Abstract
Mesenchymal progenitors such as bone marrow stromal cells (BMSCs) are an attractive cell source for fibrocartilage tissue engineering, but the types or combinations of signals required to promote fibrochondrocyte-specific differentiation remain unclear. The present study investigated the influences of cyclic tensile loading on the chondrogenesis of BMSCs and the development of engineered fibrocartilage. Cyclic tensile displacements (10%, 1 Hz) were applied to BMSC-seeded fibrin constructs for short (24 h) or extended (1–2 weeks) periods using a custom loading system. At early stages of chondrogenesis, 24 h of cyclic tension stimulated both protein and proteoglycan synthesis, but at later stages, tension increased protein synthesis only. One week of intermittent cyclic tension significantly increased the total sulfated glycosaminoglycan and collagen contents in the constructs, but these differences were lost after 2 weeks of loading. Constraining the gels during the extended culture periods prevented contraction of the fibrin matrix, induced collagen fiber alignment, and increased sulfated glycosaminoglycan release to the media. Cyclic tension specifically stimulated collagen I mRNA expression and protein synthesis, but had no effect on collagen II, aggrecan, or osteocalcin mRNA levels. Overall, these studies suggest that the combination of chondrogenic stimuli and tensile loading promotes fibrochondrocyte-like differentiation of BMSCs and has the potential to direct fibrocartilage development in vitro.
Introduction
Fibrocartilaginous tissues such as the meniscus of the knee are susceptible to damage from traumatic injuries1 and have a limited capacity for regeneration.2 Unrepaired damage to the menisci can often lead to altered joint biomechanics3 and the onset of osteoarthritis in the adjacent articular cartilage.4 Tissue engineering is a potential strategy for repairing fibrocartilage injuries; however, there are significant challenges for developing tissues with the appropriate composition and structure. Furthermore, the limited availability of autologous fibrochondrocytes presents additional obstacles for obtaining an appropriate cell source. Mesenchymal stem cells may be an alternative cell type for fibrocartilage tissue engineering, but the types or combinations of signals required to promote fibrochondrocytic differentiation and matrix production remain unknown.
It is well established that bone marrow stromal cells (BMSCs) contain a population of multipotent mesenchymal progenitors capable of chondrogenic differentiation.5–9 The combination of transforming growth factor-beta1 (TGF-β1) and the glucocorticoid dexamethasone promotes chondrogenesis within a variety of three-dimensional culture systems, including pellet culture5,9 and cell-seeded hydrogels.6,7,10 While much work has been devoted to studying the chondrogenesis of BMSCs for articular cartilage repair, few studies have explored the use of progenitors for fibrocartilage repair. Implantation of rabbit BMSCs into a partial meniscal defect resulted in tissue formation that histologically resembled fibrocartilage, but the implant ultimately failed to restore mechanical function of the meniscus or prevent osteoarthritic degradation in the joint.11 Intra-articular injection of caprine BMSCs after medial meniscectomy resulted in regeneration of meniscus-like tissue,12 although the tissue growth has been attributed to trophic effects of the BMSCs rather than direct tissue regeneration.13 Recently, the delivery of BMSCs within a meniscus-derived scaffold reduced or delayed the onset of osteoarthritis within a meniscus defect model.14 Together, these studies suggest that BMSCs could be a viable cell source for fibrocartilage repair, but additional stimuli are likely required to develop a mechanically functional tissue substitute.
Fibrocartilaginous tissues, such as the menisci of the knee, temporomandibular joint disks, and tendon and ligament insertion sites, are typically characterized by high levels of type I collagen (type I Collagen),15 as well as the presence of sulfated proteoglycans (PGs).16 Compared to articular cartilage, fibrocartilage has a higher collagen content (15–25% of wet mass)15 and a lower content of the large PG aggrecan.17 Consistent with the tissue composition, fibrochondrocytes (although there is substantial phenotypic variation within fibrocartilage tissues) express higher levels of collagen I and less collagen II and aggrecan than articular chondrocytes.18 In addition to these patterns of extracellular matrix (ECM) expression, recent studies in our laboratory have identified distinct differences in aggrecan processing between fibrochondrocytes and articular chondrocytes, with high levels of degraded aggrecan associated with fibrocartilage tissues and a fibrochondrocyte-like phenotype.19
In vivo, fibrocartilage tissues experience a wide range of compressive, tensile, and shear forces, and the tissue's composition and structure are critical for its diverse mechanical functions. In the meniscus, for example, the relatively high density of negatively charged PGs in the inner region allows the tissue to bear compressive stresses,20 whereas large, circumferential collagen I fibers in the outer region effectively withstand tensile hoop stresses during joint loading.4 In vitro, cyclic tensile loading has been shown to down-regulate PG synthesis by articular chondrocytes and induce cytoskeletal changes.21 In addition, cyclic stretching of tendon fibroblasts can stimulate collagen I expression and synthesis.22
The structure–function relationships in the meniscus and the response of articular chondrocytes to cyclic tension suggest that tensile loading may promote more fibroblastic phenotypes and help guide fibrochondrocytic differentiation of mesenchymal progenitors. Although several studies have demonstrated that appropriate compressive loading can enhance the in vitro chondrogenesis of BMSCs,23,24 the responses to tension of BMSCs undergoing chondrogenesis have been largely unexplored. Therefore, the present study investigated the influences of cyclic tensile loading on the chondrogenesis of BMSCs and development of engineered fibrocartilage constructs. Using a custom loading system, we examined the effects of short (24 h) or extended (1–2 weeks) periods of cyclic tensile loading on BMSC gene expression and matrix deposition. The results of these studies provide evidence that the combination of chondrogenic stimuli and tensile loading promotes fibrochondrocyte-like differentiation of BMSCs and has the potential to direct the development of a fibrocartilage tissue construct.
