Abstract
Cerebral infarct volume is typically smaller in premenopausal females than in age-matched males after ischemic stroke, but the underlying mechanisms are poorly understood. In this study we provide evidence in mice that this gender difference only occurs when the ischemic brain is reperfused. The limited tissue salvage achieved by reperfusion in male mice is associated with increased expression of proinflammatory proteins, including cyclooxygenase-2 (Cox-2), Nox2, and vascular cell adhesion molecule-1 (VCAM-1), and infiltration of Nox2-containing T lymphocytes into the infarcted brain, whereas such changes are minimal in female mice after ischemia–reperfusion (I-R). Infarct volume after I-R was no greater at 72 h than at 24 h in either gender. Infarct development was Nox2 dependent in male but not in female mice, and Nox2 within the infarct was predominantly localized in T lymphocytes. Stroke resulted in an ∼15-fold increase in Nox2-dependent superoxide production by circulating, but not spleen-derived, T lymphocytes in male mice, and this was ∼sevenfold greater than in female mice. These circulating immune cells may thus represent a major and previously unrecognized source of superoxide in the acutely ischemic and reperfused brain of males (and potentially in postmenopausal females). Our findings provide novel insights into mechanisms that could be therapeutically targeted in acute ischemic stroke patients who receive thrombolysis therapy to induce cerebral reperfusion.
Keywords: gender, ischemia, lymphocytes, NADPH oxidase, reactive oxygen species
Introduction
Studies of experimental stroke commonly use models of cerebral ischemia–reperfusion (I-R; i.e., transient ischemia), and report that stroke severity is lower in females than males (Alkayed et al, 1998; Park et al, 2006; Zhang et al, 1998). Studies assessing the effect of gender on outcome after cerebral ischemia with no reperfusion (I-NR; i.e., permanent ischemia) are surprisingly very few, and they report conflicting findings of whether females undergo less ischemic damage (Cai et al, 1998; Carswell et al, 1999; Loihl et al, 1999). No previous study has assessed the effect of gender on outcome after both I-R and I-NR.
The smaller cerebral infarct size in females after I-R is known to be estrogen dependent (Alkayed et al, 1998; Park et al, 2006; Zhang et al, 1998). Antiinflammatory effects of estrogen are believed to provide females protection from postischemic cerebral injury through reductions in leukocyte adhesion (Santizo et al, 2000), nuclear factor-κB activation (Wen et al, 2004), and inducible nitric oxide synthase (iNOS) expression (Park et al, 2006; Wen et al, 2004). Increased expression of the key proinflammatory proteins, cyclooxygenase-2 (Cox-2; Planas et al, 1995), Nox2 (Kusaka et al, 2004), and vascular cell adhesion molecule-1 (VCAM-1; Justicia et al, 2006), have been reported to occur in male rodents after I-R, but expression of these proteins have not yet been examined in female rodents after I-R. It is also conceivable that the brain is better perfused in female rodents during ischemia and/or reperfusion, because estrogen is known to be a cerebral vasodilator (Duckles and Krause, 2007). Because the salvage of brain tissue after ischemic reperfusion may, on the one hand, be limited by proinflammatory mechanisms occurring during reperfusion, but on the other hand be improved by restoration of blood flow to the ischemic tissue, it is important to better identify the mechanisms involved and to clarify whether female and male mice are equally vulnerable to reperfusion-induced damage.
Recent studies suggest that circulating T lymphocytes contribute to the brain damage caused by cerebral I-R (Hurn et al, 2007; Yilmaz et al, 2006). T lymphocytes are believed to enter the brain parenchyma within 24 h after stroke (Jander et al, 1995), and to produce damage through the generation of proinflammatory mediators (Arumugam et al, 2005). T lymphocytes express a functional NADPH (nicotinamide adenine dinucleotide phosphate) oxidase containing Nox2 (previously named gp91phox) (Jackson et al, 2004), and superoxide produced by this isoform of NADPH oxidase is now established to have a detrimental role in the brain after stroke (Jackman et al, 2009; Kahles et al, 2007; Walder et al, 1997), which seems to involve an effect of circulating leukocytes (Walder et al, 1997). Molecular mechanisms mediating the postischemic brain damage caused by infiltrating T lymphocytes are largely unknown, including whether their generation of Nox2-derived superoxide is altered after stroke in male or female mice.
We therefore first tested the importance of reperfusion in the protection of female mice after ischemic stroke. Second, we analyzed whether expression of key proinflammatory proteins was correlated with the gender-related degree of salvage achieved by reperfusion. Third, we assessed the role of Nox2 expression in cerebral infarct volume after I-R in both genders. Last, we tested whether Nox2-derived superoxide generation by circulating T lymphocytes is altered after stroke in male or female mice.
Materials and methods
Animals
This study was conducted in accordance with National Health and Medical Research Council of Australia guidelines for the care and use of animals in research. A total of 241 mice were studied, consisting of 116 male (weight, 23.3±0.2 g) and 99 female (18.2±0.1 g) 6- to 8-week-old C57Bl6/J mice, and 15 male (22.6±0.9 g) and 11 female (17.5±0.3 g) 6- to 8-week-old Nox2-deficient (Nox2−/−) mice. Nox2−/− (AKA gp91phox−/−) mice were originally generated in the laboratory of Professor Mary Dinauer and bred at Ozgene (Bentley DC, WA, Australia). The mice had free access to water and food pellets before and after surgery. In all, 31 mice were excluded from the study, which occurred when during the surgical procedure to induce middle cerebral artery occlusion (MCAO): (1) a significant volume of blood (>0.2 mL) was lost (n=4); (2) the filament did not stay in place for the entire 30 mins of ischemia (n=2); (3) the occluding clamp was in place for 5 mins (n=1); or (4) they died before the specified time for killing (e.g., at 23.5-h reperfusion; n=24).
Focal Cerebral Ischemia
Mice of either gender were randomly assigned to study groups (e.g., sham, I-R, and I-NR) by an investigator not performing surgical procedures or data analysis. Moreover, the investigator performing the surgical procedure or data analysis (e.g., neurological assessment, infarct volume quantification, and immunohistochemistry) was usually (and wherever possible) masked to the gender or study group to which the animal or tissue belonged.
