Abstract
Background
Autoimmune thyroid diseases (AITD), including Graves' disease and Hashimoto's thyroiditis, arise due to complex interactions between environmental and genetic factors. There are sound data coming from epidemiological, family, and twin studies demonstrating a strong genetic influence on the development of AITD. In this review we summarize the new findings on the genetic susceptibility to AITD focusing on emerging mechanisms of susceptibility.
Summary
Candidate gene analysis, whole-genome linkage screening, genome-wide association studies, and whole-genome sequencing are the major technologies that have advanced this field, leading to the identification of at least seven genes whose variants have been associated with AITD. One of the major ones is the HLA-DR gene locus. Recently, it was shown that substitution of the neutral amino acids Ala or Gln with arginine at position beta 74 in the HLA-DR peptide-binding pocket is key to the etiology of both Graves' disease and Hashimoto's thyroiditis. Several other genes have also been shown to confer susceptibility to AITD. These can be classified into two groups: (i) immune regulatory genes (cytotoxic T lymphocyte-associated protein 4, CD40, protein tyrosine phosphatase-22, and CD25) and (ii) thyroid-specific genes (thyroglobulin and thyrotropin receptor genes). The influence of individual genes on the development of AITD when assessed in a population appears to be weaker than would be expected from the data showing strong genetic susceptibility to AITD. Two possible mechanisms explaining this discrepancy are gene–gene interactions and subset effects.
Conclusions
Significant progress has been made in our understanding of the immunogenetic mechanisms leading to thyroid autoimmunity. For the first time we are beginning to unravel these mechanisms at the molecular level. It is hoped that these new data will be translated into novel therapies and prevention strategies in AITD, such as costimulatory blockade.
Introduction
Autoimmune diseases (AITD) affect many endocrine organs such as the thyroid, pancreatic islet beta cells, adrenal, and pituitary. However, by far the commonest autoimmune endocrine diseases are AITD (1). In fact, according to one series AITD are the commonest autoimmune diseases in the United States (2). Intriguingly, the autoimmune attack on the thyroid results in two opposing clinical syndromes, Hashimoto's thyroiditis (HT) and Graves' disease (GD). In HT the lymphocytic infiltration of the thyroid gland leads to apoptosis of thyroid cells and hypothyroidism (3). In contrast, in GD the lymphocytic infiltration of the thyroid leads to activation of thyrotropin receptor (TSHR)–reactive B-cells that secrete TSHR-stimulating antibodies causing hyperthyroidism (4). The etiology of HT and GD involves common pathways in which thyroid reactive T-cells escape tolerance and infiltrate the thyroid, and unique pathways in which these thyroid-reactive T-cells either cause thyroid cell death (in HT) or stimulation (in GD). Thus, it is not surprising that the genetic susceptibility to HT and GD involves shared genes, as well as unique genes (1). This review will summarize the major recent advances in our understanding of the genetic susceptibility to AITD.
The Evolution of Genetic Studies in AITD
Advances in genetic methods in the past 15 years enabled significant progress in the identification of complex disease genes. In fact the field was driven forward by the unprecedented rapid advances in the methods available for gene mapping and identification in complex diseases. The quest to identify complex disease genes, such as AITD, evolved in four phases: Phase 1, candidate gene analyses; Phase 2, whole-genome linkage studies; Phase 3, genome-wide association studies (GWAS); Phase 4, whole-genome sequencing.
Phase 1: candidate gene analyses
Candidate genes are genes that, based on their biological function, are believed to play a role in the genetic susceptibility to complex diseases. For example, the insulin gene is a candidate gene for type 1 diabetes (T1D) since T1D is characterized by antiinsulin immune response. Likewise, the major thyroid autoantigenes, TSHR, thyroid peroxidase, and thyroglobulin (Tg), are excellent candidate genes for thyroid autoimmunity. The first candidate gene to be tested in AITD was the HLA-DR gene. Indeed, HLA-DR3 was shown to be a major susceptibility gene for GD and HT. Additional AITD susceptibility genes identified by the candidate gene approach are the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) gene and the TSHR gene (see below).
