Abstract
Spreading depolarizations (SDs) occur spontaneously with high incidence in patients with acute brain injury. They can be detected by subdural electrocorticographic recordings. We here characterize the dynamic metabolic response to these events. A microdialysis catheter was inserted into perilesional cortical tissue adjacent to a strip for electrocorticography following craniotomy in 10 patients. The microdialysis catheter was connected to an online microdialysis assay measuring glucose and lactate concentrations every 30 to 60 secs. Spontaneously occurring SDs systematically caused a reduction in dialysate glucose by −32.0 μmol/L (range: −92.3 to −18.4 μmol/L, n=90) and increase in lactate by +23.1 μmol/L (range: +5.5 to +93.6 μmol/L, n=49). The changes were sustained at 20 mins after the SD events and highly significant using an area under the curve analysis (P<0.0001). Multiple and frequent SDs led to a progressive stepwise depletion of brain glucose. Hence, SD events cause a massive energy imbalance and their frequent occurrence leads to a local insufficiency of glucose supply. Such a failure would compromise cellular repolarization and hence tissue viability. The findings offer a new mechanism to account for otherwise unexplained instances of depletion of brain microdialysate glucose.
Keywords: glucose, human brain injury, lactate, online microdialysis, spreading depolarization
Introduction
Spreading depolarizations (SDs) were first described as cortical spreading depressions by Leao (1944). They have been characterized by large dynamic changes in the distribution of ions between the intracellular and extracellular compartments and dramatic changes in blood flow (Lauritzen et al, 1982). There is increasing evidence that SDs occur frequently and spontaneously in the injured human brain: the incidence in patients who have undergone craniotomy lies in the region of 50% to 60% for traumatic brain injury (Strong et al, 2002), 72% for aneurysmal subarachnoid hemorrhage (Dreier et al, 2006), and essentially 100% for malignant hemisphere stroke (Dohmen et al, 2008). The SDs are waves of transient, abnormal mass depolarizations of neurons and astrocytes that propagate from the core of the injury to the surrounding compromised but potentially salvageable tissue. When occurring adjacent to focal, core lesions in the injured human brain, depolarizations typically occur in temporal clusters of repetitive events (Dreier et al, 2006; Fabricius et al, 2006). The exact role of SDs in the development of secondary neurologic deterioration in the human brain is still unclear and the relation between SDs and patient outcome is currently under investigation by the COSBID group (Cooperative Study of Brain Injury Depolarizations: http://www.cosbid.org).
The occurrence of spontaneous SD-like events in experimental models of stroke (Branston et al, 1977) and contusional head injury (Katayama et al, 1990) has been well documented. More recent experiments have detected complex patterns of transient change in local cerebral blood flow, local tissue oxygen availability, and key energy metabolite pools, using a variety of techniques including laser speckle flowmetry and rapid-sampling microdialysis (rsMD) (Hashemi et al, 2009), two-photon microscopy (Takano et al, 2007), and oxygen microelectrodes (Piilgaard and Lauritzen, 2009). Recurrent SDs in periinfarct tissue have been shown not only to be associated with the expansion of the damage (Mies et al, 1993) but to promote it by progressive infarction of the perilesion tissue (Busch et al, 1996; Dijkhuizen et al, 1999).
Despite this flurry of findings in experimental models of brain injury, little is known about the effect of SDs on the injured human brain. The reason for that is mostly methodological. The SDs can now be detected reliably using continuous electrocorticography (ECoG) and appropriate frequency filtering (Fabricius et al, 2006) over extended periods of time, up to 10 days (Dreier et al, 2006). However, it remains difficult to study the metabolic state of the tissue when SDs occur: current sophisticated scanners, including magnetic resonance imaging and positron emission tomography, cannot in practice be used continuously for days, without compromising patient care. The current method of choice for the continuous assessment of metabolism is cerebral microdialysis. It has been used routinely to monitor head injury patients (Glenn et al, 2003; Goodman et al, 1999; Hutchinson et al, 2000) and has enabled the identification of markers for the early detection of ischemia and secondary neuronal deterioration (Sarrafzadeh et al, 2003; Vespa et al, 2003; Zauner et al, 1997). The main pitfall of microdialysis is its limited time resolution, of about 30 to 60 mins in general. This is not sufficient to study the direct and dynamic effects of SDs, as locally the passage of an SD wave may last only 2 to 5 mins. The remedy is to use rsMD, an online microdialysis technique whereby the dialysate, instead of being collected in sample vials, is directly analyzed electrochemically. Typically, microdialysis concentrations of glucose and lactate can be estimated every 30 to 60 secs (Jones et al, 2002). This technique has been used successfully in a variety of pathologies, both in the intensive care unit (Parkin et al, 2005) and in the operating room (Bhatia et al, 2006; Deeba et al, 2008).
Here, we have sought to determine the dynamic neurometabolic signature of recurrent spontaneous depolarizations in the injured human brain using online rsMD of perilesion tissue.
Materials and methods
Patient Recruitment and Monitoring
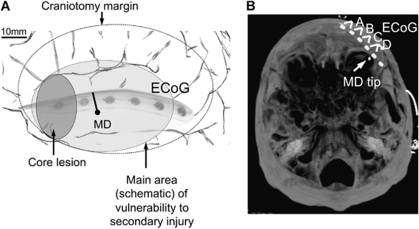
All human research procedures were approved by the local Research Ethics Committee of King's College Hospital, London, or by Cambridgeshire 4 Research Ethics Committee. Patients requiring emergency craniotomy for traumatic brain injury (n=5), aneurysmal subarachnoid hemorrhage (n=2), or intracranial hematoma (n=3) were identified and research assent obtained from relatives or the authorized surrogate. Patients under 16 years of age, and/or with a Glasgow Coma Score below 4 at admission and/or with bilateral fixed and dilated pupils were excluded from this study. Towards the completion of surgery, a sterile clinical microdialysis catheter (CMA 70, 10-cm flexible shaft, 10-mm membrane length, 20 kDa cutoff, CMA, Stockholm, Sweden) was inserted obliquely into the cortex, so far as possible, to full membrane depth through a minimal pial incision, as described earlier (Parkin et al, 2005). Along with the microdialysis catheter, a linear, six-platinum-contact ECoG recording strip (Wyler, 5 mm diameter contacts, Ad-Tech Medical, Racine, WI, USA) was placed on the surface of the cortex accessible through the craniotomy, as described earlier (Fabricius et al, 2006; Strong et al, 2002). The aim was to locate the ECoG strip and microdialysis catheter closely together; it was usually possible to site them on the same gyrus, the microdialysis probe sited in perilesion cortical tissue between contacts 4 and 5 (or 5 and 6) of the ECoG strip that was radiating away from the lesioned area. The intended positioning of the strip and microdialysis catheter are drawn in Figure 1A and can be checked on a postoperative CT, as shown in Figure 1B. We acknowledge that positioning of the MD probe in tissue ‘at risk' is crucial for the implications of our findings. Positioning of the microdialysis probe could not rely on physiologically specific imaging that could delineate a clear ‘penumbra' (as classically defined in the context of focal cerebral ischemia by marked reductions in cerebral perfusion and availability of oxygen to tissue), as none was available. Instead, pragmatic placement of the probe was guided by our initial practical experience at craniotomy and afterwards. This suggested that after evacuation of a focal TBI hematoma/contusion or an ICH, the region within about 1 to 1.5 cm of the edge of clearly contused tissue risks complete necrosis within some 24 to 36 h or less, perhaps on the basis of new microfoci of intraparenchymaml hemorrhage, and can therefore be considered as tissue at ‘high risk.' In contrast, there is tissue, apparently more remote from an obvious contusion, where spontaneous depolarizations may occur within 1 to 6 days after trauma (Fabricius et al, 2006; Hartings et al, 2009). We consider this tissue to be at ‘moderate risk,' that is, likely to be more sensitive to secondary insults and/or treatment over a longer period than 24 h, and so more clinically relevant. The aim was thus to place the probe in this tissue at ‘moderate risk,' at 1.5 to 2.5 cm from the lesion edge as seen surgically. Insertion of the probe was oblique to the cortical surface, so that the 10-mm membrane was just buried and so putatively largely in cortex. Such placement of the MD probe in the pericontusional zone is well accepted as the best localization for microdialysis measurements of early markers of deterioration of the tissue at risk (Bellander et al, 2004). ECoG and microdialysis probes were secured by double suturing onto the skin beside the point of exteriorization. In the experience of surgeons inserting identical strips for assessment of patients with intractable epilepsy, this method of fixation has proved reliable, with negligible movement of probes after placement.
