Abstract
Hydrogels that degrade at different rates were prepared by copolymerizing slowly degrading macromer poly(ethylene glycol) (PEG) dimethacrylate with a faster degrading macromer poly(lactic acid)-b-PEG-b-poly(lactic acid) dimethacrylate. A clinically relevant population of neural cells composed of differentiated neurons and multipotent precursor cells was cultured within hydrogels. Within 2 h after encapsulation, metabolic activity was higher in hydrogels prepared with increasing levels of degradable content. This improvement was accompanied by a reduction in intracellular redox state and an increase in the fraction of glutathione in the reduced state, both of which persisted throughout 7 days of culture and which may be the result of radical scavenging by lactic acid. Importantly, an increase in cellular proliferation was observed in gels prepared with increasing degradable macromer content after 7 days of growth without a shift in the cellular composition of the culture toward the glial cell phenotype. The findings of this study provide additional insight into the growth of neural cells in PEG-based hydrogels. Results suggest that lactic acid released during gel degradation may impact the function of encapsulated cells, a finding of general interest to biomaterials scientists who focus on the development of degradable polymers for cell culture and drug delivery devices.
Introduction
Neurodegenerative disorders and damage after trauma involve a large-scale loss of cell populations within the brain. Transplantation therapies are being developed to treat these conditions. Grafted neurons extend processes that re-innervate host tissue, form synaptic connections, release neurotransmitters, and improve behavioral deficits in animal models of disease or injury.1–6 Although grafting approaches are promising, an excess of cells must be transplanted to overcome the large-scale cell loss that is often observed within the first week after the surgical procedure.7–9 This cell loss has been associated in part with an acute inflammatory response that occurs immediately after the procedure.9 Efforts directed at minimizing grafted cell loss and controlling the expansion and differentiation of self-renewing multipotent neural precursor cell populations would significantly improve the availability of clinically relevant cell populations for therapeutic use.
Hydrogels prepared from both natural and synthetic materials are being developed with both of these goals in mind.10–18 Hydrogels prepared from poly(ethylene glycol) (PEG) macromers are widely studied and are particularly promising materials for this purpose19–25 as these materials produce minimal inflammation when implanted into brain tissue.26 When a clinically relevant mixed population of neurons and multipotent precursor cells is encapsulated within three-dimensional PEG hydrogels, cells survive well.20,25 Over time in degradable hydrogel culture, cells continue to proliferate or differentiate to form glia or neurons that are responsive to neurotransmitter, a characteristic necessary for functional recovery upon grafting.20 PEG hydrogels offer an additional advantage over natural materials in that the time-scale over which the network degrades can be controlled by either changing the chemistry of the degradable cross-link or by incorporating nondegradable macromer into the polymer network.19 Flexible control over degradation rate is a useful tool for treating the varied types of disease and injury that occur in the central nervous system. For example, a hydrogel that remains intact long enough to provide mechanical protection during surgery and immediately after grafting, but then degrades to facilitate cellular integration with surrounding tissue, would be useful for treating strokes that often result in focal damage to brain tissue.27 By contrast, a hydrogel that remains intact for several months to provide protection initially and over a longer time period as processes grow through the hydrogel or in contact with its surface toward more distant brain regions would be useful for treating Parkinson's disease in which neurons in the substantia nigra degenerate as well as their processes that extend to and innervate the caudate putamen.27
Although recent research has shown that PEG hydrogels support the growth and differentiation of neural cell populations composed of both neurons and multipotent precursor cells,20 permissive hydrogels that degrade over different time scales must be developed and studied for diverse application such as those mentioned above. The behavior of neural precursor cells in hydrogels that degrade over different time-scales is currently uncharacterized and is an important parameter to consider, as degradation rate and resultant changes in hydrogel properties have been shown to impact the quality of tissue that is produced and the function of individual cells within hydrogels. For example, extracellular matrix molecule deposition by chondrocytes28 and mineralization by osteoblasts29 have both been shown to be more uniformly distributed through hydrogels that contain a larger fraction of degradable macromer, likely because the gel swells to a greater extent and the mesh size of the hydrogel increases, allowing for increased diffusion of these components into the hydrogel environment.28–30 The amount of degradable macromer also impacts the function of cells within a hydrogel. In hydrogels containing increasing levels of degradable macromer, chondrocyte proliferation improves over the course of 1–8 weeks of culture.28,31 Encapsulated osteoblast functions also improve; cellular proliferation, metabolic activity, and alkaline phosphatase production over the course of 21 days of culture increase as degradable content increases.29
Therefore, the goal of this research is to characterize the growth of neural cells in hydrogels of different degradable content to assess the differential effect of degradable content on neural cell development. Experiments were designed to elucidate the role that degradable poly(lactic acid) (PLA) macromer content has on neural cells cultured within three-dimensional hydrogels. Neural cells isolated from the forebrain were utilized in part due to their demonstrated potential for the treatment of a variety of degenerative disorders affecting the central nervous system.32–37 Hydrogels that degrade to different extents during culture were prepared by copolymerizing slowly degrading macromer (PEG-DM, also referred to as nondegradable macromer) with degradable macromer (PEG-LA-DM). A mixed population of neural cells, composed of postmitotic neurons and multipotent precursor cells, was encapsulated in copolymer hydrogels, and the effect of degradable macromer content on the metabolic activity of cultures was assessed over time. Previous work has shown that lactic acid, a molecule released during degradation of the hydrogel, has antioxidant properties, reducing levels of reactive oxygen species (ROS) and the intracellular redox state, and increasing cell proliferation when presented to this cell population in monolayer culture.38 These parameters of cell function were also investigated as different levels of degradable macromer in hydrogel cultures should expose cells to different levels of lactic acid. Finally, because the cellular composition of the culture would be impacted by differences in either survival or proliferation, gene expression analysis was used to detect relative levels of precursor, neuronal, or glial cell populations in hydrogels prepared with different levels of degradable macromer.
Materials and Methods
Neural cell isolation and cell culture
Embryonic day 14–15 (E14-E15) Sprague-Dawley rat embryos were removed by Cesarean section, and the forebrain was isolated and dissociated enzymatically as described in detail elsewhere.38 NIH guidelines for the care and use of laboratory animals were observed. Immediately after isolation, immunocytochemical staining verified that the cell population is composed of a mixture of postmitotic neurons (66%) and the remainder proliferative nestin-positive cells are capable of proliferating and/or differentiating into neurons or glia.39 The culture medium consisted of Dulbecco's modified Eagle's medium/F12 (50:50) supplemented with 1% penicillin/streptomycin (Mediatech, Herndon, VA), 1% serum-free supplement, N2 (Invitrogen, Carlsbad, CA), 2.5 mM L-glutamine (Mediatech), and 10 ng/mL basic fibroblast growth factor (Sigma, St. Louis, MO). Cultures were grown at 37°C in 100% humidity with 5% CO2. The medium was replaced on day 1 and every 2–3 days thereafter.
