Abstract
Cerebral energy metabolism has been suggested to have an important function in body weight regulation. We therefore examined whether there is a relationship between body mass and adenosine triphosphate (ATP) metabolism in the human brain. On the basis of our earlier findings indicating a neuroprotective preferential energy supply of the brain, as compared with peripheral muscle on experimentally induced hypoglycemia, we examined whether this physiological response is preserved also in low-weight and obese participants. We included 45 healthy male subjects with a body mass index (BMI) ranging from 17 to 44 kg/m2. Each participant underwent a hypoglycemic glucose-clamp intervention, and the ATP metabolism, that is, the content of high-energy phosphates phosphocreatine (PCr) and ATP, was measured repeatedly by 31phosphor magnetic resonance spectroscopy (31P-MRS) in the cerebral cortex and skeletal muscle. Results show an inverse correlation between BMI and high-energy phosphate content in the brain (P<0.01), whereas there was no such relationship found between skeletal muscle and BMI. The hypoglycemic clamp intervention did not affect the ATP metabolism in both tissues. Our data show an inverse correlation between BMI and cerebral high-energy phosphate content in healthy humans, suggesting a close relationship between energetic supply of the brain and body weight regulation.
Keywords: adenosine triphosphate, glucose, obesity, 31phosphor magnetic resonance spectroscopy, skeletal muscle
Introduction
Sensations of appetite and satiety that regulate food intake behavior are processed in hypothalamic centers within the brain (Gao and Horvath, 2008; Horvath, 2005). Accordingly, a disruption in hypothalamic nutrient sensing has been shown to induce obesity (He et al, 2006). On the basis of bidirectional signaling between cerebral structures and peripheral organs, the brain controls not only calorie consumption but also the whole organism's energy homeostasis (Schwartz et al, 2000; Schwartz and Porte, 2005). Because of this key function in the overall energy regulation, the brain's own energy supply has been assumed to be a crucial factor in body weight regulation (Peters et al, 2004; Peters et al, 2007). To maintain its own energy content, the brain provides itself with energy sources, mainly circulating glucose, by a mechanism termed ‘energy on demand' (Magistretti et al, 1999). We previously found that under conditions of energetic over- or undersupply, that is, hypo- or hyperglycemia, the brain modulates its energy status, in contrast to a stable peripheral skeletal muscle energy content (Oltmanns et al, 2008).
We assumed that this fundamental physiological regulation may be disturbed in subjects with very high or low body weight, which may underlie the development of body mass index (BMI) deviations from normal values as defined by the World Health Organization. To test this hypothesis, we induced hypoglycemic conditions by the established standardized glucose-clamp technique (Ludwig et al, 2009; Oltmanns et al, 2005) in healthy male subjects of the three BMI categories: low weight (<20 kg/m2), normal weight (20 to 25 kg/m2), and obese (>30 kg/m2). High-energy phosphate content, that is, mainly adenosine triphosphate (ATP) and phosphocreatine (PCr), was measured by 31phosphor magnetic resonance spectroscopy (31P-MRS) in the cerebral cortex and skeletal muscle at baseline and repeatedly on induction of hypoglycemia, and within-subject analyses as well as group comparisons were performed.
Materials and methods
Subjects
We included 45 healthy Caucasian men aged 18 to 30 years who were classified into three different body-weight groups (n=15 for each group): low weight (BMI<20 kg/m2), normal weight (BMI between 20 and 25 kg/m2), and obese (BMI>30 kg/m2). Exclusion criteria were chronic or acute illness, alcohol or drug abuse, smoking, competitive sports, exceptional physical or mental stress, and current medication of any kind. As it has been shown that the offspring of parents with type 2 diabetes mellitus may show disturbances in insulin-induced ATP synthesis (Petersen et al, 2005), we excluded these subjects by medical history. All participants were requested to abstain from alcohol, not to perform any kind of exhausting physical activity, and to go to bed no later than 2300 hours on the day preceding the test. The study was performed in accordance with the Declaration of Helsinki (2000) of the World Medical Association and was approved by the ethics committee of the University of Luebeck. Each participant gave written informed consent.
Hyperinsulinemic–Hypoglycemic Clamp Procedure
On the days of experimental testing, subjects reported to the Department of Neuroradiology at 0800 hours after an overnight fast of at least 12 hours. A cannula was inserted into a vein on the back of the hand and a second cannula was inserted into an antecubital vein of the contralateral arm. Both cannulas were connected to long thin tubes, which enabled blood sampling and adjustment of the dextrose infusion rate beyond the subjects' visual field. After recording the baseline 31P-MR spectra, insulin (Humaninsulin, Sanofi-Aventis, Frankfurt, Germany) was infused at a rate of 5 mU per minute per kg for 2 minutes and subsequently with a continuous infusion rate of 1.5 mU per minute per kg. A 20% dextrose solution was simultaneously infused at a variable rate to control blood glucose levels, which were measured at 5-minute intervals (B-Glucose-Data-Management, HemoCue GmbH, Grossostheim, Germany). Blood glucose concentration was reduced to a niveau of 2.2 mmol/L and held stable at this level for 45 minutes to record further 31P-MR spectroscopy sequences during the hypoglycemic period. After the last spectroscopy sequence, insulin infusion was stopped and blood glucose concentration was normalized.