Figure 1.
Probe locations. (A) Schematic drawing of the insertion of the probes towards the end of the craniotomy: the ECoG strip is placed near the core lesion, sampling the transition from tissue ‘at risk' out towards healthy tissue. The MD probe is inserted in tissue ‘at risk' between contacts 4 and 5 on the same gyrus. Both probes are secured by double suturing at the skin exteriorization points. (B) Postoperative processed CT volume acquisition scan showing the location of the microdialysis catheter in relation to the ECoG strip. Maximal intensity projection of a selected set of contiguous reformatted slices, orientated to include all six electrodes on subdural strip, and (seen within the subjacent cortex) the tip of the microdialysis catheter, 2 mm below the cortical surface. The ECoG data channels (A to D) are indicated.
After surgery, patients were transferred to intensive or high dependency care. Arterial blood pressure and intracranial pressure were continuously recorded and blood gases, glucose, and electrolytes were documented hourly or 4-hourly. Where required, patients were ventilated, typically to a target partial pressure of arterial carbon dioxide (PaCO2) of 30 to 34 torr (4 to 4.5 kPa). Sedation was principally with fentanyl and midazolam, replaced with propofol during weaning. Target cerebral perfusion pressure was 60 mmHg, flexibly applied. Acute intravenous fluid therapy was typically Ringer's lactate or Hartmann's solution, 2 L/24 h, supplemented with colloid for any additional volume requirements. Once tolerated, enteral nutrition replaced this, comprising in general 20 to 25 kcal/kg/day or 60 mL/h, containing ∼50% carbohydrate, 30% lipid, and 20% protein, together with electrolytes and trace elements. Glycemic control was targeted to the range 70 to 140 mg/dL (3.9 to 8 mmol/L) using actrapid insulin as a continuous intravenous infusion, typically at 1 to 2 u/h.
Data Collection
The microdialysis catheter was perfused with sterile artificial cerebrospinal fluid (CMA perfusion fluid CNS: 147 mmol/L NaCl, 2.7 mmol/L KCl, 1.2 mmol/L CaCl2, 0.85 mmol/L MgCl2) at 2 μL/min using a CMA 100 Microinjection syringe pump (CMA Microdialysis, Stockholm, Sweden). Perfusion of the MD catheter started immediately after the end of the operation so that the blood–brain barrier was sealed and the initial baseline dialysate levels were steady when the online measurements started in the intensive care unit (typically 1 to 2 h after surgery). The outlet tubing of the probe was adapted to connect to a dual online assay system (Parkin et al, 2005). Typically, a 1-m length of low volume connection tubing (Microbiotech, Stockholm, Sweden) was used between the patient and the online assay to facilitate patient movement and nurse care. The rsMD online assay has been described elsewhere (Jones et al, 2002; Parkin et al, 2005). Briefly, it couples enzymatic recognition with electrochemical detection to yield two traces of current peaks every 30 to 60 secs. The heights of these peaks are proportional to the dialysate concentrations of glucose and lactate respectively (Figure 2, bottom two traces). The rsMD data, four channels of ECoG (Figure 2), arterial blood pressure, and intracranial pressure (when available) were digitized at 200 Hz using a Powerlab 16/SP analogue/digital converter and Chart-5.5 software (ADInstruments, Oxfordshire, UK) running on an Apple MacBook Pro (A1212, Apple, CA, USA).
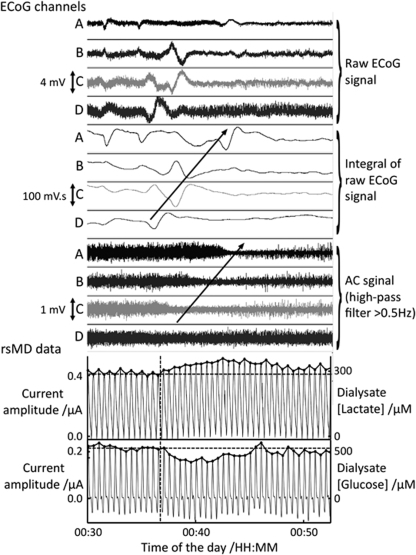
Figure 2.
The 25-min recording of four ECoG data channels and rsMD glucose and lactate during an SD in patient 4. The first 12 channels correspond to the bipolar ECoG recording along the six-electrode subdural strip, as described in Fabricius et al (2006). The raw traces are shown in the first four channels: all frequencies within the amplifier bandwidth (0.02 to 200 Hz) are recorded. The middle four channels emphasize the slow potential changes: the depolarizations are observed on all channels with a time delay between consecutive contacts, corresponding to a 4 mm/min propagation speed. The lower four channels highlight the depression of the high-frequency activity (>0.5 Hz) subsequent to the depolarization wave. The arrows indicate a spread of the SD wave from channel D to A, corresponding to contacts 6 to 2 on the subdural strip. The bottom two traces are the rsMD lactate and glucose traces (top and bottom, respectively) time aligned with the ECoG data. The actual current peaks recorded by the assay are shown with the scale on the left axes graduated in μAmperes. The corresponding dialysate concentrations after the extraction of the peak heights (indicated by the dots) are scaled on the right axes. The dotted vertical line indicates the onset of the metabolic response to SD and will define time t=0 for further analysis. The horizontal dotted lines define the dialysate pre-SD concentrations calculated as the mean concentrations over 5 mins before the SD.
A video camera (iSight, Apple, USA) and a customized event marker were used to identify changes due to necessary nursing interventions or to movement artefacts.
The whole data collection system was placed on a clinically certified trolley (Series 7000, CTL Medical, Essex, UK) situated behind the patient bed in the ICU to cause minimal disruption to clinical care during the study. The data were recorded continuously for a minimum of 24 h and a maximum of 5 days (Table 1).
