Abstract
Recent studies showed that soluble annexin A2 dramatically increases tissue plasminogen activator (tPA)-mediated plasmin generation in vitro, and reduces thrombus formation in vivo. Here, we hypothesize that combining annexin A2 with tPA can significantly enhance thrombolysis efficacy, so that lower doses of tPA can be applied in ischemic stroke to avoid neurotoxic and hemorrhagic complications. In vitro activity assays confirmed tPA-specific amplification of plasmin generation by recombinant annexin A2. In a rat focal embolic stroke model, combination therapy with tPA and recombinant annexin A2 protein at 2 h post-ischemia decreased the effective dose required for tPA by four-fold and reduced brain infarction. Combining annexin A2 with tPA also lengthened the time window for thrombolysis. Compared with tPA (10 mg/kg) alone, the combination of annexin A2 (5 mg/kg) plus low-dose tPA (2.5 mg/kg) significantly enhanced fibrinolysis, attenuated mortality, brain infarction, and hemorrhagic transformation, even when administered at 4 h post-ischemia. Combination with recombinant annexin A2, the effective thrombolytic dose of tPA can be decreased. As a result, brain hemorrhage and infarction are reduced, and the time window for stroke reperfusion prolonged. Our present findings provide a promising new approach for enhancing tPA-based thrombolytic stroke therapy.
Keywords: annexin A2, cerebral ischemia, combination therapy, thrombolysis, tPA
Introduction
Intravenous administration of recombinant tissue plasminogen activator (tPA) remains the most beneficial proven intervention for emergency treatment of stroke (Adams et al, 2007). However, hemorrhagic transformation, neurotoxicity, and short treatment time window comprise the major limitations for the thrombolytic therapy (Kaur et al, 2004; Wang et al, 2004; Yepes et al, 2009). Improving tPA stroke therapy has become one of the highest priorities in the field. Recent efforts have been aimed identifying a new strategy that might increase thrombolytic efficacy of tPA, while reducing its associated hemorrhagic transformation and neurotoxicity (Davalos, 2005).
In this study, we tested a new approach that combines annexin A2 with low-dose tPA. tPA works by enzymatically converting thrombus-bound plasminogen to plasmin, allowing plasmin to degrade cross-linked fibrin. Annexin A2 is a cell-surface receptor for both plasminogen (the inactive precursor of plasmin), and its activator, tPA (Hajjar and Menell, 1997; Kim and Hajjar, 2002). The tPA-annexin A2-plasminogen triple complex enables lower concentrations of tPA to convert plasminogen to plasmin very efficiently with a maxim of 60-fold increase in catalytic efficiency compared with the same amount of tPA alone (Hajjar and Acharya, 2000; Hajjar and Krishnan, 1999). Clinically, large amounts of tPA are required to overcome the effects of circulating protease inhibitors, whereas annexin A2 appears to protect tPA from its major antagonist, plasminogen activator inhibitor-1. Recent studies have shown that soluble recombinant annexin A2 (rA2) reduces thrombus formation in rat carotid arteries (Ishii et al, 2001) and middle cerebral arteries in vivo (Tanaka et al, 2007), indicating that this idea may be feasible. Thus in this study, we hypothesize that combining annexin A2 with tPA can significantly enhance thrombolysis efficacy so that lower doses of tPA can be applied in ischemic stroke, thus abolishing neurotoxic and hemorrhagic complications.
In this study, we tested our hypotheses in a rat model of focal embolic stroke. Results showed that rA2 (5 mg/kg) combined with low-dose tPA (2.5 mg/kg) administered at 2 h after the onset of stroke achieved therapeutic benefits similar to those observed with high-dose tPA alone (10 mg/kg). Moreover, delayed combination therapy with rA2 plus low-dose tPA initiated at 4 h after onset of stroke, also significantly improved outcome, compared with standard high-dose tPA alone at 1 day after stroke, and the combination also showed neuroprotection at 3 days after stroke. These findings provide a promising new approach for enhancing clinical outcome and lengthening the time window for thrombolytic stroke therapy with tPA.
Materials and methods
Preparation of Recombinant Human Annexin A2
Histidine-tagged rA2 was produced in Escherichia coli from a bacterial expression vector containing full-length human annexin A2 cDNA according to the method described earlier (Ishii et al, 2001). Purity of rA2 was confirmed by SDS-PAGE followed by Coomassie blue staining, and its identity was verified by western blot analysis.
Plasmin Activity Assay
The indicated individual or combined concentrations of rA2, tPA, uPA (100 Units/mL), and BSA were added directly to wells of 96-well culture plate preloaded with N-terminal lysine plasminogen (2.5 μg/mL) and a fluorogenic plasmin substrate, -Val-Leu-Lys-AMC (200 nmol/L) in a final volume of 100 μL PBS. After incubation at 37°C for 30 mins, plasmin generation or activity was read on a fluorescent plate reader at excitation 360 nm and emission 460 nm (Ishii et al, 2001). The plate readings were expressed as relative fluorescent units for each well, and the final result was represented as fold of plasmin activity generated by tPA or uPA alone.
Animal Models of Focal Embolic Cerebral Ischemia
All experiments were performed following an institutionally approved protocol in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Wistar rats (280 to 330 g) were used. Animals were anesthetized with 1% to 2% isoflurane under spontaneous respiration in a 30% oxygen/70% nitrous oxide mixture. Rectal temperatures were maintained between 37°C and 38°C with a thermostat-controlled heating pad. The right femoral artery was cannulated, and physiologic parameters including rectal temperature, mean arterial blood pressure (MABP), pH, PCO2, and PO2 were monitored throughout all experiments. The right femoral vein was cannulated for drug administration. Focal embolic strokes were induced as described earlier, except for minor modification (Asahi et al, 2000). One blood clot, 40 mm in length was used. In the appropriate groups, human recombinant tPA (Activase, Genentech Inc, San Francisco, CA, USA) and/or rA2 protein were administered intravenously. An initial 10% bolus was followed by infusion of the remaining drug over 30 mins using a syringe pump. The relatively high dose of tPA was chosen based on the ∼10-fold difference in fibrin-specific enzyme activity between human and rodent systems (Korninger and Collen, 1981).
