Abstract
The purpose of this study is to investigate the possible mechanism and the neuroprotective effect of human urinary kallidinogenase (HUK) in cerebral ischemia. The mouse middle cerebral artery occlusion (MCAO) model was used. Mice were treated with HUK (20 PNAU/g per day, intravenous) or saline as control, from the beginning of reperfusion to 72 h. Neurological deficits, infarct size, and BWC were measured at 6, 24, 48, and 72 h after MCAO, respectively. Pathological changes of brain were observed by TUNEL assay. Inflammatory factors were measured by real-time PCR and western blotting. Activation of MAPKs, Akt, and nuclear factor-κB (NF-κB) was detected by western blotting. Our results indicated that HUK significantly improved neurofunction, decreased infarct size, and suppressed edema, as well as inflammatory mediators as compared with the vehicle group. Furthermore, HUK inhibited the NF-κB pathway and activated the MAPK/ERK pathway in this neuroprotection.
Keywords: human urinary kallidinogenase, inflammation, ischemic stroke, neuroprotection, nuclear factor-κB
Introduction
Ischemia/reperfusion injury is considered the main cause of disability and high mortality in ischemic stroke (Xia et al, 2004). Mechanisms involved in ischemic brain damage could be related to excitotoxicity, oxidative stress, inflammation, and apoptosis. The significance of the inflammatory response has been shown during ischemic pathology in both animal and human stroke. (Dirnagl et al, 1999) Inflammatory mediators, such as interleukin-1 (IL-1), IL-6, or tumor necrosis factor-α (TNF-α), ELAM-1, and monocyte chemoattractant protein1 (MCP-1) could trigger the production of additional cytokines and activate leukocytes, which infiltrate into the central nervous system after stroke.
Despite advances in the understanding of the pathophysiology of stroke, little progress has been made in clinical therapy. Current therapies have shown unsatisfactory results except for thrombolysis, which has a short time window. An expanding body of research suggests that suppression of post-ischemic inflammation is useful in experimental stroke treatment (Muir et al, 2007), but initial therapeutic efforts targeting cytokines and adhesion molecules have not been as successful as expected (Sughrue et al, 2004). It is therefore important to study the upstream pathways that trigger the inflammatory signal.
The kallikrein–kinin system is composed of kallikrein, kininogen, kininase, kinin, and kinin receptors. Tissue kallikrein is widely distributed in the body, including the kidney, blood vessels, central nervous system, pancreas, gut, salivary and sweat glands, spleen, adrenal, plasma, and neutrophils. Tissue kallikrein cleaves low-molecular-weight kininogen to release the potent vasoactive kinin peptides bradykinin (BK) or Lys-BK (kallidin). Kinins have a very short half-life, which is destroyed in less than 20 secs by through the action of kininases (aminopeptidases) present in the tissues and blood. Therefore, kinins are unsuitable for use as a drug. In plasma, kininogen is in excess, leaving kallikrein as the limiting factor. Therefore, it is logical to supply kallikrein to patients. Kinin acts by binding to the B2 receptor, activating a signaling pathway that results in neuroprotection (Chao and Chao, 2005).
Tissue kallikrein has proven to be beneficial for the ischemic brain (Chao and Chao, 2006; Ling et al, 2008; Nagano et al, 1993; Xia et al, 2004, 2006; Zhang et al, 1999), as it functions as an antihypertensive, antioxidant, anti-excitotoxic, and apoptosis inhibitor. Tissue kallikrein gene therapy has anti-inflammatory effects on ischemic stroke, cardiovascular disease, and gentamicin-induced renal injury (Chao et al, 2006). This therapy could also function as neuroprotection (Xia et al, 2006). However, gene therapy for stroke is not clinically feasible. Human urinary kallidinogenase (HUK) has been used clinically for more than 4 years in China to treat ischemic stroke patients and, although the underlying mechanisms are still not well-understood, it could be useful beyond the time limit available in thrombolytic therapy.
Generally, nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) involving p38 MAPK (p38 MAPK), c-Jun N-terminal kinases (JNK kinase), but not extracellular signal-regulated kinases (ERK-1/2), contribute to activating some cytokines and inducing inflammation (Allan and Rothwell, 2001; Thompson and Van Eldik, 2009). This study investigates the role played by neuroprotection of HUK in ischemic stroke through suppression of inflammation by inhibiting the phosphorylation of NF-κB and activating the MAKP/ERK signaling pathway.
Materials and methods
Test Animals
Kunming mice (25 to 30 g, male and female=1:1, n=10 per group) were provided by the Experimental Animal Center in the Affiliated Drum Tower Hospital of Nanjing University Medical School, China. The animal study protocols were approved by the Animal Care and Use Committee at Nanjing University in Nanjing, China.
The mice were grouped on the basis of two factors: (1) treatment: a sham-operated group, a group that received only middle cerebral artery occlusion (MCAO), and a treatment group that received MCAO and HUK; and (2) time elapsed: 6, 24, 48, and 72 h. The SAS software was used to do random block design. Investigators were masked to treatment group assignments.