31Phosphor Magnetic Resonance Spectroscopy Measurements
Spectra of the cortex (occipital lobe) and skeletal muscle (trapezius muscle) were taken in a whole-body 1.5-T MR spectrometer (Magnetom Symphony, Siemens Medical, Erlangen, Germany). Participants rested in a supine position, with their head and shoulder, respectively, on a double-tuned flexible surface coil (DT Flex Coil, Lammers Medical Technology, Germany), which combines a square coil of 8 cm edge length for transmitting and receiving on the phosphorus resonance frequency (25.8 MHz) and a butterfly coil (15 cm × 30 cm) for imaging and decoupling on the hydrogen (1H) resonance frequency (63.9 MHz). The butterfly design of the 1H-coil partition allowed for nearly homogeneous radio frequency excitation of both volumes of interest, the occipital cortex and the trapezius muscle. Subjects were asked to relax and keep their eyes open. The order of brain and muscle measurements was randomized across subjects. Four 31P-MR spectroscopy sequences were measured: baseline measurement before initiation of the glucose clamp, during the glycemic drop 15 minutes after the start of blood glucose decline, when the target glucose level of 2.2 mmol/L was reached, and one last measurement after further 30 minutes of stable hypoglycemia.
Recording of spectra was performed by four dummy excitations to reach a steady state of the magnetization, followed by averaging 128 measurements (repetition time 1500 milliseconds, 1024 data points, bandwidth 4 kHz). To ensure good spectral quality and to increase the signal-to-noise ratio, we used the nuclear Overhauser effect (Bachert-Baumann et al, 1990) with a broadband proton decoupling during excitation (10 rectangular radio frequency pulses at a proton resonance frequency of 10 milliseconds duration and 10 milliseconds delay between each other to generate a 90°C flip angle on the 1H nuclei) and a wideband alternating-phase technique for the zero-residual splitting-4 (Barker et al, 2001) decoupling scheme during readout. The wideband alternating-phase technique for zero-residual splitting-4 decoupling was performed during the whole read-out time with 64 radio frequency pulses of 2 milliseconds duration followed by 2 milliseconds delay producing a 180°C decoupling angle. To localize the signal from the volumes of interest and suppress the signal coming from superficial tissues and the skull, the flip angle of rectangular excitation pulses was set to approximately 180°C in the coil plane. We localized the standardized volumes of interest by taking scout images before measurements and thereby ensured the comparability of our spectra. Volume selection was accomplished by the limited penetration depth of the used surface coil to approximately 4 cm. Renouncement of the magnetic field gradients led to a sufficient signal-to-noise ratio in the chosen acquisition time of 3 minutes and 18 seconds.
Evaluation of the spectra data was performed by Magnetic Resonance User Interface (Naressi et al, 2001). Spectral line positions and intensities were calculated using the Advanced Method for Accurate, Robust, and Efficient Spectral Fitting algorithm (Vanhamme et al, 1997).
We examined the high-energy phosphate compounds ATP and PCr. Phosphocreatine represents a high-energy reservoir linked to ATP in a bidirectional reaction in which ATP is formed by PCr and vice versa, catalyzed by the creatine-phospho-kinase, at a PCr:ATP molar ratio of 1:1. The equilibrium for this reaction favors ATP formation so that energy demands in excess of the cells' capacity for ATP synthesis are met initially through a shift in this equilibrium, whereby ATP concentrations are held constant through PCr hydrolysis. Phosphocreatine and ATP-based measurements of energetics thus appear to be appropriate because they directly reflect the overall high-energy phosphate turnover (Iosifescu and Renshaw, 2003). Because of the high resolution of our spectra, the nicotinamide-adenine-dinucleotide peak that may represent a bias because of its adjacency to the α-ATP peak could be delimited and was excluded from all analyses. The ATP was calculated as the sum of α-, β-, and γ-ATP divided by 3. Our intention was not the quantification of high-energy phosphate concentrations but to determine the relative differences between groups. In addition to PCr and ATP, the ratios of PCr/inorganic phosphate (Pi), ATP/Pi, and PCr/γ-ATP were evaluated because they are frequently used as indicators of the intracellular energy status (Iosifescu and Renshaw, 2003; Rango et al, 2001). pH was calculated from the chemical shift of Pi referenced to PCr (δPi) by using the equation according to Barker et al (1999):
Statistical Analysis
Data analysis was performed using SPSS, version 15.0. Values are presented as mean values±standard error of mean (s.e.m.). Statistical analysis was based on analyses of variance (ANOVA) for repeated measurements, including the factors ‘group' (low weight, normal weight, or obese) and ‘time' (time points of data collection). The interaction effect of these two factors was termed ‘group by time.' Correlations (of the mean values of the high-energy phosphate content at baseline with BMI) were analyzed by Pearson's bivariate correlation analysis. A P-value of <0.05 was considered significant.