Experiments and Treatment Groups
Three sets of experiments were performed; animals were randomly divided into each group of experiments. All drug treatments and outcome measurements were performed by investigators blinded to the surgical group. The first set of experiment was to test whether rA2 can make low-dose tPA effective in embolic stroke animal model. Two hours after initiation of ischemia, 48 animals (n=8 per group) were treated intravenously with saline, high-dose tPA (10 mg/kg), intermediate-dose tPA (5 mg/kg), low-dose tPA (2.5 mg/kg), rA2 alone (5 mg/kg), or a combination of low-dose tPA (2.5 mg/kg) plus rA2 (5 mg/kg). Laser-doppler flowmetry (LDF) was used to monitor regional cerebral blood flow (rCBF) for up to 1 h after treatment. Brain infarction and hemorrhage volumes were quantified at 24 h after stroke. Our initial choice of combination doses for tPA (2.5 mg/kg) and rA2 (5 mg/kg) in vivo was based on our in vitro plasmin generation data, whereby combining tPA with rA2 at a 1:2 ratio increased by almost four-fold, the plasmin-generating capability of tPA. The second set of experiment was to test whether the combination is thrombolytically effective in prolonged time window. Fifty-one rats (n=17 per group) were treated intravenously at 4 h after stroke onset with saline, high-dose tPA (10 mg/kg), or low-dose tPA (2.5 mg/kg) plus rA2 (5 mg/kg). Neurologic outcomes were measured at 24 h after initiation of stroke. The third set of experiment was to test whether the combination can improve neurologic outcomes in longer survival time after stroke. Twenty-eight rats (n=14 per group) were treated at 4 h after stroke with either saline or low-dose tPA (2.5 mg/kg) plus rA2 (5 mg/kg). Neurologic outcomes were measured at 3 days after stroke. As high-dose tPA treated at 4 h after stroke caused higher 1 day mortality (41%), to avoid unacceptable high mortality, this treatment group was not included in this set of experiment.
Laser-Doppler Flowmetry Analysis
The rCBF was monitored continuously by LDF. In brief, an LDF probe was positioned 2 mm posterior and 5 mm lateral to the bregma, thus monitoring rCBF within the middle cerebral artery territory. Preischemic baselines were set as 100% for each rat. Many laboratories including our laboratory have been using rCBF to identify animals with successful embolic middle cerebral artery occlusion, but the thresholds of baseline varied between laboratories. On the basis of our experience and others' published work, rCBF level is reliable to determine successful clot occlusion and correlated to severity of ischemic brain injury (Henninger et al, 2009). As spontaneously clot lysis may occur, thus in this study we selected a stable ≤40% rCBF of preischemic baseline at 2 h after embolization, as the exclusion criteria. Our experience and published work have shown the rCBF increase after thrombolysis is correlated to brain infarction when treated at early time (1 to 2 h) after focal embolic stroke in rats (Asahi et al, 2000). Thus, in 2 h thrombolysis experiments, rCBF was monitored for 2 h after induction of ischemia, and continuously monitored for 1 h after treatment for determining reperfusion. However, with delayed thrombolysis at 4 h, successful reperfusion is more heterogeneous and takes longer to occur, sometimes up to 3 h post-tPA. Prolonged anesthesia for this embolic clot model significantly raises mortality. So for the 4 h thrombolysis groups, continuously monitoring LDF during ischemia (4 h period) and a full reperfusion (>3 h) would necessitate anesthesia for over 7 h. This was simply not feasible for our animal model. Hence, LDF was used to monitor only the first 2 h of ischemia to ensure adequate embolic occlusions in these delayed 4 h thrombolysis experiments. Furthermore, others have reported that early LDF poorly predicts delayed reperfusion (Henninger et al, 2009), in agreement with our experience for the delayed thrombolysis in the 4-h treatment group.
Analysis of Infarct Volumes
For brain infarction at 24 h after ischemia, rats were killed with a lethal dose of sodium pentobarbital and transcardially perfused to remove all intravascular blood. Coronal brain sections (2 mm thick) were stained with 2.3.5-triphenyltetrazolium chloride (TTC, Sigma, St Louis, MO, USA). Infarct volumes were quantified using computer-assisted image analysis (Asahi et al, 2000). To eliminate confounding effects of edema and swelling, the indirect method was used (contralateral volume minus uninfarcted ipsilateral volume). For 3 days brain infarction, frozen sections (interval 2 mm) were stained with hematoxylin and eosin (H&E). Infarction volumes were quantified as described earlier (Zhao et al, 2006) and were expressed as percentage of hemisphere.
Spectrophotometric Assay of Intracerebral Hemorrhage
At 24 h after stroke, TTC-stained brain sections were used for quantification of intracerebral hemorrhage volume with spectrophotometric hemoglobin assay as described earlier (Asahi et al, 2000).
Analysis of Neurologic Deficits
At 3 days after ischemia, rats were assessed with a 4-point neurologic deficit scale that has been extensively used for rat models of stroke (Bederson et al, 1986).
Measurement of Plasma -Dimer Levels
Plasma levels of -dimer were assayed on plasma samples diluted 1:2 in an ELISA kit (American Diagnostica Inc, Stamford, CT, USA) according to the manufacturer's instructions.
In Situ Zymography
In situ zymography was conducted to detect and localize enzyme activity in tissue sections following a standard method as described earlier (Aoki et al, 2002). In brief, at 24 h after stroke onset, 20-μm cryostat brain sections from animals receiving the 4-h delayed treatment were incubated overnight at room temperature with FITC-labeled gelatin (Molecular Probes, Eugene, OR, USA). Reaction products were visualized by fluorescence microscope.
Statistical Analysis
Data were expressed as mean+s.e.m. The χ2 test was used to evaluate differences in mortality. The rCBF levels, infarct volumes, hemorrhage volumes, neurologic score, and -dimer levels were assessed with ANOVA analysis followed by Tukey–Kramer tests. Differences with P<0.05 were considered statistically significant.