MCAO in Mice
The MCAO model was designed as previously described (Xu et al, 2006). Briefly, mice were anesthetized with an intraperitoneal injection of sodium pentobarbital (1%) at a dose of 45 mg/kg. Body temperature was maintained at 37±0.5°C. A 6/0 monofilament nylon suture with a heat-rounded tip was inserted through the internal carotid artery into the beginning of the MCA. Mice were subjected to 2 h of occlusion and then the filament was withdrawn for blood reperfusion at 6, 24, 48, and 72 h. Blood pressure and heart rate were measured from pre-MCAO to 2 h after the HUK treatment using the tail-cuff method (PowerLabo/S system connected to CHART software; AD Instruments, Milford, MA, USA). Three consecutive measurements were averaged at each interval. Blood glucose was assayed in tail-vein blood using a glucometer (Omnitest plus; BBRAUN, Melsungen, Germany) and blood gas was detected from 0.5 mL of left ventricular blood using a clinical blood gas analyzer (Radiometer, Copenhagen, Denmark). Sham-operated mice underwent the same procedures mentioned above with the exception of MCAO. The mortality of mice in the model group was around 10%.
Drug Treatments
Mice were treated with HUK (Techpool Bio-Pharma Co. Ltd, Canton, China) through the tail-vein at doses of 10, 20, and 40 PNAU/g within 3 mins for 24 h. A 20-PNAU/g concentration was used according to the amount of brain edema for the following experiments. HUK was administered at reperfusion and at 24-h intervals with 0.9% saline solution as a vehicle.
Behavior Test
Neurological Severity Scores (NSS) were evaluated at 6, 24, 48, and 72 h after MCAO (Chen et al, 2001). Neurological function was graded on a scale of 0 to 18 (normal score, 0; maximal deficit score, 18). NSS is a composite of motor, sensory, reflex, and balance tests. In the severity scores of injury, one point is awarded for the inability to perform a test or for the lack of a tested reflex; thus, higher the score, the more severe is the injury. Ten mice were evaluated in each group.
Infarct Size Measurement
The brains were stained with 2,3,5-triphenyltetrazolium chloride (TTC) (Sigma, St Louis, MO, USA) to detect the infarction volume (Xu et al, 2006). Brains were removed and cut into five 2-mm-thick coronal slices and then immersed in a 2% TTC solution at 37°C for 20 m in the dark. A small pale gray color indicates the infarct area and a dark red color indicates normal tissue. Slices were photographed and analyzed using the image-analysis software (OSIRIS 4.19; Geneva, Switzerland) to calculate the infarct size. Infarct volume in all slices was expressed as a percentage of the contralateral hemisphere after correcting for edema. There were 10 mice in each group.
Measurement of Brain Water Content
The mice were anesthetized with 1% barbital and the brains were quickly removed at different time points as mentioned above. The brains were weighed to obtain the wet weight and were then dried at 100°C for 24 h before measuring dry weight. Brain moisture content (%) was calculated as follows: 100 × (wet weight−dry weight)/wet weight. Ten mice per group were used.
TUNEL Assay
The mice were anesthetized and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde in phosphate-buffered saline. The brains were then removed and immersed in 4% paraformaldehyde for 6 h, then kept in 15% sucrose for 24 h, and in 25% sucrose for an additional 24 h. The cryopreserved brains were sectioned into coronal slices measuring 14 μm and kept at −20°C.
Brain damage was assessed by terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling (TUNEL) assay (Roche, In Situ Cell Death Detection kit, POD, Mannheim, Germany). Brain sections were incubated with a blocking solution for 10 mins at room temperature and then incubated in phosphate-buffered saline for an additional 5 mins on ice three times, and then treated with a terminal deoxynucleotidyl transferase reaction mixture of DNA strand breaks for 60 mins at 37°C in a dark humidified atmosphere. The sections were then incubated with horseradish peroxidase (POD) for 30 mins at 37°C, followed by diaminobenzidine substrate to develop a brown color. The sections were then counterstained with modified Harris' hematoxylin and blue, and TUNEL-positive cells were examined using a microscope. Cells in each microscopic field were determined using Image-Pro Plus 6.0 (Media Cybernetics, Bethesda, MD, USA). Brown dots were counted as positive cells. Three mice per group were used in this test.
Nuclear Protein Extraction
Total proteins from the brain tissue were resuspended in a nuclear lysis buffer (10 mmol/L HEPES or Tris (pH 7.5), 500 mmol/L NaCl, 1% Triton X-100, 10% glycerol, 1 mmol/L NaPPi, 1 μg/mL pepstatin, 1 μg/mL aprotinin, 1 μg/mL leupeptin, 1 mmol/L NaVO4, 1 mmol/L NaF, 1 mmol/L phenylmethylsulfonyl fluoride, with water to 50 mL). The pellet was sonicated five to eight times for 30 secs, with a 1-min rest at a power setting of 5, followed by centrifugation at 14,000r.p.m. for 15 mins at 4°C. The supernatant, or nuclear protein, was saved for the NF-κB65/50 western blotting.