Results
Blood Glucose and Serum Insulin
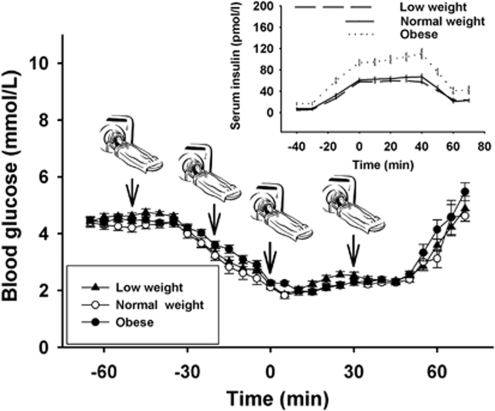
Circulating glucose concentrations were held stable on a euglycemic level for 25 minutes from baseline measurements (4.4±0.14 mmol/L in the low-weight, 4.4±0.17 mmol/L in the normal-weight, and 4.5±0.12 mmol/L in the obese group) to the start of the insulin infusion (Figure 1). On insulin infusion, glucose dropped within 40 minutes to a hypoglycemic level of 2.2±0.1, 2.1±0.08, and 2.3±0.08 mmol/L in the low-weight, normal-weight, and obese group, respectively, and was held at this plateau for another 45 minutes. Subsequently, insulin infusion was stopped and blood glucose returned to normal. There was no difference between groups with respect to glucose concentrations throughout the study. Serum insulin rose from baseline values of 4.8±0.92, 7.5±2.08, and 16.5±2.57 pmol/L to peak levels of 59.6±2.15, 67.2±5.07, and 107.2±9.16 pmol/L during the clamp session in the low-weight, normal-weight, and obese group, respectively (Figure 1, small panel). Group comparisons revealed no difference in insulin concentrations between the low- and the normal-weight group (P=0.29), whereas serum insulin was significantly higher in obese subjects as compared with normal-weight controls (P<0.001).
Figure 1.
Mean values±s.e.m. of blood glucose during glucose-clamp experiments. There was no difference in circulating glucose concentrations between low-weight (black triangles), normal-weight (white circles), and obese (black circles) subjects throughout the study (n=15 in each group). Arrows mark the time points of 31phosphor magnetic resonance spectroscopy measurements. Small panel: mean values±s.e.m. of serum insulin concentrations during the experiments. Overall, insulin levels were higher in obese participants as compared with both normal-weight and low-weight subjects (P<0.001).
Cerebral High-Energy Phosphate Content
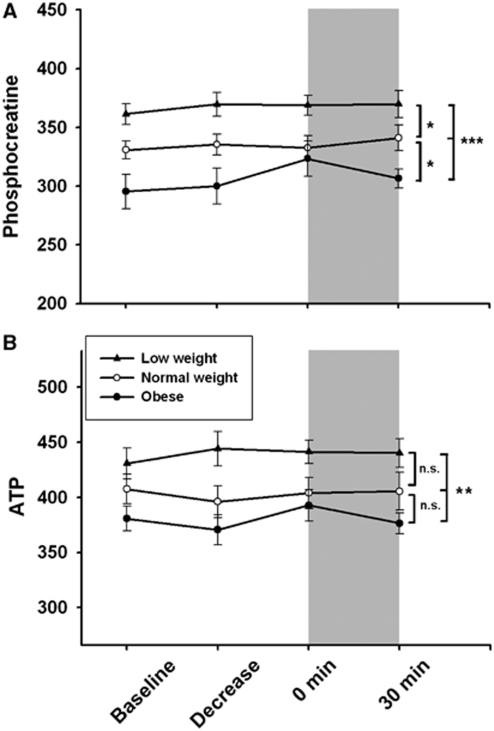
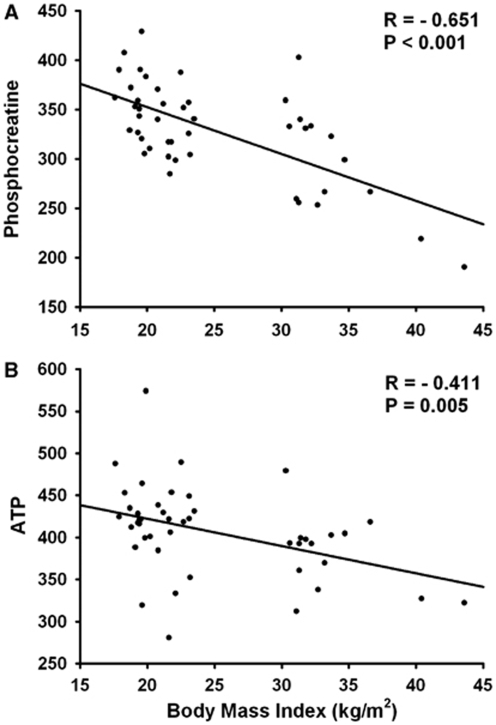
Figure 2 shows the cerebral PCr and ATP contents in the three body-weight groups. Both parameters reveal the same pattern in an ANOVA analysis comprising all time points, that is, significantly different levels in PCr and ATP between all BMI groups, with highest concentrations in low-weight and lowest levels in obese subjects (P<0.001 for PCr and P=0.004 for ATP; Figures 2A and 2B). Group comparisons between low- and normal-weight subjects reached significance in PCr values (P=0.01), but a trend was observed only for ATP data (P=0.058). Obese men showed lower cerebral PCr content than normal-weight controls (P=0.034), but values of ATP were similar in both groups (P=0.175). There was, however, no effect of the hypoglycemic intervention, neither in the course of PCr measurements nor in ATP values. Correlation analyses between merged high-energy phosphate values of all groups at baseline and BMI revealed a clear negative correlation between body weight and both PCr (P<0.001; Figure 3A) and ATP (P=0.005; Figure 3B) content in the brain. Discrete analyses of γ-ATP confirmed the ATP data, with a strong negative correlation of γ-ATP and BMI at baseline (P<0.001), and ANOVA analysis for all time points revealed also a strong group effect, with highest levels in low-weight and lowest levels in obese subjects (P<0.001; Table 1a).