Table 1. Summary of patient demographics and recordings.
| Patient ID | Age | Gender | Diagnosis | Monitoring date (post-ictus) | Duration of monitoring (per hours) | Number of SDs |
|---|---|---|---|---|---|---|
| 1 | 48 | F | ICH | Day 0 to day 2 | 72 | 5 |
| 2 | 24 | M | ICH | Day 1 to day 2 | 31 | 10 |
| 3 | 21 | M | ICH | Day 2 to day 4 | 46 | 30 |
| 4 | 57 | F | SAH | Day 11 to day 14 | 55 | 28 |
| 5 | 69 | F | SAH | Day 2 to day 5 | 94 | 22 |
| 6 | 67 | M | TBI | Day 0 to day 2 | 64 | 17 |
| 7 | 48 | M | TBI | Day 1 to day 3 | 66 | 7 |
| 8 | 41 | M | TBI | Day 1 to day 2 | 24 | 4 |
| 9a | 56 | M | TBI | Day 0 to day 5 | 97 | 33 |
| 10 | 41 | M | TBI | Day 1 to day 5 | 120 | 0 |
Abbreviations: F, female; M, male; ICH, spontaneous intracranial hematoma; SAH, spontaneous subarachnoid hemorrhage; TBI, traumatic brain injury; SDs, spreading depolarizations identified by their electrocorticographic signature.
Patient 9 was diabetic and his initial glycemia was estimated between 325 and 490 mg/dL (18 and 27 mmol/L) at 21:00 on Day 0. The hyperglycemia was subsequently controlled by continuous intravenous insulin infusion together with continuous feeding. The plasma glucose concentrations were maintained between 105 and 230 mg/dL (6 and 12.8 mmol/L) during the rest of the monitoring period.
Data Analysis
The SDs were identified by their ECoG signature, as described earlier, as the sequential onset in adjacent channels of a propagating, polyphasic slow potential change followed by a rapidly developing reduction in the power of the ECoG amplitude by at least 50% (Fabricius et al, 2006) (Figure 2, middle traces).
The rsMD data were analyzed without the knowledge of the ECoG analysis results. The traces were processed into Matlab 7.5 (MathWorks, Natick, MA, USA) according to the following procedure:
Noise removal using signal processing techniques newly developed within the group (Feuerstein et al, 2009);
Automatic peak detection using a standard local maximum algorithm;
Conversion to dialysate concentrations of glucose and lactate based on calibrations performed twice daily at the bedside throughout the monitoring period;
Filtering (three-point moving average) to yield dialysate concentration time series.
The dialysate concentration time series were then time-aligned with the ECoG trace corresponding to the closest electrode on the strip to take account of the 9 mins delay due to the 1-m length low volume connection tubing between the patient and the rsMD assay. Epochs of 5 mins before and 20 mins after ECoG-identified SDs were extracted from the whole glucose and lactate dialysate concentration data for further analysis. All epochs were characterized by their basal levels (defined as the mean of the recorded rsMD values during 5 mins before the SD event), the changes in dialysate concentrations at 10 mins and at 20 mins after SD, and the area under the curve (AUC) for 20 mins after SD. Basal levels were defined over only 5 mins so as not to include the end of an earlier SD. The choice of 20 mins after SD was determined by the fact that it was generally the minimum interval time between consecutive SDs.
Data that showed transient parallel changes on both the glucose and lactate traces were excluded as they could not be differentiated from non physiological events that could be due to the performance of the assay or the microdialysis recovery, as defined earlier (Parkin et al, 2003). Events that were likely due to nurse activities or movements of the patients observed on anonymized video camera recordings were regarded as artefacts and also excluded.
In all patients, 25-min control epochs were selected and characterized at times when the ECoG recordings showed normal background activity (no SD events or epileptiform activity).
All values are given as median dialysate values (interquartile range); results were not corrected for recovery (Parkin et al, 2003). Statistical analyses including D'Agostino-Pearson's K2 tests for normality, Pearson product-moment correlations, binomial tests, and two-tailed Wilcoxon signed-rank tests were performed with Matlab 7.5 (MathWorks, Natick, MA, USA) or Igor Pro 6 (WaveMetrics, Portland, OR, USA).
Results
Ten subjects were studied overall during the first 1 to 5 days of their stay in the intensive care unit following craniotomy. It amounted to a total of 669 h of high-quality ECoG and online microdialysis data. In nine patients, a total of 156 SD events were identified according to their ECoG signatures. The initial baseline microdialysis concentrations (an average of values recorded between 60 and 120 mins after dialysis started) ranged between 300 and 2000 μmol/L for glucose and 400 and 2000 μmol/L for lactate. These are comparable to other microdialysis studies in the injured human brain when accounting for the different recoveries of different probes and perfusion flow rates (Goodman et al, 1999; Hutchinson et al, 2000; Parkin et al, 2005; Sarrafzadeh et al, 2003; Vespa et al, 2003, 2005; Zauner et al, 1997).
After exclusion of artefacts on the rsMD trace (criteria defined in the Materials and methods section), 90 transients of rsMD glucose and 49 transients of rsMD lactate were associated with the SD events and were fully characterized. The number of lactate events is smaller than that of glucose because the data were either missing or unrecoverable for technical reasons. All 49 lactate events were associated with glucose events, according to the inclusion criteria specified earlier (Parkin et al, 2003). An example of 25-min recording of ECoG and rsMD data during a spontaneous SD event in patient 4 is given in Figure 2. The SD wave is followed by a fall in glucose concentrations and an increase in lactate concentrations for >12 mins after SD.
Metabolic Signature of Spreading Depolarizations
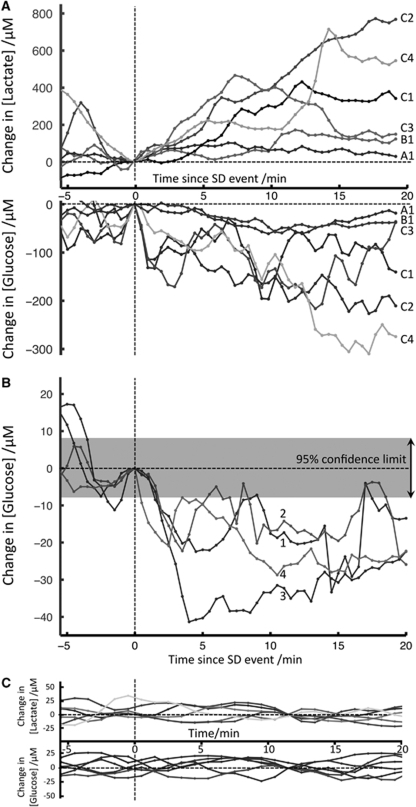
To evaluate the impact of SD events on the microdialysis concentrations of glucose and lactate, 25-min epochs were compared. Ten events are shown in Figure 3A and 3B from two patients. They are all time aligned with the onset of the rsMD response. The general pattern of changes is similar in all cases: each SD is followed by a drop in glucose and a rise in lactate concentrations. In Figure 3A (patient 4), the glucose drops ranged from −20 to −96 μmol/L and lactate rises from +130 to +300 μmol/L at 10 mins following the SDs. Some rsMD changes were very small and examples for glucose are given in Figure 3B (patient 3): the glucose changes were uniform, ranging from −17 to −33 μmol/L at 10 mins following the SDs. Although small, these changes could effectively be resolved and quantified after processing the traces with the denoising filters we have described (Feuerstein et al, 2009), as shown by the smallest resolvable change of ±8 μmol/L (given as the 95% confidence limit) for the assay when used in the ICU environment. The true variability in the baseline measurement due to patient variables is explicitly shown in Figure 3C for patient 10 who did not show any SD events. These control epochs can be considered as slow fluctuations of the microdialysis concentrations within an absolute band of 20 μmol/L changes over a stretch of 25 mins. The absolute concentrations in microdialysis glucose and lactate were stable during the whole duration of monitoring in this patient (120 h), and were around 750 to 780 μmol/L for glucose and 660 to 690 μmol/L for lactate.