Phase 2: whole-genome linkage screening
Whole-genome linkage screening is a powerful tool, as it enables scanning the entire human genome for a disease gene without any prior assumptions on disease pathogenesis. Whole-genome linkage screening is performed by testing a panel of markers that span the entire human genome for linkage with a disease in a dataset of families in which the disease aggregates.
Linkage analysis
The principle of linkage analysis is based on the premise that the likelihood of recombination between two genes is a direct function of the genetic distance between them. In fact, based on this assumption the distance between genes in linkage maps of the human genome is measured in recombination fraction units. Therefore, if two genes or polymorphisms are close together on a chromosome, they will cosegregate in families, since recombination between them will rarely occur. Similarly, if a polymorphic marker is close to a disease susceptibility gene, its alleles will cosegregate with the disease in families. The measure of the likelihood of linkage between a disease and a genetic marker is the logarithm of odds (LOD) score (5). The LOD score is the base-10 LOD ratio in favor of linkage. According to widely accepted guidelines, in complex diseases an LOD score of >1.9 is suggestive of linkage, while an LOD score of >3.3 indicates significant linkage in studies using the parametric approach. Linkage is confirmed if evidence for linkage is replicated in two separate data sets (6).
Polymorphic markers used in whole-genome linkage studies
The major genetic polymorphisms used in whole-genome linkage studies are microsatellites and single-nucleotide polymorphisms (SNPs). Microsatellites are dinucleotide repeats such as (AC)n. They are highly polymorphic because there are many possible number of repeats. They are genotyped by PCR amplification and size separation. For screening the entire human genome for linkage, 400 microsatellites provide enough density. New technologies enabled automation and rapid screening of the entire human genome for linkage with sets of microsatellite markers. SNPs are single-base positions in genomic DNA where two alleles exist, such as A/C. SNPs are very abundant and on the average there is a SNP every 300 bp. Since SNPs have two alleles, unlike microsatellites that have 5–10 alleles, more SNPs are required to screen the entire human genome for linkage with a disease, usually about 4000 SNPs are required.
Performing whole-genome linkage studies
To perform a whole-genome linkage study one needs to collect multiplex families (families in which more than one member is affected by the disease) and genotype all members for the panel of microsatellites or SNPs covering the entire human genome (7). If one or more markers in a certain locus show evidence for linkage with the disease, this locus may harbor a susceptibility gene for the disease studied. Linked regions can then be fine mapped and the genes identified (8). Many loci have been identified as linked with AITD in whole-genome linkage studies. In our own whole-genome linkage screens we have identified 7 AITD susceptibility loci on chromosomes: 2q, 6p, 8q, 10q, 12q, 14q, and 20q. Some of these loci have been fine-mapped and the genes identified. The AITD susceptibility gene on 2q is the CTLA-4 gene (also identified by the candidate gene approach), the susceptibility gene on 8q is Tg, on 14q the TSHR (also identified by the candidate gene approach), and on 20q the CD40 gene.
Phase 3: GWAS
Association analysis
Association analysis is highly sensitive and may detect genes contributing <5% of the total genetic contribution to a disease. Association analyses are performed by comparing the frequency of the allele studied (e.g., HLA-DR3) in a dataset of patients and in ethnically matched control subjects. If the allele tested is associated with the disease, it will appear significantly more frequently in patients than in control subjects. The probability of having the disease in an individual positive for the allele compared with an individual negative for the allele is estimated by the relative risk (9). There are at least two possible explanations for the existence of an association between an allele and a disease: (i) the associated allele itself is the genetic variant causing an increased risk for the disease, and (ii) the associated allele itself is not causing the disease but rather a gene in linkage disequilibrium (LD) with it (10).
