Abstract
A significant challenge of tissue engineering is to build tissues whose size is not limited by diffusion. We are investigating the use of scaffold-free lumen containing toroid-shaped microtissues as minimal building units. Monodispersed H35 cells, a rat hepatocyte cell line, were seeded onto micromolded agarose, forming self-assembled multicellular toroids within 48 h. Toroid and lumen diameter were easily controlled by micromold design, and toroid thickness was controlled by seeding density. When harvested, toroids were stable, but underwent predictable changes over time with their lumens narrowing. When brought into contact, these building units fused in the x–y plane, forming a double-lumen structure, as well as the z plane, forming a tubular structure, which completed within 72 h. Large, multi-luminal structures were assembled by multidimensional fusion of many toroids. Toroid settling was not entirely random, with most toroids lying flat with their lumens oriented along the z axis. The rapid production of toroid building units of controlled dimension and lumen size that undergo predictable changes and that can be fused to form larger structures is a step closer to tissue engineering large porous three-dimensional tissues with high cell density.
Introduction
A major challenge to tissue engineering is the in vitro fabrication of large tissue constructs with high densities of living cells, similar to natural organs and tissues.1,2 Hurdles are numerous, notably that the diffusion of oxygen, nutrients, and metabolic waste products limits cellular tissues to thicknesses of ∼100–200 μm to maintain viability.3,4 In natural organs and tissues, a branching vascular supply ensures that all cells are close to blood vessels.1,5 Tissue engineering approaches to this problem have included efforts to make an artificial vascular tree by microfabrication of degradable polymers,6 the assembly of modules of cells and collagen,7–10 and the layer-by-layer printing of cells and extracellular-matrix-like materials.11–16
To date, most successful tissue engineering applications have used thin tissues (<2 mm), in which transport of oxygen, nutrients, and metabolic waste critical for cell viability occurs by diffusion.1 In highly cellular tissues, this distance is thought to be ∼100–200 μm, challenging the field of tissue engineering to design large tissue constructs that are or can become vascularized.3,17 There are several approaches to this challenge, and this article presents data on the self-assembly and stability of a scaffold-free cellular toroid and its use as a building unit.
Scaffold-free cellular microtissues in the shape of spheroids have been used as building units.12 Spheroids of Chinese hamster ovary cells (∼500 μm diameter) prepared by extruding a larger cell pellet through a capillary tube were harvested and added to a ring-shaped mold of collagen gel. After 4–5 days, the spheroids fused to form a single large toroid (∼2.3 mm diameter), and cells in the spheroids adhered to and migrated into the surrounding collagen gel. Another group used the hanging drop method to prepare smaller spheroids of myoblasts or chondrocytes and fused them to make large macrotissue patches (mm sized).18 Likewise, spheroids of myofibroblasts, coated with human umbilical vein endothelial cells, have been fused and formed a capillary network within the macrotissue that could connect to the host vasculature after transplantation.18 Our group has used spheroids prepared in micromolded agarose as building units to control cell position, and to form small toroidal and honeycomb-shaped structures.19
Previously, we have presented a versatile approach to forming multicellular microtissues of defined sizes and geometries.20,21 Monodispersed cells seeded onto micromolds of agarose settle into the small recesses, where they are unable to attach to the agarose, allowing cell-to-cell adhesion to direct cells to aggregate and self-assemble a three-dimensional multicellular microtissue. This occurs in the absence of any added scaffold or extracellular matrix protein and is complete within 24–48 h. The shape of the microtissue is controlled by the shape of the recesses that are micromolded into the agarose. It had been thought that cells would self-assemble only a spheroid, in which surface area and surface free energy are minimized; however, we have used agarose micromolds to direct the self-assembly of complex shapes such as toroids.20,21
In this article, we investigate the use of multicellular toroids as building units, notably, the interplay of micromold design and cell behavior in toroid production, and the tissue fusion process using toroids as minimal building units. The toroid building unit, with its ring of cells in high density and open lumen space, offers possibilities for building a large tissue construct with both a high cell density and a network of interconnected lumens. We show that toroid and lumen diameters are easily controlled by micromold design, and that toroid thickness is controlled by the number of monodispersed cells seeded. When harvested, the toroids are intact and undergo predictable changes to their size and shape over time. Moreover, toroids can fuse with one another in a process that is complete within 72 h, and toroids can be used as building units to make a large multilayered multitorus structure.
Materials and Methods
Design, fabrication, and casting of micromolds
Micromolds were fabricated as previously described.20 Briefly, micromolds were designed using computer-assisted design (CAD; Solid Works Corporation, Concord, MA). Wax prototypes from the CAD files were produced with a ThermoJet® rapid prototyping machine (3D Systems Corporation, Valencia, CA) and then replicated in polydimethylsiloxane (PDMS; Dow Corning, Midland, MI).
Six different designs with toroid-shaped recesses were fabricated to fit in a six-well plate. Four micromolds were designed with peg diameters of 400, 600, 800, or 1000 μm, with circular track widths of 400 μm and peg heights of 800 μm, and 100, 81, 64, or 25 features/gel, respectively. Two micromolds were designed with circular track widths of 600 or 800 μm, with peg diameters of 600 μm and peg heights of 800 μm, and 49 or 36 features/gel, respectively.