Figure 3.
Changes in dialysate glucose and lactate concentrations following SDs. In this figure, time t=0 indicates the onset of the rsMD changes, and dialysate change=0 μmol/L corresponds to the dialysate concentration at time t=0. These are indicated by vertical and horizontal dotted lines, respectively. Ten epochs of 5 mins before and 20 mins after SD waves are shown. (A) (Patient 4): 6 epochs of rsMD lactate (top) and glucose (bottom) concentration changes following six SD waves. The basal dialysate concentrations ranged from 640 to 930 μmol/L for glucose, and between 1 and 1.5 mmol/L for lactate. The first two SD waves (A1 and B1) were isolated with SDs occurring on Day 12 between 00:30 and 02:30 with a 90-mins interval between the two events. They led to small changes in dialysate glucose and lactate. The other four SDs (C1 to C4) were observed as a temporal cluster of SDs repeating every 35 to 40 mins on Day 12 between 14:00 and 16:00 and they resulted in bigger changes in dialysate concentrations (>100 μmol/L). (B) (Patient 3): 4 epochs of small rsMD changes for glucose following four SD waves that occurred on Day 2 at regular intervals of 35 mins between 08:03 and 09:43. The basal dialysate concentrations ranged from 440 to 540 μmol/L. The changes in dialysate glucose concentrations are small (<50 μmol/L) and closely similar within these four events. The band on the top of the graph shows the smallest resolvable change, ±8 μmol/L (95% confidence limit). This is significantly smaller than the changes observed during an SD event. (C) (Patient 10): 6 control epochs of rsMD changes for glucose (bottom) and lactate (top). Time t=0 is arbitrary and the zero on the dialysate change scale was defined as the median value for the 25 mins of data. During the whole period of monitoring, the microdialysis levels were stable, around 750 to 780 μmol/L for glucose and 660 to 690 μmol/L for lactate.
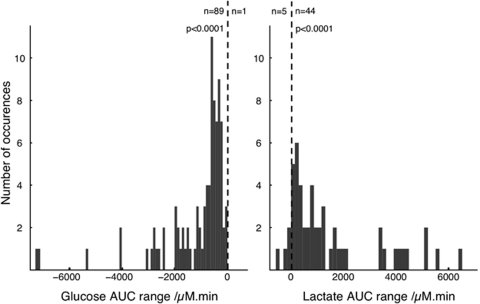
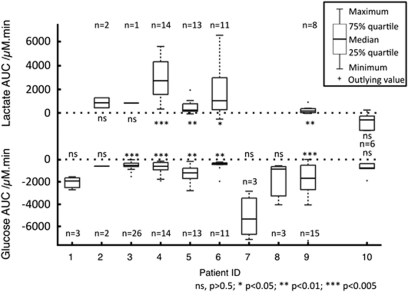
To integrate both the amplitude and the time course of the rsMD changes, an AUC analysis was performed: for each 20-min period following an SD event, the AUC of dialysis concentration changes was calculated. The distribution of these AUCs is shown in Figure 4. These distributions clearly indicate that the AUCs for glucose are negative, indicating a deficit of dialysate glucose during the 20 mins following SD events, whereas the AUC's for lactate are positive, indicating an excess of dialysate lactate during the 20 mins after SD. This is confirmed by the very high significance of the binomial test, rejecting an equal distribution of positive and negative AUC values, both for glucose and for lactate. The distribution histograms also indicate that this population cannot be described by a normal Gaussian distribution (full details given in the Supplementary Information). As a consequence, a nonparametric statistical analysis was performed on the AUCs for each patient. This is summarized in the box and whisker plot of Figure 5. Using the two-tailed Wilcoxon signed-rank test for each patient, the null hypothesis of a zero-AUC change, i.e., a zero-change of dialysate concentrations during the 20 mins following an SD, was rejected very significantly for each patient, provided there were more than three SD events. Overall, the 90 glucose AUCs and 49 lactate AUCs were very significantly different from zero (P<0.0001 for both glucose and lactate). In contrast, in patient 10, for whom no SDs were identified on the ECoG recording, we characterized 6 epochs of 25 mins of rsMD data and the 20-mins-AUCs were not significantly different from zero (Figure 5). Similarly, when pooling all the control periods across all the 10 patients in this study (n=41 for glucose and n=29 for lactate), the AUCs were again not significantly different from zero (P=0.984 and P=0.308, respectively). This confirms that the changes in glucose and lactate dialysis concentrations, though sometimes small, are due to SD events and not to random changes.
Figure 4.
The AUC distribution for the glucose and the lactate events observed in the rsMD data. The AUC for each rsMD epoch was calculated for 20 mins after SD. The distribution for all the glucose events across the nine patients is shown on the left panel and the distribution for all lactate events on the right. The dotted vertical line specifies the zero-AUC. The number of epochs with a negative AUC is indicated on the left of this line and the number of epochs with positive AUC is indicated on the right for glucose and lactate, respectively. The P-value results from a Binomial test with the expectation of a 50% distribution of positive and negative AUCs.
Figure 5.
Per-patient box and whisker plots for the 20-mins-AUCs after SDs. Lactate AUCs are plotted on the top and glucose AUCs on the bottom of the graph. The zero-AUC values are marked by dotted horizontal lines on both parts of the graph. The data were plotted for each patient. For patient 10, who showed no SD events on the ECoG recording, 6 epochs of 20 mins changes in rsMD glucose and lactate were also analyzed for their AUCs. A two-tailed Wilcoxon signed-rank test was performed for each patient, and the results are indicated on the figure with the number of events (n) for each patient.
Dynamic and Sustained Changes After the Passage of the Spreading Depolarization Wave
The median and interquartile ranges for dialysate glucose and lactate changes were, respectively, −43.4 μmol/L (−92.2 to −17.8 μmol/L) and +41.7 μmol/L (+8.2 to +93.9 μmol/L) at 10 mins following SDs across all the nine patients included in this study. The relatively wide interquartile range indicates that a variety of rsMD responses were observed. The changes in dialysate glucose at 10-mins showed a significant correlation with the duration of the suppression of ECoG AC signal (Pearson product-moment correlation: r=−0.25, P=0.05). The longer the suppression, the greater the fall in dialysate glucose seen.
In most cases, no signs of recovery to the basal values were observed at 20 mins after the SD events: overall, the concentration changes at 20 mins after SDs were of −32.0 μmol/L (−92.3 to −18.4 μmol/L) for glucose and +23.1 μmol/L (+5.5 to +93.6 μmol/L) for lactate. These values were significantly different from zero when using a two-tailed Wilcoxon signed-rank test (P<0.0001 for both glucose and lactate).