Results
rA2 Amplifies tPA-Mediated Plasmin Generation In Vitro
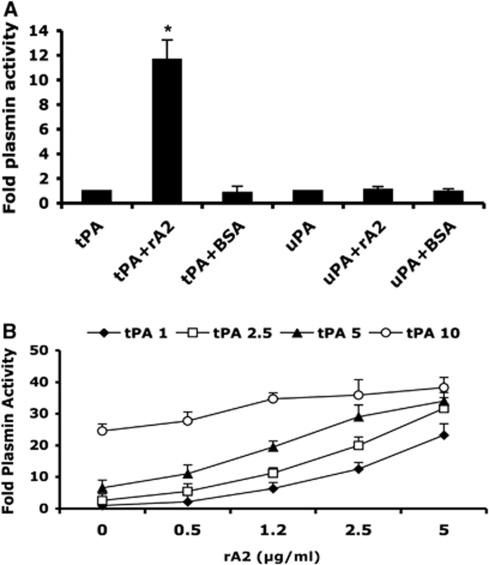
We synthesized and purified recombinant human annexin A2 protein (rA2) as described earlier (Ishii et al, 2001). The purity of the rA2 protein was confirmed by SDS-PAGE and western blotting (data not shown). In vitro plasmin activity assays showed rA2 (5 μg/mL) combined with tPA (2.5 μg/mL) significantly amplified tPA-converted plasmin generation, and this action was tPA specific because BSA protein (5 μg/mL) combined to tPA or rA2 combined with uPA did not increase plasmin activity (Figure 1A). rA2 combined with tPA significantly amplified plasmin generation in a dose-dependent manner. Equal activity of plasmin can be reached by high-dose tPA alone or different combinations with lower doses of tPA with rA2, such as tPA 5 μg/mL versus tPA 1 μg/mL plus rA2 2.5 μg/mL, or tPA 10 μg/mL versus tPA 2.5 μg/mL plus rA2 5 μg/mL. Those data indicated that combining tPA with rA2 at about 1:2 w/v ratio (or 1:4 molar ratio) increased by almost four-fold, the plasmin-generating capability of tPA in vitro. This assay data also indicated amplification of plasmin generation by rA2 plus tPA is predominantly rA2 dose dependent (Figure 1B).
Figure 1.
Effect of rA2 on tPA-dependent plasmin generation in vitro. (A) Plasmin activity was measured as described under Materials and methods and expressed as a ratio to that generated by either 2.5 μg/mL of tPA alone or 100 Units/mL of uPA alone. Concentrations of both rA2 and BSA were 5 μg/mL. Data were expressed as mean+s.e.m., *P<0.001, n=4 per group. (B) A range of concentrations of tPA (1, 2.5, 5, 10 μg/mL) with or without the indicated concentrations of rA2 (0, 1, 2.5, 5 μg/mL) were added to wells of 96-well plate. Plasmin activity was represented as fold of plasmin activity related to 1 μg/mL of tPA alone. Data were expressed as mean+s.e.m., n=4 per group.
rA2 Decreases the Dose of tPA for Focal Embolic Stroke
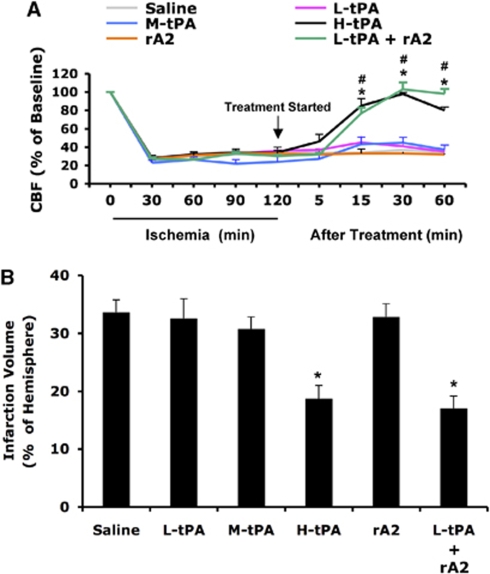
Because of species differences in fibrin specificity, the equivalent effective dose of human recombinant tPA in rat embolic stroke models is 10 mg/kg, about 10 times higher than the dose used in human (Korninger and Collen, 1981). Two hours after initiation of ischemia, animals were treated intravenously with saline, high-dose tPA (10 mg/kg), intermediate-dose tPA (5 mg/kg), low-dose tPA (2.5 mg/kg), rA2 alone (5 mg/kg), or a combination of low-dose tPA (2.5 mg/kg) plus rA2 (5 mg/kg). The LDF was used to monitor rCBF for up to 1 h after treatment. Brain infarction volume was quantified at 24 h after stroke. As expected, high-dose tPA (10 mg/kg) significantly improved reperfusion, and also decreased infarct volumes by about 45% (Figures 2A and 2B). Neither intermediate (5 mg/kg) nor low (2.5 mg/kg) doses of tPA alone, nor rA2 alone were effective for reperfusion improvement and reduction of infarction (Figures 2A and 2B). However, the combination of low-dose tPA (2.5 mg/kg) and rA2 (5 mg/kg) successfully achieved reperfusion and reduced infarct size, in a manner similar to that observed with high-dose tPA alone (Figures 2A and 2B).
Figure 2.
Effect of treating rats at 2 h after initiation of ischemia. (A) Two hours after initiation of ischemia, animals were treated intravenously with saline, high-dose tPA (10 mg/kg, H-tPA), intermediate-dose tPA (5 mg/kg, M-tPA), low-dose tPA (2.5 mg/kg, L-tPA), rA2 alone (5 mg/kg), or a combination of low-dose tPA (2.5 mg/kg) plus rA2 (5 mg/kg). Laser-doppler flowmetry was used to monitor regional cerebral blood flow (rCBF) for up to 1 h after treatment. (B) At 24 h after stroke, brain infarction was stained by TTC, and the volume was quantified using computer-assisted image analysis. Data were expressed as mean+s.e.m., *P<0.05 for L-tPA plus rAN, #P<0.05 for H-tPA, respectively, n=7 or 8 per group.