Agarose gels were cast from PDMS micromolds. Powder Ultrapure© Agarose (Invitrogen, Carlsbad, CA) was autoclaved and then dissolved via heating in sterile water to 2% (weight/volume). Molten agarose (2.75 mL) was pipetted into each PDMS micromold, and air bubbles were removed. After setting, gels were removed and transferred to six-well plates where they were equilibrated with the tissue culture medium.20,21
Side-view polyacrylamide gels with a single row of 12 recesses were cast directly from the wax prototypes to allow observation in the vertical plane. To produce gels, 10 mL of 35% prepolymer solution containing acrylamide-bis-acrylamide (29:1 mix ratio), Tris buffer (pH 6.8), and Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) was degassed and polymerized by addition of 50 μL of 10% ammonium persulfate and 100 μL N,N,N,N-tetramethylethylenediamine. Gels were equilibrated overnight, rinsed with 3 mL of the medium, and stored in the medium until use.20,21
Cell culture, toroid assembly, and fusion
The rat hepatocyte cell line (H35)21 was expanded in DMEM supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA) and 1% penicillin/streptomycin (Sigma, St. Louis, MO) and maintained at 37°C, 5% CO2.19–21 Cells were trypsinized, counted, and re-suspended in 200 μL and pipetted into the rectangular recess. An agarose gel with 81 features was seeded with 2 × 106 cells producing toroids with ∼24,700 cells/toroid. For the polyacrylamide gels, 70 μL of cell suspension was used (3.0 × 105 cells; ∼24,700 cells/toroid). Gels were incubated for 20 min before 3 mL of the medium was added. The medium was exchanged every other day.20,21
Toroids were self-assembled for 48 h and then harvested by inverting the gels in a new dish with 3 mL of the medium and gently centrifuged (700 rpm, 1 min). Harvested toroids were transferred to a 24-well plate whose wells were coated with agarose and monitored for 10 days, with images captured daily. Toroid width and lumen diameter were measured using ImageJ (National Institutes of Health, Bethesda, MD).
For stacking experiments, individual toroids were aspirated into a 1 mL micropipette and carefully transferred to a polyacrylamide gel already containing toroids that had self-assembled for 48 h. This gel contained 12 toroid-shaped recesses (trough 400 μm wide) with a cone-shaped peg (600 μm diameter, height 600 μm, and slope 85°). With the aid of a dissecting microscope, toroids were carefully stacked on the toroid self-assembled around the conical peg. Gels were given a fresh medium and returned to the incubator.
Large, multiluminal structures were assembled by multidimensional fusion of numerous toroids. Toroids self-assembled for 48 h were harvested and seeded into another agarose gel containing one large recess with a rounded bottom (6 mm diameter) that had been equilibrated in the culture medium. The medium was exchanged every other day.
Microscopy
Bright-field, phase-contrast, and fluorescent images were obtained using a Carl Zeiss Axio Observer Z1 equipped with an AxioCam MRm camera (Carl Zeiss MicroImaging, Thornwood, NY) and captured using Axiovision Software. Bright-field side-view images were captured using a Mitutoyo FS-110 microscope modified to lie horizontally, equipped with a Nikon CoolPix 990 digital camera. Side-view fluorescent images were captured with the aid of a small mirror (Thorlabs, Newton, NJ) placed adjacent to the gel.
For scanning electron micrographs, toroids were fixed in Karnofsky's solution (phosphate-buffered saline [PBS] supplemented with 2% paraformaldehyde/2% glutaraldehyde). Samples were critical point-dried (LADD Research, Williston, VT) and sputter-coated with gold/palladium (Emitech, Sussex, United Kingdom) and imaged using a Hitachi S-2700 SEM (Tokyo, Japan).
Cell tracker and live/dead viability fluorescent staining
Cell position was assessed using Cell Tracker chloromethylfluorescein dyes (Invitrogen). Cell Tracker red (CMTPX) was prepared by dissolving 50 μg of the stain in 7.1 μL of dimethyl sulfoxide (Acros, Geel, Belgium) and 14.1 mL of serum-free DMEM. Cell Tracker green (CMFDA) was prepared by dissolving 50 μg of the stain in 10.8 μL of dimethyl sulfoxide and 10.8 mL of serum-free DMEM. Cells on tissue culture plates were stained by incubation for 45 min at 37°C. Green and red dyes were observed at an excitation/detection of 492/517 nm and 577/602 nm, respectively.
Cell viability was assessed with LIVE/DEAD® Viability/Cytotoxicity Kit (L3224, Invitrogen). The medium was removed, gels were rinsed twice with 3 mL of PBS, and 300 μL of PBS containing 2 μM calcein-acetoxymethyl (AM) and 4 μM ethidium homodimer was added to the seeding chamber. Plates were protected from light and incubated at room temperature for 45 min, and then observed using wide-field fluorescence microscopy.
Statistics
Measurements were performed in triplicate, with results presented as the mean of all triplicate means. Error bars represent standard deviation. Statistical significance was evaluated using a Student's t-test at a probability of p = 0.05.
Results
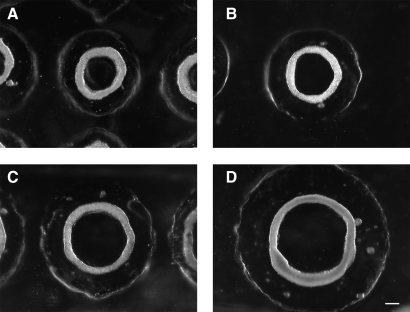
To form toroid-shaped microtissues, monodispersed H35 cells were seeded onto the agarose gels where they self-assembled multicellular toroids. To determine whether the diameter of the toroid and its lumen could be controlled by micromold design, we tested four different diameters of the peg (400, 600, 800, and 1000 μm) (Fig. 1). The width of the circular trough was kept constant (400 μm) and each gel was seeded with the same number of cells (2 × 106 cells). By 48 h, the toroids had contracted around the agarose peg. Thus, the diameter of each toroid and its lumen were dependent on the diameter of the peg.