Real-Time PCR
Real-time PCR was performed as described previously (Wang et al, 2009a). Total RNA was extracted by using the Trizol reagent (Invitrogen, Carlsbad, CA, USA) and was reverse-transcribed into cDNA using a PrimeScript RT reagent kit (Takara, Dalian, China) for Quantitative PCR (ABI 7500, USA) in the presence of a fluorescent dye (SYBR Green I; Takara). The relative abundance of mRNA was calculated after normalization to glyceraldehyde-3-phosphate dehydrogenase ribosomal RNA. The primers (Invitrogen USA) are as follows: inducible nitric oxide synthase (iNOS): F: CAGCTGGGCTGTACAAACCTT, R: CATTGGAAGTGAAGCGTTTCG; cyclooxygenase-2 (COX-2): F:GATGACTGCCCAACTCCC, R: AACCCAGGTCCTCGCTTA; ELAM-1: F: CTCACTCCTGACATCGTCCTC, R: ACGTTGTAAGAAGGCACATG, IL-1β: F: AAGCCTCGTGCTGTCGGACC, R: TGAGGCCCAAGGCCACAGGT, IL-6: F: GCTGGTGACAACCACGGCCT, R: AGCCTCCGACTTGTGAAGTGGT, MCP-1: F: CCAGCACCAGCACCAGCCAA; R: TGGATGCTCCAGCCGGCAAC, TNF-α: F: CAAGGGACAAGGCTGCCCCG, R: GCAGGGGCTCTTGACGGCAG, glyceraldehyde-3-phosphate dehydrogenase: F: GCCAAGGCTGTGGGCAAGGT, R: TCTCCAGGCGGCACGTCAGA.
Western Blot
The procedure used was published previously (Xu et al, 2006). Proteins from brain tissue were extracted and then quantified using a Coomassie Blue Fast Staining Solution according to the manufacturer's instructions. Equal amounts of total protein samples were separated by sodium dodecyl sulfate-PAGE and blotted onto polyvinylidene fluoride membranes. The membranes were probed with primary antibodies against COX-2 (1:300; Santa Cruz Biotechnology, Santa Cruz, CA, USA), NOS-2 (1:300; Santa Cruz Biotechnology) IκBα (1:1,500; Cell Signaling, Boston, MA, USA), phospho-IκBα, phospho-SAPK/JNK, phospho-p38, phospho-p44/42(ERK-1/2), phospho-Akt, SAPK/JNK, p38 MAPK, p44/42(ERK-1/2), Akt (1:1,000; Cell Signaling), and glyceraldehyde-3-phosphate dehydrogenase (1:2,000; Bioworld, Minneapolis, MN, USA). Nuclear proteins were detected with primary antibodies NF-κB p65 and p50 (1:1,000, Cell Signaling). Horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibodies were then used and the reaction was observed using chemiluminescence reagents provided with the ECL kit (Amersham Pharmacia Biotech, Piscataway, NJ, USA) and exposed to a film. The intensity of blots was quantified by densitometry. Total p38, SAPK/JNK, ERK, Akt, and glyceraldehyde-3-phosphate dehydrogenase were used as loading controls.
Statistical Analysis
The data are expressed as mean±s.e.m. and analyzed using the SPSS 13.0 statistical analytical software (SPSS, Chicago, IL, USA). Differences in infarct size, blood gas, blood glucose, heart rate, BWC, NSS, mRNA, and proteins at different time points after MCAO were analyzed by analysis of variance and the change between MCAO and the treatment group was analyzed by Student's t-test (two-tailed). The comparative difference was considered significant at P<0.05.
Results
HUK Reduces Brain Damage in Experimental Stroke
To determine whether HUK could reduce mouse brain injury after MCAO, a behavior test was performed and brain water content (BWC) and other pathological changes were determined. As shown in Figure 1A, increase of neurological score observed in the MACO-only group was 11.67±0.56 at 6 h and 9.33±0.84 at 72 h, with greatest neurological damage at 24 h (11.8±0.60, P<0.05) The MCAO+HUK group showed improved neurological function, with lower NSS mean scores at 24 h (9.83±0.54, P<0.05), 48 h (8.17±0.40, P<0.05), and 72 h (7.17±0.48, P<0.05). There was no significant change in physiological parameters (blood gas, blood glucose, heart rate) during the experiments. HUK induced a slightly lower blood pressure at the 15-min time point, but there was no significant difference between the HUK treatment and the MCAO-only group (see Supplementary Table 1).
Figure 1.
NSS and BWC at different time points. (A) NSS increased at different time points after MCAO versus that in the sham group, and HUK reverse it at 24, 48, and 72 h; *P<0.05 versus the MCAO group. (B) BWC as a measure of brain edema of the ischemic hemisphere. HUK could significantly reduce the ischemic brain edema at 6, 24, 48, and 72 h caused by MCAO; *P<0.05 versus the sham group and #P<0.05 versus the MCAO group. Values are mean±s.e.m., n=10.