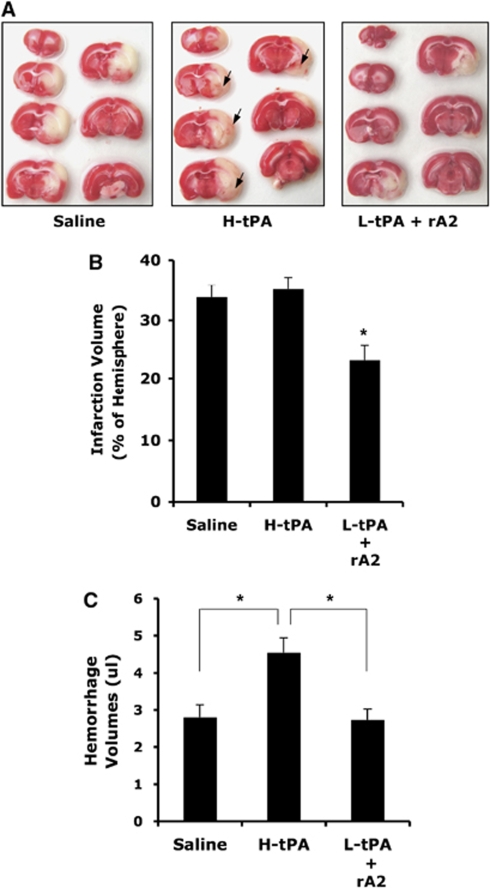
Low-Dose tPA, in Combination with rA2, Extends the Thrombolytic Window for Treatment of Focal Embolic Stroke in Rats
In the current rat model of embolic focal cerebral ischemia, earlier studies have documented that treatment with tPA is effective only when given within 2 to 3 h of onset of stroke (Morris et al, 2001). Here, we asked whether combination therapy with tPA plus rA2 can also extend the time window for thrombolysis. At 4 h after stroke onset, three groups of rats were treated intravenously with saline, high-dose tPA (10 mg/kg), or low-dose tPA (2.5 mg/kg) plus rA2 (5 mg/kg). At 24 h after initiation of stroke, this combination also significantly reduced infarction volume, compared with either saline controls or rats treated with high-dose tPA alone (Figures 3B and 3C). As expected, high-dose tPA administered at the delayed 4 h time point induced significant hemorrhagic transformation at 24 h (Figures 3C and 3D). The combination of low-dose tPA plus rA2 significantly ameliorated the severity of hemorrhagic transformation (Figure 3D). Finally, in concert with these beneficial effects on cerebral perfusion, infarction, and hemorrhage, combination therapy of low-dose tPA plus rA2 also reduced mortality. Mortality rates were 24% (4/17) in saline-treated rats and 41% (7/17) in the high-dose tPA group, whereas combination therapy with low-dose tPA plus rA2 significantly reduced mortality to 18% (3/17). In addition, physiologic parameters measured before ischemia, after ischemia, and 30 mins following thrombolysis remained within normal range in all groups (Table 1).
Figure 3.
Effect of treating rats at 4 h after initiation of ischemia. (A) Representative images of brain sections after TTC staining at 24 h after initiating ischemia. At 4 h after stroke onset, three groups of rats were treated intravenously with saline, high-dose tPA (10 mg/kg, H-tPA), or low-dose tPA (2.5 mg/kg, L-tPA) plus rA2 (5 mg/kg). Ischemic infarctions (white color area) were detected in all three groups; however, large areas of grossly visible hemorrhage appeared only on the brain sections of H-tPA-treated rats pointed by arrows. (B) At 24 h after stroke, brain infarction was quantified using computer-assisted image analysis. (C) Volumes of intracerebral hemorrhage ware quantified with hemoglobin assay at 24 h after stroke. Data were expressed as mean+s.e.m., *P<0.05, n=13 for saline, n=10 for H-tPA, n=14 for the combination.
Table 1. Measurements of physiological parameters.
| Saline | H-tPA | L-tPA + rA2 | |
|---|---|---|---|
| Weight (g) | 291±7.91 | 287±6.17 | 295±8.22 |
| Rectal temperature (°C) | 37.1±0.23 | 37.0±0.13 | 36.8±0.28 |
| MABP (mm Hg) | 123±4.23 | 120±3.05 | 121±4.21 |
| pH | 7.36±0.04 | 7.41±0.04 | 7.38±0.05 |
| PCO2 (mm Hg) | 39.5±2.87 | 39.8±3.66 | 40.1±4.24 |
| PO2 (mm Hg) | 125±4.7 | 127±4.8 | 126±4.6 |
MABP, mean arterial blood pressure; tPA, tissue plasminogen activator.
Physiological parameters were measured at 30 mins after thrombolysis at 4 h after the onset of stroke. All measurements remained within normal range in three groups. Mean±s.e.m. n=4.
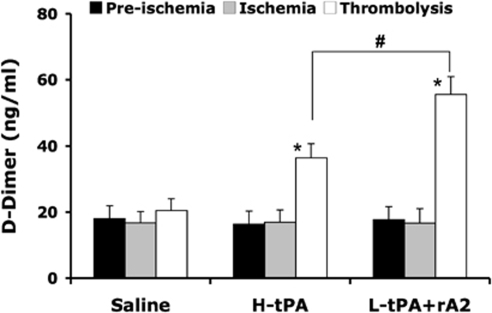
Combination of rA2 Plus Low-Dose tPA Increases Plasma Levels of Fibrin Degradation Product -Dimer
To further evaluate the thrombolytic profiles of animals treated at 4 h after the initiation of stroke, we examined plasma levels of the fibrin degradation product -dimer. Samples were collected before ischemia, just before thrombolytic therapy, and 1 h after treatment. ELISA showed that plasma levels of -dimer did not differ from those observed at the prestroke baseline, consistent with the lack of effective thrombolysis. However, both high-dose tPA and low-dose tPA plus rA2 significantly increased -dimer after thrombolysis for 2.2-fold and 3.2-fold, respectively, and the increase in low-dose tPA plus rA2 combination was significantly greater compared with high-dose tPA-only treatment (Figure 4). These data indicate that this combination thrombolytic therapy was more effective and specific for fibrinolysis.
Figure 4.
Effect of tPA alone or in combination with rA2 on plasma levels of -dimer. Plasma samples were collected before ischemia, just before thrombolytic therapy, and 1 h after treatment. Concentrations of -dimer in plasma were quantified by ELISA analysis. Data were expressed as mean+s.e.m., *P<0.01 versus ischemia, #P<0.01, n=6 per group.
Combination of rA2 Plus Low-Dose tPA does not Amplify MMP Activation in the Brain
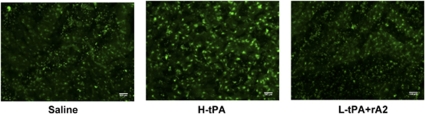
One of the potential mediators of tPA-associated hemorrhagic complications may be the neurovascular protease matrix metalloproteinases (MMP)-9. In delayed 4 h treatments, MMP activity of brain sections was examined with in situ zymography at 24 h after stroke. Cerebral ischemia increased MMP activity in the ischemic hemisphere compared with nonischemic hemisphere in all three treatments. By comparing MMP activity on the front cortex of periinfarction areas, we found, as expected, tPA treatment alone showed brighter signals for activated MMP compared with saline treatment, but the combination of low-dose tPA plus rA2 had similar or even slightly less positive signals of MMP activity compared with tPA alone treatment (Figure 5).