Hydrogel preparation
Using the method first developed by Sawhney et al.,40 PLA was grafted to linear PEG chains (MW 4600) to form fast-degrading triblock copolymers. Macromers were end-capped with methacrylate functionalitites and purified via a combination of iterative crystallization and filtration, and then precipitated in ice-cold ethyl ether (Fisher Scientific, Fair Lawn, NJ). Linear PEG chains (MW 4600) were also end-capped with methacrylate groups to form slowly degrading macromer that were purified by precipitation into ice-cold ethyl ether followed by dialysis in diH2O (10 wt% solution, MWCO:1000, [Spectra/Por Biotech]) cellulose ester membrane. [Spectrum Laboratories, Rancho Dominguez, CA]. The purified nondegradable macromer solution was then frozen at −80°C and lyophilized until dry. All macromers were stored at 4°C under argon. Lactide addition and methacrylation efficiency were characterized by proton NMR spectroscopy. Similar methacrylation efficiencies were obtained for both fast-degrading (73%) and nondegradable (71%) macromers. The fast-degrading macromer had an average of 2.5 lactic acid units per side.
Hydrogels were prepared with a 7.5 wt% macromer solution in a sterile culture medium containing cells (1 × 107 cells/mL) and 0.025 wt% photoinitiator (Irgacure 2959, Ciba, Tarrytown, NY). The composition of the macromer solution was varied such that it was composed of 0, 15, 30, 50, 70, or 100 wt% degradable macromer, the remaining fraction being slow-degrading macromer. Forty microliters of gels was formed by exposing the macromer–cell solution in cylindrical molds to 365 nm UV light (∼4 mW/cm2) for 10 min. Photopolymerized hydrogels were rinsed in sterile phosphate-buffered saline (PBS) (Mediatech) and cultured in six-well tissue culture plates as described in the section Neural cell isolation and cell culture. For comparison measurements, in some cases a solution of lactic acid with a pH of 7.4 was added during the polymerization process. For other comparisons, pH 7.4 lactic acid or N-acetyl-L-cysteine (NAC, Sigma) were added to the culture medium for 30-min incubation before analysis.
Hydrogel mechanical property measurement
Mechanical properties of the hydrogels were measured to determine the relative integrity and degradation of hydrogels with different degradable contents. Hydrogels laden with cells were weighed at discrete time points and were then subjected to mechanical property measurement. Compressive modulus was measured on a mechanical testing system (MTS Synergie 100, MTS, Eden Prairie, MN) in unconfined compression using a low strain method (strain endpoint = 0.15 mm/mm). Collected samples were lyophilized. Mass swelling ratio was calculated as final wet mass before compression divided by final dry mass. Mass loss was calculated by comparing final dry masses to dry masses of the day 0 hydrogels. Four to five hydrogels were analyzed at each time point.
Measurement of DNA, ATP, and glutathione content
Total DNA and ATP content were used to assess cell number and mitochondrial activity, respectively, in culture. Reduced glutathione (GSH) is a potent intracellular scavenger of ROS. Reduced GSH content was therefore used to determine the extent to which cells in hydrogels prepared with different degradable content were undergoing oxidative stress, a condition that can potentially lead to apoptosis and cell death. Cells encapsulated into hydrogels of varying degradable content may be exposed to different levels of lactic acid, a free radical scavenger,38 thus altering reduced GSH content and cell function in hydrogel cultures. Hydrogels were collected at discrete time points and stored at −80°C until analysis. Hydrogels were immersed in 300 μL of cell lysis buffer (20 mM Tris [Bio-Rad, Hercules, CA], 2 mM EDTA [Bio-Rad], 150 mM NaCl, and 0.5% Triton X-100 [Fisher Scientific] in diH2O). Gels were crushed and sonicated on ice using a Misonix ultrasonic cell disruptor for ∼30 s at an output power of 4–5 watts. DNA was measured using the Quant-iT PicoGreen dsDNA assay kit (Invitrogen), ATP was quantified using the CellTiter-Glo cell viability assay (Promega, Madison, WI), and reduced GSH and oxidized GSSG (glutathione disulfide) content were assessed using the GSH-Glo kit (Promega). Briefly, sample was combined with an equal volume of reagent in a 384-well assay plate and mixed well. After 10 min of incubation, fluorescence (DNA assay) or luminescence (ATP and GSH assays) was quantified using a Fluostar Optima plate reader. Data were obtained from six different experiments for ATP and DNA, or two different experiments for GSH, with three to five hydrogel samples per time point.
Measurement of intracellular redox state
Intracellular redox state is an effective measure of the abundance of ROS within a cell,41,42 and dissociated cells often undergo an inflammatory process associated with high levels of ROS. Cells encapsulated into hydrogels of varying degradable content may be exposed to different levels of lactic acid during degradation, which may scavenge ROS and prevent oxidative stress as well as reduce the baseline intracellular redox state.38 Such a shift in intracellular redox state has been shown to impact neural precursor cell fate where more oxidized cellular states lead to differentiation and more reduced states lead to self-renewal.42,43 At discrete time points, hydrogels were removed from the medium and intracellular redox state was quantified by utilizing a dye that is internalized within cells and fluoresces upon oxidation.41,42 To facilitate dye penetration into the interior regions of the hydrogel, gels were cut into two sections before staining in a 150 nM solution of Mitotracker Orange CM-H2TMRos (Invitrogen 7511) in the phenol red-free medium for 30 min. Hydrogels were fixed overnight in 4% paraformaldehyde at 4°C, rinsed 2 × in PBS, and stored at 4°C for less than 48 h until imaging. All images were collected using a Ziess LSM Pascal confocal microscope and a 40 × water immersion objective (Zeiss Achroplan 40 × ). Z-series image stacks were collected from the interior surface of the gel fragment every 5 μm up to a maximum depth of 130 μm. Within this range, no change in dye penetration or fluorescence signal was detected. Using NIH ImageJ software, 8–15 cells per image stack were traced on a minimum of 4 image stacks per condition using the bright-field setting and the average fluorescent intensity was calculated per cell. Background fluorescence of each hydrogel was subtracted from each average fluorescent intensity measurement. At least 38 total cells were measured per experimental condition.