Materials and Methods
Materials
Immature bovine hind limbs were from Research 87 (Marlborough, MA). Recombinant human TGF-β1 and basic-fibroblast growth factor were from Peprotech (Rocky Hill, NJ). Bovine fibrinogen, dexamethasone, aprotinin, 1,9 dimethyl methylene blue, Direct Red, Chondroitinase ABC, and Hoechst dye 33258 were from Sigma Aldrich (St. Louis, MO). The ITS+ premix (insulin, transferrin and selenium) and Proteinase K were from BD Biosciences (San Jose, CA). Fetal bovine serum (FBS) was from Hyclone (Logan, UT). Dulbecco's modified Eagle's medium (DMEM), antibiotic/antimycotic, trypsin, nonessential amino acids, and phosphate-buffered saline (PBS) were from Invitrogen (Carlsbad, CA). The 35S-sodium sulfate and bovine thrombin were from MP Biomedicals (Irvine, CA). The 3H-proline was from GE Healthcare (Piscataway, NJ). The porous polyethylene was from Porex (Fairburn, GA). The RNeasy mini kit was from Qiagen (Valencia, CA), and the AMV reverse transcriptase kit was from Promega (Madison, WI). The Sybr Green master mix was from Applied Biosystems (Foster City, CA). The anti-type I collagen and anti-type II collagen antibodies were from Abcam (Cambridge, United Kingdom), and the Alexafluor 488 and 555 secondary antibodies were from Invitrogen.
BMSC isolation and expansion
BMSCs were isolated as previously described.23,25 Bone marrow was harvested from the tibiae and femora of an immature calf and physically disrupted by passage through 50 and 10 mL serological pipettes, followed by 16, 18, and 20 gauge needles. The marrow was then separated by centrifugation, and the fatty layer was removed. The remaining heterogeneous mixture was rinsed with PBS and preplated for 30 min to remove the rapidly adherent cells. The remaining cells were then re-plated at approximately 250,000 cells/cm2 on tissue culture plastic and cultured in low-glucose DMEM with 1% antibiotic/antimycotic, 10% FBS, and 1 ng/mL basic-fibroblast growth factor. After 3 days, the nonadherent cells were removed during the first medium change. The remaining adherent BMSCs were expanded until nearly confluent, at which time they were trypsinized and replated at 6000 cells/cm2. Cells were expanded twice more to near-confluence before seeding in fibrin.
Fibrin gel seeding and culture
As in our previous studies with chondrocytes and fibrochondrocytes, cell-seeded fibrin hydrogels were used as a model system for studying effects of tensile loading.21 BMSCs were seeded into fibrin gels (50 mg/mL) at a density of 10 × 106 cells/mL by combining a fibrinogen/cell suspension in DMEM with bovine thrombin (50 U/mL final concentration). For an initial differentiation time-course study, fibrin gels were cast in cylindrical molds (Ø 11 mm × 3 mm). For tensile loading studies, gels were cast in rectangular molds between porous (15–45 μm) polyethylene end-blocks (Fig. 1). Infiltration of the fibrinogen/cell suspension into the polyethylene before gelation fixed the gels to the end blocks and provided a rigid support for applying controlled tensile strains to the gels.
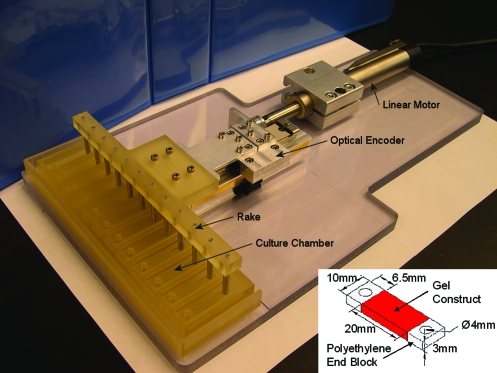
FIG. 1.
Tensile loading system. The tensile loading system consisted of a linear motor that applied cyclic tensile displacements to the fibrin constructs via a linear sliding table and rake attachment. Motion was controlled by a Trio Motion controller and feedback from an optical encoder. The fibrin constructs (inset) were cast between porous polyethylene end blocks for rigid fixation and connection to the rake attachment. Color images available online at www.liebertonline.com/ten.
Fibrin gel constructs were cultured either in basal medium consisting of high-glucose DMEM with ITS + premix, nonessential amino acids, antibiotic/antimycotic, ascorbate, and 0.1 TIU/mL aprotinin, or in chondrogenic medium consisting of basal medium supplemented with 10 ng/mL TGF-β1 and 100 nM dexamethasone. Media were collected and changed every 2 days. For tensile loading studies, sinusoidal tensile displacements of 10% (peak-peak) were applied to the constructs at 1 Hz using a custom loading device (Fig. 1). Unconstrained controls remained in free-swelling (FS) culture throughout the study, whereas constrained controls were maintained at a fixed length during the loading phase of the long-term loading study.
Study design
To examine patterns of BMSC chondrogenesis in fibrin gels, an unloaded differentiation study was first performed using cylindrical gels. Fibrin gel constructs were cultured in basal or chondrogenic medium for 2, 4, 7, or 12 days. At each time point, collagen II, aggrecan, and sox-9 mRNA levels were measured via real time reverse transcription polymerase chain reaction (RT-PCR), and sulfated glycosaminoglycan (sGAG) content was determined as an indicator of ECM accumulation. Portions of the gels were examined to qualitatively assess cell morphology via f-actin staining.
To assess the initial response to tensile loading at different stages of differentiation, a short-term loading study was performed. Fibrin constructs were cultured unconstrained for 4 or 12 days in either basal or chondrogenic medium. Constructs were then loaded for 3 h at 10% displacement and 1 Hz frequency, followed by 3 h recovery at 0% displacement (50% duty cycle), with the load/recovery cycle repeated four times for 24 h. Unloaded controls were cultured in parallel. Matrix synthesis rates during the 24 h loading period were measured by incorporation of radiolabeled precursors, and construct sGAG and DNA contents were measured.