Figure 5.
MMP activation in ischemic brains from rats treated 4 h after stroke onset. At 24 h after stroke onset, we performed in situ zymography to examine MMP activation in ischemic brains at delayed 4 h treatments. In the cortex of the periinfarction zone, brains from animals treated with tPA alone showed brighter activated MMP signals compared with saline treatment, but the combination of low-dose tPA plus rA2 had similar or even slightly less positive signals compared with tPA alone treatment. Similar observations were obtained from three individual experiments.
Combination of rA2 Plus Low-Dose tPA Improves Neurologic Outcomes at 3 Days After Stroke
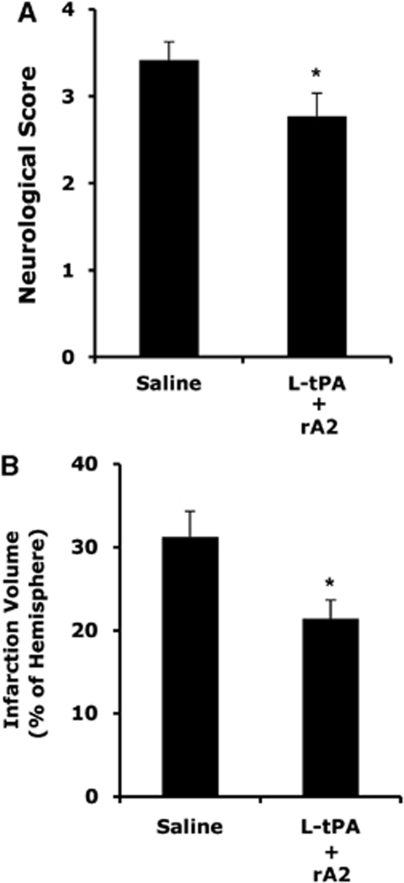
To test whether the combination can improve neurologic outcomes in longer survival time after stroke, 28 rats (n=14 per group) were treated at 4 h after stroke with either saline or a combination of low-dose tPA (2.5 mg/kg) plus rA2 (5 mg/kg). Neurologic outcomes were measured at 3 days after stroke. The combination of low-dose tPA (2.5 mg/kg) plus rA2 (5 mg/kg) significantly decreased neurologic deficits (Figure 6A) and brain infarction (Figure 6B). Furthermore, mortality of saline-treated animals (42%, 6/14) was significantly reduced by the combination therapy (21%, 3/14).
Figure 6.
Three days neurological outcomes in rats treated at 4 h after stroke onset. (A) Two groups of rats were treated intravenously at 4 h after stroke with either saline, or a combination of low-dose tPA (2.5 mg/kg, L-tPA) plus rA2 (5 mg/kg). Three days after stroke, neurological scores were significantly improved in treated rats. (B) Ischemic infarctions on H&E-stained sections were quantified using computer-assisted image analysis. Infarction volumes were significantly reduced in the treated rats. *P<0.05, n=8 for saline, n=11 for the combination.
Discussion
Recent clinical investigations have shown the potential for improving tPA therapy. For instance, PWI-DWI mismatch in magnetic resonance imaging studies suggest that some individual patients may benefit from treatment beyond 3 h time window, if there is low bleeding risk and salvageable mismatch (Marks et al, 2008; Thomalla et al, 2006). The ECASS III, a randomized phase III trial to test tPA at 3 to 4.5 h, showed that intravenous tPA given during this time window still improved clinical outcomes in patients with somewhat milder stroke scores (Hacke et al, 2008). However, there is still a large difference in odds ratios between early reperfusion within 90 mins (∼2.8 odds ratio) versus delayed reperfusion (∼1.4 odds ratio). The benefits of thrombolysis are still heavily dependent on time to treatment, and use of tPA may still be associated with intracranial hemorrhage and reperfusion injury. Hence, it is imperative that we seek combination therapies to truly broaden the therapeutic window, reduce the risk of tPA-associated hemorrhagic transformation, and enhance thrombolytic efficacy. We believe that the combination of low-dose tPA with rA2 might be promising.
In this study, we tested the hypothesis that combining Annexin A2 with tPA can significantly enhance thrombolytic efficacy so that lower doses of tPA can be applied in ischemic stroke to avoid neurotoxic and hemorrhagic complications. Our results show that this novel approach successfully achieved improvements in thrombolysis, and brain tissue protection in focal embolic stroke of rats. Importantly, the risk of intracerebral hemorrhage was also significantly reduced. These beneficial effects on infarct volume, hemorrhage, and mortality suggest that rA2 might safely augment the efficacy and lengthen the treatment window of tPA stroke therapy.
As the combination of tPA and A2 may generate amounts of plasmin that are equal to or greater than those produced with high-dose tPA alone, one concern is that this regimen might cause side effects similar to those seen with high-dose tPA. Both tPA and plasmin are serine proteases, they can initiate microglial activation (Gravanis and Tsirka, 2008; Sheehan and Tsirka, 2005), trigger MMP activation, and extracellular proteolytic dysfunction (Gravanis and Tsirka, 2008). To address this concern, we performed in situ zymography to examine MMP activation in ischemic brains at delayed 4 h treatments. At 24 h after stroke onset, increased MMP activity in ischemic brain area was observed in all three groups, but the combination treatment did not increase MMP activity in ischemic brain above that seen with tPA alone. Furthermore, a number of reports have suggested new strategies for reducing the tPA dose, but enhancing plasmin generation or activity to improve safety of tPA thrombolytic therapy. These include the findings that (1) local infusion of plasmin into the thrombus does not cause excessive bleeding within six-fold greater than the effective thrombolytic dose of tPA used (Marder et al, 2001); (2) antiplasmin quickly (in 1 sec) neutralizes plasmin that appears in the circulation (Marder et al, 2001), whereas tPA has longer half life (4 to 5 mins), and can cross the blood–brain barrier, whether damaged or intact, through low-density lipoprotein receptor–related protein-dependent and independent mechanisms, further weaking blood–brain barrier integrity and worsening brain damage (Benchenane et al, 2005; Wang et al, 2003; Yepes et al, 2003); (3) tPA can induce plasmin-independent MMP-9 up-regulation and microglia activation in stroke animal models (Aoki et al, 2002; Wang et al, 2003; Zhang et al, 2009). In addition, initial experimental evidence suggested that rA2 administration alone in vivo has not exhibited any organ specific or systematic complications (Ishii et al, 2001; Tanaka et al, 2007). These data, together with findings from this study, suggest, but do not prove, that optimization of the tPA-mediated fibrinolytic process may greatly improve the efficacy and safety of this form of stroke therapy. It is clear that safety issues will need to be carefully investigated before any future preclinical evaluation.