Focal cerebral ischemia was induced by transient or permanent intraluminal filament occlusion of the right MCA. Mice were anesthetized with ketamine–xylazine (80 and 10 mg/kg, respectively; intraperitoneal). Rectal temperature was monitored and maintained at 37.5°C±0.5°C throughout the procedure and until animals regained consciousness through an electronic temperature controller (Extech Equipment, Boronia, Victoria, Australia) linked to a heat lamp. Under a dissecting microscope (Leica MZ6, Wetzlar, Germany), the right carotid bifurcation was exposed through a ventral midline neck incision and was carefully dissected free from surrounding connective tissue. A branch of the external carotid artery was cauterized, and the external carotid artery (ECA) was ligated distal to the bifurcation of the common carotid artery (CCA) and cut, forming an ECA stump. The CCA and internal carotid artery (ICA) were carefully separated from the adjacent vagus nerve, the CCA was clamped, and tension applied to the ICA using a suture bridge. A small nick was made in the ECA stump, and a 6-0 nylon monofilament with silicone-coated tip (Doccol Co., Redlands, CA, USA) was inserted and advanced distally along the ICA (11 to 12 mm distal to the carotid bifurcation), causing MCAO at its junction with the circle of Willis. Severe (∼75%) reduction in regional cerebral blood flow (rCBF) was confirmed using transcranial laser-Doppler (Perimed, Järfälla, Sweden) in the area of cerebral cortex supplied by the MCA (∼2 mm posterior and ∼5 mm lateral to bregma). The filament was then tied in place, the CCA clamp was removed, and the suture bridge released. In the protocol for transient ischemia (i.e., I-R), occlusion was maintained for 30 mins, and the monofilament was then retracted to allow reperfusion for either 23.5 or 71.5 h. Reperfusion was confirmed by an immediate increase in rCBF, which reached the preischemic level within 5 mins. For permanent ischemia (i.e., I-NR), the monofilament was kept in place and the occlusion maintained for 24 h. Sham-operated mice were anesthetized and the right carotid bifurcation was exposed and dissected free from surrounding connective tissue but no filament was inserted. Regional CBF was recorded for 1 h after the induction of ischemia. The neck and head wounds were then closed, covered with betadine and spray dressing, and when the mice regained consciousness, they were then returned to their cages.
Neurological Assessment
After 24 or 72 h, neurological assessment was performed using the hanging wire test (Hattori et al, 2000), in which mice were suspended from a wire 30 cm high for up to 60 secs, and the average time of 3 trials with 5-min rest in between was recorded.
Evaluation of Cerebral Infarct Volume
Mice were then killed at 24 or 72 h by inhalation of CO2 and O2 (80:20), followed by decapitation. The brains were immediately removed, snap frozen in liquid nitrogen, and stored at −80°C. Evenly spread (separated by ∼420 μm) coronal sections (30-μm thick) were obtained spanning the infarct (which equated to ∼15 sections/brain in transient MCAO, and ∼20 sections/brain in permanent MCAO), thaw-mounted onto poly--lysine coated glass slides (0.1% poly--lysine in dH2O) and stained with thionin (0.1%) to delineate the infarct. Images of the sections were captured with a CCD camera (Cohu Inc., San Diego, CA, USA) mounted above a light box (Biotec-Fischer Colour Control 5000, Reiskirchin, Germany). Infarct volume was quantified using image analysis software (ImageJ, NIH, Bethesda, MD, USA), correcting for brain edema, according to the following formula: CIV=[LHA−(RHA−RIA)] × (thickness of section+distance between sections); in which CIV is corrected infarct volume, LHA is left hemisphere area, RHA is right hemisphere area, and RIA is right hemisphere infarct area (Tsuchiya et al, 2003; Xia et al, 2006). Edema volume was calculated using the formula: [RHA−LHA] × (thickness of section+distance between sections). Edema-corrected infarct volumes of individual brain sections were then added giving a three-dimensional approximation of the total infarct volume.
Protein Expression of Cox-2, Nox2, and VCAM-1
In separate mice, expression of the proinflammatory proteins, Cox-2, NADPH-oxidase catalytic subunit Nox2, and VCAM-1, were measured in homogenates of the ischemic (right) hemisphere using Western blotting. Anti-Cox-2 and anti-Nox2 mouse monoclonal antibodies were purchased from BD Biosciences (North Ryde, Australia). The anti-VCAM-1 mouse monoclonal antibody was purchased from Santa Cruz (Santa Cruz, CA, USA). Mice were killed and brains were removed and frozen as for ‘evaluation of cerebral infarct volume' (see above). Brains were cut into hemispheres, and the ischemic hemisphere was homogenized and prepared in Laemmli buffer (25% glycerin, 12.5% β-mercaptoethanol, 7.5% sodium docedyl sulfate, 25% 1 mol/L Tris-HCl pH 8.0, and 0.25 mg/mL bromophenol blue) in liquid nitrogen. Homogenates were sonicated, heated (at 37°C for 10 mins), and then centrifuged (15,700g for 10 mins at 4°C). The supernatant was removed and stored at −20°C and protein concentration was determined using the RCDC protein assay (Bio-Rad, Hercules, CA, USA). Equal quantities of protein were loaded onto 7.5% (Cox-2 and Nox2) or 10% (VCAM-1) polyacrylamide gels and transferred to a nitrocellulose membrane. Membranes were blocked in 5% skim milk for 1 h (room temperature) and then incubated overnight (4°C) with the appropriate primary antibody (1:500 for Cox-2, 1:1,000 for Nox2, 1:250 for VCAM-1, and 1:2,000 for β-actin) in 5% skim milk. Membranes were then incubated with a horseradish peroxidase-conjugated anti-mouse (Cox-2 and Nox2; purchased from ImmunoResearch, West Grove, PA, USA), anti-rat (VCAM-1; purchased from Santa Cruz) or anti-rabbit (β-actin; purchased from DakoCytomation, Glostrup, Denmark) immunoglobulin G (IgG) for 1 h (room temperature). Immunoreactive bands were detected using enhanced chemiluminescence (GE Healthcare, Waukesha, WI, USA) and quantified using a ChemiDoc XRS molecular imager (Bio-Rad). Relative intensities were normalized to intensity of corresponding bands for β-actin and, within a single gel, bands of samples from male sham or female sham mice were taken to be equal to 1, and all other bands were quantitated relative to their corresponding gender sham.