To assess the effects of sustained tensile loading on construct maturation, a long-term loading study was performed. Based on the results of the time-course and short-term loading study, fibrin constructs were cultured in chondrogenic medium under FS conditions for 7 days before loading, at which time they were randomly assigned to one of three loading conditions. The unconstrained FS constructs were placed in chambers and allowed to freely contract over the course of the experiment. Another group was cultured in the loading chambers but constrained at the day 7 length of 14 mm (0% displacement) to control for possible effects of static stress induced by restricting further contraction. Cyclic tensile strains were intermittently applied to the third group of constructs for up to 2 weeks using the custom loading system. Sinusoidal displacements of 10% (peak–peak) were applied to the constructs at 1 Hz for 1 h, followed by 3 h of recovery at 0% displacement (25% duty cycle) and repeated continuously (6 times daily) for the remaining culture period. Samples were taken after the initial 7 day preculture and at days 14 and 21 (after 7 and 14 days of loading, respectively). One half of each construct was analyzed to determine the sGAG, hydroxyproline (as an indicator of collagen), and DNA contents, and a slice from the central region was taken for histology. The remaining portions of the constructs were analyzed for collagen II, aggrecan, collagen I, and osteocalcin mRNA levels by real-time RT-PCR. Conditioned media were analyzed for sGAG release. The length of the constructs under FS conditions was also measured at days 7, 14, and 21.
Radiolabel incorporation and matrix accumulation
During the final 24 h of culture in the short-term loading study, 5 μCi/mL 35S-sodium sulfate and 10 μCi/mL 3H-proline were included in the culture media to measure sGAG and protein synthesis, respectively. After loading, radiolabel incorporation was quenched with four sequential 30 min washes in PBS plus 0.8 mM sodium sulfate and 1.0 mM L-proline at 4°C. The gels were weighed, lyophilized, reweighed, and digested with Proteinase K (1 mg per 80 mg construct dry mass) at 60°C. Radiolabel contents were measured using a liquid scintillation counter. The total sGAG contents were measured using the 1,9 dimethyl methylene blue assay,26 and the DNA contents were measured using the Hoechst dye assay.27 In addition, portions of the digests were hydrolyzed in 6M HCl at 100°C and the total collagen contents were measured using the pDAB and chloramine-T assay for hydroxyproline.
Real-time RT-PCR
RNA was isolated from the fibrin gel constructs using the Tri-spin method.28 Gels were immediately dissociated in Qiagen lysis buffer plus 2-mercaptoethanol. RNA was extracted from the fibrin using the Trizol reagent and chloroform and precipitated with 100% isopropanol. The RNA was further purified using the Qiagen RNeasy kit according to the manufacturer's protocol. Total RNA (1 μg) was reverse transcribed to cDNA using the AMV reverse transcriptase kit. Gene expression was measured by real-time RT-PCR using the SybrGreen master mix and custom primers for collagen II, aggrecan, sox-9, collagen I, and osteocalcin.29 The PCR reactions and detection were performed with the ABI Prism 7700 (Applied Biosystems), and mRNA levels were determined using absolute standard curves generated with known concentrations of the PCR product.
Immunofluorescence staining and histology
For the unloaded time course experiment, portions of the fibrin gels were fixed in 10% neutral buffered formalin for 20 min and stored in PBS at 4°C. The gels were blocked with 5% FBS and 1% Triton-X100 for 30 min and labeled with Alexa Fluor 594-phalloidin for 90 min at 37°C. The gels were rinsed thoroughly and imaged using a laser scanning confocal microscope (LSM 510; Zeiss, Heidelberg, Germany).
After the long-term loading studies, gel constructs were fixed in 10% neutral buffered formalin for 4 h, rinsed in PBS, and dehydrated in 70% ethanol. Samples were subsequently embedded in paraffin and sectioned at 5 μm onto glass slides. Sections were deparaffinized and rehydrated in water. sGAGs were stained with 0.5% Safranin-O. Additional sections were stained for collagen with picrosirius red (1 mg/mL Direct Red in saturated picric acid). For immunoflurescence staining, sections were de-glycosylated with 0.1 U/mL chondroitinase ABC for 2 h at 37°C and blocked with 10% FBS for 1 h at room temperature. Sections were then labeled with a mouse monoclonal antibody against type I collagen and rabbit polyclonal against type II collagen. Secondary detection was performed with anti-mouse IgG (Alexafluor 488) and anti-rabbit IgG (Alexafluor 555). DNA was labeled with 4′,6-diamidino-2-phenylindole (DAPI). Negative controls without primary antibodies were stained in parallel. Immunofluorescent slides were preserved with aqueous gel mount and imaged using a Zeiss Axiovert 200 fluorescence microscope.
Data analysis
All data are presented as the mean ± standard error of the mean. Gene expression levels were transformed by a Box-Cox transformation for normality. Data were analyzed by a two- or three-factor general linear model. For the unloaded time course, the factors were time point and medium formulation. For the short-term loading experiments, the factors were preculture period, medium formulation, and loading condition, and for the long-term loading, the factors were time point and loading condition. All pairwise comparisons were performed with Tukey's test. Significance was at p < 0.05 with n = 6 constructs per group.
Results
Unloaded time course
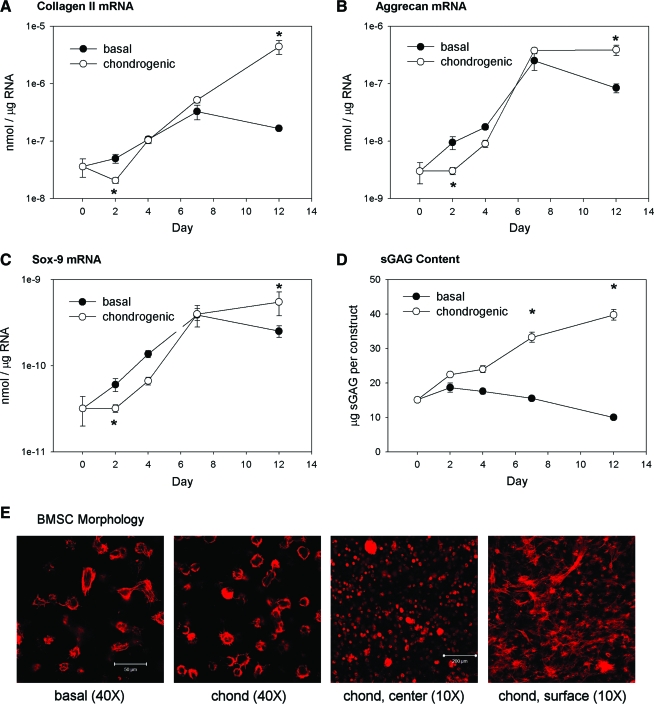
A time course for BMSC chondrogenesis in fibrin gels was first established in FS culture. At day 2, BMSCs cultured with TGF-β1 and dexamethasone expressed significantly lower levels of the chondrocytic genes, collagen II, aggrecan, and sox-9, than those cultured in the basal medium (Fig. 2A–C). This initial inhibitory response may be due to integrin-mediated adhesion to the fibrin matrix.29 By day 7, however, cells in the basal and chondrogenic media expressed similar levels of the chondrocytic genes, and by day 12 the chondrogenic group expressed significantly higher levels. Collagen I expression was not significantly different between medium conditions during the unloaded time course (not shown). Addition of the chondrogenic supplements stimulated sGAG accumulation within the fibrin construct, with significant increases over basal medium at days 7 and 12 (Fig. 2D). Stimulation of sGAG accumulation but not aggrecan gene expression over the first week in chondrogenic medium suggests that aggrecan may not be the dominant PG during the early stages of chondrogenesis, consistent with previous observations.30 BMSCs displayed similar morphologies in both basal and chondrogenic media (Fig. 2E). A mix of round cells and spread cells with distinct f-actin stress fibers and cytoskeletal projections could be observed within the constructs. BMSCs within the center of the construct were more rounded, whereas those on the surfaces had primarily spread morphologies. Overall, these results demonstrate that BMSCs are capable of chondrogenic differentiation within fibrin gels and that TGF-β1 and dexamethasone enhance this process.
