJ. For better or worse? Stress inoculation effects for implicit but not explicit anxiety. Depress Anxiety. 2009;26:831–837. doi: 10.1002/da.20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand J, Hellsten J, Tingstrom A. Environmental enrichment, exercise and corticosterone affect endothelial cell proliferation in adult rat hippocampus and prefrontal cortex. Neurosci Lett. 2008;442:203–207. doi: 10.1016/j.neulet.2008.06.085. [DOI] [PubMed] [Google Scholar]
- Hellsten J, West MJ, Arvidsson A, Ekstrand J, Jansson L, Wennstrom M, Tingstrom A. Electroconvulsive seizures induce angiogenesis in adult rat hippocampus. Biol Psychiatry. 2005;58:871–878. doi: 10.1016/j.biopsych.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Katychev A, Wang X, Duffy A, Dore-Duffy P. Glucocorticoid-induced apoptosis in CNS microvascular pericytes. Dev Neurosci. 2003;25:436–446. doi: 10.1159/000075669. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Chavez JC, Pichiule P. Structural and functional adaptation to hypoxia in the rat brain. J Exp Biol. 2004;207:3163–3169. doi: 10.1242/jeb.00976. [DOI] [PubMed] [Google Scholar]
- Mouton PR. Principles and Practices of Unbiased Stereology: An Introduction for Bioscientists. Baltimore: The Johns Hopkins University Press; 2002. [Google Scholar]
- Neigh GN, Gillespie CF, Nemeroff CB. The neurobiological toll of child abuse and neglect. Trauma Violence Abuse. 2009;10:389–410. doi: 10.1177/1524838009339758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. Am J Physiol Endocrinol Metab. 2004;287:E863–E870. doi: 10.1152/ajpendo.00137.2004. [DOI] [PubMed] [Google Scholar]
- Pijlman FT, Wolterink G, Van Ree JM. Physical and emotional stress have differential effects on preference for saccharine and open field behaviour in rats. Behav Brain Res. 2003;139:131–138. doi: 10.1016/s0166-4328(02)00124-9. [DOI] [PubMed] [Google Scholar]
- Vogt CJ, Schmid-Schonbein GW. Microvascular endothelial cell death and rarefaction in the glucocorticoid-induced hypertensive rat. Microcirculation. 2001;8:129–139. [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res. 2003;965:290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauner A, Daugherty WP, Bullock MR, Warner DS.2002Brain oxygenation and energy metabolism: part I-biological function and pathophysiology Neurosurgery 51289–301.discussion 302 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.