Localization of T Lymphocytes and Nox2
The localization of CD3+ cells (T lymphocytes) was performed using immunohistochemistry. Brains were removed, snap frozen in liquid nitrogen, and stored at −80°C. Multiple serial coronal sections of 30 μm were taken at the paraventricular nucleus (bregma −0.58 mm) and the mid hippocampus (bregma −1.82 mm) and thaw-mounted onto poly--lysine-coated glass slides (0.1% poly--lysine in dH2O). Tissue sections were fixed in acetone for 15 mins and washed in 0.5 mol/L tris-buffered saline (TBS, pH 8.4; 3 × 5 mins) before incubation in a humid chamber in 10% goat serum in TBS for 2 h to block nonspecific binding. Sections were then incubated for 1 h in primary rabbit polyclonal anti-CD3 IgG antibody (1:250; Abcam, Cambridge, MA, USA). Sections were washed in TBS (3 × 5 mins), blocked with a peroxidase blocking agent for 15 mins and stained using the DAKO EnVision+ system (DAKO). Sections were then incubated for 45 mins with a peroxidase-labeled polymer conjugated to goat anti-rabbit Igs, washed in TBS (3 × 5 mins), followed by incubation with diaminobenzidine for 5 mins. Sections were then washed in TBS (3 × 5 mins) and mounted in Aquatex (Merck; Darmstadt, Germany). Staining was analyzed using an Olympus light microscope (Hamburg, Germany) by two masked observers who counted CD3+ cells within five high-power ( × 200) fields within the infarct and periinfarct zones of the ipsilateral striatum and cortex. Infarct and periinfarct zones were verified from thionin-stained adjacent sections.
To determine whether CD3+ cells were colocalized with Nox2 protein in the infarct core of the striatum, acetone-fixed brain sections from sham and stroke animals were simultaneously incubated with two primary antibodies raised in different species. Tissue sections were incubated in a monoclonal antibody raised against mouse gp91phox (1:1,000; BD Biosciences) and a polyclonal antibody raised against rabbit CD3 (1:50, Abcam) overnight in a humid box. The next day, the tissues were washed in 0.01 mol/L phosphate-buffered saline (PBS, pH 7.4; 3 × 10 mins) to remove any excess antibody, and incubated in a fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (1:200) (Zymed Laboratories, South San Francisco, CA, USA) and Texas Red-labeled goat anti-rabbit IgG (1:200) for 3 to 4 h in a humid box. The sections were then washed in 0.01 mol/L PBS (3 × 10 mins) and cover slipped. Tissue-mounted slides were viewed and photographed on a Leica confocal scanning laser system. The appropriate control experiments were performed to ensure that no cross-reactivity occurred. This involved incubating brain sections after stroke with all of the possible combinations of primary and secondary antibodies. Our control experiments showed that FITC fluorescence was not visible using the Texas Red filter set and vice versa. Thus, the Nox2 and CD3+ immunoreactivity was concluded to be specific.
Isolation of Leukocytes from Blood and Spleen
Mice were killed at 24 h by isofluorane inhalation and exsanguination. A midline abdominal incision was made and blood was withdrawn from the inferior vena cava into a 1 mL syringe containing 0.1 mL Clexane (400 U/mL), and was transferred to an eppendorf tube. The spleen was then removed, and placed in media (RPMI 1640 + 10% fetal calf serum (FCS)). Single cell suspensions were prepared and red cells were lysed with NH4Cl buffer (2 × 5 mins at 37°C).
Analysis of Cell Populations
Fluorescence flow cytometry analyses were performed to determine the numbers of T lymphocytes (CD3+) in the blood and spleen. Cells (∼1 × 106) were labeled using optimal dilutions of FITC-conjugated anti-CD3 (clone 145-2C11; BD Biosciences, Pharmingen, San Diego, CA, USA). Flow cytometry was performed using a BD FACS Canto II flow cytometer and CELLQuest 8.0 software (BD Biosciences). Data were analyzed using FlowJo (V8.8.2, Tree Star, Inc., Ashland, OR, USA).
Measurement of Superoxide Production by T Lymphocytes
After isolation of leukocytes, a purified suspension of T cells was obtained using the Dynal Mouse T Cell Negative Isolation Kit (Invitrogen, Carlsbad, CA, USA). Fluorescence-activated cell sorting analysis was used to confirm purity of CD3+ T lymphocytes in samples. Basal and phorbol 12,13-dibutyrate (PDB; 1 μmol/L)-stimulated superoxide production by T lymphocytes from blood and spleens of mice after I-R was measured by 100 μmol/L L-012-enhanced chemiluminescence. In all experiments, background counts were subtracted and superoxide production was normalized to T-lymphocyte numbers.
Statistical Analysis
All data are presented as mean±s.e.. Statistical analyses were performed using GraphPad Prism version 5 (GraphPad Software Inc., San Diego, CA, USA). Between-group comparisons were compared using one-way analysis of variance (ANOVA) or Student's unpaired t test, as appropriate. Mortality data were analyzed using a log-rank test. Group numbers are shown in parentheses. Statistical significance was accepted when P<0.05.
Results
Effect of Reperfusion and Gender on Cerebral Infarct Volume
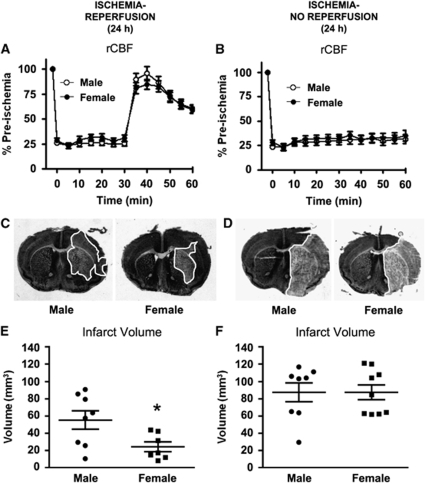
First, cerebral I-R was produced by 30-min MCAO and 23.5-h reperfusion. Regional cerebral blood flow decreased to ∼25% of the preischemia level for the duration of MCAO (Figure 1A). Regional CBF then increased initially to ∼100% on removal of the monofilament, and then stabilized at ∼60% after 30 mins of reperfusion (Figure 1A). Second, in mice subjected to permanent MCAO (i.e., no reperfusion; I-NR), rCBF remained at 25% to 30% of the preischemia level for 60 mins (Figure 1B). No differences in rCBF profiles were observed between male and female mice in either protocol. At 24 h, mortality rates were similar in male and female mice after I-R (13% in male and 14% in female mice) or I-NR (15% in both genders). Similarly, hanging wire times did not differ between genders after I-R (males=22±5 secs and females=24±7 secs), I-NR (males=25±5 secs and females=19±6 secs), or after sham surgery (males=53±3 secs and females=55±2 secs).
Figure 1.
Regional cerebral blood flow (rCBF) and infarct volume at 24 h in wild-type mice. rCBF was recorded during and after 30-min MCAO (A) with reperfusion (n=39 male and 28 female mice; I-R) or (B) without reperfusion (n=25 male and 25 female mice; I-NR). Representative coronal brain sections are shown from male and female mice after I-R (C) or I-NR (D) with the infarct area outlined in white. Cerebral infarct volumes measured in male and female mice after (E) I-R (n= 8 male and 7 female mice; *P<0.05, unpaired t-test) or (F) I-NR (n=8 male and 9 female mice) are also shown. Infarct volume data are presented as mean±s.e.m.