Characterization of cell composition by quantitative real-time-polymerase chain reaction
In the event that cell survival, proliferation, and/or differentiation are altered by culture in hydrogels with different degradable content, the final cell composition of the culture would be impacted. Quantitative real-time-polymerase chain reaction was utilized to identify such an effect on the cell composition of the hydrogel cultures. After 7 days of culture, standard phenol:chloroform RNA extraction was performed on hydrogel cultures using TriReagent (Sigma). After isolation, RNA was treated with DNAse (Ambion, Austin, TX) and quantified using the Quant-iT RiboGreen RNA Assay Kit (Invitrogen) as described in detail elsewhere.25 Glia were identified by glial fibrillary acidic protein (GFAP) expression, neurons by β-tubulin-III, and neural precursors by nestin expression. Primer sequences are provided elsewhere.25
Imaging of neural cells in PEG hydrogel culture
Cell morphology in hydrogel cultures was assessed in two ways. Live cells within hydrogels were imaged using a fluorescence-based membrane integrity assay (LIVE/DEAD Viability/Cytotoxicity Kit for mammalian cells; Invitrogen) to determine cell viability and some degree of microtissue development. For imaging on both days 1 and 7 of culture, hydrogel were sectioned into 500-μm-thick slices and stained in a solution of 2 μM calcien AM and 4 μM ethidium homodimer-1 in Dulbecco's modified Eagle's medium without phenol red for 30 min. Hydrogel slices were imaged via confocal microscopy in a custom imaging chamber containing sterile PBS. All images were collected using a Ziess LSM Pascal confocal microscope. Fluorescent images were captured with a 10 × or 40 × water immersion objective (Zeiss Achroplan 10 × or 40 × ). Two to three images were collected every 1 μm to create a projection.
Further analysis of morphology and cell composition was performed via immunocytochemistry. After 7 days of culture, hydrogels were rinsed and fixed in 4% paraformaldehyde. Hydrogels were cut into 40 μm section using a cryostat. Standard immunocytochemical techniques were employed to observe encapsulated cells. Neurons were identified by β-tubulin-III and glia were identified by GFAP-positive labeling. Unless otherwise noted, all antibodies were purchased from Chemicon, Temecula, CA. Secondary antibodies conjugated to Alexafluor 633 were purchased from Invitrogen. Sytox green (Invitrogen) was utilized as a nuclear counterstain.
Measurement of lactic acid concentration
Degradable materials containing PLA are expected to release lactic acid during the course of their degradation. Previous work has shown that lactic acid significantly affects the population of cells used in this work,38 and thus it was necessary to determine the maximum amount of lactic acid that may be released from these hydrogels. Fifteen hydrogels were incubated at 37°C in 4 mL of the culture medium. Medium samples were collected just before culture medium exchange, which occurred on days 1, 3, 6, and 8 and upon degradation at day 10. Samples were stored at −80°C until analysis. Twenty milligrams of β-nicotinamide adenine dinucleotide was reconstituted in 4 mL of glycine buffer and 8 mL of distilled water with 200 μL lactate dehydrogenase suspension (Sigma). A 20 μL sample or standard was combined with 280 μL β-nicotinamide adenine dinucleotide reagent and incubated at 37°C for 30 min. Absorbance at 355 nm was determined using a Fluostar Optima plate reader and was compared to a standard curve prepared from stocks of lactate (Sigma).
Statistical analysis
For all data, statistical significance was determined using single factor analysis of variance (alpha = 0.05). Statistically significant differences from controls are denoted with an asterisk unless otherwise indicated.
Results
Macroscopic properties of hydrogels
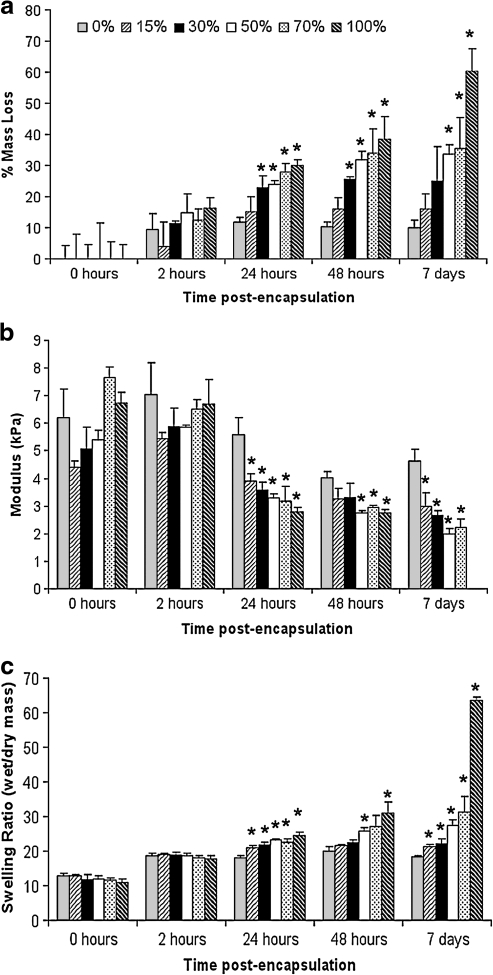
Although hydrogels made from PEG-DM macromers form cross-links that to not degrade on short time-scales in culture, lactic-acid containing cross-links within a hydrogel are susceptible to hydrolysis. Although the 100% degradable gels were intact on day 7, they were near the point of complete degradation, or reverse gelation, having achieved greater than 60% mass loss (Fig. 1a). In the 30, 50, and 70 wt% degradable hydrogels, a considerably lower level of degradation was observed, whereas minimal degradation was observed in 0% and 15% degradable hydrogels (Fig. 1a). As lactic acid containing cross-links were degraded, macroscopic properties of the polymer network also changed with time. At 2 h postpolymerization, values for mass loss, swelling ratio, and compressive modulus were not statistically different across formulations as low levels of gel degradation and subsequent mass loss occur at this early time point (Fig. 1). By 24 h, an increase in the rate of mass loss and swelling ratio and a decrease in the compressive modulus was observed as the fraction of degradable macromer in the gel increased. These results are consistent with prior work showing that over time, in hydrogels containing increasing amounts of degradable macromer, the degradation rate and swelling ratio increase while compressive modulus decreases.44
FIG. 1.
Degradation and macroscopic properties of hydrogels prepared with poly(ethylene glycol)-DM (0% degradable) and different levels of poly(ethylene glycol)-poly(lactic acid)-DM (degradable) macromer. Mass loss (a), compressive modulus (b), and swelling ratio (c) vary with degradable macromer content. *Values significantly different from the 0% degradable condition (*p < 0.01). Compressive modulus of 100% condition was immeasurable at 7 days due to extensive degradation.
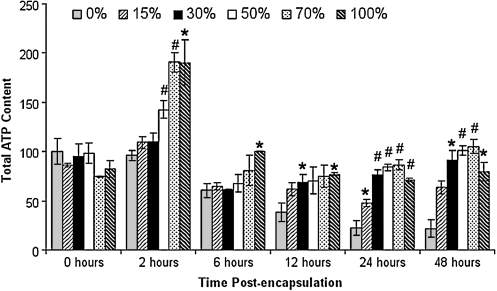
Effect of degradable macromer content on total ATP levels
To determine whether or not the amount of degradable macromer present within the hydrogel impacted cell survival and/or metabolic activity at early time points of culture (<48 h), before the onset of significant cell proliferation20 total ATP content was measured in hydrogels prepared with different amounts of degradable macromer (Fig. 2). At these early time points, cell number was constant across formulations as reflected by DNA measurements (data not shown). Thus, differences in ATP content reflected a change in the viability or metabolic activity of cells in culture.