FIG. 1.
Toroid diameter and lumen diameter can be controlled by micromold design. Monodispersed cells (2 × 106) were seeded onto agarose micromolds containing toroid-shaped recesses of varying dimensions. The width of the circular tracks were the same (400 μm), but the diameter of the peg varied, 400, 600, 800, or 1000 μm (A–D). Forty-eight hours after seeding, monodispersed cells self-assembled multicellular toroids that contracted around the peg. Scale bar is 200 μm.
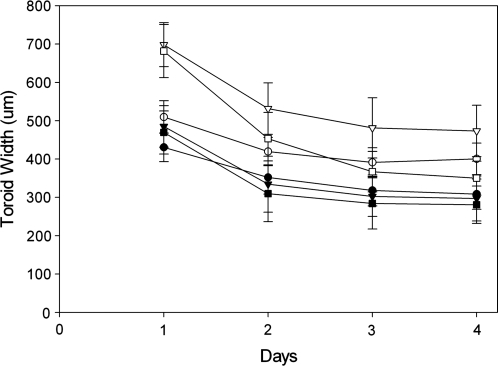
To determine whether the toroid width could be controlled by the width of the circular track, we designed features whose pegs were the same (600 μm), but whose circular tracks varied in width (400, 600, and 800 μm) (Fig. 2). Each gel had different numbers of toroidal recesses, with cell seeding number normalized to two unique circumferences of the peg (20 or 40 cells/μm of circumference). As the cells self-assembled, the widths (x–y) of the toroids decreased each day. At both seeding densities, toroid width was independent of the circular track width. Additionally, doubling the number of cells per micrometer of circumference did not double toroid width, suggesting that cells contribute to the z-thickness of the toroid as it self-assembles.
FIG. 2.
Toroid width is independent of trough width. Monodispersed cells (20 [closed symbols] or 40 [open symbols] cells/μm of circumference) were seeded onto micromolds containing toroid-shaped recesses whose peg had the same diameter (600 μm) but whose circular tracks had varying widths of 400 (•, ○), 600 (▾, ▿), or 800 (▪, □) μm. Width of the toroids as a function of time is plotted for each design. n = 39 (400 μm, 20 cells/μm, •,), 47 (400 μm, 40 cells/μm, ○), 45 (600 μm, 20 cells/μm, ▾), 30 (600 μm, 40 cells/μm, ▿), 26 (800 μm, 20 cells/μm, ▪), and 11 (800 μm, 40 cells/μm, □). Error bars represent standard deviation.
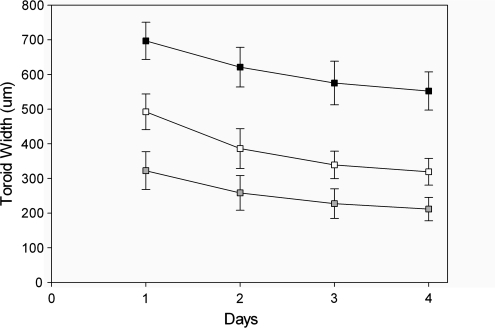
To determine whether the width of the toroids could be controlled by the cell seeding number, we picked one toroid design (trough width 600 μm, peg diameter 600 μm) and seeded it with varying numbers of cells (10 20 or 50 cells/μm of circumference) (Fig. 3). As the cells self-assembled, the widths of the toroids decreased. When normalized, the widths of the toroids with 10 and 20 cells/μm of circumference had decreased ∼35% by day 4, whereas the toroids with 50 cells/μm of circumference decreased only ∼20%, suggesting that an upper threshold had been reached for efficient self-assembly.
FIG. 3.
Toroid width is dependent on the number of cells seeded into the micromold. Toroids were self-assembled from monodispersed cells seeded at three unique seeding densities of 10 (▪), 20 (□), and 50 (▪) cells per micrometer of circumference, and observed daily for a period of 4 days. Error bars represent standard deviation.
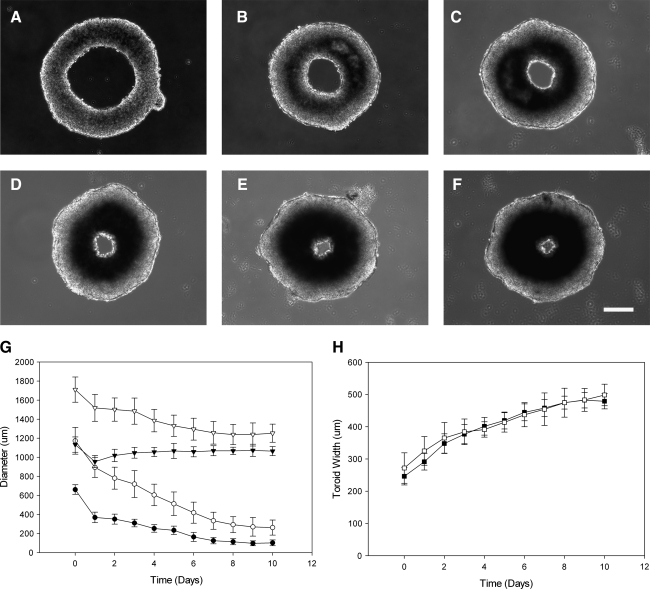
To determine their stability, toroids were harvested and placed on flat agarose and allowed to contract over time. This was done for toroids assembled in micromolds with a 600 μm peg diameter (600 μm toroid) or 1000 μm peg diameter (1000 μm toroid), both with 400 μm circular track widths. The diameters of the toroid, its lumen, and the width of the toroid were measured over time (Fig. 4). At 10 days, the outer diameter of the 600 μm toroid had decreased 6% and its lumen diameter decreased 85%, with the largest decrease (44%) occurring in the first 24 h after harvest. Thereafter, outer diameter decreased at a rate of approximately 1% per day and lumen diameter decreased at a rate of about 16% per day, until day 7, when the rate slowed. Likewise, the outer diameter of the 1000 μm toroids decreased 27% and the lumen diameter decreased 77% by 10 days, with the largest decrease (24%) also occurring in the first 24 h after harvesting. Thereafter, outer diameter decreased at a rate of approximately 2% per day and lumen diameter decreased at a rate of about 15%, per day, until day 7, when the rate slowed.