Edema is one of the earliest pathological changes after ischemic neuronal damage, which significantly increases as early as 20 to 45 mins after MCAO (Gerriets et al, 2009). This study indicated that BWC was enhanced in a time-dependent manner after MCAO-only treatment (6 h: 82.07±0.16% 24 h: 83.30±0.25% 48 h: 84.48±0.35% 72 h: 84.96±0.21% Figure 1B) as compared with that in the sham-treated group (79.29±0.18%). However, administration of 20 PNAU/g HUK, the most appropriate dose in related dose–course studies (data not shown), decreased the BWC by 1.88% (6 h), 2.17% (24 h), 2.53% (48 h), and 2.36% (72 h) as compared with that in the MCAO-only group (Figure 1B).
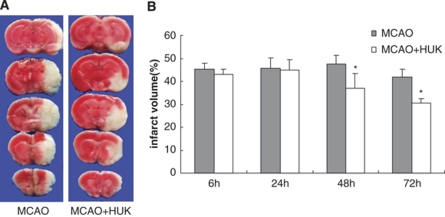
The infarct volume was measured by TTC at different time points after MCAO. HUK decreased the infarct size as compared with that in the vehicle group at 48 and 72 h after ischemia (Figure 2, MCAO+HUK versus MCAO: 6 h: 42.91±2.54% versus 45.36±2.43% 24 h: 44.79±4.60% versus 45.56±4.72% 48 h: 36.96±6.36% versus 47.50±3.76% and 72 h: 30.66±1.98% versus 41.91±3.49% P<0.05 at 48 and 72 h).
Figure 2.
Infarct size was measured by TTC. (A) Infarct images obtained by TTC staining at 48 h after MCAO. The normal tissue was stained deep red and the infarct area was stained pale gray. (B) Infarct size was expressed by bar graphs at 6, 24, 48, and 72 h after MCAO with HUK or vehicle, respectively; *P<0.05 versus MCAO with vehicle. Values are mean±s.e.m., n=10.
To further verify that HUK could reverse brain damage after MCAO, apoptotic neurons were detected by TUNEL staining in the penumbra area after MCAO following 24 h of reperfusion (Figure 3). As expected, there were few TUNEL-positive cells in the normal brain and TUNEL-positive cells in the HUK group were reduced as compared with that in the MCAO-only group (3.11±0.68% versus 8.39 ±1.81%, P<0.05).
Figure 3.
Apoptosis determined by TUNEL. TUNEL-positive cells significantly increased at 24 h after MCAO (B) versus that in the sham group (A), and HUK could reverse it (C). The percent of TUNEL-positive was 8.39±1.81% in the MCAO group and decreased to 3.11±0.68% after treatment with HUK (D). Six representative microscopic fields were analyzed for each group; *P<0.05 versus the sham group and #P<0.05 versus the HUK group.
HUK Attenuates Inflammatory Response in Ischemic Brain Injury
The mRNA expression of cytokines (IL-1, IL-6, and TNF-α), chemokine (MCP-1), adhesion molecules (ELAM-1), as well as the mRNA and protein levels of iNOS and COX-2, in the cortex of mice subjected to MCAO were estimated. The result indicated that the protective effect of HUK was associated with decrease in the level of proinflammatory mediators.
Real-time PCR (Figure 4) showed that all the inflammatory factors, except COX-2, evaluated at 6 h in the MCAO-only group showed significantly higher values over that in the sham group (IL-1: 2,201.7±142.3% IL-6: 747.9±15.2% TNF-α: 2,380.2±277.4% MCP-1: 259.5±82.1% ELAM-1: 542.1±112.1% iNOS: 71.3±12.6%, P<0.05; values at 24 h included the following: IL-1: 3,210±167.1% IL-6: 130±41.3% TNF-α: 2,170±269.7% MCP-1: 440±87.1% ELAM-1: 630±127.4% iNOS: 71.3±12.6%, P<0.05). After 72 h of reperfusion, the mRNA levels of IL-1, IL-6, and TNF-α began to return to lower levels, near the sham baseline, whereas that of MCP-1, iNOS, and ELAM-1 remained higher than that of the sham. Importantly, in the whole time-course experiment, COX-2 continued to rise, peaking at 72 h (by 5.4-fold over sham; P<0.05). However, as expected, all these effects were reversed by HUK (Figure 4), whereas, IL-6 mRNA expression was enhanced (6 h: 211.4±6.44% 24 h: 1,489.3±50.42% 48 h: 398.0±72.1% 72 h: 188.5±16.2%) compared with that in the MCAO-only control group.
Figure 4.
Expression of mice mRNA for IL-1β (A), IL-6 (B), ELAM-1 (C), iNOS (D), TNF-α (E), COX-2 (F), and MCP-1 (G) at 6, 24, 48, and 72 h after MCAO or treated with HUK. Six independent samples were used in the experiments per group; *P<0.05, **P<0.01 versus the sham group and #P<0.05, ##P<0.01 versus the MCAO group.
The protein levels of COX-2 and iNOS were also increased after MCAO. However, in the presence of HUK, COX-2 was attenuated by 48.3% (6 h), 5.2% (24 h), 84.7% (48 h), 42.0% (72 h), and iNOS was decreased by 17.6% (6 h), 22.0% (24 h), 14.1% (48 h), 48.7% (72 h) (see Figure 5).
Figure 5.
Expression of protein level for iNOS and COX-2 at 6, 24, 48, and 72 h after MCAO in the treated or untreated group. Three independent experiments were performed. *P<0.05 versus the sham group and #P<0.05, ##P<0.01 versus the MCAO group.