FIG. 2.
BMSC chondrogenesis in fibrin gels. Constructs were cultured up to 12 days under free-swelling (FS) conditions in basal or chondrogenic medium and examined for mRNA expression of (A) collagen II, (B) aggrecan, and (C) sox-9 and (D) sGAG accumulation. (E) Cell morphology within the fibrin gels was examined by staining for the f-actin cytoskeleton and imaging with a confocal microscope (representative images from day 4). Levels of mRNA are expressed on a logarithmic scale as nmol of transcript per μg of total RNA. n = 6/condition, *p < 0.05 with basal medium. BMSC, bone marrow stromal cell; sGAG, sulfated glycosaminoglycan. Color images available online at www.liebertonline.com/ten.
Short-term tensile loading
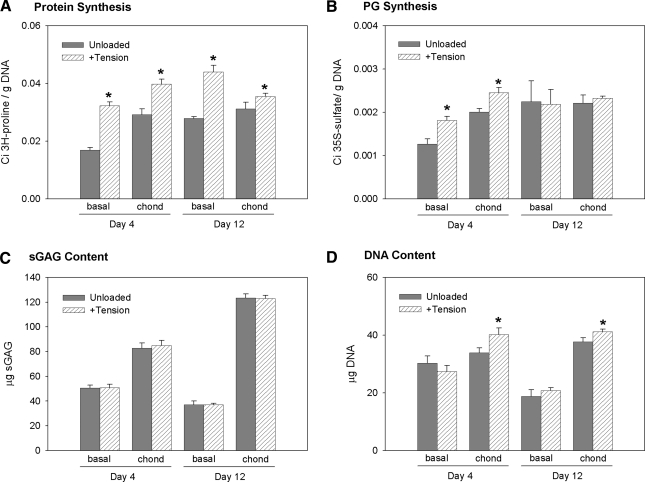
The initial response to cyclic tensile loading was examined at early (day 4) and later (day 12) stages of in vitro chondrogenesis. At day 4, before significant changes in gene expression and matrix synthesis, intermittent tensile loading significantly increased both protein and PG synthesis for BMSCs in both basal and chondrogenic media (Fig. 3A, B). At day 12, however, tension increased protein synthesis only. Short-term loading had no effect on total sGAG content. Cyclic tension also slightly increased the DNA content of constructs in chondrogenic medium at days 4 and 12 (Fig. 3D). Intermittent tensile loading did not appear to have an effect on BMSC morphology (not shown) given the large variations in cell morphology similar to the FS conditions (Fig. 2E). The stimulatory effects of loading on protein but not PG synthesis at day 12 suggested that cyclic tension encourages a more fibrous tissue phenotype at later stages of construct development. On the basis of this observation and the gene expression patterns in the time-course study, subsequent experiments included a 7 day preculture period before introducing tensile loading.
FIG. 3.
Matrix synthesis rates during short-term loading. Constructs were cultured in basal or chondrogenic medium for 4 or 12 days and loaded intermittently (3 h on–3 h off) for 24 h. (A) Protein and (B) proteoglycan (PG) synthesis rates were measured during the 24 h loading period by incorporation of 3H-proline and 35S-sulfate, respectively. The total (C) sGAG and (D) DNA contents were also measured in the constructs. n = 6/condition, *p < 0.05 with unloaded.
Long-term loading
The tensile loading period was next extended to 1–2 weeks in chondrogenic medium to investigate the effects on longer-term tissue development. During the 7 day preculture under FS conditions, BMSCs contracted the fibrin matrix by 30% to an average length of 13.87 mm (Table 1). The 0% displacement group (included to distinguish the effects of dynamic loading from static tensile stresses generated by constraining the gels against contraction) was held at this position for the remaining 2 weeks, and 1.4 mm displacements (10% of day 7 length) were applied to the cyclic tension group. BMSCs in the FS group continued to contract the gels to 9.68 and 8.75 mm after 14 and 21 days, respectively (Table 1). By day 21, this contraction resulted in a 37.5% difference in the nominal construct length between the FS and constrained gels.
Table 1.
Fibrin Gel Contraction and Length Differences Compared To Constrained Gels
| Day | FS length (mm) | Contraction (%) | Δ FS vs. 0% (mm) | Δ FS vs. 0% (%) | Dynamic Disp (mm) | Dynamic Disp (%) |
|---|---|---|---|---|---|---|
| 0 | 20 | 0 | 0 | 0 | 0 | 0 |
| 7 | 13.87 ± 0.35 | 30.7 ± 1.74 | 0.13 ± 0.35 | 0.9 ± 2.52 | 1.4 | 10 |
| 14 | 9.68 ± 0.20 | 51.6 ± 1.00 | 4.32 ± 0.20 | 31.6 ± 2.06 | 1.4 | 10 |
| 21 | 8.75 ± 0.13 | 56.3 ± 0.66 | 5.25 ± 0.13 | 37.5 ± 1.50 | 1.4 | 10 |
The free-swelling (FS) construct length was measured over the 21 day experiment. Starting at day 7, loaded constructs were constrained at an approximate FS length of 14 mm. All data are mean ± standard error of the mean, n = 6 constructs.