Performing a GWAS
Up until recently, whole-genome scanning was possible only using linkage because the linkage intervals needed between markers are 10–20 Mb, whereas for genome-wide screening by association analysis one would need to employ ∼500,000 markers at much shorter distances (∼<50 kb). The completion of the HapMap project (11) has made whole-genome scanning by association studies feasible (12). The HapMap project genotyped more than 1 million SNPs spanning the entire human genome in four ethnically distinct human populations and tested these SNPs for LD (11). The HapMap analysis demonstrated that the human genome is highly organized into discrete LD blocks that are flanked by recombination hot spots, or areas at which recombinations are much more likely to occur. Recombinations are much rarer at the LD blocks with all the markers in each block in tight LD. This enabled the utilization of tag-SNPs (each SNP representing an entire LD block) to test the entire human genome for association with disease. Moreover, microarray-based genotyping technology enabled the typing of up to 500,000 SNPs in a single experiment. Thus, today it is possible to scan the entire human genome using densely spaced SNPs.
So far, no full GWAS have been reported in AITD. However, one limited nonsynonymous SNP GWAS was reported in GD (13). Nonsynonymous SNPs are SNPs that cause amino acid changes in the protein coded for by genes. In this study 14,500 nonsynonymous SNPs were genotyped in 900 AITD patients and 1466 control subjects. The study confirmed the TSHR gene as a susceptibility gene for GD and identified the FCRL3 as an additional putative susceptibility gene for GD (13).
Phase 4: whole-genome sequencing
Very recently, the field of complex disease genetics was revolutionized again by the development of rapid and affordable platforms for sequencing the entire human genome. While the price is still high, it is expected that sequencing the entire human genome will cost around $1000 per sample within the next few years making whole-genome sequencing a feasible approach to identify complex disease genes. Whole-genome sequencing has already been utilized successfully in two patients, one with Carcot-Marie-Tooth disease (14) and the other with acute myeloid leukemia (15). The main challenge of whole-genome sequencing is developing robust methods for analyzing the sequence data and sorting out normal variations between individuals from those that are responsible for disease susceptibility. However, once the sequence changes responsible for susceptibility to various complex diseases are mapped, it will be possible to offer to individuals a genetic risk assessment whereby a whole-genome sequence of the individual will provide a panel of genetic risks for the individual prompting prophylactic and life style interventions. Moreover, the availability of whole-genome sequencing will lead to better predictions of responses to medications, including idiosyncratic responses such as agranulocytosis from antithyroid medications. This will truly lead to a personalized approach to the treatment of complex diseases such as AITD.
AITD Susceptibility Genes Identified
Several recent reviews summarized thoroughly the data on susceptibility genes for thyroid autoimmunity (1,16–18). Therefore, here we will give only a brief overview of the major susceptibility genes for AITD.
Immunological synapse genes
The immunological synapse is the interface between antigen-presenting cells (APCs) and T-cells that is formed during T-cell activation. The immunological synapse is a complex interface involving peptide antigen bound to an HLA class II molecule and to the T-cell receptor, costimulatory molecules, and receptors on the APC and T-cells, integrins, and other molecules (19). Interestingly, several of the AITD susceptibility genes participate in the immunological synapse, suggesting that abnormalities in antigen presentation are important mechanisms leading to AITD.
The HLA-DR gene
The major histocompatibility complex region, encoding the HLA glycoproteins, is a highly polymorphic genetic region that includes many genes and is located on chromosome 6p21 (16). Initial studies analyzed the major HLA class II alleles in AITD (16). These studies demonstrated a significant association of GD with HLA-DR3 (20,21). However, data on HLA alleles in HT have been less definitive than in GD, with several HLA-DR alleles reported to be associated (22–26).