FIG. 2.
Total ATP content in hydrogels prepared with different levels of degradable macromer expressed as percent of the 0% degradable condition at the 0 h time point. Cells incorporated into higher percent degradable hydrogels do not experience the same loss of viability as cells incorporated into a nondegrading (0%) hydrogel, which decrease in viability over time. Values significantly different from the time-matched 0% degradable condition are indicated as follows: *p < 0.05; #p < 0.01.
Immediately after encapsulation (0 h), no difference in total ATP content was observed between conditions. By 2 h significant differences were present with an overall increase in ATP concentration in hydrogels containing degradable content. Over the course of 48 h ATP content decreased, in the 0% degradable hydrogels and to a lesser extent in 15% degradable hydrogels, reflecting a reduction in metabolic activity and/or an increase in cell death (Fig. 2). In 30–50% degradable hydrogels, a transient decrease in ATP content was observed 6 h postencapsulation, which may be indicative of cellular damage or stress after photopolymerization. In these conditions, however, ATP levels began to recover by 12 h and were equivalent to that at 0 h by 48 h postencapsulation. In 70% and 100% degradable hydrogels total ATP content was constant over 0–48 h, suggesting that cells experienced very little acute toxicity when cultured in these formulations. When compared to levels in the 0% degradable condition, at each time point, total ATP content was significantly higher in gels containing degradable macromer (Fig. 2). This trend was observed at all time points, suggesting that the inclusion of degradable macromer improved the viability and/or metabolic function of encapsulated cells. This effect is likely due to lactic acid released by the degradable portions of the hydrogel. Treating cells in nondegradable gels with lactic acid results in similar effects. Treating 0% degradable hydrogels with 0.5 mg/mL of pH 7.4 lactic acid during polymerization led to improved metabolic activity. At 48 h after encapsulation, there was a 67% increase in ATP content when 0.5 mg/mL of lactic acid was used to scavenge ROS during encapsulation (data not shown).
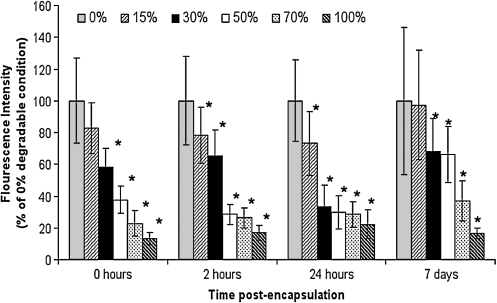
Effect of degradable macromer content on intracellular redox state
Reduced Mitotracker Orange, a dye that fluoresces upon oxidation, was utilized to determine the extent by which the level of degradable content of a hydrogel decreases intracellular ROS, thereby shifting the intracellular redox state to a more reduced level. At each time point tested, redox state was most oxidized in the 0% degradable condition as indicated by a greater intensity of measured fluorescence (Fig. 3). As degradable content of the hydrogel increased, the intracellular redox state became more reduced. The magnitude of reduction observed as degradable content increased was relatively consistent for each time point examined (i.e., 100% degradable condition ranged from 13% to 22% of control over time). It is expected that lactic acid is at least partially responsible for this effect, as 0.5 mg/mL of pH 7.4 lactic acid added to the culture medium of a 0% degradable hydrogel (no degradable PLA) for 30 min reduced the redox state of the cells to 55% of the control using standard media (data not shown). In the same way, pH-balanced lactic acid from 0.005 to 5 mg/mL reduced the redox state to 63–54% of the control. As a comparison, a 30 min application of 1 mM NAC (a potent antioxidant) to the culture medium shifted the intracellular redox state of cells in a 0% degradable hydrogel to 43% of controls (data not shown).
FIG. 3.
Intracellular redox state in hydrogels prepared with different levels of degradable macromer expressed as percent of the 0% degradable condition at each time point. Increases in degradability reduced the intracellular redox state of cells incorporated into the hydrogel, with a sustained reduction for up to 7 days. Values significantly different from the time-matched 0% degradable condition (p < 0.0001) are indicated with asterisks (*).
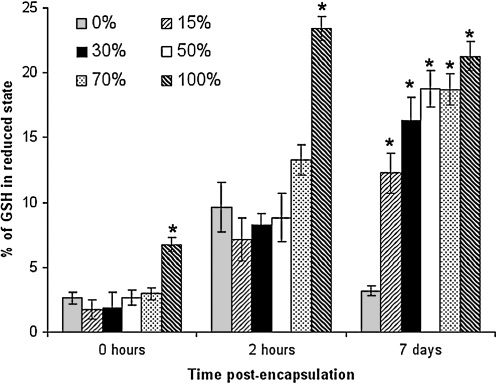
Effect of degradable macromer content on reduced GSH levels
Intracellular redox state can also be assessed by quantifying levels of reduced and oxidized species for dominant redox pairs found within the cell. The GSH/GSSG redox pair is the major thiol-disulfide redox pair found within the cell and is present at much higher concentrations than other redox pairs.45 A decrease in reduced GSH content renders a cell more susceptible to oxidant injury as the cell has a reduced capacity to scavenge free radicals.
At each time point the fraction of GSH in the reduced state increased as the amount of degradable macromer within the hydrogel increased (Fig. 4). The effects of degradable content after encapsulation on reduced GSH content were apparent at short time points (0 and 2 h postencapsulation), immediately after cells were exposed to oxidizing radicals, which are a product of the radical polymerization process. The effects were also observed at long time points (7 days), where endogenous ROS play a dominant role. At 7 days, all degradable hydrogel formulations significantly impacted the levels of reduced GSH. For frame of reference, a 30 min application of 1 mM NAC (a potent antioxidant), or 0.5 mg/mL of pH 7.4 lactic acid to the culture medium increased reduced GSH content of a 100% degradable hydrogel by 10.5 ± 1.5% or 2.3 ± 0.2%, respectively (data not shown).
FIG. 4.
Fraction of glutathione (GSH) in the reduced state for hydrogel cultures prepared with different levels of degradable macromer. This fraction of GSH in the reduced state for hydrogel cultures prepared with different levels of degradable macromer indicates that the higher levels of degradable hydrogel increase the reduced GSH levels at all time points. Values significantly different from the 0% degradable condition at each time point are indicated with asterisks (*) (p < 0.05).
Effect of degradable macromer content on total DNA content
To determine the extent to which the level of degradable macromer present within the hydrogel impacted cellular proliferation in the hydrogel after 7 days of culture, total DNA content was measured in hydrogels prepared with different levels of degradable macromer. Increase in DNA content is a standard measure of cell proliferation,24,28,46 and previous experiments have demonstrated via both DNA increase and bromodeoxyuridine (BRDU) staining that proliferation occurs in similar hydrogels.20 On day 0 of culture, total DNA content was not statistically different for hydrogels prepared with different levels of degradable macromer, suggesting that all gel compositions initially contained equivalent numbers of cells (data not shown). After 7 days of culture, total DNA content increased in all hydrogel conditions. Total DNA content was higher in gels containing degradable macromer relative to the 0% degradable condition (Fig. 5). The extent of the increase observed on day 7 depended on the amount of degradable macromer in the hydrogel. A moderate increase in DNA content was observed for hydrogels containing 15% degradable macromer (170% of the 0% degradable condition at the same 7 day time point). A higher increase was observed in gels with 30% or more degradable macromer (201–242% of the control).