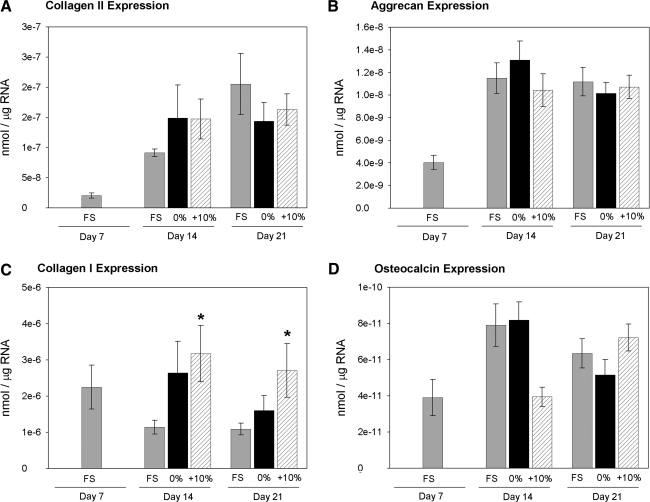
Collagen II mRNA levels increased over the course of the long-term loading period for all conditions (Fig. 4A), and aggrecan expression increased from day 7 to 14 but did not change through day 21 (Fig. 4B). There were no significant differences in chondrocytic gene expression among the loading conditions at days 14 or 21. Under FS conditions, collagen I expression decreased from day 7 to 14 and did not change through day 21 (Fig. 4C). Cyclic tensile loading significantly increased collagen I expression over the FS group at days 14 and 21. Osteocalcin expression remained low, near the detection limits of the real-time PCR assay, throughout the experiment (Fig. 4D).
FIG. 4.
BMSC gene expression. The mRNA expression levels of (A) collagen II, (B) aggrecan, (C) collagen I, and (D) osteocalcin were measured by real time reverse transcription polymerase chain reaction. *p < 0.05 for 10% vs. FS, n = 6/group.
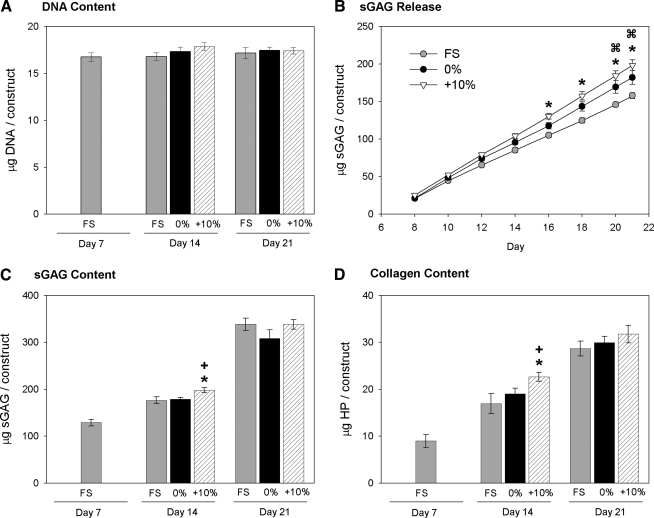
The DNA contents of the fibrin constructs did not significantly vary over the course of the 2 week loading period (Fig. 5A). The 0% and cyclic tension groups released significantly higher amounts of sGAG to the media (Fig. 5B). After 7 days, cyclic tensile loading significantly increased the total sGAG content within the constructs compared to the FS (12.5% higher) and 0% (10.6% higher) conditions; however, this effect was lost after 14 days of loading, with no significant differences in sGAG content between any of the loading conditions (Fig. 5C). Similarly, 7 days of cyclic tensile loading increased the total collagen content over FS (33.7% higher) and 0% (19.0% higher), but not after 14 days of loading.
FIG. 5.
Matrix accumulation with long-term loading. Fibrin gel constructs were analyzed for (A) DNA, (C) sGAG, and (D) collagen contents following culture under FS conditions, fixed at the day 7 length (0%), or cyclically loaded (+10%). (B) Conditioned medium samples were analyzed for sGAG content and reported as the cumulative sGAG released. *p < 0.05 for 10% vs. FS, +p < 0.05 for 10% vs. 0%,  p < 0.05 for 0% vs. FS. n = 6/group.
p < 0.05 for 0% vs. FS. n = 6/group.
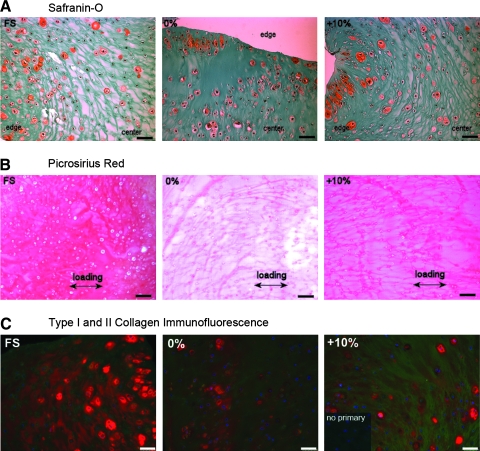
Safranin-O staining of day 21 cross sections indicated that the sulfated PGs were localized to the pericellular matrix of cells at the periphery of the constructs (Fig. 6A). Picrosirius red staining of day 14 transverse sections revealed distinct protein fibers (most likely fibrin and newly synthesized collagen) in the 0% and cyclic tension constructs, and there appeared to be a preferential alignment with the loading direction, whereas the free-swell samples had a denser, more homogeneous matrix (Fig. 6B).
FIG. 6.