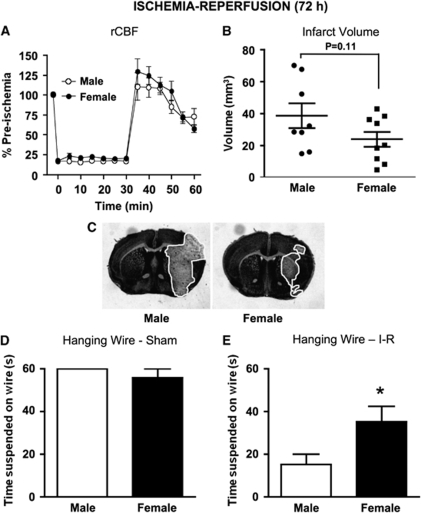
In addition, 72 h cerebral I-R was produced by 30-min MCAO and 71.5-h reperfusion. No differences in rCBF profiles were observed between males and female mice (Figure 2A). Mortality rates in the 72 h I-R studies were similar for the 24 h I-R protocol (17% in male and 8% in female mice; P=0.55). Hanging wire times of female mice were significantly longer than male mice after 72 h I-R (males=15±5 secs and females=35±7 secs; P<0.05; Figure 2E), whereas no gender difference was observed after sham surgery (males=60±0 secs and females=56±4 secs; Figure 2D).
Figure 2.
Regional cerebral blood flow (rCBF), infarct volume, and hanging wire at 72 h in wild-type mice. (A) rCBF was recorded during and after 30-min MCAO (n=8 male and 9 female mice), and (B) cerebral infarct volumes were measured in male and female mice at 72 h after I-R (n=8 male and 9 female mice; P=0.11, unpaired t-test). Representative coronal brain sections are shown from male and female mice after 72 h I-R (C) with the infarct area outlined in white. Hanging wire data from male and female mice at 72 h after (D) sham surgery (n=3 male and 3 female mice) and I-R (E) are also shown (*P<0.05, unpaired t-test). Data are presented as mean±s.e.m.
Representative coronal sections of 24 h infarcted brain are shown in Figures 1C and 1D. At 24 h after I-R, significantly larger infarct volumes were produced in male compared with female mice (P<0.05; Figure 1E). After 24 h of I-NR, infarct volumes were larger than in mice subjected to I-R (Figure 1F versus Figure 1E), and there was no gender difference in infarct volume produced in these mice (Figure 1F). Edema values were: I-R: males=34±6 mm3, females=30±5 mm3; and I-NR: males=63±6 mm3, females=54±5 mm3.
At 72 h after I-R, mean infarct volume was ∼70% larger in male (40±8 mm3, n=8) versus female mice (24±5 mm3, n=9; Figures 2B and 2C), although this difference did not reach statistical significance (P=0.11, unpaired t-test). Importantly, infarct volumes at 72 h were not larger in either gender than at 24 h (i.e., males=55±11 mm3 and females=24±6 mm3; see Figure 1E for comparison). Edema values after 72 h I-R also tended to be larger in male compared with female mice (males=32±12 mm3 and females=14±4 mm3; P=0.14).
Effect of Gender on Proinflammatory Protein Expression After Ischemia–Reperfusion
To analyze molecular mechanisms underlying the gender difference in infarct volume that occurred because of reperfusion, we examined expression of several proinflammatory proteins, including Cox-2, the NADPH oxidase catalytic subunit Nox2, and VCAM-1, in the ischemic hemisphere after I-R. These proteins were each expressed at significantly greater levels in male mice after I-R compared with sham-operated male controls (Figures 3B, 3E, and 3H). In contrast, compared with sham-operated female mice, none of these proteins were expressed at higher levels in female mice after I-R (Figures 3C, 3F and 3I).
Figure 3.
Proinflammatory protein expression. Representative Western blots and densitometric analyses of immunoreactive band intensities showing expression of (A–C) cyclo-oxygenase-2 (Cox-2), (D–F) NADPH-oxidase catalytic subunit Nox2, and (G–I) the vascular cell adhesion molecule-1 (VCAM-1) in ipsilateral hemispheres of male and female mice subjected to sham surgery or ischemia–reperfusion (I-R). Values are expressed as relative intensity normalized to β-actin intensity, and then normalized relative to gender-matched control, and are presented as mean±s.e.m. (*P<0.05 versus matched sham group, unpaired t-test; n=6 to 11).
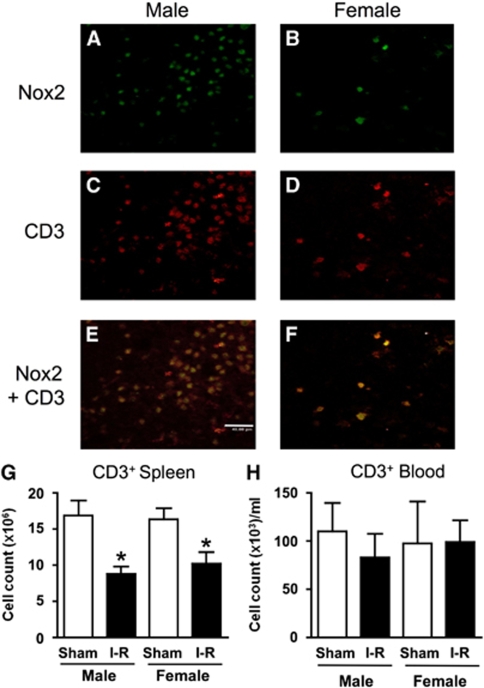
Localization of Nox2 and T Lymphocytes
After 24 h I-R, CD3+ cells (i.e., T lymphocytes) were found to be present within cerebral infarct and also in periinfarct areas at a frequency that was ∼7-fold greater in male than in female mice (70±21 versus 10±5 cells per high power field; n=15 fields from 3 mice per group; P=0.01). Immunofluorescence studies confirmed the presence of Nox2-containing cells (Figures 4A and 4B) and also CD3+ cells (Figures 4C and 4D) within the striatal infarct core of male and female mice at 24 h after I-R. When these images were overlayed, it was apparent that, at 24 h in the infarcted core tissue of both genders, Nox2 was frequently colocalized with CD3 (e.g., see Figures 4E and 4F). The frequency of Nox2-expressing cells in the infarct core that also expressed CD3 was similarly high in male (95% to 99%, n=3) and female mice (94% to 100%, n=3) and overall was estimated to be 97.5%±1.1% (n=6).
Figure 4.