Cumulative Effects of Recurrent Spreading Depolarizations
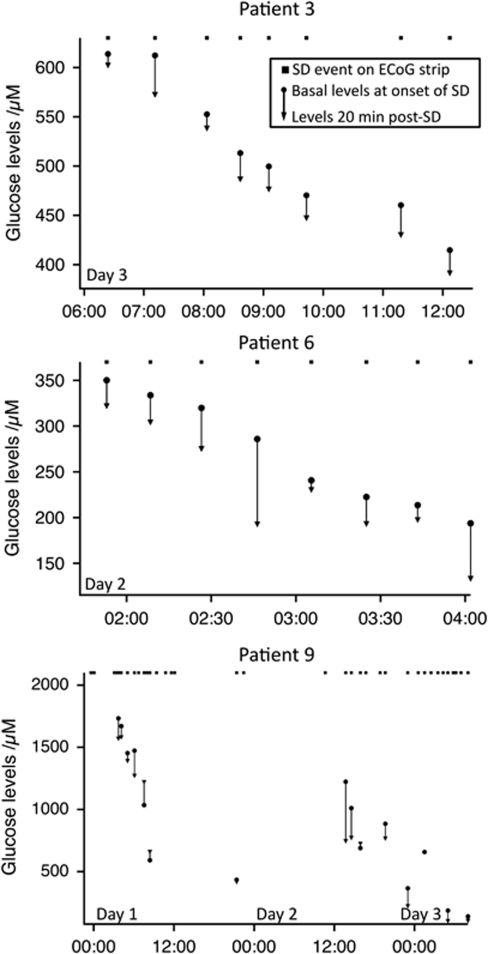
The SDs typically occur as clusters of regularly repeating events. We have investigated the effect of frequently occurring SDs on the microdialysis glucose concentrations, and the results are plotted for three patients in Figure 6. Overall, we found that when SD events repeated within <50-min interval, the glucose concentrations did not recover fully to their initial pre-SD levels before the onset of the following SD events, thus leading to a progressive stepwise fall in glucose concentrations. Specific narratives for the three patients in Figure 6 are given in the Supplementary Information.
Figure 6.
Cumulative effect on glucose concentrations following frequently occurring SDs in three patients. For each patient, the square indicates an SD event detected on the ECoG subdural strip. For each SD event associated with an rsMD change in glucose, the basal levels and 20 mins levels are plotted. Patient 3: Eight SD events on Day 3. The first two events were separated by 50 mins, with an initial 613 μmol/L dialysate glucose down to 574 μmol/L. The next four SD events repeated at a regular interval of 35 mins (thus constituting a cluster) and the glucose levels fell from 553 to 448 μmol/L, with no recovery to basal concentrations between subsequent SD events. Finally, after a gap of 1 h and 30 mins, by which time the glucose levels nearly recovered the basal levels of the preceding SD, two SDs occurred with 50-min interval and the glucose levels were just below 400 μmol/L. The glucose levels did not fall further and patient 3 recovered well (extended Glasgow Outcome score of 6). Patient 6: During this cluster of eight SD events repeating every 15 to 20 mins at the end of the monitoring period, the glucose dialysate concentrations fell from 334 to 134 μmol/L. The falls in glucose concentrations varied between 15 and 92 μmol/L for all the eight SDs. The glucose levels remained below 150 μmol/L for the rest of the monitoring period and patient 6 died on Day 4. Note that in the case when the SDs were observed at <20-min intervals, the 20-min value was defined as the basal levels just preceding the next SD. Patient 9 (diabetic): three initial temporal clusters of SD events until 12:00 on Day 1 repeated every 20 to 35 mins and drove the glucose concentrations down from 2.0 mmol/L to 591 μmol/L, with no recovery in between the events. After a gap of 9 h and an apparent stabilization of the glucose levels, two SDs at 21:30 on Day 1, repeating at an hour interval, led to a small −14 μmol/L change in glucose concentrations. During 12 h with no SD events, the glucose concentrations recovered to a high level of 1.2 mmol/L. Another cluster of six SDs repeating every 50 to 60 mins between 13:44 and 16:44 on Day 2 resulted in a drop of glucose down to 768 μmol/L. Two isolated SDs followed a few hours later, and a final cluster of 13 SDs early on Day 3 led to a fall of glucose down to 106 μmol/L. Patient 9 never recovered and deceased on Day 6.
Discussion
There are four principal findings from this study. First, SD waves in the perilesion zone in the human brain lead to a fall in extracellular glucose concentrations and a rise in extracellular lactate concentrations. Second, the effect of SDs on glucose and lactate could be resolved dynamically using rsMD in the ICU and showed a wide range of patterns of changes. Third, the metabolic response to the passage of the SD wave is sustained, with no sign of recovery at 20 mins after SD. Finally, spontaneous SDs occurring frequently in perilesion zones in the injured human brain lead to a stepwise progressive depletion of the extracellular glucose pool.
Fall in Glucose and Increase in Lactate Following Spreading Depolarization Waves
We have described here a characteristic metabolic response to SD waves in the human brain: a fall in dialysate glucose and an increase in dialysate lactate concentrations. Such changes in extracellular glucose and lactate levels have been observed in experimental models of stroke with induced or spontaneous SD waves. Mies et al (1993) found a decline in glucose in brain slices following the passage of a single SD wave, and a microdialysis study showed a marked increase in lactate during the repolarization phase in the rat striatum (Taylor et al, 1994). Selman et al (2004) observed a marked decrease in extracellular glucose concentration and increase in lactate concentration at the SD wavefront and after the passage of the SD in rats using a freeze trapping technique. More recently, the metabolic response to induced SD was quantified in cats using the same rsMD assay, with results (Hashemi et al, 2009) very similar to our values now described for the human brain. This same study showed that the fall in microdialysate glucose follows rather than causes depolarization.
Clinically, Parkin et al (2005) previously demonstrated a clear correlation between the total number of SD events and a reduction of dialysate glucose at 1 to 2 h after the last SD compared with initial values. However, although the association between low glucose concentrations and SD events was observed, the causation could not be demonstrated, for example, from a typical time course of metabolic changes linked to single depolarization events. Here, we provide the first evidence that SD waves in the injured human brain do indeed lead to declines in extracellular glucose and increases in extracellular lactate concentrations. The increase in lactate represents an increase in local production (possibly due to a local lack of oxygen). The levels of dialysate glucose represent a balance between supply from the blood and utilization by the brain. Hence, a fall could either be due to increased utilization with an invariant supply or a falling supply for a given utilization.
Dynamic Changes in the Metabolic Response
We observed a wide variety of glucose and lactate responses to SD in terms of amplitude and time course of the changes as exemplified in Figure 3A (e.g., trace A1 compared with traces C2 and C4). The variability of these responses is much greater than the random noise from the rsMD assay following denoising techniques (Feuerstein et al, 2009), and illustrated in Figure 3B. We conclude that the observed variety in the responses is physiological and likely to reflect different responses of the injured tissue to SD waves.