Studies from other investigators have attempted to develop different new approaches for tPA combination therapy in animal stroke models. These have shown a reduction in intracerebral hemorrhage and brain damage by combining tPA with certain reagents (Armstead et al, 2006; Asahi et al, 2000; Cheng et al, 2006; Lapchak et al, 2002; Pfefferkorn and Rosenberg, 2003; Strbian et al, 2007; Zhang et al, 2003, 2006), suggesting that many mechanisms contribute to the deleterious effects of tPA. If successful, the combination of rA2 plus low-dose tPA might therefore synergize other combination approaches to further optimize tPA-based thrombolytic therapy. Furthermore, as tPA is broadly used in treating a range of thrombotic disorders, besides acute ischemic stroke, this new combination approach for lowering the minimum effective tPA dose may improve thrombolytic treatments for other conditions such as acute myocardial infarction, pulmonary embolism, deep venous thrombosis, and thrombosed catheters, and lysis of intracerebral hematomas.
However, there are several limitations for this study. One limitation was that we were unable to determine the plasmin concentration in rat plasma due to the lack of reliable quantitative assays. In the circulation, newly generated plasmin is quickly neutralized by plasma α-2-antiplasmin, resulting in the formation of an inactive protease-inhibitor complex. Although commercial ELISAs for plasmin-α-2-antiplasmin complex provide an index of the rate of plasmin generation, these assays were not available for the rat plasmin-α-2-antiplasmin complex, and the available human assays are not reliable due to high nonspecific baselines (data not shown). To address this problem, we evaluated the thrombolytic profiles in the delayed treatment group by examining plasma levels of the fibrin degradation product -dimer. Degradation of cross-linked fibrin, which is enriched in thrombi, produces a number of fragments containing the -dimer epitope, an indicator of the extent of fibrin clot lysis. ELISA data showed that both H-tPA and L-tPA plus rA2 significantly increased -dimer levels after treatments, but it was significantly higher in the combination group compared with H-tPA alone. These data suggested that this combination was pharmacologically more effective and specific for fibrin clot lysis, and further supports the idea of improved plasmin-dependent fibrinolysis. In fact, it is tempting to speculate that increased efficiency of fibrinolysis by the rA2-tPA combination might even generate more plasmin locally in the clot site. Earlier findings have shown that annexin A2 accelerates the activation of plasmin by complexing with tPA and with the plasmin precursor plasminogen, which binds the endothelial cell surface and is enriched in the clot (Hajjar and Krishnan, 1999; Sakharov and Rijken, 1995). Therefore, combination of tPA plus rA2 might locally bind plasminogen and consequently amplify plasmin generation in the vicinity of the clot, resulting in more effective fibrinolysis where it counts.
One more limitation was that we only examined infarction and neurologic outcomes of the combination treatment at 1 and 3 days after stroke. It remains to be rigorously determined whether the acute neuroprotection may sustain for longer periods in neurofunctional and tissue recovery, and how it interfaces with tPA effects in delayed times post-stroke.
Finally, the molecular mechanisms of the combination are incompletely understood from the results of this study. However, there are a number of mechanisms that may be speculated. First, rA2 may optimize tPA stroke therapy by increasing the thrombolytic efficacy of tPA to benefit reperfusion, while reducing its associated neurotoxicity and hemorrhagic complications. Experimental and clinical studies have well demonstrated the tPA-dose dependent side effects of hemorrhage and neurotoxicity (Kaur et al, 2004; Wang et al, 1999). Theoretically, by lowering tPA dose in combining with rA2, it might reduce higher dose tPA-mediated neurotoxicity if equal or greater thrombolytic activity can be achieved. Second, our central idea is based on a well-established basic concept that biologically tPA converts plasminogen to clot-dissolving plasmin efficiently relies on fibrinolytic assembly via a triple complex formation of tPA-annexin A2-plasminogen (Hajjar and Menell, 1997). However, clinically given large amount of tPA alone but lacking fibrinolytic assembly of tPA-annexin A2-plasminogen complex formation, which makes tPA-converting plasminogen to plasmin insufficiently, resulting requirement of higher tPA dose for reperfusion, but clinically even within 3 h time window, reperfusion efficacy was insufficient with recanalization rate of about 50% during the first 6 to 24 h, among them about a third of cases had reocclusion (tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group, 1995). Thus, the rA2 plus low-dose tPA combination may improve recanalization and cerebral blood flow recovery. Third, despite clot lysis and reperfusion, experimental studies using advanced ‘closed cranial window technique' have clearly shown that exogenous tPA may impair physiological responses of cerebral vasodilatation to hypoxia/ischemia, and as well cause vasoconstriction after hypoxic/ischemic injury in a dose-dependent manner (Armstead et al, 2005, 2009; Nassar et al, 2004). So by lowering tPA dose, the combination might eliminate tPA direct vasoactivity effect. Fourth, disturbed extracellular proteolysis after stroke targets multiple brain cells, cell–cell communication and matrix integration within the neurovascular unit (Lee et al, 2004). Emerging experimental evidence have documented that extracellular proteolysis dysregulation represents a key pathological cascade underlying blood–brain barrier disruption and hemorrhagic transformation after ischemic stroke and tPA thrombolysis (Lee et al, 2004; Lo et al, 2004; Wang and Lo, 2003; Wang et al, 2004, 2008). Our data showed that the combination reduced high-dose tPA-induced MMP activity elevation (Figure 5), suggesting rA2 plus low-dose tPA might attenuate tPA-associated dysregulated neurovascular matrix proteolysis and blood–brain barrier damage (Wang et al, 2008). Finally, by improving blood flow and decreasing neurovascular side effects, rA2 plus low-dose tPA combination might lead to brain tissue salvage and improved long-term functional outcomes. There may be more mechanisms involved, all together with anticipated translational aspects of the combination need further investigations.