T-lymphocyte localization and counts. Representative photomicrographs showing immunofluorescent staining within the core of striatal infarcts of a male and a female mouse after ischemia–reperfusion (I-R). Approximately sevenfold more Nox2+ and CD3+ cells (i.e., T lymphocytes) were observed in infarcted brains of male (A and C, respectively) than female mice (B and D, respectively). Overlayed images revealed substantial colocalization of Nox2 and CD3 (E and F). The scale bar represents 40 μm. Flow cytometric analyses are shown for CD3+ cells isolated from the spleen (G) and the blood (H) after sham surgery and I-R (*P<0.05 versus matched sham group, unpaired t-test with Welch's correction; n=5 to 9).
Effect of Ischemia–Reperfusion on T-Lymphocyte Levels
There were significantly fewer T lymphocytes in the spleen at 24 h after I-R in both male and female mice in comparison to sham-operated mice (P<0.05, Figure 4G). However, circulating levels of T lymphocytes were not significantly altered at 24 h after I-R in either male or female mice (Figure 4H).
Effect of Ischemia–Reperfusion on Generation of Nox2-Derived Superoxide by T Lymphocytes
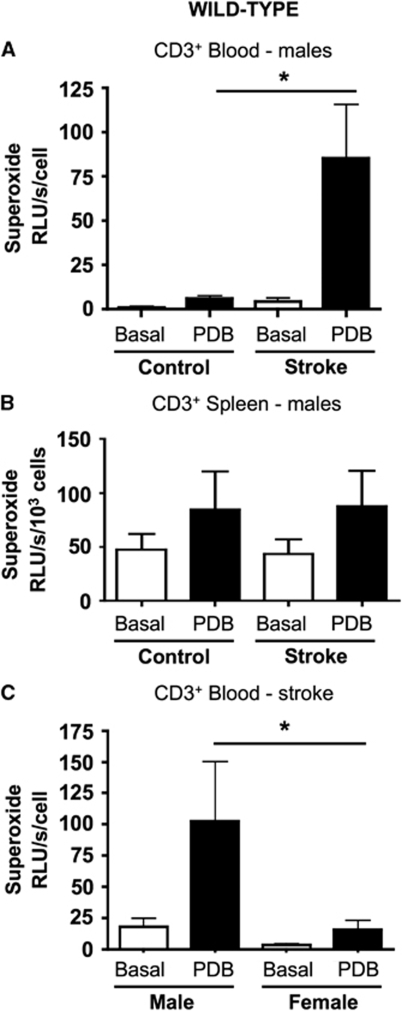
Superoxide release from T lymphocytes isolated from the blood of control male wild-type mice was slightly augmented (by ∼fourfold above basal) by incubation with the Nox2 activator, PDB (Figure 5A). However, PDB-stimulated superoxide release was profoundly greater (a further ∼15-fold) from T lymphocytes isolated from the blood of male wild-type mice at 24 h after I-R (Figure 5A). Moreover, in separate experiments we found that superoxide production after I-R was markedly greater (∼sevenfold) in circulating T lymphocytes from male versus female mice (Figure 5C). The effect of PDB to stimulate superoxide release from circulating T lymphocytes was subsequently found to be Nox2 dependent (see Figure 6D). After stroke, PDB-induced superoxide release was up to ∼1,000-fold higher in T lymphocytes from blood versus spleen (see Figures 5A and 5B). However, neither stroke nor PDB had any significant effect on superoxide release from spleen-derived T lymphocytes from wild-type male mice (Figure 5B).
Figure 5.
Superoxide generation by T lymphocytes. Basal and PDB-stimulated superoxide generation by T lymphocytes from control wild-type mice and from mice at 24 h after I-R. Data are shown for cells isolated from blood (A) and spleen (B) of male mice. Note the different y axis scales used in A and B. *P<0.05, ANOVA and Bonferroni's post hoc test; n=5 per group. (C) Superoxide generation by T lymphocytes isolated from blood of male and female wild-type mice at 24 h after I-R. *P<0.05, ANOVA and Bonferroni's post hoc test; n=6 per group.
Figure 6.
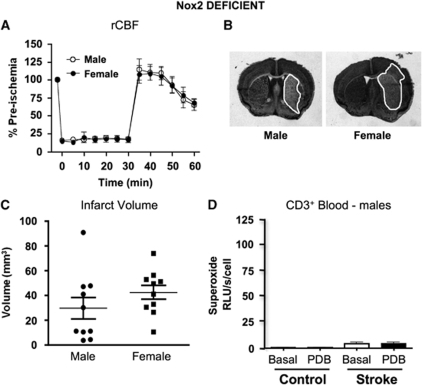
Stroke outcome and superoxide generation in Nox2-deficient mice. (A) Regional cerebral blood flow (rCBF) recorded in Nox2-deficient mice during and after 30-min MCAO and 23.5-h reperfusion (n=10 male and 10 female mice). Representative coronal brain sections are shown from a male and a female Nox2-deficient mouse after I-R (B) with the infarct area outlined in white. (C) Cerebral infarct volumes measured in male and female mice after I-R (n= 10 male and 10 female mice).. (D) Basal and PDB-stimulated superoxide generation by T lymphocytes isolated from the blood of male Nox2-deficient mice (control, n=3; I-R, n=5).
Effect of Gender on Cerebral Infarct Volume in Nox2-Deficient Mice
There was no difference in rCBF profile between genders of Nox2-deficient mice subjected to 30-min MCAO followed by 23.5-h reperfusion (Figure 6A). There was no mortality in either group (both n=10). Hanging wire times were not statistically different between genders, although male mice tended to hold on longer (males=25±5 secs and females=16±6 secs; P=0.22). There was also no significant difference between genders of Nox2-deficient mice in the infarct volume produced by I-R (Figures 6B and 6C), although there was a tendency for female mice to have larger infarcts (P=0.24). Edema volumes were similar between genders (males=23±6 mm3 and females=31±5 mm3; P=0.32). Neither PDB nor stroke had any significant effect on superoxide release from T lymphocytes isolated from the blood of Nox2-deficient mice (Figure 6D).
Discussion
This study has primarily assessed the effect of gender on the infarct volume that develops within 24 h after cerebral ischemia in adult mice with or without reperfusion, and on the associated molecular and cellular changes. We have established for the first time that the larger infarct volume present in male mice after I-R is in fact dependent on both reperfusion and Nox2. Importantly, mean infarct volume was not larger in either gender at 72 h than at 24 h after I-R, and neurological function remained significantly greater in female versus male mice at 72 h. Furthermore, in the ischemic hemisphere of male mice after I-R we found an increased expression of the proinflammatory proteins, Cox-2, Nox2, and VCAM-1, and an infiltration of T lymphocytes, whereas there was little or no change in these parameters in female mice. A reduction in T-lymphocyte levels in the spleen occurred similarly in both genders, but there was no significant change in circulating T-lymphocyte levels at 24 h. Interestingly, Nox2 protein was largely colocalized with T lymphocytes (CD3+ cells) within the infarct core after I-R. Moreover, we found circulating T lymphocytes to be a potentially important source of Nox2-derived superoxide after cerebral I-R especially in male mice, with Nox2-dependent superoxide production by these cells ∼15-fold greater at 24 h after I-R versus controls. Superoxide production by circulating T cells after stroke was substantially greater in male than in female mice. Our findings are compatible with the concept that salvage of brain tissue during postischemic reperfusion is limited in male mice at least in part due to release of Nox2-derived superoxide from T lymphocytes infiltrating the inflamed brain after stroke.