FIG. 4.
Toroids are stable building units, whose lumens narrow with predictable kinetics. Toroids were self-assembled and cultured for 48 h before being harvested from the micromolds. Toroids were placed on flat nonadherent agarose and observed for 10 days. Representative bright-field images of toroids self-assembled in recesses with a 600 μm peg diameter and 400 μm circular track width are shown at days 0, 2, 4, 6, 8, and 10 (A–F). Lumen diameter (•, ○) and total diameter (▾, ▿) were measured as a function of time for toroids self-assembled in recesses with a 600 μm peg diameter (closed symbols) and in recesses with a 1000 μm peg diameter (open symbols) (G). Toroid width for the same samples (▪, □) was also measured as a function of time (H). Error bars represent standard deviation. Scale bar is 200 μm.
In contrast, the widths of both the 600 and 1000 μm toroids increased. After 10 days, the widths of the 600 and 1000 μm toroids had increased 95% and 83%, respectively. The width of the 600 and 1000 μm toroids increased at rates of 7.1% and 6.4% per day, respectively. Despite their different starting diameters and different cell densities along their circumferences (600 and 1000 μm toroids; 13 and 25 cells/μm of circumference, respectively), the widths of the 600 and 1000 μm toroids increased with similar kinetics. In addition to increases in width, the 600 and 1000 μm toroids also increased in thickness (z dimension). This was evident from the bright-field images, which showed a decrease in transparency due to an increase in cell density. Increased cell density was not uniformly distributed around the toroid and was found closer to the lumen rather than the outer rim.
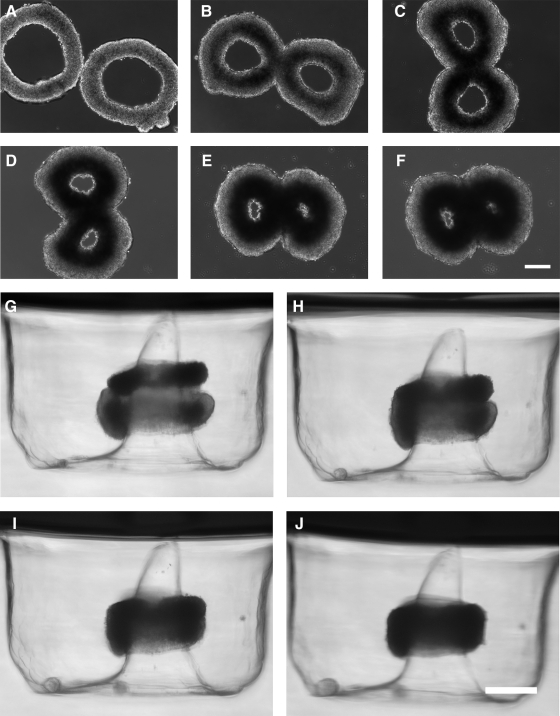
To determine whether the outer circumference of toroids was capable of undergoing fusion, two toroids were placed apposed on flat agarose (Fig. 5). After making contact, the toroids fused, forming a double-lumen structure. Similar to the behavior of individual units, toroid and lumen diameters decreased, and toroid width increased over time. Additionally, cell density, which was initially uniform, became more dense toward the lumen for both units. However, at the junction of the two units, the areas of high cell density fused to form a continuous internal figure-eight structure of high cell density.
FIG. 5.
Toroids undergo fusion in multiple dimensions. Toroids were self-assembled and cultured for 48 h before being harvested and tested for fusion. Two toroids placed opposed to one another on flat nonadhesive agarose fused in the x–y plane into a double-lumen structure. Bright-field images at days 0, 2, 4, 6, 8, and 10 are shown (A–F). A harvested toroid was stacked onto another that had previously self-assembled on a conical peg. Both toroids fused in the z plane. Bright-field side-view images at 0, 24, 48, and 72 h are shown (G–J). Scale bars are 200 μm.
To determine whether toroids could be fused on their top and bottom surfaces, we used a second micromold to guide fusion. Toroids were harvested after 48 h and transferred to a second micromold containing toroid-shaped recesses, but with a cone-shaped peg, in which another toroid had also self-assembled for 48 h. Toroids from the first micro-mold were carefully stacked on the second toroid, and side-view bright-field images taken to observe fusion (Fig. 5).