HUK Inhibits Ischemia-Induced NF-κB Activation and Enhances ERK-1/2 Phosphorylation
NF-κB activation occurs through phosphorylation of IκBα, resulting in the proteasome-dependent degradation of IκBα with release and nuclear translocation of NF-κB (Silverman and Maniatis, 2001). Evaluations were completed to determine whether HUK would suppress NF-κB activation. As shown on Figure 6, MCAO increased the expression of nuclear NF-κB p65 and p50 after 6 h, whereas HUK reduced this increase (Figure 6). NF-κB activity was also determined indirectly by western blotting indicating rapid phosphorylation of IκBα after MCAO, but HUK repressed the activation, with the maximum at 6 h.
Figure 6.
(A) Effect of HUK on activation of the NF-κB pathway as analyzed by western blotting. (B) A graphical representation of the densitometric measurements showing the ratio of p-IκBα and IκBα in the cytoplasm and (C, D) graphical representation of the densitometric measurements showing the protein level of p65 and p50 in the nucleus at 6, 24, 48, and 72 h after MCAO in the treated or untreated group. Three independent experiments were performed. **P<0.01 versus the sham group and #P<0.05, ##P<0.01 versus the MCAO group.
At the same time, MAPK and Akt pathways were determined. Results showed that the expression of phosphorylated ERK-1/2 in the cortex was increased after MCAO and the peak appeared at 24 h, and then gradually returned near the baseline level. HUK enhanced such effects at 6 and 24 h (Figure 7C). SAPK/JNK was also activated at 24 and 48 h (Figure 7A), but HUK had no effect on it. The expression of another subgroup of MAPKs p38 showed no significant change (Figure 7B), nor did the Akt signal pathway (Figure 7D).
Figure 7.
Effect of HUK on activation of the MAPK pathway and the Akt pathway as analyzed by western blotting. (A–C) A graphical representation of the densitometric measurements showing the ratio of p-JNK and JNK; p-p38 and p38; and pERK and ERK; and (D) graphical representation of the densitometric measurements showing the ratio of p-AKT and AKT at 6, 24, 48, and 72 h after MCAO in the treated or untreated group. Three independent experiments were performed. *P<0.05 versus the sham group and #P<0.05 versus the MCAO group.
Discussion
The experiment results prove that (1) HUK may protect against ischemic brain injury by improving neurological dysfunction, reducing infarct size, edema, and pathological changes at different time points after MCAO; (2) HUK may suppress inflammatory cascades after MCAO; and (3) the underlying mechanism of this neuroprotection may be involved in the activation of MAPK/ERK and the downregulation of the NF-κB pathways.
Inflammatory response significantly contributes to ischemic brain damage, which leads to infiltration and activation of microglia/macrophages. Activation of astrocytes induces a more severe inflammatory response. Inflammatory cells and their mediators have already been identified through experimental investigations of brain edema (Simard et al, 2007). Some lines of evidence implicate inflammation as a cause of secondary cerebral injury in ischemia/reperfusion (Mocco et al, 2006), with a further worsening of edema. Progressive edema would result in more serious neurological deterioration leading to cell death. This study shows that edema begins in mice at 6 h lasting until 72 h after MCAO, accompanied by increasing infarct size and cell death (Figures 1 and 2). HUK reduces the infarct size and edema, and suppresses pathological injury after stroke, which is in agreement with previous reports about the effects of transfer of the kallikrein gene (Chao and Chao, 2006). Kallikrein has been shown to protect against ischemic stroke in rats. Similarly, transgenic expression of tissue kallikrein in rats attenuates ischemic cardiac damage, whereas icatibant, a specific BK B2-receptor antagonist, aggravates the damage (Yoshida et al, 2000).
Some reports conflict with this study, reporting that BK/kinin-B2 receptors mediate inflammatory responses resulting in edema formation and secondary brain damage after cerebral ischemia and that BK B2-receptor antagonists show reduced brain edema and infarct formation after MCAO in rats (Klasner et al, 2006). However, increasing evidence indicates that the kallikrein–kinin system mediates neuroprotection in ischemic brain injury (Chao and Chao, 2005, 2006; Zausinger et al, 2002). This type of neuroprotection may be a direct effect of the B2 receptor on neuronal survival (Groger et al, 2005), or of direct activation of the kinin B2 receptor without kinin formation (Chao et al, 2008), or the suppression of mitochondrial pore opening and reducing oxidative stress, or the activation of Akt-mediated signaling pathways (Chao and Chao, 2005). Further studies are needed to investigate this in more detail.