FIG. 5.
Total DNA content in hydrogel cultures prepared with different levels of degradable macromer after 7 days of growth. DNA content was used as a measure of proliferation as all hydrogels were initially loaded equally. Cell proliferation elevated when cells were cultured with a hydrogel that was at least 30% degradable, as indicated by a higher percent of DNA compared with the 0% degradable cultures. Data are expressed as a percentage of the level present in 0% degradable hydrogels. *Statistical difference from control values (p < 0.05).
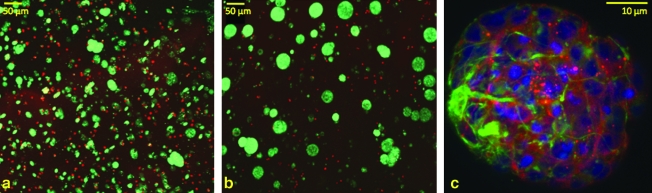
Effect of degradable macromer content on cell composition and morphology
Initially upon encapsulation single cells are present throughout the hydrogel culture (Fig. 6a). By day 7 cells proliferate to form small neurospheres (Fig. 6b) that are composed of both neurons and glial cells (Fig. 6c), and some nestin-positive precursors (data not shown). At this early stage of degradation neural processes penetrate and grow throughout the neurosphere interior. As the material degrades at later time points, processes do penetrate throughout the hydrogel.20 In Figure 6 cell morphology in 100% degradable hydrogels is presented; however, cell behavior in hydrogels with different degradable content is similar over this time frame and is not presented. Although no statistical differences were observed, quantitative real-time-polymerase chain reaction revealed a slight increase in β-tubulin and nestin gene expression with increasing degradable content after 7 days in culture (Table 1). There were no changes in GFAP expression across hydrogel conditions.
FIG. 6.
Cell morphology in hydrogel culture. On day 1 (a) or day 7 (b) cells in degradable hydrogels were observed with fluorescent live (green)–dead (red) indicators. Initially, single cells are present in gel cultures. On day 7 small clusters are apparent (c). Immunocytochemistry performed on gel cultures demonstrates that these clusters are composed of neurons (green) and glial cells (red). Nuclei are counterstained with sytox green (blue). Cell behavior in hydrogels of different degradable content is similar to that pictured here for degradable gels at these time points. Color images available online at www.liebertonline.com/ten.
Table 1.
Effect of Degradable Macromer Content on the Cellular Composition of Hydrogel Cultures
| |
Gene |
||
|---|---|---|---|
| Hydrogel degradability | GFAP | Beta-tubulin | Nestin |
| 0% | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.0 ± 0.2 |
| 15% | 0.9 ± 0.1 | 1.7 ± 0.5 | 1.5 ± 0.2 |
| 30% | 1.4 ± 0.2 | 2.3 ± 1.6 | 1.6 ± 0.7 |
| 50% | 1.0 ± 0.1 | 1.7 ± 0.4 | 1.6 ± 0.3 |
| 70% | 1.4 ± 0.2 | 3.3 ± 1.3 | 2.0 ± 0.6 |
| 100% | 0.7 ± 0.03 | 2.4 ± 0.6 | 1.9 ± 0.3 |
Cell composition was assessed by quantitative real-time-polymerase chain reaction data for GFAP, beta-III-tubulin, and nestin and is expressed as fold change in a gene expression relative to the nondegradable condition on day 7 of culture. Although slight increases in beta-tubulin and nestin expression were observed in more degradable gels, changes in the degree of degradability did not significantly alter the relative cell populations within the culture, suggesting that the different hydrogels do not preferentially support or inhibit one cell type over another. Data represent the mean ± standard error of the mean. No statistical differences were identified.
GFAP, glial fibrillary acidic protein.
Lactic acid concentration in the culture medium containing degradable PEG hydrogels
Over the course of the culture period, the concentration of lactate (ionic form of lactic acid) detected in the culture medium ranged between 0.06 and 0.17 mg/mL (Table 2) with levels decreasing at later stages of degradation. Although lactic acid concentration data were only collected for purely degradable gels, it is expected that similar trends were likely occurring in the other hydrogel formulations although as degradable content decreased, it would be expected that the maximum lactic acid concentration in the medium would decrease. Additionally, while substantial concentrations of lactic acid were found in the medium, concentrations experienced by the cells within the gel microenvironment may have been higher given diffusional limitations.
Table 2.
Concentration of Lactic Acid Measured in the Culture Medium Containing 100% Degradable Gels at Discrete Time Points
| Time point (days) | Lactic acid concentration (mg/mL) |
|---|---|
| 0 | 0.149 ± 0.022 |
| 1 | 0.134 ± 0.004 |
| 3 | 0.136 ± 0.003 |
| 6 | 0.172 ± 0.001 |
| 8 | 0.087 ± 0.002 |
| 10 | 0.063 ± 0.001 |
The culture medium removed on day indicated was tested for lactic acid concentration and indicates that lactic acid was released from the hydrogels at substantial concentrations. Data represent mean ± standard error of the mean.
Discussion
Hydrogels are of considerable interest in the development of regenerative therapies for treating disease and injuries of the central nervous system. When prepared from synthetic materials, the rate at which the hydrogel degrades can be controlled, a particularly useful tool as materials that degrade over short or long time scales both have clinical relevance. However, because the rate of hydrogel degradation has been shown to impact the quality of other types of tissue produced within the hydrogel, it is important to first understand how neural tissue develops within hydrogels with different mass loss profiles. Toward this end, the focus of this work was to characterize neural cell growth within hydrogels prepared by combining slow-degrading (nondegradable) macromer with different levels of degradable macromer containing PLA subunits. Over time, degradable cross-links are hydrolyzed, releasing lactic acid until a network composed of only PEG cross-links remains. Consistent with previous reports, as the gel degrades, hydrogels utilized in this work imbibe more water, becoming more open and swollen (mesh size increases) and, as a result, less stiff as reflected by a decrease in compressive modulus (Fig. 1).