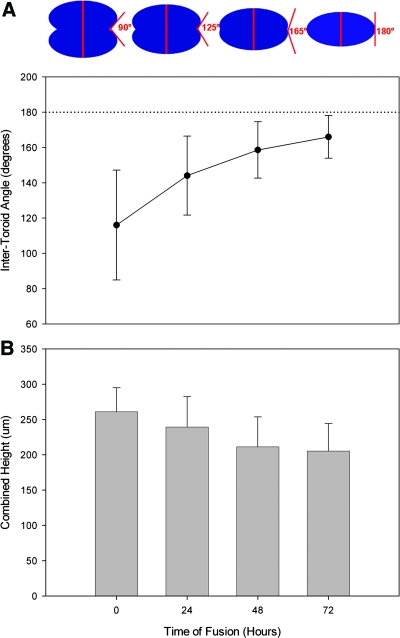
To quantify the kinetics of fusion, the intertoroid angle was measured on each oblique side with an angle of 180° representing total fusion (Fig. 6). Variance in the intertoroid angle was greatest at the start of the experiment due to the challenges of perfectly aligning the stacked toroids. As fusion progressed, the variance declined as the intertoroid angle increased toward 180°. The combined z height or thickness of the stacked toroids was also measured. Thickness of the toroids harvested from the agarose micromold was 113.6 ± 21.2 μm and 147.5 ± 23.9 μm from the polyacrylamide micromold. After 72 h, the thickness of two stacked toroids was 205 μm, a decrease of 21%. Thickness decreased 10% per day for the first 48 h, after which it remained stable.
FIG. 6.
Stacked toroids undergo fusion. From side-view bright-field images, the kinetics of fusion of stacked toroids were assessed by measuring the intertoroid angle (diagram) (A). A reference dotted line is shown at 180°. The change in z thickness of the stacked toroids was also measured from the side-view images (B). Error bars represent standard deviation. Color images available online at www.liebertonline.com/ten.
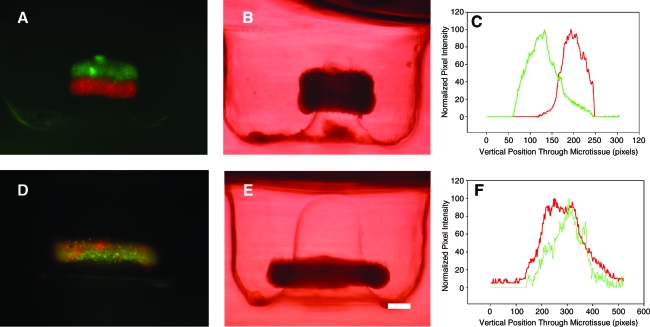
To determine the extent of cell mixing between building units, the stacking assay was repeated using fluorescently labeled toroids (Fig. 7). A red-labeled toroid was stacked on a green-labeled toroid, and fluorescent side-view images were taken after 48 h of fusion. The images and a representative vertical profile of fluorescence intensity indicated fusion, but minimal cell mixing between toroids.
FIG. 7.
Toroids fuse and undergo minimal cell mixing. One fluorescently labeled red and one green toroid were cultured for 48 h, stacked, and fused for 48 h. Fluorescent (A) and bright-field images (B) demonstrate fusion, but minimal cell mixing as confirmed by the fluorescence intensity profile (C). A control toroid (1:1 mix of monodispersed red and green cells) was cultured for 96 h. Fluorescent (D) and bright-field images (E) demonstrate mixing of fluorescent signals as confirmed by the fluorescence intensity profile (F). Scale bar is 200 μm. Color images available online at www.liebertonline.com/ten.
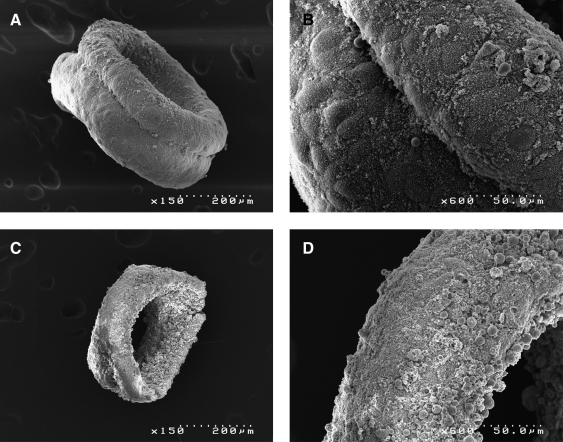
Scanning electron micrograph was used to observe fusion of toroids (Fig. 8). After 12 h of fusion, the images show a fusion furrow at the junction of the two toroids. At 72 h, a furrow can no longer be identified and the units have undergone a seamless fusion.
FIG. 8.
Scanning electron micrographs of toroid fusion. Toroids were cultured for 48 h, and allowed to fuse around a peg, and then fixed approximately 12 h (A, B) and 72 h (C, D) after initial fusion. An obvious furrow is observed throughout between initially distinct toroids after 12 h, and appears as a coherent tissue after 72 h.