Extracellular matrix composition and organization. Portions of the fibrin gel constructs were formalin fixed, paraffin embedded, and sectioned to 5 μm. (A) Cross sections from day 21 constructs from FS, unloaded (0%), or cyclically loaded (+10%) groups were stained for sGAG with Safranin-O and counterstained with Fast Green. (B) Transverse sections from day 14 samples were stained with Picrosirius Red. Arrows indicate the long axis and loading direction of the constructs. (C) Type I collagen (green) and type II collagen (red) were detected by immunofluorescence staining of day 21 cross sections, and inset shows the absence of staining without the primary antibody. All scale bars are 50 μm.
The specific collagen composition of the engineered constructs was examined by immunofluorescence staining for types I and II collagen. At day 21, type II collagen could be detected in the pericellular region in all samples, and the FS constructs displayed a higher density within the extracellular space. Consistent with the effects on gene expression, cyclic tension increased the amount of type I collagen detected within the gel (Fig. 6C). These changes in collagen composition were consistent with fibrocartilage tissues, which contain less type II collagen and more type I collagen than does articular cartilage.15
Discussion
The objective of the present study was to examine the potential for tensile loading to promote fibrochondrocytic differentiation of BMSCs within engineered tissue constructs. The results of our experiments indicate that tensile loading influences BMSC chondrogenesis, but the specific responses depend on the differentiation and/or developmental state of the cell-seeded constructs. At early time points, short periods of cyclic tension stimulated both protein and PG synthesis, whereas at later time points tension increased protein synthesis only. Similarly, extended loading periods had a greater effect on total collagen accumulation and specifically stimulated type I collagen gene expression and protein deposition. Some differences in the loading between the short-term and long-term loading studies should be considered in integrating the results of the two studies. Specifically, the short-term study involved a 50% duty cycle for 1 day, whereas the long-term loading study involved a 25% duty cycle applied for up to 2 weeks. Additionally, the long-term loading was initiated at a time point (7 days) in-between the two times examined in the short-term study (4 and 12 days), and additional studies would be required to identify the ideal preculture period before initiating loading. Nevertheless, the results of these studies provide initial evidence that the application of cyclic tensile loading during the initial stages of differentiation directs chondrogenic BMSCs toward a more fibrochondrocytic phenotype.
Our results are consistent with previous reports in which cyclic tensile strains stimulated expression and synthesis of type I collagen in MSCs.31,32 While most of these studies were conducted in the absence of chondrogenic stimuli, one recent report found that cyclic tension upregulated PG synthesis rates over the first 7 days of loading.33 This response in human cells is consistent with the observed increases in PG synthesis at day 4 in the present study. However, our longer loading study indicated that the early stimulatory effect may diminish as differentiation and tissue development progress. In contrast to the effects of tension, dynamic compressive loading has been reported to stimulate PG synthesis and chondrocytic gene expression in a variety of scaffold materials and for BMSCs from several different species, including bovine,23,34 equine,35 caprine,36 and human.37 The consistencies across these studies suggest that BMSC mechanobiology is similar for multiple species and aged donors; however, direct confirmation of these findings in humans will be required in future work. In one particular report, 7 days of preculture followed by 7 days of compressive loading increased chondrocytic gene expression for human MSCs in fibrin gels.37 The distinct responses to tension and compression for similar hydrogels and loading periods raise the possibility that different loading modes can direct tissue-specific differentiation.
The apparent changes in mechanosensitivity that occur during BMSC chondrogenesis may be due to several factors. For example, differences in gene regulation during differentiation could potentially influence the sensitivity of ECM genes to mechanical stimuli.34,38 Alternatively, changes in the cells' microenvironments that result from accumulation of a newly synthesized matrix could influence the response to loading. Several studies have shown that adhesion to specific proteins and integrin activation can modulate the effects of tensile strains.39,40 Similarly, accumulation of PGs can affect the availability of growth factors that may be released during deformational loading.41,42
Although cyclic tension increased sGAG and collagen accumulation after the first week of loading, these effects were lost after the second week. BMSCs may become less sensitive to mechanical stimuli at later time points due to matrix remodeling or stress shielding43; however, the elevated collagen I mRNA at day 21 suggests that the cells are still responsive to loading at this time point. It is also possible that the strong chondrogenic response to TGF-β1 and dexamethasone is the dominating factor regulating matrix synthesis at the later time points. Sustained exposure to TGF-β is not required to maintain chondrogenic differentiation and matrix production,44 and eliminating or reducing the TGF-β dose may enhance the sensitivity to tensile loading and allow mechanical stimuli to play a larger role in guiding construct development. On the basis of these findings, future studies are likely required to optimize the specific growth conditions and loading parameters for directing construct development during longer culture periods.
In addition to regulating matrix synthesis, tensile stresses had a marked effect on matrix organization during the long-term loading experiments. Constraining the gels from day 7 to 21 prevented cellular contraction of the matrix and resulted in a preferential alignment of the protein matrix with the loading direction. These types of physical cues have also been used to direct matrix orientation within collagen gels45 and provide a useful strategy for controlling ECM organization and mechanical anisotropy in engineered tissues. Constraining the constructs also increased sGAG released to the media, possibly reflecting an increased catabolic response to static tension. Such an effect would be consistent with previous reports where static stresses, both compressive and tensile, elicited catabolic degradation of the ECM.46–48
In summary, the results of this study indicate that combinations of chondrogenic stimuli and tensile stresses encourage a fibrochondrocyte-like phenotype and development of a fibrocartilage matrix. While the soluble chondrogenic factors promoted PG production and chondrocytic gene expression, cyclic tensile loading specifically stimulated collagen I expression. For the purposes of fibrocartilage engineering, tuning the relative levels of tensile forces in vitro, as well as the concentration, timing, or specific combinations of soluble chondrogenic stimuli, may be advantageous for modulating tissue development and mechanical properties. Moreover, graded levels of these different stimuli may provide control over the spatial variations in matrix composition and organization found in heterogeneous tissues such as the meniscus.
Footnotes
Portions of this work were presented in abstract form at the 2008 Annual Meeting of the Orthopaedic Research Society.
Acknowledgments
This work was funded by the Georgia Tech/Emory Center for the Engineering of Living Tissues, an Engineering Research Center program of the National Science Foundation under award number EEC 9731643, and by the National Institutes of Health through NIAMS R03AR048253. J.T.C., E.J.V., and J.K.M. received National Science Foundation Graduate Research Fellowships, E.J.V. received an ARCS Foundation fellowship, and C.G.W. was supported by the Cellular and Tissue Engineering Training Grant Program through National Institutes of Health award number T32 GM008433.