In this work, within 2 h of formation an improvement in cell viability and/or metabolic function was observed as degradable content of the hydrogel increased (Fig. 2). In addition, intracellular redox state was more reduced and the level of reduced GSH content was increased in gels containing more degradable macromer. Differences in macroscopic properties are not likely to be responsible for the improvements in cell function observed at this early time point (2 h) as statistical differences in compressive modulus and swelling ratio (thus, hydrogel mesh size) were not present until later (24 h). Instead, the lactic acid product of hydrogel degradation, which is a free radical scavenger47 and can protect cells from damage due to photoinitiator-generated and endogenously present free radicals,38 may positively impact encapsulated cell function. Indeed lactic acid is present during polymerization and is released into the hydrogel culture medium at levels that scavenge radicals and modify intracellular redox state in monolayer culture (>0.005 mg/mL).38 Effects are likely due only to free lactic acid released from the polymer network. Although the PLA form is too bulky to enter the cell, both lactic acid and the ionized-form lactate are capable of transport into cells. Lactic acid is uncharged and small enough to permeate through the lipid membrane; lactate is capable of entering cells via the monocarboxylate transporter protein shuttle system.48
Once inside the cell, lactic acid is also capable of undergoing oxidation to pyruvate, another potent antioxidant, thus increasing intracellular pyruvate levels. Pyruvate is then capable of decreasing quantities of intracellular ROS, leading to a reduced intracellular redox state and, as a result, an increase in reduced GSH content and ultimately an improvement in cell survival and/or metabolic activity. The transient increase in ATP content observed at the 2 h time point may be the combined effect of lactic acid as well as endogenous upregulation of ATP, as others have documented an increase in ATP content when neural cells are under stress.49
After 7 days of culture, intracellular redox state was more reduced in hydrogels with degradable content (Fig. 3) and reduced GSH content (Fig. 4) approached preencapsulation levels (46.2 ± 1.0%, data not shown), effects which may be similarly related to the presence of lactic acid. A shift in intracellular redox state has been shown to impact neural precursor cell fate where more oxidized cellular states lead to differentiation and more reduced states lead to self-renewal.42,43 Thus, when exposed to lactic acid released from a biomaterial, neural precursor cells may be protected from damage due to naturally occurring ROS; intracellular redox state may shift to one that is more reduced, thus maintaining cells in a proliferative state.38 At the 7 day time point total DNA content in hydrogels increased with increasing degradable macromer content (Fig. 5). The increase in DNA content may be related to a difference in the number of surviving, proliferative cells present in gels at 24–48 h where conditions with lower degradable content would result in fewer surviving cells and lower DNA content after 7 days of growth. Lactic acid may also directly impact cell proliferation, as entry into the S phase of the cell cycle (DNA replication) is facilitated by the presence of lactate (the ionized form of lactic acid).50
In addition to being exposed to varying levels of lactic acid, at these longer time points cells are gradually exposed to three-dimensional polymer networks of different mechanical strength. To the best of our knowledge, there are no data in the literature to suggest that mechanical properties would directly impact intracellular redox state and reduced GSH content. However, other aspects of neural cell function have been shown to be impacted by mechanical properties. For example, the stiffness of a substrate has been shown to impact cellular proliferation and/or the cellular composition of cultures. In the case of neural cells, when cultured for extended periods of time on two-dimensional surfaces, less stiff surfaces tend to promote the differentiation and/or survival of neurons while discouraging the growth of glia.51 In this work proliferation was improved in gels with reduced stiffness without altering cell composition. Trends similar to what has been reported in the literature were not observed in this work perhaps because the time-scale over which the stiffness of the surrounding polymer network is dramatically lower is confined to a brief window during late stages of culture. Instead, the improvement in proliferation is more likely related to exposure to lactic acid as previously discussed. Importantly the improvement in proliferation is not accompanied by an increase in the growth of glial cells (Table 1), which if implanted in the brain tissue could contribute to the formation of a glial scar.
PEG comprised the bulk of the mass of hydrogels prepared in this work with minor mass contributions from the hydrophobic PLA subunits within the degradable crosslinks. Although hydrogels prepared with different levels of degradable macromer may exhibit differences in hydrophobicity, these differences were negligible given the relatively low molar percentage of PLA incorporated (<7 mol%) and the dominant hydrophilicity of the major component, PEG. Thus, differences in hydrophobicity were not likely to substantially underlie the findings reported in this study. When encapsulated in gels with different levels of degradable macromer, the pH of the gel microenvironment is also likely to vary, with more acidic conditions arising in faster degrading gels, as alluded to in previous work.29 In this work, the rate of ATP hydrolysis would have been impacted by a change in pH as ATP has been shown to be rapidly degraded outside of the 6.8 to 7.4 pH range. Because higher levels of ATP were observed in gels containing higher levels of degradable macromer, an increase in the rate of ATP hydrolysis cannot account for trends reported in this study. Thus, hydrogel degradable content must preserve ATP, improving the viability of surrounding neural cells.
Conclusions
To summarize, in this study neural cell survival and/or metabolic function was improved in hydrogels prepared with increasing degradable macromer content immediately after encapsulation. This improvement was accompanied by a reduction in intracellular redox state and an increase in reduced GSH content, both of which persisted throughout 7 days of culture and which may be the direct or indirect result of radical scavenging by slowly released lactic acid. Importantly, an improvement in cell proliferation was observed in gels prepared with increasing degradable macromer content after 7 days of growth without a shift in the cellular composition of the culture toward the glial cell phenotype. Effects of degradable macromer content on cell viability and/or metabolic function observed here are consistent with previous reports involving other cell types, suggesting that cell growth is generally improved by culture in hydrogels containing degradable lactic acid cross-links. Released lactic acid is expected to be at least partially responsible for improved metabolic activity, redox state, and reduced GSH content because lactic acid added to nondegradable hydrogels resulted in similar improvements. As low levels of reduced GSH content are an early indicator of dopaminergic cell stress and ultimately cell loss, lactic-acid-releasing hydro-gels are chemically advantageous in treating Parkinson's disease.52 By using degradable hydrogels for cell transplantation therapy, the loss of transplanted cells may be reduced due to effects of degradable hydrogels on reduced GSH content, an effect that may hold true more generally for other clinically relevant neuronal cell populations susceptible to injury from ROS.
Acknowledgments
The authors would like to thank NIH for their support (R01 NS052597-02) and the U.S. Department of Education's Graduate Assistantships in Areas of National Need Program for fellowship to K.J.L. They would also like to thank Dr. H.K. Heywood for assistance in developing the lactate quantification protocol and Enrique Gonzalez-Moore for assistance in viability and proliferation quantification.
Disclosure Statement
No competing financial interests exist.