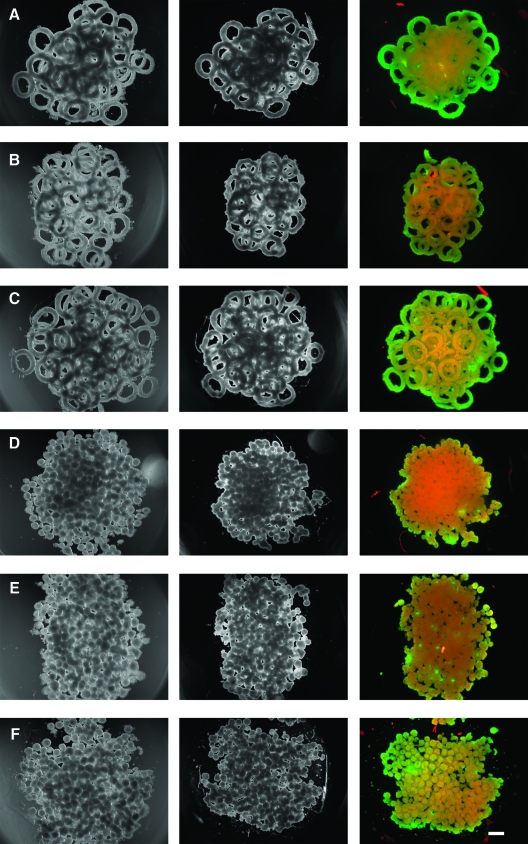
To determine whether toroids could be used to form a larger structure, toroids (600 μm) were harvested (micromolds containing 81 toroids each) after 48 h of self-assembly. The toroids were combined and added to a single large well cast in agarose (Fig. 9). The toroids settled and formed a multilayered pile of toroids at the bottom of the well. Settling was not entirely random, with most toroids lying flat with their lumens oriented along the z axis. Seven days later in wells that were not perfused, the toroids and a control of spheroids (∼200 μm, micromolds containing 822 spheroids each) were stained for viable cells. Seeding density was kept uniform for toroid and spheroid gels, with a total seeding density of 2 × 106 cells/gel, with comparable yields upon harvesting. Compared to the spheroids, the toroids showed more evidence of luminal space and increased cell viability.
FIG. 9.
Toroids will fuse to form large multiluminal structures. Toroids (A–C) (∼75, 600 μm) or spheroids (D–F) (∼800, 200 μm) were harvested after 48 h of self-assembly and added to a single large well cast in agarose. The microtissues settled and formed a pile at the bottom of the well at day 0 (left column). Toroids overlapped randomly, but their lumens were oriented along the z axis. After 7 days (center column) samples were stained for viability with a live/dead assay (right column), which revealed higher proportions of viable cells (green) than dead cells (red) in toroid samples, as compared to spheroid samples. Scale bar is 500 μm. Color images available online at www.liebertonline.com/ten.
Discussion
Several groups are using scaffolds as part of a biofabrication or bioprinting approach.11,13–16 This approach is a modification of ink jet printing or rapid prototyping technologies, with tissue constructs made layer-by-layer by printing monodispersed cells or spheroids along with an extracellular-matrix-like material. Computer control of the deposition process facilitates the fabrication of large, complex-shaped structures. In another scaffold-based approach, cells are cast within small (submillimeter) gels and these are used as building units.7–10 HepG2, a human hepatoma cell line, was encapsulated in cylindrical collagen gels and the capsules were subsequently coated with human umbilical vein endothelial cells. These building units were packed into a larger vessel where they created a luminal network via the space between the building units, with the endothelial cells reducing thrombogenicity when the construct was perfused with blood in vitro.7–10 Another group has shown that the shape of cell-containing microgels can help direct the assembly of these building units and their orientations.22
In this article, we used agarose micromolds to direct the self-assembly of monodispersed cells into toroid-shaped multicellular microtissues, and we show that these scaffold-free toroids can be used as building units to form larger tissue structures by the process of tissue fusion. Further, lumen and toroid size can be easily controlled, and their fusion proceeds with predictable kinetics. Unlike the spheroid shape, where diffusion limits its maximum size, toroid building units can be made over a range of diameters without compromising cell viability, provided the thickness of the tissue does not exceed the diffusion limit. The viability of the toroid stems from its open lumen with access to the medium. Although the outermost diameter of the toroid is large, the cross section of the cellular portion of the toroid does not exceed the critical diffusion distance needed to maintain cell viability. If the same number of cells were self-assembled into a single spheroid, its diameter would exceed the critical distance needed to maintain the viability of the cells in the core of the spheroid. The fundamental shape of a toroid, with its open lumen structure, provides new possibilities as a building unit that when fused can produce dense cellular tissues with a network of interconnected lumens.
There are straightforward ways to control the size of the toroid and its lumen. The first step is the design of the micromold, and we were able to produce micromolds where cells self-assembled toroids with lumens that ranged in diameter from 1000 μm down to 400 μm. Self-assembly by monodispersed cells is rapid and occurs within 48 h. Lumen size is controlled by the diameter of the agarose peg in the micromold, and 300–400 μm is the smallest diameter that can be reliably made with our rapid prototyping machine. Other technologies, such as photolithography, could make molds with smaller pegs that could create toroids with even smaller lumens.
Thickness of the toroid can be controlled by the number of monodispersed cells seeded into the micromolds. After seeding, the cells self-assemble and contract around the peg, forming the toroid-shaped microtissue, and x–y thickness could be varied from ∼250 to ∼600 μm after 4 days of self-assembly (Fig. 2), depending on the number of cells seeded. There are a minimal number of cells needed to form a toroid, which is probably dependent on cell type and mold design, but we estimate from other studies that this number is in the range of 5–10 cells/μm of circumference of the peg.
Once released from the micromolds, the toroids remained intact, but underwent predictable changes to their size and shape. These changes are mediated by cellular processes, and an understanding of the types of changes that occur as well as their kinetics is another level of control over the size and shape of the toroid building unit. After release from the micromold, the lumen diameter of the 600 μm toroid narrowed to a minimum of 100 μm after 10 days, whereas its outer diameter decreased by only 6%. The largest change in lumen diameter (44%) occurred within the first 24 h and is probably due to the release of cellular tension built up as the toroid contracts around the peg. These forces of self-assembly involve not only cell surface adhesion molecules, but also the action of the cytoskeleton.23,24 The H35 toroids do not undergo complete closure in the time frame measured because they are a cell type that has a slow rate of self-assembly.21
The toroid's lumen undergoes significant narrowing, but changes to its outer diameter are minimal due to the fact that the toroid's x–y thickness increases. When first harvested, the z dimension of the 600 μm toroid was 113.6 ± 21.2 μm and the thickness in the x–y dimensions was 246.3 ± 26.8 μm. Over 10 days, the x–y thickness increased steadily to a maximum of 479.2 ± 23.6 μm. This thickening may be due to cellular migration and spreading of the toroid and/or cell proliferation. The rate of thickening was nearly the same for the 600 μm and the 1000 μm toroids, suggesting that the process is independent of toroid diameter. Cell density in the toroid was initially uniform, but with time, cell density increased in a nonuniform way with high cell density localized to a central ring closer to the lumen rather than the outermost circumference. It would be interesting to determine whether cell migration and/or proliferation vary at these locations.