This is the first study to compare the effects of both gender and reperfusion on cerebral infarct volume produced after focal cerebral ischemia. The data clearly indicate that although smaller infarcts develop in female mice within 24 h after I-R, and that this difference is maintained at 72 h, there is no such gender difference in outcome if reperfusion is not instituted. It is clear that early (0.5 h) reperfusion leads to a reduction in infarct volume in both genders, but the degree of salvage is equivalent to only ∼35% in male mice whereas it is ∼75% in female mice. The smaller infarct volume in female mice after I-R is thought to be either because of the antiinflammatory effects of estrogen (Park et al, 2006; Santizo et al, 2000; Wen et al, 2004) or its vasodilatory effects (Duckles and Krause, 2007). However, in our study, the neuroprotection observed in female mice is less likely to be due to different reductions in regional cerebral blood flow, because both genders were exposed to a similar relative ischemic insult and underwent a similar relative level of reperfusion. Importantly, our data indicate that protection against infarct development in female mice is maintained for at least 72 h and only applies if the ischemic brain is reperfused.
Increased expression of Cox-2 (Planas et al, 1995), Nox2 (Kusaka et al, 2004), and VCAM-1 (Justicia et al, 2006) have been reported to occur in the brain after I-R in male mice and rats. Moreover, the inhibition or genetic deletion of Cox-2 and Nox2 confers protection after focal cerebral I-R (Iadecola et al, 2001; Jackman et al, 2009; Kahles et al, 2007; Nogawa et al, 1997; Walder et al, 1997), indicating their detrimental roles after stroke in male mice. We confirmed that protein expression of Cox-2, Nox2, and VCAM-1 are all increased in the brains of male mice after cerebral I-R, and to our knowledge, for the first time, these key proinflammatory molecules were also examined after cerebral I-R in female mice. In contrast to male mice, we found no significant change in expression of these proteins in female mice after I-R, consistent with the smaller infarct volume and with a protective, antiinflammatory action of estrogen.
To better understand the inflammatory consequences of stroke to the brain, we were interested to explore further our finding of increased VCAM-1 expression. VCAM-1 promotes adhesion of leukocytes, including lymphocytes, and is strongly expressed on activated vascular endothelial cells in response to pro-inflammatory cytokines (Marui et al, 1993; Stins et al, 1997), oxidative stress (Marui et al, 1993), and after I-R (Justicia et al, 2006). VCAM-1 expression is critical for infiltration of T lymphocytes into the brain (Baron et al, 1993; Engelhardt et al, 1995). Recent studies in T-lymphocyte-deficient mice have revealed that these cells of the adaptive immune system are surprisingly important contributors to the acute brain infarction after cerebral I-R (Hurn et al, 2007; Yilmaz et al, 2006). However, the precise actions of T lymphocytes in the brain after stroke are not well understood and are likely to be complex and somewhat specific for the different T-cell subpopulations. After migration into the ischemic brain, certain subsets of T lymphocytes may either directly elicit cell necrosis (i.e., CD8+), or release proinflammatory cytokines and chemokines that propagate the cellular immune response by activating resident cells and upregulating the expression of adhesion molecules, thus promoting further brain injury (i.e., CD4+) (Arumugam et al, 2005). In contrast, the regulatory T-lymphocyte subpopulation (CD4+CD25+Foxp3+) was very recently found to have a protective role in the brain after stroke, which includes secretion of the antiinflammatory cytokine interleukin-10 (Liesz et al, 2009).
Using immunohistochemistry and immunofluorescence we documented the presence of T lymphocytes throughout the infarcted male mouse brain after I-R, as previously reported in male rats (Jander et al, 1995). We noted that there were ∼sevenfold more T lymphocytes in infarct and periinfarct brain areas of male than female mice, consistent with the gender-dependent expression of proinflammatory proteins after stroke, including the T-cell adhesion molecule VCAM-1. We found little or no evidence of T lymphocytes in the brains of sham-operated mice or within the core of ischemic, but non-reperfused infarcts (data not shown), indicating that these cells do not normally reside in the brain and that their infiltration into the infarct core occurred because of postischemic reperfusion.
T lymphocytes express a functional Nox2-containing NADPH oxidase (Jackson et al, 2004), and interestingly, superoxide generated by the activity of this enzyme in T lymphocytes was recently reported to contribute to angiotensin II-induced hypertension (Guzik et al, 2007). We and others have found that Nox2-containing NADPH oxidase contributes to infarct development in the male brain after stroke (Jackman et al, 2009; Kahles et al, 2007; Walder et al, 1997). The cellular source(s) of the enzyme responsible for this brain damage is not well defined but may include circulating, bone-marrow-derived cells (Walder et al, 1997). Having confirmed by Western blotting that Nox2 expression was increased in male brains after I-R, we next explored whether Nox2 in infiltrating T lymphocytes might be relevant to postischemic changes in the brain. Using immunofluorescence we found that, at least in the infarct core within the striatum, Nox2 was mostly localized in CD3+ cells (i.e., infiltrated T lymphocytes), suggesting that T lymphocytes may be an important source of Nox2-derived superoxide in the infarcted brain after I-R. We cannot exclude the possibility that other cell types also contributed to the increased Nox2 expression but our observations suggest that in mice, T lymphocytes may be an important source of Nox2 within brain infarcts after 24 h of I-R (see below for further discussion of the role of various Nox2-containing circulating cells).
We next determined whether superoxide production could be elicited in isolated T lymphocytes in response to a stimulus of Nox2-containing NADPH oxidase (i.e., PDB), and whether a previous (24 h) stroke had any effect on such release. We thus used L-012-enhanced chemiluminescence to detect superoxide release from T lymphocytes isolated from the blood and spleens of control and poststroke mice. The most profound finding was the ∼15-fold increase in superoxide generation from T lymphocytes isolated from the blood of poststroke male versus control mice. This increase in superoxide production by circulating T lymphocytes after I-R also occurred in cells from female mice, but to a much lesser degree. Importantly, PDB did not elicit superoxide production from T lymphocytes isolated from control or poststroke Nox2-deficient mice, confirming that the source of superoxide generated in wild-type mice was a Nox2-containing NADPH oxidase. Furthermore, we found that neither PDB nor stroke had any effect on superoxide production by T lymphocytes isolated from the spleen, suggesting that only when these cells reach the circulation are they able to generate much larger amounts of Nox2-derived superoxide, and that this ability increases markedly after stroke. Such findings are consistent with the possibility of an acute detrimental effect in brain of infiltrating T lymphocytes after I-R, especially in male mice. Further studies are required to identify the precise stimuli and molecular signaling leading to increased Nox2-derived superoxide generation by circulating T lymphocytes after I-R.