Figure 2.
Mean values±s.e.m. of cerebral high-energy phosphates. Phosphocreatine (A) and adenosine triphosphate (ATP; α- + β- + γ-ATP/3) (B) content in low-weight (black triangles), normal-weight (white circles), and obese (black circles) subjects are shown at baseline, during the glycemic decrease, and at the hypoglycemic plateau (n=15 in each group). As values are determined by calculating the area under the spectral peak, no units are indicated. Gray areas mark the hypoglycemic period; asterisks mark significant analyses of variance group effects (*P<0.05; **P<0.01; ***P<0.001).
Figure 3.
Correlation analyses between body mass index (BMI) and baseline phosphocreatine (A) and adenosine triphosphate (ATP; α- + β- + γ-ATP/3) (B) levels in the brain including all participants (n=45). Both measures indicate the same pattern. BMI correlates inversely with cerebral high-energy phosphate content. As phosphate values are determined by calculating the area under the spectral peak, no units are indicated.
Table 1a. Mean values±s.e.m. of γ-ATP and inorganic phosphate (Pi) content, as well as calculated pH in the brain.
| Brain | Baseline | Decrease | 0 minute | 30 minutes | P-value (effect of time) | P-value (group effect) |
|---|---|---|---|---|---|---|
| γ-ATP | ||||||
| Low weight | 372.9±11.0 | 382.8±7.8 | 373.6±8.3 | 380.4±7.8 | ||
| Normal weight | 352.9±7.0 | 343.7±6.5 | 353.6±11.1 | 352.1±11.1 | 0.657 | <0.001*** |
| Obese | 318.6±12.8 | 313.5±14.0 | 332.5±14.3 | 319.0±10.0 | ||
| Pi | ||||||
| Low weight | 189.8±14.4 | 184.2±11.6 | 174.7±11.3 | 163.4±10.0 | ||
| Normal weight | 172.3±9.6 | 188.0±21.1 | 178.5±10.7 | 149.3±8.7 | 0.022* | 0.194 |
| Obese | 167.1±19.2 | 140.4±8.4 | 161.4±10.5 | 147.4±8.1 | ||
| pH | ||||||
| Low weight | 7.09±0.01 | 7.10±0.01 | 7.09±0.01 | 7.08±0.01 | ||
| Normal weight | 7.11±0.01 | 7.11±0.02 | 7.10±0.01 | 7.09±0.01 | 0.064 | 0.779 |
| Obese | 7.11±0.00 | 7.18±0.02 | 7.10±0.01 | 7.08±0.01 | ||
ATP, adenosine triphosphate. Asterisks mark significant analyses of variance effects (*P<0.05; ***P<0.001).
Inorganic phosphate declined during hypoglycemia (P=0.022 including all groups; Table 1a), whereas there was a clear rise in PCr/Pi ratio (P=0.002; Table 1b). However, there was no group effect when looking at these parameters (P>0.1 for both). The ATP/Pi ratios showed no significant changes on hypoglycemia induction (P=0.074) and were equal in all groups (P=0.625; Table 1b). In addition, PCr/γ-ATP ratios showed no differences between groups in ANOVA analyses including all time points (group effect P=0.827; Table 1b). Cerebral pH values remained stable over all conditions and groups (P>0.05 for both; Table 1a).