References
- 1.Kordower J.H. Freeman T.B. Snow B.J. Vingerhoets F.J.G. Mufson E.J. Sanberg P.R. Hauser R.A. Smith D.A. Nauert G.M. Perl D.P. Olanow C.W. Neuropathological evidence of graft-survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with parkinsons-disease. N Engl J Med. 1995;332:1118. doi: 10.1056/NEJM199504273321702. [DOI] [PubMed] [Google Scholar]
- 2.Clarkson E.D. Zawada W.M. Adams F.S. Bell K.P. Freed C.R. Strands of embryonic mesencephalic tissue show greater dopamine neuron survival and better behavioral improvement than cell suspensions after transplantation in parkinsonian rats. Brain Res. 1998;806:60. doi: 10.1016/s0006-8993(98)00717-3. [DOI] [PubMed] [Google Scholar]
- 3.Yang D.L. Zhang Z.J. Oldenburg M. Ayala M. Zhang S.C. Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in Parkinsonian rats. Stem Cells. 2008;26:55. doi: 10.1634/stemcells.2007-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yasuhara T. Matsukawa N. Hara K. Yu G.L. Xu L. Maki M. Kim S.U. Borlongan C.V. Transplantation of human neural stem cells exerts neuroprotection in a rat model of Parkinson's disease. J Neurosci. 2006;26:12497. doi: 10.1523/JNEUROSCI.3719-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendez I. Sanchez-Pernaute R. Cooper O. Vinuela A. Ferrari D. Bjorklund L. Dagher A. Isacson O. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson's disease. Brain. 2005;128:1498. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redmond D.E. Bjugstad K.B. Teng Y.D. Ourednik V. Ourednik J. Wakeman D.R. Parsons X.H. Gonzalez R. Blanchards B.C. Kim S.U. Gu Z. Lipton S.A. Markakis E.A. Roth R.H. Elsworth J.D. Sladek J.R. Sidman R.L. Snyder E.Y. Behavioral improvement in a primate Parkinson's model is associated with multiple homeostatic effects of human neural stem cells. Proc Natl Acad Sci USA. 2007;104:12175. doi: 10.1073/pnas.0704091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zawada W.M. Zastrow D.J. Clarkson E.D. Adams F.S. Bell K.P. Freed C.R. Growth factors improve immediate survival of embryonic dopamine neurons after transplantation into rats. Brain Res. 1998;786:96. doi: 10.1016/s0006-8993(97)01408-x. [DOI] [PubMed] [Google Scholar]
- 8.Emgard M. Karlsson J. Hansson O. Brundin P. Patterns of cell death and dopaminergic neuron survival in intrastriatal nigral grafts. Exp Neurol. 1999;160:279. doi: 10.1006/exnr.1999.7198. [DOI] [PubMed] [Google Scholar]
- 9.Barker R.A. Dunnett S.B. Faissner A. Fawcett J.W. The time course of loss of dopaminergic neurons and the gliotic reaction surrounding grafts of embryonic mesencephalon to the striatum. Exp Neurol. 1996;141:79. doi: 10.1006/exnr.1996.0141. [DOI] [PubMed] [Google Scholar]
- 10.Park K.I. Teng Y.D. Snyder E.Y. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat Biotechnol. 2002;20:1111. doi: 10.1038/nbt751. [DOI] [PubMed] [Google Scholar]
- 11.Schense J.C. Bloch J. Aebischer P. Hubbell J.A. Enzymatic incorporation of bioactive peptides into fibrin matrices enhances neurite extension. Nat Biotechnol. 2000;18:415. doi: 10.1038/74473. [DOI] [PubMed] [Google Scholar]
- 12.Bellamkonda R. Ranieri J.P. Aebischer P. Laminin oligopeptide derivatized agarose gels allow 3-dimensional neurite extension in vitro. J Neurosci Res. 1995;41:501. doi: 10.1002/jnr.490410409. [DOI] [PubMed] [Google Scholar]
- 13.Yu X.J. Dillon G.P. Bellamkonda R.V. A laminin and nerve growth factor-laden three-dimensional scaffold for enhanced neurite extension. Tissue Eng. 1999;5:291. doi: 10.1089/ten.1999.5.291. [DOI] [PubMed] [Google Scholar]
- 14.Beaty C.E. Saltzman W.M. Controlled growth-factor delivery induces differential neurite outgrowth in 3-dimensional cell-cultures. J Control Release. 1993;24:15. [Google Scholar]
- 15.Hynes S.R. McGregor L.M. Rauch M.F. Lavik E.B. Photopolymerized poly(ethylene glycol)/poly(L-lysine) hydrogels for the delivery of neural progenitor cells. J Biomater Sci Polym Ed. 2007;18:1017. doi: 10.1163/156856207781494368. [DOI] [PubMed] [Google Scholar]
- 16.Ren Y.J. Zhou Z.Y. Cui F.Z. Hyaluronic acid/polylysine hydrogel as a transfer system for transplantation of neural stem cells. J Bioact Compat Polym. 2009;24:56. [Google Scholar]
- 17.Zhong Y.H. Bellamkonda R.V. Biomaterials for the central nervous system. J Royal Soc Interface. 2008;5:957. doi: 10.1098/rsif.2008.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nisbet D.R. Crompton K.E. Horne M.K. Finkelstein D.I. Forsythe J.S. Neural tissue engineering of the CNS using hydrogels. J Biomed Mater Res B Appl Biomater. 2008;87B:251. doi: 10.1002/jbm.b.31000. [DOI] [PubMed] [Google Scholar]
- 19.Anseth K.S. Metters A.T. Bryant S.J. Martens P.J. Elisseeff J.H. Bowman C.N. In situ forming degradable networks and their application in tissue engineering and drug delivery. J Control Release. 2002;78:199. doi: 10.1016/s0168-3659(01)00500-4. [DOI] [PubMed] [Google Scholar]
- 20.Mahoney M.J. Anseth K.S. Three-dimensional growth and function of neural tissue in degradable polyethylene glycol hydrogels. Biomaterials. 2006;27:2265. doi: 10.1016/j.biomaterials.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Burdick J.A. Ward M. Liang E. Young M. Langer R. Controlled delivery of neurotrophic factors from photocrosslinkable and degradable PEG hydrogels. J Neurotrauma. 2004;21:1311. [Google Scholar]
- 22.Burdick J.A. Ward M. Liang E. Young M.J. Langer R. Stimulation of neurite outgrowth by neurotrophins delivered from degradable hydrogels. Biomaterials. 2006;27:452. doi: 10.1016/j.biomaterials.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 23.Gunn J.W. Turner S.D. Mann B.K. Adhesive and mechanical properties of hydrogels influence neurite extension. J Biomed Mater Res A. 2005;72A:91. doi: 10.1002/jbm.a.30203. [DOI] [PubMed] [Google Scholar]
- 24.Mahoney M.J. Anseth K.S. Contrasting effects of collagen and bFGF-2 on neural cell function in degradable synthetic PEG hydrogels. J Biomed Mater Res A. 2007;81A:269. doi: 10.1002/jbm.a.30970. [DOI] [PubMed] [Google Scholar]