No previous study has examined the role of Nox2 in female mice after stroke. Unlike in wild-type mice, we found that cerebral infarct volume after I-R was not smaller in female versus male Nox2-deficient mice. We speculate that the salvage of ischemic brain by reperfusion is limited by inflammation, including Nox2-derived superoxide generated by infiltrating T lymphocytes. Moreover, we further speculate that the reduced infarct volume and infiltration of Nox2-containing T lymphocytes in female mice reflects an inhibitory effect(s) of estrogen on these processes. It is unclear from the present data what role, if any, Nox2 has in female mice after stroke. Although not statistically significant, the tendency for a worsened stroke outcome in female mice lacking Nox2 raises the intriguing possibility that this protein could in some way be beneficial in female mice after cerebral I-R.
It might be suggested that neutrophils and monocytes–macrophages—blood cells involved in innate immunity that are well known to express Nox2 (Selemidis et al, 2008)—are more likely candidates than T lymphocytes as sources of oxidative and inflammatory damage after cerebral I-R. However, there is evidence against a detrimental role of neutrophils in the brain after acute stroke in that neutrophil depletion does not reduce infarct volume (Beray-Berthat et al, 2003; Harris et al, 2005; Yilmaz et al, 2006). Neutrophils instead seem to enter the brain because of damage rather than being a cause of it (Emerich et al, 2002), and this does not occur to a significant degree for at least 3 days after stroke (Gelderblom et al, 2009). We did detect a reduction in both circulating and splenic monocytes and neutrophils after I-R, although no gender difference was present (data not shown). Although we cannot completely exclude a role for neutrophils or circulating monocytes in the poststroke Nox2-dependent brain damage, our data indicate that at 24 h Nox2 seems to be predominantly colocalized with T lymphocytes at least in the infarct core, and Nox2-dependent superoxide production is markedly augmented in these cells present in the circulation. It is also possible that activated microglia could be Nox2 positive in the brain after I-R, including outside of the infarct core. However, in this study, our colocalization studies were only performed in regions of the striatal infarct core and therefore might not be representative of cell types expressing Nox2 around and beyond the infarct. Overall, we suggest that a possible scenario is that the damaging Nox2-derived superoxide production in the male brain in the first 24 h after cerebral I-R originates to some degree from T lymphocytes that infiltrate the brain. Furthermore, because Nox2-deficient female mice do not have a smaller infarct volume than Nox2-deficient male mice after cerebral I-R, Nox2-derived superoxide generated by infiltrating T lymphocytes could account for the limited salvage achieved by reperfusion in male versus female wild-type mice.
In this study we have analyzed the effect of a 30-min ischemic duration that produces a moderate infarct volume. Further studies will be necessary to assess whether similar findings are evident after other clinically relevant ischemic durations that are either shorter (e.g., in transient ischemic attack patients) or longer (e.g., in patients treated with recombinant tissue plasminogen activator (rt-PA) after up to 4.5 h ischemia) than 30 mins.
In summary, our study provides the first evidence that the smaller infarct volume that develops in adult female versus male mice after cerebral I-R is reperfusion dependent, and moreover, this gender difference is dependent on Nox2 expression in male mice. Our data suggest that the salvage obtained by reperfusion may be greater in female mice because of limited expression of proinflammatory proteins, including VCAM-1, resulting in markedly less damage caused by superoxide from infiltrating Nox2-containing T lymphocytes. These circulating immune cells could represent a major source of superoxide in the ischemic and reperfused brain. Viable new therapies for acute stroke are desperately needed. Our findings raise the possibility that short-term therapies to reduce T lymphocyte infiltration into the brain after transient ischemic attack, or in acute ischemic stroke patients who receive rt-PA, might be useful for reducing reperfusion injury not only in younger males, but potentially in aged patients of both genders.
Acknowledgments
This research was supported by a Project Grant (491133) from the National Health and Medical Research Council of Australia (NHMRC). VHB was supported by a Monash Postgraduate Research Scholarship. CGS is an NHMRC Senior Research Fellow (350327) and GRD is a Career Development Award Fellow (465109) of the NHMRC. KAJ was supported by an NHMRC Dora Lush Biomedical Research Scholarship. BRSB is Postdoctoral Fellow of the National Heart Foundation of Australia (PF07M3289). The authors acknowledge the facilities, scientific, and technical assistance of Monash Micro Imaging, Monash University, Victoria, Australia.
The authors declare no conflict of interest.