Recent findings by the COSBID group indicate that SDs in SAH patients can be associated with different patterns of hemodynamic response: either physiological or inverse (Dreier et al, 2009). The former corresponds to a hyperemic response that delivers enough oxygen and energy metabolites to repolarize the tissue and hence sustain recurrent SDs. This could correspond to small changes in the rsMD data both in amplitude and in duration. The inverse response is seen as a transient (but sometimes sustained) oligemia that spreads across the tissue in a similar pattern as the SD waves (and has been termed ‘cortical spreading ischemia' (Dreier et al, 2009)). This vascular change, reasonably designated as microvascular spasm (of varying severities and durations), has been shown to follow rather than lead to the depolarization event (Dreier et al, 2000). This inverse coupling results in tissue hypoxia and could account for the larger prolonged responses observed in the rsMD data. Another factor that could account for the variability of the rsMD responses is the duration of depolarizations. In support of this, we found a positive correlation between the duration of the depression of the background ECoG activity and the amplitude of the glucose drop at 10 mins after SD. These findings suggest that the amplitude and duration of the glucose and lactate changes could serve as measures of the insult severity associated with SD waves in cortical perilesion boundary zones in human brain injury.
Sustained Changes After the Passage of the Spreading Depolarization Wave
Despite variability in the dynamic responses, the observed metabolic changes were on average still present 20 mins after SD, with no sign of recovery. Increased utilization of glucose in cortical tissue during the depolarization phase of the SD wave has been demonstrated (Shinohara et al, 1979). It has been attributed to the activation of the ATP-dependent ionic pumps to restore ionic homeostasis following the depolarization. However, this alone cannot explain the sustained decrease in glucose at 20 mins since at this time, cells are no longer depolarized, but are hyperpolarized. Alternatively, glucose may be used at this stage to replenish the astrocytic glycogen. Hertz et al (2007) suggested that glycogenolysis offers a higher anaerobic yield of ATP than fresh glucose and is thus likely to dominate ATP supply when there is acute energy demand. A significant reduction of glycogen levels after the passage of an SD wave has indeed been observed (Selman et al, 2004) and glycogen resynthesis is therefore likely to be required between 10 and 20 mins after SD. Glucose utilization by activated microglia may constitute a further drain on the glucose pool: whether microglial glucose utilization increases with the same time course as glucose microdialysate declines with an individual SD is unknown, but upregulated background microglial (and/or astrocytic) utilization would likely render the brain glucose pool more labile in the face of an abrupt increase in utilization linked to an SD.
The concomitant sustained increase in lactate concentrations suggests a possible lack of oxygen locally and/or a resort to nonoxidative metabolism to sustain the additional metabolic workload following SDs in perilesion tissue. Tissue hypoxia has been observed to coincide with the passage of SD waves (Piilgaard and Lauritzen, 2009; Takano et al, 2007; Tsacopoulos and Lehmenkühler, 1977). It has also been suggested that the early increased aerobic metabolism in response to massive depolarization (Rosenthal and Somjen, 1973) could switch to glycolysis at a later stage (Hashimoto et al, 2000). Hyperglycolysis was also observed in other microdialysis studies in head injury patients (Glenn et al, 2003; Goodman et al, 1999) and ultimately leads to increased lactate concentrations, as observed here. Such increases in lactate were recently found to be the most sensitive biochemical marker for predicting spontaneous episodes of intracranial hypertension in TBI patients (Adamides et al, 2009).
Cumulative Stepwise Fall of Glucose with Recurrent Spreading Depolarizations
We show that the sustained drop in glucose concentrations after depolarizations, combined with their capacity to recur in clusters at intervals of <50 mins leads in the injured brain to a progressive depletion of the cerebral extracellular glucose pool. We estimated from our data that changes in the levels of plasma glucose could influence the microdialysis glucose concentrations with a sensitivity of 40.8±10.7 μmol/L per mmol/L plasma glucose change (mean±s.e. of the mean, n=8) (please see Supplementary Information for details). This suggests that large changes in blood glucose are a confounding variable. However, plasma glucose changes alone are not sufficient to explain the changes reported here.
A stepwise decrease in glucose concentrations similar to those reported here was observed linked to spontaneous periinfarct SDs following experimental middle cerebral artery occlusion (Hopwood et al, 2005). Similarly, Takeda et al (1993) described a depletion of tissue glucose and production of lactate with recurrent SDs in rats.
There is also evidence from in vivo experiments for a reverse chain of causality, in which a higher incidence of spontaneous PIDs is associated with relatively mild reductions in plasma glucose, which was interpreted as the independent variable here (Hopwood et al, 2005; Strong et al, 2000). This combination of findings led to the suggestion of a vicious circle (Hopwood et al, 2005) in which recurrent SDs (or, more certainly, peri-infarct SDs) drive brain glucose down, in turn increasing the frequency of SDs as proposed earlier (Nedergaard and Astrup, 1986), and in turn further lowering tissue glucose concentrations. The interaction of frequency of recurrence of depolarizations with delayed restoration of tissue glucose that we have demonstrated here suggests that this concept of a vicious circle, strengthened by mild hypoglycemia, can indeed be applied to patients. To this threatening combination of factors (perhaps present to some degree in injured tissue even without depolarizations) must now be added the impact of microvascular spasm promoted by depolarizations in ischemic brain, as first characterized experimentally as ‘cortical spreading ischemia' (Dreier et al, 2000) and recently demonstrated in patients with SAH (Dreier et al, 2009). However, low tissue/plasma glucose is by no means the only factor likely to influence depolarization frequency, with clear evidence for an effect of lower cerebral perfusion pressure (Hartings et al, 2009), with suspicion falling also on pyrexia, seizures, choice of sedation, PaO2, and ICU nursing procedures.
Several clinical studies have demonstrated a correlation between low brain glucose concentrations and poor patient outcomes. An initial study reported ‘undetectable' (lower than 50 μmol/L) dialysate glucose in most patients who died after severe head injury (Zauner et al, 1997). More recently, a strong relationship between periods of low brain glucose concentrations (lower than 200 μmol/L for 2 h) and poor outcome was demonstrated in TBI patients (Vespa et al, 2003) and in aneurysmal SAH patients (Schlenk et al, 2008). In all these studies, clinical factors (e.g., seizures, hypoglycemia, activated macrophages or neutrophils, or elevated glutamate concentrations) either did not have a time course that corresponded well to the observed increased glucose utilization (Zazulia et al, 2009) or simply accounted for only a minority of the observed prolonged periods of low glucose values (Vespa et al, 2003). We suggest that clusters of frequent SDs are an important mechanism leading to the low brain glucose concentrations measured in these studies, augmenting the likely effect of mitochondrial failure (Verweij et al, 2000).
Given the small number of patients (n=9) and the diversity of their primary pathologies (TBI, SAH, and ICH), we cannot presently conclude that repetitive SDs are the dominant cause for low brain glucose. A prospective study would be required to address this question, together with, for example (1) whether there is a difference in the responses to SDs when they occur at early versus late days following injury, (2) whether there is a discernible pattern in the metabolic response to SDs depending on the primary pathology and its location (requiring interval imaging, preferably with MR-diffusion-weighted imaging), and (3) whether the magnitudes of the glucose drops are correlated with the frequency of SD events.
Conclusions
For the first time, it has been possible to resolve the dynamic metabolic response to SDs in the injured human brain using rapid sampling of cerebral microdialysate for glucose and lactate, together with noise-reduction postprocessing. We here show reductions of tissue glucose in association with depolarization events. Our findings suggest that clusters of frequent SDs are one mechanism that leads to the low brain glucose concentrations that are often observed. The resulting accumulated failure of glucose supply to meet the energetic demand for the restoration and maintenance of cell membrane polarization could compromise the viability of the tissue and hence lead to further expansion of the lesion and poor neurologic outcome.