Recent studies shifted the focus from the association of the major HLA-DR allele groupings with AITD to the molecular structure of the peptide binding pocket and its association with disease (27,28). This mechanistic-based approach proved very fruitful. Since T-cells recognize and respond to peptide antigens when presented by APCs bound to HLA class II pockets, it was hypothesized that certain HLA-DR alleles may permit autoantigenic peptides to fit into the peptide binding pocket and to be presented more efficiently to T-cells (29). Indeed in several autoimmune diseases, most notable T1D, this hypothesis was confirmed (30). In T1D it was found that the amino acid residue at position 57 of the DQβ chain plays a key role in the genetic susceptibility to T1D (31,32). We have discovered that a similar mechanism is key to the etiology of AITD. We recently identified arginine at position 74 of the HLA-DRβ1 chain (DRβ-Arg74) as the critical DR amino acid conferring susceptibility to GD (27). In contrast, the presence of glutamine at position 74 of the DRβ1 chain was protective. These data were replicated in an independent dataset (33). Similarly, we have identified a pocket HLA-DR amino acid signature that conferred strong risk for HT (28). As in GD, the key pocket amino acid was also DRβ-Arg74. Structural analysis demonstrated that this pocket amino acid signature resulted in a unique pocket structure that is likely to influence pathogenic peptide binding and presentation to T-cells. Further studies enabled us to identify those Tg peptides that could be presented by HLA-DR pockets containing arginine at position beta 74 (34). Thus the peptide binding pocket structure plays a major role in the etiology of AITD, as has been shown in T1D (35).
CTLA-4
The CTLA-4 gene is a major negative regulator of T-cell activation (36). Since CTLA-4 suppresses T-cell activation to control normal T-cell responses, it was postulated that CTLA-4 polymorphisms that reduce its expression and/or function might predispose to autoimmunity by creating overreactive T-cells.
The first demonstration of an association between CTLA-4 and GD was reported by DeGroot and colleagues (37). In fact, their study showing a significant association between a microsatellite in the 3′ untranslated region (3′UTR) of CTLA-4 and GD was the first report of an association between CTLA-4 and any autoimmune condition. By now CTLA-4 is established as an autoimmunity gene shown to be linked and/or associated with various autoimmune conditions. These include both GD and HT (38–43), as well as the production of thyroid antibodies (TAb) alone without clinical disease (44–46). The association has been consistent across ethnic and geographic groups (37,40,47–52).
Recent analysis by our group showed that the involvement of CTLA-4 in the genetic susceptibility to AITD is more complex than originally thought. While CTLA-4 alone predisposes to the development of TAb, CTLA-4 may play a role in the susceptibility to high levels TAb and clinical AITD when interacting with other loci (53). Moreover, both the G allele (previously reported to be associated with AITD) and the A allele (reported to be protective) of the A/G49 SNP of CTLA-4 may predispose to AITD when interacting with different loci (53).
It is still not known which CTLA-4 variant is the causative variant and by what mechanism it confers susceptibility to autoimmunity. Three main CTLA-4 variants have been studied: an AT-repeat microsatellite at the 3'UTR of the CTLA-4 gene (37,40); an A/G SNP at position 49 in the signal peptide resulting in an alanine/threonine substitution (A/G49) (39,51,54–56); and an A/G SNP located downstream and outside of the 3′UTR of the CTLA-4 gene (designated CT60) (42). To identify the causative variant, functional studies are needed as all associated variants are in tight LD. Mechanistically, a polymorphism that reduced CTLA-4 expression/function would be expected to augment T-cell activation leading to autoimmunity. Indeed, Kouki et al. (57) have shown an association between the G allele of the A/G49 SNP and reduced inhibition of T-cell proliferation, results that were later replicated by us (43). However, this association could be due to a direct effect of the A/G49 SNP on CTLA-4 expression/function, or due to the effects of another variant in LD with the A/G49 SNP. Further studies did not support a role for the A/G49 SNP in reducing expression and function of CTLA-4. They suggested that another variant in LD with A/G49 is causative (58). At this point there are data to support either the CT60 (42) or the 3′UTR (AT)n (59,60) as the causative variants, and further studies are needed to determine which one is causative.