References
- Alkayed NJ, Harukuni I, Kimes, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Granger DN, Mattson MP. Stroke and T-cells. Neuromolecular Med. 2005;7:229–242. doi: 10.1385/NMM:7:3:229. [DOI] [PubMed] [Google Scholar]
- Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA., Jr Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beray-Berthat V, Palmier B, Plotkine M, Margaill I. Neutrophils do not contribute to infarction, oxidative stress, and NO synthase activity in severe brain ischemia. Exp Neurol. 2003;182:446–454. doi: 10.1016/s0014-4886(03)00106-7. [DOI] [PubMed] [Google Scholar]
- Cai H, Yao H, Ibayashi S, Uchimura H, Fujishima M. Photothrombotic middle cerebral artery occlusion in spontaneously hypertensive rats: influence of substrain, gender, and distal middle cerebral artery patterns on infarct size. Stroke. 1998;29:1982–1986. doi: 10.1161/01.str.29.9.1982. [DOI] [PubMed] [Google Scholar]
- Carswell HV, Anderson NH, Clark JS, Graham D, Jeffs B, Dominiczak AF, Macrae IM. Genetic and gender influences on sensitivity to focal cerebral ischemia in the stroke-prone spontaneously hypertensive rat. Hypertension. 1999;33:681–685. doi: 10.1161/01.hyp.33.2.681. [DOI] [PubMed] [Google Scholar]
- Duckles SP, Krause DN. Cerebrovascular effects of oestrogen: multiplicity of action. Clin Exp Pharmacol Physiol. 2007;34:801–808. doi: 10.1111/j.1440-1681.2007.04683.x. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Dean RL, III, Bartus RT. The role of leukocytes followingcerebral ischemia: pathogenic variable or bystander reaction to emerging infarct. Exp Neurol. 2002;173:168–181. doi: 10.1006/exnr.2001.7835. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Conley FK, Kilshaw PJ, Butcher EC. Lymphocytes infiltrating the CNS during inflammation display a distinctive phenotype and bind to VCAM-1 but not to MAdCAM-1. Int Immunol. 1995;7:481–491. doi: 10.1093/intimm/7.3.481. [DOI] [PubMed] [Google Scholar]
- Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AK, Ergul A, Kozak A, Machado LS, Johnson MH, Fagan SC. Effect of neutrophil depletion on gelatinase expression, edema formation and hemorrhagic transformation after focal ischemic stroke. BMC Neurosci. 2005;6:49. doi: 10.1186/1471-2202-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori K, Lee H, Hurn PD, Crain BJ, Traystman RJ, DeVries AC. Cognitive deficits after focal cerebral ischemia in mice. Stroke. 2000;31:1939–1944. doi: 10.1161/01.str.31.8.1939. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27:1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Niwa K, Nogawa S, Zhao X, Nagayama M, Araki E, Morham S, Ross ME. Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc Natl Acad Sci USA. 2001;98:1294–1299. doi: 10.1073/pnas.98.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman KA, Miller AA, De Silva TM, Crack PJ, Drummond GR, Sobey CG. Reduction of cerebral infarct volume by apocynin requires pretreatment and is absent in Nox2-deficient mice. Br J Pharmacol. 2009;156:680–688. doi: 10.1111/j.1476-5381.2008.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase thatis activated after T cell receptor stimulation. Nat Immunol. 2004;5:818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- Jander S, Kraemer M, Schroeter M, Witte OW, Stoll G. Lymphocytic infiltration and expression of intercellular adhesion molecule-1 in photochemically induced ischemia of the rat cortex. J Cereb Blood Flow Metab. 1995;15:42–51. doi: 10.1038/jcbfm.1995.5. [DOI] [PubMed] [Google Scholar]
- Justicia C, Martin A, Rojas S, Gironella M, Cervera A, Panes J, Chamorro A, Planas AM. Anti-VCAM-1 antibodies did not protect against ischemic damage either in rats or in mice. J Cereb Blood Flow Metab. 2006;26:421–432. doi: 10.1038/sj.jcbfm.9600198. [DOI] [PubMed] [Google Scholar]
- Kahles T, Luedike P, Endres M, Galla HJ, Steinmetz H, Busse R, Neumann-Haefelin T, Brandes RP. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke. 2007;38:3000–3006. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- Kusaka I, Kusaka G, Zhou C, Ishikawa M, Nanda A, Granger DN, Zhang JH, Tang J. Role of AT1 receptors and NAD(P)H oxidase in diabetes-aggravated ischemic brain injury. Am J Physiol Heart Circ Physiol. 2004;286:H2442–H2451. doi: 10.1152/ajpheart.01169.2003. [DOI] [PubMed] [Google Scholar]
- Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- Loihl AK, Asensio V, Campbell IL, Murphy S. Expression of nitric oxide synthase (NOS)-2 followingpermanent focal ischemia and the role of nitric oxide in infarct generation in male, female and NOS-2 gene-deficient mice. Brain Res. 1999;830:155–164. doi: 10.1016/s0006-8993(99)01388-8. [DOI] [PubMed] [Google Scholar]
- Marui N, Offermann MK, Swerlick R, Kunsch C, Rosen CA, Ahmad M, Alexander RW, Medford RM. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J Clin Invest. 1993;92:1866–1874. doi: 10.1172/JCI116778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogawa S, Zhang F, Ross ME, Iadecola C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EM, Cho S, Frys KA, Glickstein SB, Zhou P, Anrather J, Ross ME, Iadecola C. Inducible nitric oxide synthase contributes to gender differences in ischemic brain injury. J Cereb Blood Flow Metab. 2006;26:392–401. doi: 10.1038/sj.jcbfm.9600194. [DOI] [PubMed] [Google Scholar]
- Planas AM, Soriano MA, Rodriguez-Farre E, Ferrer I. Induction of cyclooxygenase-2 mRNA and protein followingtransient focal ischemia in the rat brain. Neurosci Lett. 1995;200:187–190. doi: 10.1016/0304-3940(95)12108-g. [DOI] [PubMed] [Google Scholar]
- Santizo RA, Anderson S, Ye S, Koenig HM, Pelligrino DA. Effects of estrogen on leukocyte adhesion after transient forebrain ischemia. Stroke. 2000;31:2231–2235. doi: 10.1161/01.str.31.9.2231. [DOI] [PubMed] [Google Scholar]
- Selemidis S, Sobey CG, Wingler K, Schmidt HH, Drummond GR. NADPH oxidases in the vasculature: molecular features, roles in disease and pharmacological inhibition. Pharmacol Ther. 2008;120:254–291. doi: 10.1016/j.pharmthera.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Stins MF, Gilles F, Kim KS. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J Neuroimmunol. 1997;76:81–90. doi: 10.1016/s0165-5728(97)00036-2. [DOI] [PubMed] [Google Scholar]
- Tsuchiya D, Hong S, Kayama T, Panter SS, Weinstein PR. Effect of suture size and carotid clip application uponblood flow and infarct volume after permanent and temporary middle cerebral artery occlusion in mice. Brain Res. 2003;970:131–139. doi: 10.1016/s0006-8993(03)02300-x. [DOI] [PubMed] [Google Scholar]
- Walder CE, Green SP, Darbonne WC, Mathias J, Rae J, Dinauer MC, Curnutte JT, Thomas GR. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke. 1997;28:2252–2258. doi: 10.1161/01.str.28.11.2252. [DOI] [PubMed] [Google Scholar]
- Wen Y, Yang S, Liu R, Perez E, Yi KD, Koulen P, Simpkins JW. Estrogen attenuates nuclear factor-kappa B activation induced by transient cerebral ischemia. Brain Res. 2004;1008:147–154. doi: 10.1016/j.brainres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Xia CF, Smith RS., Jr, Shen B, Yang ZR, Borlongan CV, Chao L, Chao J. Postischemic brain injury is exacerbated in mice lacking the kinin B2 receptor. Hypertension. 2006;47:752–761. doi: 10.1161/01.HYP.0000214867.35632.0e. [DOI] [PubMed] [Google Scholar]
- Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Shi J, Rajakumar G, Day AL, Simpkins JW. Effects of gender and estradiol treatment on focal brain ischemia. Brain Res. 1998;784:321–324. doi: 10.1016/s0006-8993(97)00502-7. [DOI] [PubMed] [Google Scholar]