Critical to the usefulness of a building unit is its ability to be used to build larger structures. In biofabrication, spheroids, along with an extracellular-matrix-like material, are melded together to build a tissue, and our toroids may be useful in this approach. Scaffold-free building units, such as spheroids, can also undergo cell-mediated fusion to build larger structures.12,19,25 Our data show that toroids can fuse at points of contact along their outer rim as well as their top and bottom surfaces. The kinetics of fusion shows that this process is largely complete after 48–72 h with some compaction when fusion occurs along the top and bottom surfaces. Similar to spheroid fusion, our fluorescent-labeled toroids show that fusion occurs with minimal cell mixing between the building units.25 Cell–cell adhesion is critical to fusion,26 and surface adhesion molecules, such as cadherins and integrins, are involved as well as the cytoskeleton to which these proteins are linked.27
As a proof of principle, we show that toroids can be fused to form a large multitorus structure. Toroids were added to a single well and allowed to settle to form a random pile of toroids; however, the settling was not random. Overlap of toroids was random, but the majority of toroids had their lumens oriented along the z axis. This bias is probably due to the shape of the toroid and would provide a dominant orientation to the network of lumens created after fusion. Moreover, toroids that are randomly overlapped would create a range of lumen sizes after fusion, all smaller than the lumen of the building unit. Although this approach may not be useful for creating lumens that approximate neither the density nor the diameter of capillaries (∼10 μm), it may be useful for mimicking the range of vessels that connect capillaries to small-diameter arteries and veins (∼0.1–5.0 mm).28 However, strategies to endothelialize the lumens of these structures are needed.
Future work with toroid building units can take advantage of the fact that it is possible to make toroids with two or more different cell types. Previously, we have shown that when two cell types are mixed and added to the micromolds, the cells will self-assemble a mixed cell toroid.21 The cells also self-sorted so that one cell type was located in the inner core of the toroid and the other cell type formed an outer coating. The self-sorting phenomenon has been observed for many years in spheroids and patterns vary with cell types.29,30 Another variation on the toroid building unit is a larger structure with multiple lumens. We have shown that cells can self-assemble a stable honeycomb structure with 13 lumens.21,31 Like toroids, these honeycomb parts were self-assembled within 48 h and could likely be fused with other parts in a similar time frame. Parts of different sizes, geometries, and cell types could be mixed or directed by secondary molds to control the size, shape, cell position, and lumen sizes of a large tissue construct.
In summary, we have shown that monodispersed cells can be self-assembled into scaffold-free multicellular building units in the shape of a toroid. The diameter and lumen size of these units are controlled by micromold geometry, and both undergo predictable changes when harvested. By the process of tissue fusion, these building units can be assembled into larger structures with interconnected lumens.
Acknowledgment
This work was funded, in part, by the NIH R01EB008664-01A1.
Disclosure Statement
No competing financial interests exist.
References
- 1.Griffith C.K. Miller C. Sainson R.C. Calvert J.W. Jeon N.L. Hughes C.C. George S.C. Diffusion limits of an in vitro thick prevascularized tissue. Tissue Eng. 2005;11:257. doi: 10.1089/ten.2005.11.257. [DOI] [PubMed] [Google Scholar]
- 2.Khademhosseini A. Langer R. Borenstein J. Vacanti J.P. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci USA. 2006;103:2480. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colton C.K. Implantable biohybrid artificial organs. Cell Transplant. 1995;4:415. doi: 10.1177/096368979500400413. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P. Jain R.K. Angiogenesis in cancer and other diseases. Nature. 2000;407:249. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 5.Ko H.C. Milthorpe B.K. McFarland C.D. Engineering thick tissues—the vascularisation problem. Eur Cell Mater. 2007;14(1) doi: 10.22203/ecm.v014a01. discussion 9. [DOI] [PubMed] [Google Scholar]
- 6.Fidkowski C. Kaazempur-Mofrad M.R. Borenstein J. Vacanti J.P. Langer R. Wang Y. Endothelialized microvasculature based on a biodegradable elastomer. Tissue Eng. 2005;11:302. doi: 10.1089/ten.2005.11.302. [DOI] [PubMed] [Google Scholar]
- 7.McGuigan A.P. Sefton M.V. Design criteria for a modular tissue-engineered construct. Tissue Eng. 2007;13:1079. doi: 10.1089/ten.2006.0245. [DOI] [PubMed] [Google Scholar]
- 8.McGuigan A.P. Sefton M.V. Design and fabrication of sub-mm-sized modules containing encapsulated cells for modular tissue engineering. Tissue Eng. 2007;13:1069. doi: 10.1089/ten.2006.0253. [DOI] [PubMed] [Google Scholar]