Table 1b. Mean values±s.e.m. of ratios of PCr/Pi, ATP/Pi, and PCr/γ-ATP in the brain.
| Brain | Baseline | Decrease | 0 minute | 30 minutes | P-value (effect of time) | P-value (group effect) |
|---|---|---|---|---|---|---|
| PCr/Pi | ||||||
| Low weight | 2.05±0.2 | 2.13±0.2 | 2.23±0.1 | 2.39±0.2 | ||
| Normal weight | 1.99±0.1 | 2.02±0.2 | 1.95±0.1 | 2.42±0.2 | 0.002** | 0.838 |
| Obese | 2.01±0.2 | 2.20±0.1 | 2.10±0.1 | 2.17±0.1 | ||
| ATP/Pi | ||||||
| Low weight | 2.45±0.12 | 2.41±0.14 | 2.59±0.12 | 2.85±0.14 | ||
| Normal weight | 2.43±0.10 | 2.37±0.19 | 2.34±0.11 | 2.84±0.18 | 0.074 | 0.625 |
| Obese | 2.67±0.30 | 2.75±0.17 | 2.59±0.20 | 2.67±0.18 | ||
| PCr/γ-ATP | ||||||
| Low weight | 0.98±0.03 | 0.97±0.02 | 1.00±.02 | 0.97±0.03 | ||
| Normal weight | 0.94±0.02 | 0.98±0.02 | 0.94±0.02 | 0.98±0.04 | 0.354 | 0.827 |
| Obese | 0.93±0.03 | 0.96±0.03 | 0.99±0.06 | 0.97±0.02 | ||
ATP, adenosine triphosphate; PCr, phosphocreatine; Pi, inorganic phosphate. Asterisks mark significant analyses of variance effects (**P<0.01).
Muscular High-Energy Phosphate Content
In skeletal muscle, PCr content decreased on hypoglycemia induction (P=0.044 including all data; Table 2a), whereas there was no such effect observed in ATP measures. Group comparisons including all the three groups showed a difference, with the highest ATP concentrations in normal-weight and lowest content in obese subjects (P=0.039; Table 2a), an effect mainly reflecting significantly lower ATP levels in obese subjects as compared with normal-weight controls (P=0.014), as low-weight and normal-weight subjects did not differ (P=0.184). Discrete ANOVA analyses of the γ-ATP content confirmed this effect (P=0.017 for all groups, P=0.008 in obese subjects as compared with normal-weight controls). Phosphocreatine content, in contrast, did not show any significant differences between groups (P=0.239), although a comparison of obese and normal-weight controls confirmed the difference seen in ATP concentrations by trend (P=0.075). Correlation analyses between BMI and high-energy phosphates were nonsignificant in the muscle tissue.
Table 2a. Mean values±s.e.m. of PCr, ATP, γ-ATP, and Pi content, as well as calculated pH in skeletal muscle.
| Muscle | Baseline | Decrease | 0 minute | 30 minutes | P-value (effect of time) | P-value (group effect) |
|---|---|---|---|---|---|---|
| PCr | ||||||
| Low weight | 2272.7±106.6 | 2270.7±116.3 | 2170.0±96.6 | 2211.3±103.5 | ||
| Normal weight | 2424.7±110.4 | 2460.0±125.4 | 2395.3±89.8 | 2346.7±89.0 | 0.044* | 0.239 |
| Obese | 2208.0±95.8 | 2178.0±79.5 | 2153.3±84.2 | 2106.7±87.9 | ||
| ATP | ||||||
| Low weight | 714.7±28.3 | 734.2±33.0 | 698.8±26.0 | 707.6±28.3 | ||
| Normal weight | 789.5±37.0 | 778.0±36.1 | 771.0±27.2 | 760.0±28.1 | 0.221 | 0.039* |
| Obese | 665.±28.3 | 669.4±32.3 | 653.6±27.3 | 662.7±40.3 | ||
| γ-ATP | ||||||
| Low weight | 728.2±30.4 | 784.3±47.0 | 718.5±32.3 | 758.8±41.2 | ||
| Normal weight | 807.5±52.8 | 784.0±39.0 | 783.4±27.3 | 766.6±33.4 | 0.380 | 0.017* |
| Obese | 653.5±34.0 | 652.7±53.8 | 621.4±38.3 | 638.9±43.2 | ||
| Pi | ||||||
| Low weight | 412.5±17.1 | 439.6±15.7 | 437.3±19.2 | 427.0±24.5 | ||
| Normal weight | 382.3±19.5 | 421.1±20.8 | 413.1±20.7 | 394.7±18.8 | 0.011* | 0.051 |
| Obese | 355.2±20.3 | 383.0±18.6 | 380.1±19.2 | 363.9±19.7 | ||
| pH | ||||||
| Low weight | 6.97±0.01 | 6.96±0.01 | 6.96±0.01 | 6.96±0.01 | ||
| Normal weight | 6.96±0.01 | 6.97±0.01 | 6.96±0.01 | 6.97±0.01 | 0.392 | 0.417 |
| Obese | 6.94±0.01 | 6.96±0.00 | 6.96±0.00 | 0.95±0.01 | ||
ATP, adenosine triphosphate; PCr, phosphocreatine; Pi, inorganic phosphate. Asterisks mark significant analyses of variance effects (*P<0.05).
On analyzing changes in Pi content, we found an increase during the hypoglycemic session (P=0.011; Table 2a). The PCr/Pi and ATP/Pi ratios in contrast dropped from baseline to the end of the intervention (P<0.001 for PCr/Pi ratio and P=0.007 for ATP/Pi ratio comprising all time points; Table 2b). The ANOVA group comparisons comprising all groups revealed a significant difference in PCr/Pi and ATP/Pi ratios, with lowest concentrations in low-weight participants and highest levels in normal-weight controls (P=0.025 for the PCr/Pi ratio and P=0.014 for the ATP/Pi ratio). Moreover, PCr/γ-ATP ratios showed no differences between groups (P=0.105; Table 2b) and there was no effect of hypoglycemia (P=0.210; Table 2b). Muscle pH values remained stable over all conditions and groups (P>0.1 for both; Table 2a).