In summary, this study shows that addition of the ‘tPA amplifier' rA2 can decrease the effective thrombolytic dose of tPA, reduce hemorrhage and brain infarction, and prolong the reperfusion time window for stroke. Our present findings provide a promising new approach for enhancing tPA thrombolytic stroke therapy.
Acknowledgments
This work was supported in part by Scientist Development Grant 0435087N from the American Heart Association, National Institute of Health grant R01-NS049476 (to XW), National Institute of Health grants R01-HL042493 and P01-046403 (to KAH), National Institute of Health grants R01-NS37074, R01-NS48422, R01-NS53560, and P50-NS10828 (to EHL).
The authors declare no conflict of interest.
References
- Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation. 2007;115:e478–e534. doi: 10.1161/CIRCULATIONAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- Aoki T, Sumii T, Mori T, Wang X, Lo EH. Blood-brain barrier disruption and matrix metalloproteinase-9 expression during reperfusion injury: mechanical versus embolic focal ischemia in spontaneously hypertensive rats. Stroke. 2002;33:2711–2717. doi: 10.1161/01.str.0000033932.34467.97. [DOI] [PubMed] [Google Scholar]
- Armstead WM, Cines DB, Higazi AA. Plasminogen activators contribute to impairment of hypercapnic and hypotensive cerebrovasodilation after cerebral hypoxia/ischemia in the newborn pig. Stroke. 2005;36:2265–2269. doi: 10.1161/01.STR.0000181078.74698.b0. [DOI] [PubMed] [Google Scholar]
- Armstead WM, Ganguly K, Kiessling JW, Chen XH, Smith DH, Higazi AA, Cines DB, Bdeir K, Zaitsev S, Muzykantov VR. Red blood cells-coupled tPA prevents impairment of cerebral vasodilatory responses and tissue injury in pediatric cerebral hypoxia/ischemia through inhibition of ERK MAPK activation. J Cereb Blood Flow Metab. 2009;29:1463–1474. doi: 10.1038/jcbfm.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstead WM, Nassar T, Akkawi S, Smith DH, Chen XH, Cines DB, Higazi AA. Neutralizing the neurotoxic effects of exogenous and endogenous tPA. Nat Neurosci. 2006;9:1150–1155. doi: 10.1038/nn1757. [DOI] [PubMed] [Google Scholar]
- Asahi M, Asahi K, Wang X, Lo EH. Reduction of tissue plasminogen activator-induced hemorrhage and brain injury by free radical spin trapping after embolic focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2000;20:452–457. doi: 10.1097/00004647-200003000-00002. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Benchenane K, Berezowski V, Fernandez-Monreal M, Brillault J, Valable S, Dehouck MP, Cecchelli R, Vivien D, Touzani O, Ali C. Oxygen glucose deprivation switches the transport of tPA across the blood-brain barrier from an LRP-dependent to an increased LRP-independent process. Stroke. 2005;36:1065–1070. doi: 10.1161/01.STR.0000163050.39122.4f. [DOI] [PubMed] [Google Scholar]
- Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, Liu D, Maggirwar SB, Deane R, Fernandez JA, LaRue B, Griffin JH, Chopp M, Zlokovic BV. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med. 2006;12:1278–1285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- Davalos A. Thrombolysis in acute ischemic stroke: successes, failures, and new hopes. Cerebrovasc Dis. 2005;20 (Suppl 2:135–139. doi: 10.1159/000089367. [DOI] [PubMed] [Google Scholar]
- Gravanis I, Tsirka SE. Tissue-type plasminogen activator as a therapeutic target in stroke. Expert Opin Ther Targets. 2008;12:159–170. doi: 10.1517/14728222.12.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D. Thrombolysis with alteplase 3 to 4.5 h after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- Hajjar KA, Acharya SS. Annexin II and regulation of cell surface fibrinolysis. Ann NY Acad Sci. 2000;902:265–271. doi: 10.1111/j.1749-6632.2000.tb06321.x. [DOI] [PubMed] [Google Scholar]
- Hajjar KA, Krishnan S. Annexin II: a mediator of the plasmin/plasminogen activator system. Trends Cardiovasc Med. 1999;9:128–138. doi: 10.1016/s1050-1738(99)00020-1. [DOI] [PubMed] [Google Scholar]
- Hajjar KA, Menell JS. Annexin II: a novel mediator of cell surface plasmin generation. Ann NY Acad Sci. 1997;811:337–349. doi: 10.1111/j.1749-6632.1997.tb52013.x. [DOI] [PubMed] [Google Scholar]
- Henninger N, Bouley J, Bratane BT, Bastan B, Shea M, Fisher M. Laser Doppler flowmetry predicts occlusion but not tPA-mediated reperfusion success after rat embolic stroke. Exp Neurol. 2009;215:290–297. doi: 10.1016/j.expneurol.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Ishii H, Yoshida M, Hiraoka M, Hajjar KA, Tanaka A, Yasukochi Y, Numano F. Recombinant annexin II modulates impaired fibrinolytic activity in vitro and in rat carotid artery. Circ Res. 2001;89:1240–1245. doi: 10.1161/hh2401.101066. [DOI] [PubMed] [Google Scholar]
- Kaur J, Zhao Z, Klein GM, Lo EH, Buchan AM. The neurotoxicity of tissue plasminogen activator. J Cereb Blood Flow Metab. 2004;24:945–963. doi: 10.1097/01.WCB.0000137868.50767.E8. [DOI] [PubMed] [Google Scholar]
- Kim J, Hajjar KA. Annexin II: a plasminogen-plasminogen activator co-receptor. Front Biosci. 2002;7:d341–d348. doi: 10.2741/kim. [DOI] [PubMed] [Google Scholar]
- Korninger C, Collen D. Studies on the specific fibrinolytic effect of human extrinsic (tissue-type) plasminogen activator in human blood and in various animal species in vitro. Thromb Haemost. 1981;46:561–565. [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM, Song D, Wei J, Purdy R, Zivin JA. Effects of the spin trap agent disodium-[tert-butylimino)methyl]benzene-1,3-disulfonate N-oxide (generic NXY-059) on intracerebral hemorrhage in a rabbit Large clot embolic stroke model: combination studies with tissue plasminogen activator. Stroke. 2002;33:1665–1670. doi: 10.1161/01.str.0000017145.22806.aa. [DOI] [PubMed] [Google Scholar]