This study shows the protective efficacy of HUK against cerebral ischemic injury, attributed, at least in part, to lessening of ischemia-induced proinflammatory factors. A certain number of factors related to proinflammatory responses, such as cytokines (IL-1, TNF-α), chemokines (MCP-1), adhesion molecules (ELAM-1), and complement proteins like iNOS and COX-2, were induced by ischemia and downregulated by HUK. Cyto/chemokines formed immediately after ischemic injury, stimulate the expression of adhesion molecules on leukocytes and endothelial cells, causing extravasation and adherence of leukocytes to brain parenchyma. The timing and type of inflammatory factors, as well as the extent of stimulation, determine the balance between the positive and negative effects of inflammation (Barone and Feuerstein, 1999). From previous studies of cerebral ischemia, it appears that cyto/chemokines like IL-1, MCP-1, and TNF-α exacerbate cerebral injury. For example, increased expression of IL-1β and TNF-α augments infarct size and leads to neuronal death after MCAO. Mice deficient in MCP-1 were protected from ischemic injury (Hughes et al, 2002). However, IL-6, IL-10, and transforming growth factor-β may be neuroprotective (Benveniste et al, 1995). Previously, IL-6 was thought of as a proinflammatory cytokine, but whether it has a significant role in ischemic stroke is far from clear. These data show that IL-6 is an anti-inflammatory cytokine and HUK enhances its effect while attenuating the others. IL-6 decreases ischemic brain infarct in rats and protects against N-methyl--aspartate-induced toxicity in cortical neurons. Inhibition of IL-6 signaling by treatment with monoclonal antibodies aggravates ischemic cerebral injury in mice (Ali et al, 2000; Wang et al, 2009b). Expression of IL-6 mRNA first appeared at 3 h, peaked at 12 h, and gradually decreased thereafter (Wang et al, 1995). This is similar to the current experimental results.
In addition, this study shows that HUK reduces the induction of COX-2 and iNOS, two downstream effectors after NF-κB activation, on both mRNA and protein levels. This suggests that HUK has a direct anti-inflammatory effect on cells. As opposed to the crucial calcium-dependent regulation of constitutive NOS enzymes (nNOS and eNOS), iNOS has been described as calcium-insensitive. Among these three, iNOS targets inflammatory cells and may contribute to ischemic injury through NO. In fact, expression of iNOS is thought to be restricted to cells involved in inflammatory responses such as circulating leukocytes, microglia, and astrocytes. The ischemia-induced upregulation of iNOS mRNA and protein is associated with increases in iNOS enzymatic activity and NO production, whereas its activity and infarct volume were reduced after treatment with iNOS inhibitor (Iadecola et al, 1995a, 1995b).
COX-2, a rate-limiting enzyme for prostanoid synthesis, is upregulated and present at the border of the ischemic territory (Nogawa et al, 1997). Although the roles of various COX metabolites are protean, accumulated data suggest that those downstream from COX-2 are likely deleterious. Treatment with COX-2 inhibitors improves neurological outcome after stroke, whereas COX-2 overexpression exacerbates brain injury (Sugimoto and Iadecola, 2003). Furthermore, recent work has shown that prostaglandin-E-(2) receptor type-I may be the downstream effector responsible for neurotoxicity in ischemic stroke (Kawano et al, 2006).
It is worth mentioning that these data show that (Figure 4), IL-1, IL-6, TNF-α, MCP-1, ELAM-1, and expression of mRNA peaked no later than 24 h, which is called the acute phase of ischemic stroke, and then weakened or returned to the baseline at about 72 h. Similar results have been reported in the previous literature (Bowen et al, 2006).
It has been observed that one cluster of factors induced by stroke and reversed by HUK was primarily comprised of inflammation and oxidative stress-related factors that are known to trigger inflammatory responses.
HUK acts on inflammatory factors following ischemia. So far, three major subgroups of MAPKs, involved in inflammation, have been identified: p38 MAPK, JNK kinase, and ERK-1/2, as well as the Akt pathway (De Keulenaer et al, 2000). Several transcription factors are also activated, NF-κB being the most important up to now. In this study, it was found that p38 MAPK, JNK kinase, ERK-1/2, Akt, and NF-κB in the cortex were activated after MCAO. However, HUK enhanced the activation of ERK rather than the p38 MAPK, JNK kinase, or Akt. In addition, HUK inhibited NF-κB activity through blockade of IκBα phosphorylation, suggesting that HUK may inhibit IκBα phosphorylation and prevent NF-κB activation, leading to downregulation of the level of inflammatory factors, including TNF-α, IL-1, IL-6, iNOS, intracellular adhesion molecule-1, and matrix metallopeptidase-9, COX-2 (Herrmann et al, 2005; Wang et al, 2007), inhibiting inflammatory responses in the ischemic area.