- 9.McGuigan A.P. Sefton M.V. The thrombogenicity of human umbilical vein endothelial cell seeded collagen modules. Biomaterials. 2008;29:2453. doi: 10.1016/j.biomaterials.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGuigan A.P. Sefton M.V. Vascularized organoid engineered by modular assembly enables blood perfusion. Proc Natl Acad Sci USA. 2006;103:11461. doi: 10.1073/pnas.0602740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boland T. Mironov V. Gutowska A. Roth E.A. Markwald R.R. Cell and organ printing 2: fusion of cell aggregates in three-dimensional gels. Anat Rec A Discov Mol Cell Evol Biol. 2003;272:497. doi: 10.1002/ar.a.10059. [DOI] [PubMed] [Google Scholar]
- 12.Jakab K. Neagu A. Mironov V. Markwald R.R. Forgacs G. Engineering biological structures of prescribed shape using self-assembling multicellular systems. Proc Natl Acad Sci USA. 2004;101:2864. doi: 10.1073/pnas.0400164101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mironov V. Boland T. Trusk T. Forgacs G. Markwald R.R. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003;21:157. doi: 10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 14.Mironov V. Visconti R.P. Kasyanov V. Forgacs G. Drake C.J. Markwald R.R. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009;12:2164. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith C.M. Stone A.L. Parkhill R.L. Stewart R.L. Simpkins M.W. Kachurin A.M. Warren W.L. Willows S.K. Three-dimensional bioassembly tool for generating viable tissue-engineered constructs. Tissue Eng. 2004;10:1566. doi: 10.1089/ten.2004.10.1566. [DOI] [PubMed] [Google Scholar]
- 16.Wilson W.C., Jr. Boland T. Cell and organ printing 1: protein and cell printers. Anat Rec A Discov Mol Cell Evol Biol. 2003;272:491. doi: 10.1002/ar.a.10057. [DOI] [PubMed] [Google Scholar]
- 17.Griffith L.G. Naughton G. Tissue engineering—current challenges and expanding opportunities. Science. 2002;295:1009. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 18.Kelm J.M. Djonov V. Ittner L.M. Fluri D. Born W. Hoerstrup S.P. Fussenegger C.P. Design of custom-shaped vascularized tissues using microtissue spheroids as minimal building units. Tissue Eng. 2006;12:2151. doi: 10.1089/ten.2006.12.2151. [DOI] [PubMed] [Google Scholar]
- 19.Rago A.P. Dean D.M. Morgan J.R. Controlling cell position in complex heterotypic 3D microtissues by tissue fusion. Biotechnol Bioeng. 2009;102:1231. doi: 10.1002/bit.22162. [DOI] [PubMed] [Google Scholar]
- 20.Napolitano A.P. Chai P. Dean D.M. Morgan J.R. Dynamics of the self-assembly of complex cellular aggregates on micromolded nonadhesive hydrogels. Tissue Eng. 2007;13:2087. doi: 10.1089/ten.2006.0190. [DOI] [PubMed] [Google Scholar]
- 21.Dean D.M. Napolitano A.P. Youssef J. Morgan J.R. Rods, tori, and honeycombs: the directed self-assembly of microtissues with prescribed microscale geometries. FASEB J. 2007;21:4005. doi: 10.1096/fj.07-8710com. [DOI] [PubMed] [Google Scholar]
- 22.Du Y. Lo E. Ali S. Khademhosseini A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc Natl Acad Sci USA. 2008;105:9522. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dean D.M. Morgan J.R. Cytoskeletal-mediated tension modulates the directed self-assembly of microtissues. Tissue Eng Part A. 2008;14:1989. doi: 10.1089/ten.tea.2007.0320. [DOI] [PubMed] [Google Scholar]
- 24.Krieg M. Arboleda-Estudillo Y. Puech P.H. Kafer J. Graner F. Muller D.J. Heisenberg C.P. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol. 2008;10:429. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- 25.Jakab K. Norotte C. Damon B. Marga F. Neagu A. Besch-Willford C.L. Kachurin A. Church K.H. Park H. Mironov V. Markwald R. Vunjak-Novakovic G. Forgacs G. Tissue engineering by self-assembly of cells printed into topologically defined structures. Tissue Eng Part A. 2008;14:413. doi: 10.1089/tea.2007.0173. [DOI] [PubMed] [Google Scholar]
- 26.McLaughlin M.E. Kruger G.M. Slocum K.L. Crowley D. Michaud N.A. Huang J. Magendantz M. Jacks T. The Nf2 tumor suppressor regulates cell-cell adhesion during tissue fusion. Proc Natl Acad Sci USA. 2007;104:3261. doi: 10.1073/pnas.0700044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Discher D.E. Janmey P. Wang Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 28.Silverthorn D.U. Human Physiology: An Integrated Approach. 3rd. San Francisco, CA: Pearson Education, Inc.; 2004. [Google Scholar]
- 29.Foty R.A. Pfleger C.M. Forgacs G. Steinberg M.S. Surface tensions of embryonic tissues predict their mutual envelopment behavior. Development. 1996;122:1611. doi: 10.1242/dev.122.5.1611. [DOI] [PubMed] [Google Scholar]
- 30.Moscona A. Moscona H. The dissociation and aggregation of cells from organ rudiments of the early chick embryo. J Anat. 1952;86:287. [PMC free article] [PubMed] [Google Scholar]
- 31.Napolitano A.P. Dean D.M. Man A.J. Youssef J. Ho D.N. Rago A.P. Lech M.P. Morgan J.R. Scaffold-free three-dimensional cell culture utilizing micromolded nonadhesive hydrogels. Biotechniques. 2007;43(494)(500):496. doi: 10.2144/000112591. [DOI] [PubMed] [Google Scholar]