Table 2b. Mean values±s.e.m. of ratios of PCr/Pi, ATP/Pi, and PCr/γ-ATP in skeletal muscle.
| Muscle | Baseline | Decrease | 0 minute | 30 minutes | P-value (effect of time) | P-value (group effect) |
|---|---|---|---|---|---|---|
| PCr/Pi | ||||||
| Low weight | 5.60±0.3 | 5.17±0.2 | 5.00±0.2 | 5.26±0.2 | ||
| Normal weight | 6.62±0.5 | 5.95±0.3 | 5.96±0.3 | 6.07±0.3 | <0.001*** | 0.025* |
| Obese | 6.31±0.2 | 5.76±0.2 | 5.74±0.1 | 5.90±0.2 | ||
| ATP/Pi | ||||||
| Low weight | 1.81±0.08 | 1.71±0.06 | 1.69±0.08 | 1.74±0.07 | ||
| Normal weight | 2.15±0.16 | 1.89±0.09 | 1.92±0.09 | 1.96±0.07 | 0.007** | 0.014* |
| Obese | 1.90±0.06 | 1.75±0.04 | 1.74±0.05 | 1.85±0.09 | ||
| PCr/γ-ATP | ||||||
| Low weight | 3.12±0.13 | 2.90±0.16 | 3.02±0.14 | 2.91±0.11 | ||
| Normal weight | 3.00±0.13 | 3.13±0.09 | 3.06±0.08 | 3.06±0.10 | 0.210 | 0.105 |
| Obese | 3.38±0.09 | 3.34±0.16 | 3.47±0.21 | 3.30±0.22 | ||
ATP, adenosine triphosphate; PCr, phosphocreatine; Pi, inorganic phosphate. Asterisks mark significant analyses of variance effects (*P<0.05; **P<0.01; ***P<0.001).
Discussion
Our data show for the first time that cerebral high-energy phosphate content is related to body mass and directly correlates inversely with BMI in healthy young men. The ATP and PCr content is distinctly increased in low-weight and decreased in obese subjects, both as compared with normal-weight controls. This finding argues for the assumption that the brain's own energy supply may be involved in the regulation of the organism's body weight and overall energy turnover in humans (Peters et al, 2007).
The key question in this context is whether lower cerebral high-energy phosphates with increasing body mass are a consequence of weight gain or vice versa. In any case, it seems reasonable that suppressed high-energy phosphate levels may be due to decreased cellular glucose uptake. Crucial, at least for the insulin-dependant pathway of this process, is the cerebral insulin receptor. In this context, it has been repeatedly shown that central insulin action has an important function in regulating peripheral fat mass and glucose metabolism (Koch et al, 2008), and inactivation of the cerebral insulin receptor in turn is known to lead to obesity, increases in body fat, and elevated plasma insulin levels in mice (Bruning et al, 2000). Thus, one could speculate that our observation of decreased high-energy phosphates with rising body weight could be due to a disturbed insulin receptor function, which can particularly be implied in our obese-subject data, wherein high serum insulin levels were observed. Further evidence for the central function of cerebral insulin action supporting our speculations in this regard is the knowledge that insulin regulates hypothalamic glucose sensing via modulation of ATP-sensitive potassium channels, a mechanism that has been shown to be defective in obese rodents (Spanswick et al, 2000). Similar to a primary defect in glucose sensing, also decreased ATP levels physiologically lead to increased activation of ATP-sensitive potassium channels in glucose-sensing brain cells, making them less active and less responsive to glucose, an effect that has previously been implied to underlie the pathogenesis of obesity and type 2 diabetes mellitus (Parton et al, 2007). Therefore, it seems conceivable that a diminished glucose sensing based on low ATP levels in appetite-regulating hypothalamic structures cannot be compensated by insulin sensitizing in obesity because this mechanism is defective. In consequence, this cycle would lead to chronically activated appetite centers, further weight gain, and a compensatory rise in insulin secretion. However, the exact cause-and-effect chain is difficult to evaluate within the scope of a brief intervention study in humans without any long-term data and therefore remains to be clarified. Notwithstanding, it is generally known that ATP-sensitive potassium channels sense the current energy status in various brain areas and thereby enable the brain to react to distinct changes in its energy supply, which has some direct consequences on the regulation of hypothalamic food intake centers (Levin et al, 1999) as well as peripheral glucose disposal (Pocai et al, 2005). In case of a chronic cerebral undersupply of energies, for whatever reason, chronically augmented food intake beyond the actual energetic need could be explained (Sandoval et al, 2008). Under chronic energetic oversupply, as suggested here in our low-weight subjects, hypothalamic appetite centers may be inactivated in turn. This reasoning, however, remains speculative and to be addressed in further experiments.