Acknowledgments
We thank the patients, clinical staff, and nurses from King's College Hospital who contributed to this study and the members of the COSBID group for their support and discussions.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Adamides AA, Rosenfeldt FL, Winter CD, Pratt NM, Tippett NJ, Lewis PM, Bailey MJ, Cooper DJ, Rosenfeld JV. Brain tissue lactate elevations predict episodes of intracranial hypertension in patients with traumatic brain injury. J Am Coll Surg. 2009;209:531–539. doi: 10.1016/j.jamcollsurg.2009.05.028. [DOI] [PubMed] [Google Scholar]
- Bellander B-M, Cantais E, Enblad P, Hutchinson P, Nordström C-H, Robertson C, Sahuquillo J, Smith M, Stocchetti N, Ungerstedt U, Unterberg A, Olsen NV. Consensus meeting on microdialysis in neurointensive care. Intensive Care Med. 2004;30:2166–2169. doi: 10.1007/s00134-004-2461-8. [DOI] [PubMed] [Google Scholar]
- Bhatia R, Hashemi P, Razzaq A, Parkin MC, Hopwood SE, Boutelle MG, Strong AJ. Application of rapid sampling, online microdialysis to the monitoring of brain metabolism during aneurysm surgery. Neurosurgery. 2006;58:313–321. doi: 10.1227/01.NEU.0000208963.42378.83. [DOI] [PubMed] [Google Scholar]
- Branston NM, Strong AJ, Symon L. Extracellular potassium activity, evoked potential and tissue blood flow. Relationships during progressive ischaemia in baboon cerebral cortex. J Neurol Sci. 1977;32:305–321. doi: 10.1016/0022-510x(77)90014-4. [DOI] [PubMed] [Google Scholar]
- Busch E, Gyngell ML, Eis M, Hoehn-Berlage M, Hossmann K-A. Potassium-induced cortical spreading depressions during focal cerebral ischemia in rats: contribution to lesion growth assessed by diffusion-weighted NMR and biochemical imaging. J Cereb Blood Flow Metab. 1996;16:1090–1099. doi: 10.1097/00004647-199611000-00002. [DOI] [PubMed] [Google Scholar]
- Deeba S, Corcoles EP, Hanna BG, Pareskevas P, Aziz O, Boutelle MG, Darzi A. Use of rapid sampling microdialysis for intraoperative monitoring of bowel ischemia. Dis Colon Rectum. 2008;51:1408–1413. doi: 10.1007/s10350-008-9375-4. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen RM, Beekwilder JP, Van der Worp HB, Van der Sprenkel JWB, Tulleken KAF, Nicolay K. Correlation between tissue depolarizations and damage in focal ischemic rat brain. Brain Res. 1999;840:194–205. doi: 10.1016/s0006-8993(99)01769-2. [DOI] [PubMed] [Google Scholar]
- Dohmen C, Sakowitz OW, Fabricius M, Bosche B, Reithmeier T, Ernestus R-I, Brinker G, Dreier JP, Woitzik J, Strong AJ, Graf R, Depolarisations MotC-OSoBI Spreading depolarizations occur in human ischemic stroke with high incidence. Ann Neurol. 2008;63:720–728. doi: 10.1002/ana.21390. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Ebert N, Priller J, Megow D, Lindauer U, Klee R, Reuter U, Imai Y, Km E, Victorov I, Dirnagl U. Products of hemolysis in the subarachnoid space inducing spreading ischemia in the cortex and focal necrosis in rats: a model for delayed ischaemic neurological deficits after subarachnoid hemorrhage. J Neurosurg. 2000;93:658–666. doi: 10.3171/jns.2000.93.4.0658. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Major S, Manning A, Woitzik J, Drenckhahn C, Steinbrink J, Tolias C, Oliveira-Ferreira AI, Fabricius M, Hartings JA, Vajkoczy P, Lauritzen M, Dirnagl U, Bohner G, Strong AJ, for the Csg Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 2009;132 (Pt 7:1866–1881. doi: 10.1093/brain/awp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier JP, Woitzik J, Fabricius M, Bhatia R, Major S, Drenckhahn C, Lehmann T-N, Sarrafzadeh A, Willumsen L, Hartings JA, Sakowitz OW, Seemann JH, Thieme A, Lauritzen M, Strong AJ. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129:3224–3237. doi: 10.1093/brain/awl297. [DOI] [PubMed] [Google Scholar]
- Fabricius M, Fuhr S, Bhatia R, Boutelle M, Hashemi P, Strong AJ, Lauritzen M. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain. 2006;129:778–790. doi: 10.1093/brain/awh716. [DOI] [PubMed] [Google Scholar]
- Feuerstein D, Parker KH, Boutelle MG. Practical methods for noise removal: applications to spikes, non-stationary quasi-periodic noise and baseline drift. Anal Chem. 2009;81:4987–4994. doi: 10.1021/ac900161x. [DOI] [PubMed] [Google Scholar]
- Glenn T, Kelly D, Boscardin WJ, McArthur D, Vespa P, Oertel M, Hovda D, Bergsneider M, Hillered L, Martin N. Energy dysfunction as a predictor of outcome after moderate or severe head injury: indices of oxygen, glucose, and lactate metabolism. J Cereb Blood Flow Metab. 2003;23:1239–1250. doi: 10.1097/01.WCB.0000089833.23606.7F. [DOI] [PubMed] [Google Scholar]
- Goodman JC, Valadka AB, Gopinath SP, Uzura M, Robertson CS. Extracellular lactate and glucose alterations in the brain after head injury measured by microdialysis. Crit Care Med. 1999;27:1965–1973. doi: 10.1097/00003246-199909000-00041. [DOI] [PubMed] [Google Scholar]
- Hartings JA, Strong AJ, Fabricius M, Manning A, Bhatia R, Dreier JP, Mazzeo AT, Tortella FC, Bullock MR. Spreading depolarizations and late secondary insults after traumatic brain injury. J Neurotrauma. 2009;26:1857–1866. doi: 10.1089/neu.2009.0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi P, Bhatia R, Nakamura H, Dreier JP, Graf R, Strong AJ, Boutelle MG. Persisting depletion of brain glucose following cortical spreading depression, despite apparent hyperaemia: evidence for risk of an adverse effect of Leão's spreading depression. J Cereb Blood Flow Metab. 2009;29:166–175. doi: 10.1038/jcbfm.2008.108. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Takeda Y, Sato T, Kawahara H, Nagano O, Hirakawa M. Dynamic changes of NADH fluorescence images and NADH content during spreading depression in the cerebral cortex of gerbils. Brain Res. 2000;872:294–300. doi: 10.1016/s0006-8993(00)02509-9. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Hopwood S, Parkin M, Bezzina E, Boutelle M, Strong A. Transient changes in cortical glucose and lactate levels associated with peri-infarct depolarisations, studied with rapid-sampling microdialysis. J Cereb Blood Flow Metab. 2005;25:391–401. doi: 10.1038/sj.jcbfm.9600050. [DOI] [PubMed] [Google Scholar]
- Hutchinson PJ, al-Rawi PG, O'Connell MT, Gupta AK, Maskell LB, Hutchinson DB, Pickard JD, Kirkpatrick PJ. On-line monitoring of substrate delivery and brain metabolism in head injury. Acta Neurochir Suppl (Wien) 2000;76:431–435. doi: 10.1007/978-3-7091-6346-7_89. [DOI] [PubMed] [Google Scholar]