Disclosure Statement
No competing financial interests exist.
References
- 1.Drosos G.I. Pozo J.L. The causes and mechanisms of meniscal injuries in the sporting and non-sporting environment in an unselected population. Knee. 2004;11:143. doi: 10.1016/S0968-0160(03)00105-4. [DOI] [PubMed] [Google Scholar]
- 2.Hede A. Treatment of meniscal lesions in the knee. Epidemiological, clinical and experimental aspects. Dan Med Bull. 1993;40:317. [PubMed] [Google Scholar]
- 3.Shoemaker S.C. Markolf K.L. The role of the meniscus in the anterior-posterior stability of the loaded anterior cruciate-deficient knee. Effects of partial versus total excision. J Bone Joint Surg Am. 1986;68:71. [PubMed] [Google Scholar]
- 4.Messner K. Gao J. The menisci of the knee joint. Anatomical and functional characteristics, and a rationale for clinical treatment. J Anat. 1998;193(Pt 2):161. doi: 10.1046/j.1469-7580.1998.19320161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosnakovski D. Mizuno M. Kim G. Ishiguro T. Okumura M. Iwanaga T. Kadosawa T. Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells in pellet cultural system. Exp Hematol. 2004;32:502. doi: 10.1016/j.exphem.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Ma H.L. Hung S.C. Lin S.Y. Chen Y.L. Lo W.H. Chondrogenesis of human mesenchymal stem cells encapsulated in alginate beads. J Biomed Mater Res A. 2003;64:273. doi: 10.1002/jbm.a.10370. [DOI] [PubMed] [Google Scholar]
- 7.Steinert A. Weber M. Dimmler A. Julius C. Schutze N. Noth U. Cramer H. Eulert J. Zimmermann U. Hendrich C. Chondrogenic differentiation of mesenchymal progenitor cells encapsulated in ultrahigh-viscosity alginate. J Orthop Res. 2003;21:1090. doi: 10.1016/S0736-0266(03)00100-1. [DOI] [PubMed] [Google Scholar]
- 8.Aung T. Miyoshi H. Tun T. Ohshima N. Chondroinduction of mouse mesenchymal stem cells in three-dimensional highly porous matrix scaffolds. J Biomed Mater Res. 2002;61:75. doi: 10.1002/jbm.10144. [DOI] [PubMed] [Google Scholar]
- 9.Johnstone B. Hering T.M. Caplan A.I. Goldberg V.M. Yoo J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 10.Worster A.A. Brower-Toland B.D. Fortier L.A. Bent S.J. Williams J. Nixon A.J. Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-beta1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J Orthop Res. 2001;19:738. doi: 10.1016/S0736-0266(00)00054-1. [DOI] [PubMed] [Google Scholar]
- 11.Walsh C.J. Goodman D. Caplan A.I. Goldberg V.M. Meniscus regeneration in a rabbit partial meniscectomy model. Tissue Eng. 1999;5:327. doi: 10.1089/ten.1999.5.327. [DOI] [PubMed] [Google Scholar]
- 12.Murphy J.M. Fink D.J. Hunziker E.B. Barry F.P. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 13.Caplan A.I. Dennis J.E. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 14.Yamasaki T. Deie M. Shinomiya R. Yasunaga Y. Yanada S. Ochi M. Transplantation of meniscus regenerated by tissue engineering with a scaffold derived from a rat meniscus and mesenchymal stromal cells derived from rat bone marrow. Artif Organs. 2008;32:519. doi: 10.1111/j.1525-1594.2008.00580.x. [DOI] [PubMed] [Google Scholar]
- 15.Eyre D.R. Muir H. The distribution of different molecular species of collagen in fibrous, elastic and hyaline cartilages of the pig. Biochem J. 1975;151:595. doi: 10.1042/bj1510595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roughley P.J. McNicol D. Santer V. Buckwalter J. The presence of a cartilage-like proteoglycan in the adult human meniscus. Biochem J. 1981;197:77. doi: 10.1042/bj1970077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mow V.C. Ratcliffe A. Structure and function of articular cartilage and meniscus. In: Mow V.C., editor; Hayes W.C., editor. Basic Orthopaedic Biomechanics. Philadelphia, PA: Lippincott Williams & Wilkins; 1997. pp. 113–77. [Google Scholar]
- 18.Wildey G.M. McDevitt C.A. Matrix protein mRNA levels in canine meniscus cells in vitro. Arch Biochem Biophys. 1998;353:10. doi: 10.1006/abbi.1998.0647. [DOI] [PubMed] [Google Scholar]
- 19.Wilson C.G. Nishimuta J.F. Levenston M.E. Chondrocytes and meniscal fibrochondrocytes differentially process aggrecan during de novo extracellular matrix assembly. Tissue Eng Part A. 2009;15:1513. doi: 10.1089/ten.tea.2008.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fithian D.C. Kelly M.A. Mow V.C. Material properties and structure-function relationships in the menisci. Clin Orthop Relat Res. 1990;252:19. [PubMed] [Google Scholar]
- 21.Vanderploeg E.J. Imler S.M. Brodkin K.R. Garcia A.J. Levenston M.E. Oscillatory tension differentially modulates matrix metabolism and cytoskeletal organization in chondrocytes and fibrochondrocytes. J Biomech. 2004;37:1941. doi: 10.1016/j.jbiomech.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 22.Yang G. Crawford R.C. Wang J.H. Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions. J Biomech. 2004;37:1543. doi: 10.1016/j.jbiomech.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Mouw J.K. Connelly J.T. Wilson C.G. Michael K.E. Levenston M.E. Dynamic compression regulates the expression and synthesis of chondrocyte-specific matrix molecules in bone marrow stromal cells. Stem Cells. 2007;25:655. doi: 10.1634/stemcells.2006-0435. [DOI] [PubMed] [Google Scholar]