CD40
CD40 is expressed primarily on B-cells and other APCs (61), and plays a fundamental role in B-cell activation and antibody secretion (62,63). Our whole-genome linkage study identified a locus on chromosome 20q that was linked with GD (7). Fine mapping identified the CD40 gene as the GD susceptibility gene in this locus, and further sequencing studies of the CD40 gene demonstrated that a C/T SNP in the Kozak sequence of CD40 was the likely causative variant (64–70). The CC genotype of this SNP was associated with GD (65,67–70). One study did not find the association, possibly due to ethnic differences among populations (71). However, a metaanalysis confirmed the association (69). Intriguingly, we have recently shown that the association of the CC genotype was stronger in a subset of GD patients who had persistently high levels of TAb (72). Mechanistically, the CC genotype, being located in the Kozak sequence of CD40, can alter CD40 translation and expression. Indeed, we and others have shown that the C-allele of the polymorphism increased the translation of CD40 mRNA transcripts by 20%–30% compared to the T-allele (73,74). Since CD40 is expressed on B-cells (61) and on thyroid follicular cells (72,75,76), both involved in the development of GD, it is possible that increased CD40 expression on B-cells and or thyrocytes driven by the C-allele predisposes to disease (73). Thus, increased expression of CD40 on B-cells can result in enhanced production of anti-TSHR-stimulating antibodies, whereas increased expression of CD40 on thyrocytes can trigger an autoimmune response to the thyroid by resident T-cells. These mechanisms are not mutually exclusive and could both be operating.
Since CD40 is a major APC and B-cell costimulatory molecule, it seems plausible that CD40 will play a role in the genetic susceptibility to other autoimmune diseases. Indeed, recent data have shown that CD40 was associated and/or linked with high IgE levels in asthma (74), rheumatoid arthritis (77,78), systemic lupus erythematosus (79), and multiple sclerosis (80).
The protein tyrosine phosphatase-22 gene
The lymphoid tyrosine phosphatase, encoded by the protein tyrosine phosphatase-22 (PTPN22) gene, is a negative regulator of T-cell activation (81). The PTPN22 gene was found to be associated with AITD, including both GD (82) and HT (83), as well as with other autoimmune diseases (84–87). The causative SNP is a tryptophan/arginine variant at position 620. The disease associated variant is a gain-of-function variant and therefore, the mechanism by which it predisposes to autoimmunity is not trivial as it would be expected to suppress T-cell activation (88). It is possible that the decreased activation of T-cells enables self-reactive T-cells to escape the thymic central tolerance mechanisms, but this theoretical possibility awaits experimental confirmation.
Regulatory T-cells genes
Regulatory T-cells (Treg) are an important subset of T-cells that regulate T-cell activation (89). They play a major role in peripheral tolerance to self-antigens. Indeed, upregulation of Treg cells suppressed experimental autoimmune thyroiditis in mice (90). Moreover, depletion of Treg in mice increased their susceptibility to experimental GD (91). In another recent study, McLachlan et al. have demonstrated that Treg depletion in mice induced with experimental GD caused a switch in the mice disease to a Hashimoto's-like disease (92). Several subtypes of Treg cells have been identified. One subtype, the natural Treg cells, are characterized by expressing constitutively CD25, CTLA-4, and glucocorticoid-induced tumor necrosis factor receptor. In addition their development is regulated by the FOXP3 gene. Interestingly, both FOXP3 and CD25 have been found to be associated with AITD.