- Jones DA, Parkin MC, Langermann H, Landolt H, Hopwood SE, Strong AJ, Boutelle MG. On-line monitoring in neurointensive care. Enzyme based electrochemical assay for simultaneous, continuous monitoring of glucose and lactate from critical care patients. J Electroanal Chem. 2002;538:243–252. [Google Scholar]
- Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Jorgensen MB, Diemer NH, Gjedde A, Hansen AJ. Persistent oligemia of rat cerebral cortex in the wake of spreading depression. Ann Neurol. 1982;12:469–474. doi: 10.1002/ana.410120510. [DOI] [PubMed] [Google Scholar]
- Leao AAP. Spreading depression of activity in the cerebral cortex. J Neurophysiol. 1944;7:390. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- Mies G, Iijima T, Hossmann K-A. Correlation between peri-infarct DC shifts and ischaemic neuronal damage in rat. Neuroreport. 1993;4:709–711. doi: 10.1097/00001756-199306000-00027. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Astrup J. Infarct rim: effect of hyperglycemia on direct current potential and [14C]2-deoxyglucose phosphorylation. J Cereb Blood Flow Metab. 1986;6:607–615. doi: 10.1038/jcbfm.1986.108. [DOI] [PubMed] [Google Scholar]
- Parkin M, Hopwood S, Jones DA, Hashemi P, Landolt H, Fabricius M, Lauritzen M, Boutelle MG, Strong AJ. Dynamic changes in brain glucose and lactate in pericontusional areas of the human cerebral cortex, monitored with rapid sampling on-line microdialysis: relationship with depolarisation-like events. J Cereb Blood Flow Metab. 2005;25:402–413. doi: 10.1038/sj.jcbfm.9600051. [DOI] [PubMed] [Google Scholar]
- Parkin MC, Hopwood SE, Boutelle MG, Strong AJ. Resolving dynamic changes in brain metabolism using biosensors and on-line microdialysis. Trends Analyt Chem. 2003;22:487–497. [Google Scholar]
- Piilgaard H, Lauritzen M. Persistent increase in oxygen consumption and impaired neurovascular coupling after spreading depression in rat neocortex. J Cereb Blood Flow Metab. 2009;29:1517–1527. doi: 10.1038/jcbfm.2009.73. [DOI] [PubMed] [Google Scholar]
- Rosenthal M, Somjen GG. Spreading depression, sustained potential shifts, and metabolic activity of cerebral cortex of cats. J Neurophysiol. 1973;36:739–749. doi: 10.1152/jn.1973.36.4.739. [DOI] [PubMed] [Google Scholar]
- Sarrafzadeh A, Haux D, Sakowitz O, Benndorf G, Herzog H, Kuechler I, Unterberg AW. Acute focal neurological deficits in aneurysmal subarachnoid hemorrhage relation of clinical course, CT findings, and metabolite abnormalities monitored with bedside microdialysis. Stroke. 2003;34:1382–1388. doi: 10.1161/01.STR.0000074036.97859.02. [DOI] [PubMed] [Google Scholar]
- Schlenk F, Nagel A, Graetz D, Sarrafzadeh AS. Hyperglycemia and cerebral glucose in aneurysmal subarachnoid hemorrhage. Intensive Care Med. 2008;34:1200–1207. doi: 10.1007/s00134-008-1044-5. [DOI] [PubMed] [Google Scholar]
- Selman WR, Lust WD, Pundik S, Zhou Y, Ratcheson RA. Compromised metabolic recovery following spontaneous spreading depression in the penumbra. Brain Res. 2004;999:167–174. doi: 10.1016/j.brainres.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Dollinger B, Brown G, Rapoport S, Sokoloff L. Cerebral glucose utilization: local changes during and after recovery from spreading cortical depression. Science. 1979;203:188–190. doi: 10.1126/science.758688. [DOI] [PubMed] [Google Scholar]
- Strong AJ, Fabricius M, Boutelle MG, Hibbins SJ, Hopwood SE, Jones R, Parkin MC, Lauritzen M. Spreading and synchronous depressions of cortical activity in acutely injured human brain. Stroke. 2002;33:2738–2743. doi: 10.1161/01.str.0000043073.69602.09. [DOI] [PubMed] [Google Scholar]
- Strong AJ, Smith SE, Whittington DJ, Meldrum BS, Parsons AA, Krupinski J, Hunter AJ, Patel S, Robertson C. Factors influencing the frequency of fluorescence transients as markers of peri-infarct depolarizations in focal cerebral ischemia editorial comment. Stroke. 2000;31:214–222. doi: 10.1161/01.str.31.1.214. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian G-F, Peng W, Lou N, Lovatt D, Hansen A, Kasischke K, Nedergaard M. Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci. 2007;10:754–762. doi: 10.1038/nn1902. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Jacewicz M, Nowak T, Pulsinelli W. DC-potential and energy metabolites in the focal ischaemia. J Cereb Blood Flow Metab. 1993;13:S450. [Google Scholar]
- Taylor DL, Richards DA, Obrenovitch TP, Symon L. Time course of changes in extracellular lactate evoked by transient K+-induced depolarisation in the rat striatum. J Neurochem. 1994;62:2368–2374. doi: 10.1046/j.1471-4159.1994.62062368.x. [DOI] [PubMed] [Google Scholar]
- Tsacopoulos M, Lehmenkühler A. A double-barrelled Pt-microelectrode for simultaneous measurement of PO2 and bioelectrical activity in excitable tissues. Cell Mol Life Sci. 1977;33:1337–1338. doi: 10.1007/BF01920167. [DOI] [PubMed] [Google Scholar]
- Verweij BH, Muizelaar JP, Vinas FC, Peterson PL, Xiong Y, Lee CP. Impaired cerebral mitochondrial function after traumatic brain injury in humans. J Neurosurg. 2000;93:815–820. doi: 10.3171/jns.2000.93.5.0815. [DOI] [PubMed] [Google Scholar]
- Vespa P, Bergsneider M, Hattori N, Wu H-M, Huang S-C, Martin NA, Glenn TC, McArthur DL, Hovda DA. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab. 2005;25:763–774. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa PM, McArthur D, O'Phelan K, Glenn T, Etchepare M, Kelly D, Bergsneider M, Martin NA, Hovda DA. Persistently low extracellular glucose correlates with poor outcome 6 months after human traumatic brain injury despite a lack of increased lactate: a Microdialysis study. J Cereb Blood Flow Metab. 2003;23:866–877. doi: 10.1097/01.WCB.0000076701.45782.EF. [DOI] [PubMed] [Google Scholar]
- Zauner A, Doppenberg E, Woodward JJ, Choi SC, Young HF, Bullock R. Continuous monitoring of cerebral substrate delivery and clearance: initial experience in 24 patients with severe acute brain injuries. Neurosurgery. 1997;41:1082–1093. doi: 10.1097/00006123-199711000-00011. [DOI] [PubMed] [Google Scholar]
- Zazulia AR, Videen TO, Powers WJ. Transient focal increase in perihematomal glucose metabolism after acute human intracerebral hemorrhage. Stroke. 2009;40:1638–1643. doi: 10.1161/STROKEAHA.108.536037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.