Our data did not entirely confirm our earlier observation that acute hypoglycemia increases cerebral high-energy content (Oltmanns et al, 2008). As observed earlier, ATP levels remained constant, but in contrast to our former study, in which PCr concentrations were augmented with hypoglycemia, our current data do not show this effect. Notwithstanding, in line with the earlier study, PCr/Pi ratio was significantly increased, which is mainly based on a decrease in Pi content. The deviant effects from our former study, however, may be explained by the differences in the applied methods to induce hypoglycemic conditions. In our earlier study, we applied an insulin bolus causing a very rapid decrease in blood glucose levels and examined the high-energy phosphate content only at baseline and acutely when a hypoglycemic level <2.2 mmol/L was achieved. To perform repeated spectroscopic measurements during hypoglycemia, in this study, we created a stable hypoglycemic plateau by the established method of the glucose-clamp technique. The advantage of this method is a higher degree of glycemic control throughout the study, but the time interval needed to reach a glycemic level <2.2 mmol/L is extended when concomitantly infusing glucose instead of insulin only. It thereby seems conceivable that the brain may activate some neuroprotective mechanisms during this interval to prevent an energetic decrease in response to a lack of peripheral glucose (Oltmanns et al, 2008). However, our current data are in line with other observations of stable high-energy phosphate content during hypoglycemia in healthy subjects and type 1 diabetes mellitus patients (Bischof et al, 2004; Hilsted et al, 1988). Moreover, during starvation, ATP levels seem to be held constant in normal-weight participants (Cahill, 1970). Only under conditions of severe hypoglycemia of 1.4 mmol/L in animal studies were decreased levels of ATP and PCr found (Prichard et al, 1983). However, these extreme glycemic levels cannot be applied within the scope of an experimental study in human subjects.
Another interesting finding of our study was the fact that PCr levels, PCr/Pi, and ATP/Pi ratios in skeletal muscle decreased significantly on hypoglycemia, whereas the ATP content remained constant. As ATP concentrations are held constant through PCr hydrolysis, these data may reflect the decline of available energies in muscle tissue, a decrease that is apparently prevented in the brain by some neuroprotective mechanisms. This effect is in line with the view that the brain aims to secure its own energy supply (Peters et al, 2007). The ANOVA group comparisons in muscle mainly revealed a trend for lower PCr levels in obese as compared with normal-weight subjects, an effect that reached significance in ATP values. As obese subjects in our study showed significantly higher serum insulin levels as compared with the other groups, one could assume an underlying insulin resistance, which has been shown to be associated with reduced mitochondrial ATP synthesis in the offspring of parents with type 2 diabetes (Petersen et al, 2005). In our study, however, we excluded subjects with diabetic relatives in their medical history. Moreover, PCr/Pi ratio revealed higher values in normal-weight as compared with low-weight subjects, whereas there was no difference in this parameter between normal-weight and obese subjects. However, the underlying mechanism for our results in case of muscular high-energy phosphate content is obscure and remains to be clarified in further studies. As normal-weight subjects overall showed the highest content in ATP, PCr, PCr/Pi ratio, and ATP/Pi ratio, one could only speculate that muscular high-energy phosphate content may be within an optimized range in this group. Overall, our data confirm our earlier results of a divergent energetic response of the brain and muscle on hypoglycemia. Moreover, we show a distinct inverse relationship between cerebral high-energy phosphate content and body mass in healthy male subjects. This finding indicates that the brain's energy content per se may have a central function in the interplay between appetite regulation, food intake, and overall energy turnover, and thereby may underlie the development of disturbances in body-weight regulation such as obesity and the metabolic syndrome.
Acknowledgments
The authors thank Kai-Uwe Duysen for organizational work and invaluable assistance during the glucose-clamp experiments.
The authors declare no conflict of interest.