- 24.Mauck R.L. Yuan X. Tuan R.S. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage. 2006;14:179. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 26.Farndale R.W. Buttle D.J. Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 27.Kim Y.J. Sah R.L. Doong J.Y. Grodzinsky A.J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174:168. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 28.Chomczynski P. Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 29.Connelly J.T. Garcia A.J. Levenston M.E. Inhibition of in vitro chondrogenesis in RGD-modified three-dimensional alginate gels. Biomaterials. 2007;28:1071. doi: 10.1016/j.biomaterials.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Connelly J.T. Wilson C.G. Levenston M.E. Characterization of proteoglycan production and processing by chondrocytes and BMSCs in tissue engineered constructs. Osteoarthritis Cartilage. 2008;16:1092. doi: 10.1016/j.joca.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juncosa-Melvin N. Matlin K.S. Holdcraft R.W. Nirmalanandhan V.S. Butler D.L. Mechanical stimulation increases collagen type I and collagen type III gene expression of stem cell-collagen sponge constructs for patellar tendon repair. Tissue Eng. 2007;13:1219. doi: 10.1089/ten.2006.0339. [DOI] [PubMed] [Google Scholar]
- 32.Altman G.H. Horan R.L. Martin I. Farhadi J. Stark P.R. Volloch V. Richmond J.C. Vunjak-Novakovic G. Kaplan D.L. Cell differentiation by mechanical stress. FASEB J. 2002;16:270. doi: 10.1096/fj.01-0656fje. [DOI] [PubMed] [Google Scholar]
- 33.McMahon L.A. Reid A.J. Campbell V.A. Prendergast P.J. Regulatory effects of mechanical strain on the chondrogenic differentiation of MSCs in a collagen-GAG scaffold: experimental and computational analysis. Ann Biomed Eng. 2008;36:185. doi: 10.1007/s10439-007-9416-5. [DOI] [PubMed] [Google Scholar]
- 34.Mauck R.L. Byers B.A. Yuan X. Tuan R.S. Regulation of cartilaginous ECM gene transcription by chondrocytes and MSCs in 3D culture in response to dynamic loading. Biomech Model Mechanobiol. 2007;6:113. doi: 10.1007/s10237-006-0042-1. [DOI] [PubMed] [Google Scholar]
- 35.Kisiday J.D. Frisbie D.D. McIlwraith C.W. Grodzinsky A.J. Dynamic compression stimulates proteoglycan synthesis by mesenchymal stem cells in the absence of chondrogenic cytokines. Tissue Eng Part A. 2009;15:2817. doi: 10.1089/ten.tea.2008.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang C.Y. Hagar K.L. Frost L.E. Sun Y. Cheung H.S. Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells. 2004;22:313. doi: 10.1634/stemcells.22-3-313. [DOI] [PubMed] [Google Scholar]
- 37.Li Z. Yao S.J. Alini M. Stoddart M.J. Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin-polyurethane composites is modulated by frequency and amplitude of dynamic compression and shear stress. Tissue Eng Part A. 2009. [Epub ahead of print.] [DOI] [PubMed]
- 38.Xie J. Han Z.Y. Matsuda T. Mechanical compressive loading stimulates the activity of proximal region of human COL2A1 gene promoter in transfected chondrocytes. Biochem Biophys Res Commun. 2006;344:1192. doi: 10.1016/j.bbrc.2006.03.243. [DOI] [PubMed] [Google Scholar]
- 39.Katsumi A. Naoe T. Matsushita T. Kaibuchi K. Schwartz M.A. Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J Biol Chem. 2005;280:16546. doi: 10.1074/jbc.C400455200. [DOI] [PubMed] [Google Scholar]
- 40.MacKenna D.A. Dolfi F. Vuori K. Ruoslahti E. Extracellular signal-regulated kinase and c-Jun NH2-terminal kinase activation by mechanical stretch is integrin-dependent and matrix-specific in rat cardiac fibroblasts. J Clin Invest. 1998;101:301. doi: 10.1172/JCI1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Droguett R. Cabello-Verrugio C. Riquelme C. Brandan E. Extracellular proteoglycans modify TGF-beta bio-availability attenuating its signaling during skeletal muscle differentiation. Matrix Biol. 2006;25:332. doi: 10.1016/j.matbio.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 42.Tschumperlin D.J. Dai G. Maly I.V. Kikuchi T. Laiho L.H. McVittie A.K. Haley K.J. Lilly C.M. So P.T. Lauffenburger D.A. Kamm R.D. Mechanotransduction through growth-factor shedding into the extracellular space. Nature. 2004;429:83. doi: 10.1038/nature02543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knight M.M. Lee D.A. Bader D.L. The influence of elaborated pericellular matrix on the deformation of isolated articular chondrocytes cultured in agarose. Biochim Biophys Acta. 1998;1405:67. doi: 10.1016/s0167-4889(98)00102-5. [DOI] [PubMed] [Google Scholar]
- 44.Huang A.H. Stein A. Tuan R.S. Mauck R.L. Transient exposure to TGF-beta3 improves the mechanical properties of msc-laden cartilage constructs in a density dependent manner. Tissue Eng Part A. 2009;15:3461. doi: 10.1089/ten.tea.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomopoulos S. Fomovsky G.M. Holmes J.W. The development of structural and mechanical anisotropy in fibroblast populated collagen gels. J Biomech Eng. 2005;127:742. doi: 10.1115/1.1992525. [DOI] [PubMed] [Google Scholar]
- 46.Fitzgerald J.B. Jin M. Dean D. Wood D.J. Zheng M.H. Grodzinsky A.J. Mechanical compression of cartilage explants induces multiple time-dependent gene expression patterns and involves intracellular calcium and cyclic AMP. J Biol Chem. 2004;279:19502. doi: 10.1074/jbc.M400437200. [DOI] [PubMed] [Google Scholar]
- 47.Imler S.M. Doshi A.N. Levenston M.E. Combined effects of growth factors and static mechanical compression on meniscus explant biosynthesis. Osteoarthritis Cartilage. 2004;12:736. doi: 10.1016/j.joca.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Upton M.L. Chen J. Guilak F. Setton L.A. Differential effects of static and dynamic compression on meniscal cell gene expression. J Orthop Res. 2003;21:963. doi: 10.1016/S0736-0266(03)00063-9. [DOI] [PubMed] [Google Scholar]