- Lee SR, Wang X, Tsuji K, Lo EH. Extracellular proteolytic pathophysiology in the neurovascular unit after stroke. Neurol Res. 2004;26:854–861. doi: 10.1179/016164104X3806. [DOI] [PubMed] [Google Scholar]
- Lo EH, Broderick JP, Moskowitz MA. tPA and proteolysis in the neurovascular unit. Stroke. 2004;35:354–356. doi: 10.1161/01.STR.0000115164.80010.8A. [DOI] [PubMed] [Google Scholar]
- Marder VJ, Landskroner K, Novokhatny V, Zimmerman TP, Kong M, Kanouse JJ, Jesmok G. Plasmin induces local thrombolysis without causing hemorrhage: a comparison with tissue plasminogen activator in the rabbit. Thromb Haemost. 2001;86:739–745. [PubMed] [Google Scholar]
- Marks MP, Olivot JM, Kemp S, Lansberg MG, Bammer R, Wechsler LR, Albers GW, Thijs V. Patients with acute stroke treated with intravenous tPA 3 to 6 h after stroke onset: correlations between MR angiography findings and perfusion- and diffusion-weighted imaging in the DEFUSE study. Radiology. 2008;249:614–623. doi: 10.1148/radiol.2492071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DC, Zhang L, Zhang ZG, Lu M, Berens KL, Brown PM, Chopp M. Extension of the therapeutic window for recombinant tissue plasminogen activator with argatroban in a rat model of embolic stroke. Stroke. 2001;32:2635–2640. doi: 10.1161/hs1101.097390. [DOI] [PubMed] [Google Scholar]
- Nassar T, Akkawi S, Shina A, Haj-Yehia A, Bdeir K, Tarshis M, Heyman SN, Higazi AA. In vitro and in vivo effects of tPA and PAI-1 on blood vessel tone. Blood. 2004;103:897–902. doi: 10.1182/blood-2003-05-1685. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn T, Rosenberg GA. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 2003;34:2025–2030. doi: 10.1161/01.STR.0000083051.93319.28. [DOI] [PubMed] [Google Scholar]
- Sakharov DV, Rijken DC. Superficial accumulation of plasminogen during plasma clot lysis. Circulation. 1995;92:1883–1890. doi: 10.1161/01.cir.92.7.1883. [DOI] [PubMed] [Google Scholar]
- Sheehan JJ, Tsirka SE. Fibrin-modifying serine proteases thrombin, tPA, and plasmin in ischemic stroke: a review. Glia. 2005;50:340–350. doi: 10.1002/glia.20150. [DOI] [PubMed] [Google Scholar]
- Strbian D, Karjalainen-Lindsberg ML, Kovanen PT, Tatlisumak T, Lindsberg PJ. Mast cell stabilization reduces hemorrhage formation and mortality after administration of thrombolytics in experimental ischemic stroke. Circulation. 2007;116:411–418. doi: 10.1161/CIRCULATIONAHA.106.655423. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Ishii H, Hiraoka M, Miyasaka N, Kuroiwa T, Hajjar KA, Nagaoka T, Duong TQ, Ohno K, Yoshida M. Efficacy of recombinant annexin 2 for fibrinolytic therapy in a rat embolic stroke model: A magnetic resonance imaging study. Brain Res. 2007;1165:135–143. doi: 10.1016/j.brainres.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomalla G, Schwark C, Sobesky J, Bluhmki E, Fiebach JB, Fiehler J, Zaro Weber O, Kucinski T, Juettler E, Ringleb PA, Zeumer H, Weiller C, Hacke W, Schellinger PD, Rother J. Outcome and symptomatic bleeding complications of intravenous thrombolysis within 6 h in MRI-selected stroke patients: comparison of a German multicenter study with the pooled data of ATLANTIS, ECASS, and NINDS tPA trials. Stroke. 2006;37:852–858. doi: 10.1161/01.STR.0000204120.79399.72. [DOI] [PubMed] [Google Scholar]
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Wang X, Asahi M, Lo EH. Tissue type plasminogen activator amplifies hemoglobin-induced neurotoxicity in rat neuronal cultures. Neurosci Lett. 1999;274:79–82. doi: 10.1016/s0304-3940(99)00682-5. [DOI] [PubMed] [Google Scholar]
- Wang X, Lee SR, Arai K, Lee SR, Tsuji K, Rebeck GW, Lo EH. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003;9:1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- Wang X, Lo EH. Triggers and mediators of hemorrhagic transformation in cerebral ischemia. Mol Neurobiol. 2003;28:229–244. doi: 10.1385/MN:28:3:229. [DOI] [PubMed] [Google Scholar]
- Wang X, Rosell A, Lo EH. Targeting extracellular matrix proteolysis for hemorrhagic complications of tPA stroke therapy. CNS Neurol Disord Drug Targets. 2008;7:235–242. doi: 10.2174/187152708784936635. [DOI] [PubMed] [Google Scholar]
- Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, Lo EH. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35:2726–2730. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- Yepes M, Roussel BD, Ali C, Vivien D. Tissue-type plasminogen activator in the ischemic brain: more than a thrombolytic. Trends Neurosci. 2009;32:48–55. doi: 10.1016/j.tins.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, An J, Strickland DK, Yepes M. The low-density lipoprotein receptor-related protein 1 mediates tissue-type plasminogen activator-induced microglial activation in the ischemic brain. Am J Pathol. 2009;174:586–594. doi: 10.2353/ajpath.2009.080661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Liu X, Hozeska A, Stagliano N, Riordan W, Lu M, Chopp M. Treatment of embolic stroke in rats with bortezomib and recombinant human tissue plasminogen activator. Thromb Haemost. 2006;95:166–173. [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Zhang R, Morris D, Lu M, Coller BS, Chopp M. Adjuvant treatment with a glycoprotein IIb/IIIa receptor inhibitor increases the therapeutic window for low-dose tissue plasminogen activator administration in a rat model of embolic stroke. Circulation. 2003;107:2837–2843. doi: 10.1161/01.CIR.0000068374.57764.EB. [DOI] [PubMed] [Google Scholar]
- Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]