References
- Bachert-Baumann P, Ermark F, Zabel HJ, Sauter R, Semmler W, Lorenz WJ. In vivo nuclear Overhauser effect in 31P-(1H) double-resonance experiments in a 1.5-T whole-body MR system. Magn Reson Med. 1990;15:165–172. doi: 10.1002/mrm.1910150119. [DOI] [PubMed] [Google Scholar]
- Barker PB, Butterworth EJ, Boska MD, Nelson J, Welch KM. Magnesium and pH imaging of the human brain at 3.0 Tesla. Magn Reson Med. 1999;41:400–406. doi: 10.1002/(sici)1522-2594(199902)41:2<400::aid-mrm26>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Barker PB, Golay X, Artemov D, Ouwerkerk R, Smith MA, Shaka AJ. Broadband proton decoupling for in vivo brain spectroscopy in humans. Magn Reson Med. 2001;45:226–232. doi: 10.1002/1522-2594(200102)45:2<226::aid-mrm1031>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Bischof MG, Mlynarik V, Brehm A, Bernroider E, Krssak M, Bauer E, Madl C, Bayerle-Eder M, Waldhausl W, Roden M. Brain energy metabolism during hypoglycaemia in healthy and type 1 diabetic subjects. Diabetologia. 2004;47:648–651. doi: 10.1007/s00125-004-1362-2. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body-weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Cahill GF., Jr Starvation in man. N Engl J Med. 1970;282:668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- Gao Q, Horvath TL. Neuronal control of energy homeostasis. FEBS Lett. 2008;582:132–141. doi: 10.1016/j.febslet.2007.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Lam TK, Obici S, Rossetti L. Molecular disruption of hypothalamic nutrient sensing induces obesity. Nat Neurosci. 2006;9:227–233. doi: 10.1038/nn1626. [DOI] [PubMed] [Google Scholar]
- Hilsted J, Jensen KE, Thomsen C, Larsen S, Henriksen O. Maintenance of high-energy brain phosphorous compounds during insulin-induced hypoglycemia in men. 31P nuclear magnetic resonance spectroscopy study. Diabetes. 1988;37:760–762. doi: 10.2337/diab.37.6.760. [DOI] [PubMed] [Google Scholar]
- Horvath TL. The hardship of obesity: a soft-wired hypothalamus. Nat Neurosci. 2005;8:561–565. doi: 10.1038/nn1453. [DOI] [PubMed] [Google Scholar]
- Iosifescu DV, Renshaw PE. 31P-magnetic resonance spectroscopy and thyroid hormones in major depressive disorder: toward a bioenergetic mechanism in depression. Harv Rev Psychiatry. 2003;11:51–63. doi: 10.1080/10673220303959. [DOI] [PubMed] [Google Scholar]
- Koch L, Wunderlich FT, Seibler J, Konner AC, Hampel B, Irlenbusch S, Brabant G, Kahn CR, Schwenk F, Bruning JC. Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest. 2008;118:2132–2147. doi: 10.1172/JCI31073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Routh VH. Brain glucose sensing and body energy homeostasis: role in obesity and diabetes. Am J Physiol. 1999;276:R1223–R1231. doi: 10.1152/ajpregu.1999.276.5.R1223. [DOI] [PubMed] [Google Scholar]
- Ludwig AK, Goharian LG, Dietze T, Tauchert S, Rudolf S, Diedrich K, Schweiger U, Oltmanns KM. Impact of glycemic variations on the regulation of androgen metabolism in obese women with polycystic ovary syndrome. Fertil Steril. 2009;92:271–276. doi: 10.1016/j.fertnstert.2008.05.052. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de BR, Graveron-Demilly D. Java-based graphical user interface for the MRUI quantitation package. MAGMA. 2001;12:141–152. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- Oltmanns KM, Melchert UH, Scholand-Engler HG, Howitz MC, Schultes B, Schweiger U, Hohagen F, Born J, Peters A, Pellerin L. Differential energetic response of brain vs. skeletal muscle upon glycemic variations in healthy humans. Am J Physiol Regul Integr Comp Physiol. 2008;294:R12–R16. doi: 10.1152/ajpregu.00093.2007. [DOI] [PubMed] [Google Scholar]
- Oltmanns KM, Peters A, Kern W, Fehm HL, Born J, Schultes B. Preserved inhibitory effect of recurrent hypoglycaemia on the male gonadotrophic axis. Clin Endocrinol (Oxf) 2005;62:217–222. doi: 10.1111/j.1365-2265.2005.02203.x. [DOI] [PubMed] [Google Scholar]
- Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- Peters A, Pellerin L, Dallman MF, Oltmanns KM, Schweiger U, Born J, Fehm HL. Causes of obesity: looking beyond the hypothalamus. Prog Neurobiol. 2007;81:61–88. doi: 10.1016/j.pneurobio.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Peters A, Schweiger U, Pellerin L, Hubold C, Oltmanns KM, Conrad M, Schultes B, Born J, Fehm HL. The selfish brain: competition for energy resources. Neurosci Biobehav Rev. 2004;28:143–180. doi: 10.1016/j.neubiorev.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Shulman GI. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med. 2005;2:e233. doi: 10.1371/journal.pmed.0020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- Prichard JW, Alger JR, Behar KL, Petroff OA, Shulman RG. Cerebral metabolic studies in vivo by 31P NMR. Proc Natl Acad Sci USA. 1983;80:2748–2751. doi: 10.1073/pnas.80.9.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rango M, Bozzali M, Prelle A, Scarlato G, Bresolin N. Brain activation in normal subjects and in patients affected by mitochondrial disease without clinical central nervous system involvement: a phosphorus magnetic resonance spectroscopy study. J Cereb Blood Flow Metab. 2001;21:85–91. doi: 10.1097/00004647-200101000-00011. [DOI] [PubMed] [Google Scholar]
- Sandoval D, Cota D, Seeley RJ. The integrative role of CNS fuel-sensing mechanisms in energy balance and glucose regulation. Annu Rev Physiol. 2008;70:513–535. doi: 10.1146/annurev.physiol.70.120806.095256. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Porte D., Jr Diabetes, obesity, and the brain. Science. 2005;307:375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci. 2000;3:757–758. doi: 10.1038/77660. [DOI] [PubMed] [Google Scholar]
- Vanhamme L, van den BA, Van HS. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129:35–43. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]