- 25.Namba R.M. Cole A.A. Bjugstad K.B. Mahoney M.J. Development of porous PEG hydrogels that enable efficient, uniform cell-seeding and permit early neural process extension. Acta Biomater. 2009;5:1884. doi: 10.1016/j.actbio.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 26.Bjugstad K.B. Redmond D.E. Lampe K.J. Kern D.S. Sladek J.R. Mahoney M.J. Biocompatibility of PEG-based hydrogels in primate brain. Cell Transplant. 2008;17:409. [PubMed] [Google Scholar]
- 27.Kandel E.R. Schwartz J.H. Jessell T.M. Principles of Neural Science. Fourth. New York: McGraw-Hill; 2000. [Google Scholar]
- 28.Bryant S.J. Anseth K.S. Controlling the spatial distribution of ECM components in degradable PEG hydrogels for tissue engineering cartilage. J Biomed Mater Res A. 2003;64A:70. doi: 10.1002/jbm.a.10319. [DOI] [PubMed] [Google Scholar]
- 29.Benoit D.S.W. Durney A.R. Anseth K.S. Manipulations in hydrogel degradation behavior enhance osteoblast function and mineralized tissue formation. Tissue Eng. 2006;12:1663. doi: 10.1089/ten.2006.12.1663. [DOI] [PubMed] [Google Scholar]
- 30.Bryant S.J. Anseth K.S. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59:63. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 31.Rice M.A. Anseth K.S. Encapsulating chondrocytes in copolymer gels: bimodal degradation kinetics influence cell phenotype and extracellular matrix development. J Biomed Mater Res A. 2004;70A:560. doi: 10.1002/jbm.a.30106. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong R.J.E. Watts C. Svendsen C.N. Dunnett S.B. Rosser A.E. Survival, neuronal differentiation, and fiber outgrowth of propagated human neural precursor grafts in an animal model of Huntington's disease. Cell Transplant. 2000;9:55. doi: 10.1177/096368970000900108. [DOI] [PubMed] [Google Scholar]
- 33.Oka S. Honmou O. Akiyama Y. Sasaki M. Houkin K. Hashi K. Kocsis J.D. Autologous transplantation of expanded neural precursor cells into the demyelinated monkey spinal cord. Brain Res. 2004;1030:94. doi: 10.1016/j.brainres.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 34.Gage F.H. Coates P.W. Palmer T.D. Kuhn H.G. Fisher L.J. Suhonen J.O. Peterson D.A. Suhr S.T. Ray J. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci USA. 1995;92:11879. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindvall O. Brundin P. Widner H. Rehncrona S. Gustavii B. Frackowiak R. Leenders K.L. Sawle G. Rothwell J.C. Marsden C.D. Bjorklund A. Grafts of fetal dopamine neurons survive and improve motor function in Parkinsons-disease. Science. 1990;247:574. doi: 10.1126/science.2105529. [DOI] [PubMed] [Google Scholar]
- 36.Pluchino S. Quattrini A. Brambilla E. Gritti A. Salani G. Dina G. Galli R. Del Carro U. Amadio S. Bergami A. Furlan R. Comi G. Vescovi A.L. Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- 37.Studer L. Tabar V. McKay R.D.G. Transplantation of expanded mesencephalic precursors leads to recovery in parkinsonian rats. Nat Neurosci. 1998;1:290. doi: 10.1038/1105. [DOI] [PubMed] [Google Scholar]
- 38.Lampe K.J. Namba R.M. Silverman T.R. Bjugstad K.B. Mahoney M.J. Impact of lactic acid on cell proliferation and free radical induced cell death in monolayer cultures of neural precursor cells. Biotechnol Bioeng. 2009;103:1214. doi: 10.1002/bit.22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lampe K.J. Namba R.M. Bjugstad K.B. Mahoney M.J. Effect of macromer weight percent on neural cell growth in 2D and 3D nondegradable PEG hydrogel culture. J Biomed Mater Res A. doi: 10.1002/jbm.a.32787. (In press) [DOI] [PubMed] [Google Scholar]
- 40.Sawhney A.S. Pathak C.P. Hubbell J.A. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(alpha-hydroxy acid) diacrylate macromers. Macromolecules. 1993;26:581. [Google Scholar]
- 41.Kweon S.M. Kim H.J. Lee Z.W. Kim S.J. Kim S.I. Paik S.G. Ha K.S. Real-time measurement of intracellular reactive oxygen species using mito tracker orange (CMH(2)TMRos) Biosci Rep. 2001;21:341. doi: 10.1023/a:1013290316939. [DOI] [PubMed] [Google Scholar]
- 42.Smith J. Ladi E. Mayer-Proschel M. Noble M. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci USA. 2000;97:10032. doi: 10.1073/pnas.170209797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsatmali M. Walcott E.C. Makarenkova H. Crossin K.L. Reactive oxygen species modulate the differentiation of neurons in clonal cortical cultures. Mol Cell Neurosci. 2006;33:345. doi: 10.1016/j.mcn.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis K.A. Burdick J.A. Anseth K.S. Photoinitiated crosslinked degradable copolymer networks for tissue engineering applications. Biomaterials. 2003;24:2485. doi: 10.1016/s0142-9612(02)00582-3. [DOI] [PubMed] [Google Scholar]
- 45.Schafer F.Q. Buettner G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 46.Martens P.J. Bryant S.J. Anseth K.S. Tailoring the degradation of hydrogels formed from multivinyl poly(ethylene glycol) and poly(vinyl alcohol) macromers for cartilage tissue engineering. Biomacromolecules. 2003;4:283. doi: 10.1021/bm025666v. [DOI] [PubMed] [Google Scholar]
- 47.Groussard C. Morel I. Chevanne M. Monnier M. Cillard J. Delamarche A. Free radical scavenging and antioxidant effects of lactate ion: an in vitro study. J Appl Physiol. 2000;89:169. doi: 10.1152/jappl.2000.89.1.169. [DOI] [PubMed] [Google Scholar]
- 48.Philp A. Macdonald A.L. Watt P.W. Lactate—a signal coordinating cell and systemic function. J Exp Biol. 2005;208:4561. doi: 10.1242/jeb.01961. [DOI] [PubMed] [Google Scholar]
- 49.Olah J. Klivenyi P. Gardian G. Vecsei L. Orosz F. Kovacs G.G. Westerhoff H.V. Ovadi J. Increased glucose metabolism and ATP level in brain tissue of Huntington's disease transgenic mice. FEBS J. 2008;275:4740. doi: 10.1111/j.1742-4658.2008.06612.x. [DOI] [PubMed] [Google Scholar]
- 50.Rutz H.P. Little J.B. Exogenous lactate interferes with cell-cycle control in balb/3t3 mouse fibroblasts. Int J Radiat Oncol Biol Phys. 1995;31:525. doi: 10.1016/0360-3016(94)00362-O. [DOI] [PubMed] [Google Scholar]
- 51.Georges P.C. Miller W.J. Meaney D.F. Sawyer E.S. Janmey P.A. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J. 2006;90:3012. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gotz M.E. Kunig G. Riederer P. Youdim M.B.H. Oxidative stress—free-radical production in neural degeneration. Pharmacol Ther. 1994;63:37. doi: 10.1016/0163-7258(94)90055-8. [DOI] [PubMed] [Google Scholar]