In conclusion, HUK may protect against ischemic brain injury by inhibiting inflammatory response and this may occur through activation of the MAPK/ERK pathway or downregulation of the NF-κB pathway.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (30971010, 30670739), the Doctoral Program Foundation of the Ministry of Education of China (20060284044), Outstanding Researcher Program (RC2007006) and the National Natural Science Foundation (BK2009037) of Jiangsu Province of China, funding from the State Key Laboratory of Pharmaceutical Biotechnology (KF-GN-200901), and the 973 Program from the Ministry of Science and Technology of China (2009CB21906). We thank Marilyn White for editing the paper.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Ali C, Nicole O, Docagne F, Lesne S, MacKenzie ET, Nouvelot A, Buisson A, Vivien D. Ischemia-induced interleukin-6 as a potential endogenous neuroprotective cytokine against NMDA receptor-mediated excitotoxicity in the brain. J Cereb Blood Flow Metab. 2000;20:956–966. doi: 10.1097/00004647-200006000-00008. [DOI] [PubMed] [Google Scholar]
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- Benveniste EN, Tang LP, Law RM. Differential regulation of astrocyte TNF-alpha expression by the cytokines TGF-beta, IL-6 and IL-10. Int J Dev Neurosci. 1995;13:341–349. doi: 10.1016/0736-5748(94)00061-7. [DOI] [PubMed] [Google Scholar]
- Bowen KK, Naylor M, Vemuganti R. Prevention of inflammation is a mechanism of preconditioning-induced neuroprotection against focal cerebral ischemia. Neurochem Int. 2006;49:127–135. doi: 10.1016/j.neuint.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Chao J, Bledsoe G, Yin H, Chao L. The tissue kallikrein–kinin system protects against cardiovascular and renal diseases and ischemic stroke independently of blood pressure reduction. Biol Chem. 2006;387:665–675. doi: 10.1515/BC.2006.085. [DOI] [PubMed] [Google Scholar]
- Chao J, Chao L. Kallikrein–kinin in stroke, cardiovascular and renal disease. Exp Physiol. 2005;90:291–298. doi: 10.1113/expphysiol.2004.028464. [DOI] [PubMed] [Google Scholar]
- Chao J, Chao L. Experimental therapy with tissue kallikrein against cerebral ischemia. Front Biosci. 2006;11:1323–1327. doi: 10.2741/1886. [DOI] [PubMed] [Google Scholar]
- Chao J, Yin H, Gao L, Hagiwara M, Shen B, Yang ZR, Chao L. Tissue kallikrein elicits cardioprotection by direct kinin b2 receptor activation independent of kinin formation. Hypertension. 2008;52:715–720. doi: 10.1161/HYPERTENSIONAHA.108.114587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- De Keulenaer GW, Ushio-Fukai M, Yin Q, Chung AB, Lyons PR, Ishizaka N, Rengarajan K, Taylor WR, Alexander RW, Griendling KK. Convergence of redox-sensitive and mitogen-activated protein kinase signaling pathways in tumor necrosis factor-alpha-mediated monocyte chemoattractant protein-1 induction in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000;20:385–391. doi: 10.1161/01.atv.20.2.385. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Gerriets T, Walberer M, Ritschel N, Tschernatsch M, Mueller C, Bachmann G, Schoenburg M, Kaps M, Nedelmann M. Edema formation in the hyperacute phase of ischemic stroke. J Neurosurg. 2009;111:1036–1042. doi: 10.3171/2009.3.JNS081040. [DOI] [PubMed] [Google Scholar]
- Groger M, Lebesgue D, Pruneau D, Relton J, Kim SW, Nussberger J, Plesnila N. Release of bradykinin and expression of kinin B2 receptors in the brain: role for cell death and brain edema formation after focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005;25:978–989. doi: 10.1038/sj.jcbfm.9600096. [DOI] [PubMed] [Google Scholar]
- Herrmann O, Baumann B, de Lorenzi R, Muhammad S, Zhang W, Kleesiek J, Malfertheiner M, Kohrmann M, Potrovita I, Maegele I, Beyer C, Burke JR, Hasan MT, Bujard H, Wirth T, Pasparakis M, Schwaninger M. IKK mediates ischemia-induced neuronal death. Nat Med. 2005;11:1322–1329. doi: 10.1038/nm1323. [DOI] [PubMed] [Google Scholar]
- Hughes PM, Allegrini PR, Rudin M, Perry VH, Mir AK, Wiessner C. Monocyte chemoattractant protein-1 deficiency is protective in a murine stroke model. J Cereb Blood Flow Metab. 2002;22:308–317. doi: 10.1097/00004647-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Xu S, Casey R, Ross ME. Inducible nitric oxide synthase gene expression in brain following cerebral ischemia. J Cereb Blood Flow Metab. 1995a;15:378–384. doi: 10.1038/jcbfm.1995.47. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Xu X. Inhibition of inducible nitric oxide synthase ameliorates cerebral ischemic damage. Am J Physiol. 1995b;268:R286–R292. doi: 10.1152/ajpregu.1995.268.1.R286. [DOI] [PubMed] [Google Scholar]
- Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, Kunz A, Cho S, Orio M, Iadecola C. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med. 2006;12:225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- Klasner B, Lumenta DB, Pruneau D, Zausinger S, Plesnila N. Therapeutic window of bradykinin B2 receptor inhibition after focal cerebral ischemia in rats. Neurochem Int. 2006;49:442–447. doi: 10.1016/j.neuint.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Ling L, Hou Q, Xing S, Yu J, Pei Z, Zeng J. Exogenous kallikrein enhances neurogenesis and angiogenesis in the subventricular zone and the peri-infarction region and improves neurological function after focal cortical infarction in hypertensive rats. Brain Res. 2008;1206:89–97. doi: 10.1016/j.brainres.2008.01.099. [DOI] [PubMed] [Google Scholar]
- Mocco J, Mack WJ, Ducruet AF, Sosunov SA, Sughrue ME, Hassid BG, Nair MN, Laufer I, Komotar RJ, Claire M, Holland H, Pinsky DJ, Connolly ES., Jr Complement component C3 mediates inflammatory injury following focal cerebral ischemia. Circ Res. 2006;99:209–217. doi: 10.1161/01.RES.0000232544.90675.42. [DOI] [PubMed] [Google Scholar]
- Muir KW, Tyrrell P, Sattar N, Warburton E. Inflammation and ischaemic stroke. Curr Opin Neurol. 2007;20:334–342. doi: 10.1097/WCO.0b013e32813ba151. [DOI] [PubMed] [Google Scholar]
- Nagano H, Suzuki T, Tomoguri T, Hayashi M, Tsurumi K. [Pharmacological studies on human urinary kallidinogenase (SK-827): effects on cerebral metabolism] Yakugaku Zasshi. 1993;113:825–828. doi: 10.1248/yakushi1947.113.11_825. [DOI] [PubMed] [Google Scholar]
- Nogawa S, Zhang F, Ross ME, Iadecola C. Cyclooxygenase-2 gene expression in neurons contributes to ischemic brain damage. J Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman N, Maniatis T. NF-kappaB signaling pathways in mammalian and insect innate immunity. Genes Dev. 2001;15:2321–2342. doi: 10.1101/gad.909001. [DOI] [PubMed] [Google Scholar]
- Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sughrue ME, Mehra A, Connolly ES, Jr, D'Ambrosio AL. Anti-adhesion molecule strategies as potential neuroprotective agents in cerebral ischemia: a critical review of the literature. Inflamm Res. 2004;53:497–508. doi: 10.1007/s00011-004-1282-0. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Iadecola C. Delayed effect of administration of COX-2 inhibitor in mice with acute cerebral ischemia. Brain Res. 2003;960:273–276. doi: 10.1016/s0006-8993(02)03805-2. [DOI] [PubMed] [Google Scholar]
- Thompson WL, Van Eldik LJ. Inflammatory cytokines stimulate the chemokines CCL2/MCP-1 and CCL7/MCP-7 through NFkappaB and MAPK dependent pathways in rat astrocytes. Brain Res. 2009;1287:47–57. doi: 10.1016/j.brainres.2009.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang L, Chen ZB, Wu JY, Zhang X, Xu Y. Icariin enhances neuronal survival after oxygen and glucose deprivation by increasing SIRT1. Eur J Pharmacol. 2009a;609:40–44. doi: 10.1016/j.ejphar.2009.03.033. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yue TL, Young PR, Barone FC, Feuerstein GZ. Expression of interleukin-6, c-fos, and zif268 mRNAs in rat ischemic cortex. J Cereb Blood Flow Metab. 1995;15:166–171. doi: 10.1038/jcbfm.1995.18. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Peng YP, Lu JH, Cao BB, Qiu YH. Neuroprotection of interleukin-6 against NMDA attack and its signal transduction by JAK and MAPK. Neurosci Lett. 2009b;450:122–126. doi: 10.1016/j.neulet.2008.11.051. [DOI] [PubMed] [Google Scholar]
- Xia CF, Yin H, Borlongan CV, Chao L, Chao J. Kallikrein gene transfer protects against ischemic stroke by promoting glial cell migration and inhibiting apoptosis. Hypertension. 2004;43:452–459. doi: 10.1161/01.HYP.0000110905.29389.e5. [DOI] [PubMed] [Google Scholar]
- Xia CF, Yin H, Yao YY, Borlongan CV, Chao L, Chao J. Kallikrein protects against ischemic stroke by inhibiting apoptosis and inflammation and promoting angiogenesis and neurogenesis. Hum Gene Ther. 2006;17:206–219. doi: 10.1089/hum.2006.17.206. [DOI] [PubMed] [Google Scholar]
- Xu Y, Zhang W, Klaus J, Young J, Koerner I, Sheldahl LC, Hurn PD, Martinez-Murillo F, Alkayed NJ. Role of cocaine- and amphetamine-regulated transcript in estradiol-mediated neuroprotection. Proc Natl Acad Sci USA. 2006;103:14489–14494. doi: 10.1073/pnas.0602932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Zhang JJ, Chao L, Chao J. Kallikrein gene delivery attenuates myocardial infarction and apoptosis after myocardial ischemia and reperfusion. Hypertension. 2000;35:25–31. doi: 10.1161/01.hyp.35.1.25. [DOI] [PubMed] [Google Scholar]
- Zausinger S, Lumenta DB, Pruneau D, Schmid-Elsaesser R, Plesnila N, Baethmann A. Effects of LF 16-0687 Ms, a bradykinin B(2) receptor antagonist, on brain edema formation and tissue damage in a rat model of temporary focal cerebral ischemia. Brain Res. 2002;950:268–278. doi: 10.1016/s0006-8993(02)03053-6. [DOI] [PubMed] [Google Scholar]
- Zhang JJ, Chao L, Chao J.1999Adenovirus-mediated kallikrein gene delivery reduces aortic thickening and stroke-induced death rate in Dahl salt-sensitive rats Stroke 301925–1931.discussion 31–32 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.