FOXP3
The FOXP3 gene is the key gene to the differentiation of T-cells into natural Treg cells. Indeed FOXP3 knockout mice develop a fatal lymphoproliferative disorder (93). Therefore, the FOXP3 is an excellent candidate gene for AITD. Moreover, FOXP3 is located in a region on chromosome Xp11.23 that has been shown to be linked with AITD. Therefore, we have tested the FOXP3 gene in two cohorts of AITD patients: U.S. Caucasians and a Japanese cohort. Our results demonstrated an association of a microsatellite inside the FOXP3 gene with AITD in the Caucasians but not in the Japanese, demonstrating ethnic differences in disease susceptibility (94). Further analysis demonstrated that the association of FOXP3 with AITD was mostly in the subset of patients with juvenile GD (95).
CD25
Natural Treg cells are characterized by constitutive expression of high levels of the alpha chain of the IL-2 receptor (CD25) (89). Recent studies have found association between the CD25 gene and T1D (96), as well as with GD (97). However, the mechanisms by which CD25 can predispose to autoimmunity are unclear since the levels of expression of CD25 on Treg cells do not correlate with their suppressive activity (98).
Thyroid-specific genes
Thyroglobulin
Tg is one of the main targets of the immune response in AITD (99). Therefore, it was an obvious candidate gene for AITD. Indeed, our whole-genome linkage study identified the Tg gene as a major AITD susceptibility gene (7). These findings were replicated by several other studies in several ethnic groups (100–105). Sequencing of the Tg gene identified three amino acid variants that were associated with AITD, A734S, V1027M, and W1999R (106). Mechanistically, it is possible that the Tg variants may predispose to disease by altering Tg degradation in endosomes resulting in a pathogenic Tg peptide repertoire. Supporting this mechanism are our data showing a genetic interaction between HLA-DRβ-Arg74 and one of the Tg variant, W1999R, resulting in a high odds ratio (OR) for GD (107). Further, we have now shown that only a small group of unique Tg peptides can bind to the HLA-DRβ-Arg74 pockets (34), and one of these peptides was shown to represent a major T-cell epitope (108).
TSHR gene
GD is defined by the presence of stimulating TSHR antibodies (4). Not surprisingly, the TSHR was the first gene (after HLA) to be tested for association with GD. Earlier studies that tested three nonsynonymous SNPs in the TSHR gene for association with GD, D36H, P52T, and D727E, gave mixed results (109–114). However, studies from Japan consistently reported associations of the TSHR with GD in the Japanese (48,52). Finally, more recently, it was found that noncoding intronic SNPs in the TSHR are associated with GD (115,116). The most consistent association in Caucasians has been with an intron 1 SNP (117,118). Mechanistically, intron 1 SNPs in the TSHR can alter its splicing. Indeed, several splice variants of the TSHR exist. The major splice variant of the TSHR, identified by us in 1992 (119), is a 1.3 Kb variant, which includes most of the extracellular domain of the TSHR. Other minor splice variants have also been discovered (120).
From Genes to Mechanisms
How many susceptibility genes are needed to develop AITD?
One of the many unexpected findings of genetic studies in complex diseases was that most genes identified had a very minor effects (121). Indeed, with the exception of the DRβ1-Arg74 HLA variant, which gave an OR for GD of >5, all other AITD genes gave very low ORs of <1.5 (17). How can we reconcile the overwhelming data on the strong genetic susceptibility to AITD, contributing about 80% of the liability to disease (122), with the very weak effects of most genes found? The dogma is that for there to be a strong genetic effect on disease, an individual needs to inherit many genes with small effect. Some have estimated that >20 different susceptibility variants need to be inherited to develop complex diseases such as AITD. However, as illustrated in Figure 1, the dogma is clearly wrong as it cannot explain the high prevalence of AITD in the general population. To demonstrate this we simulated five genetic variants predisposing to AITD, each giving an OR of 1.2 individually and each having a population frequency of 0.2 (20%). Therefore, the combined OR if an individual inherited all five variants, assuming an additive effect is 1.2 × 1.2 × 1.2 ×1.2 × 1.2 = 2.5. The frequency in the population of the combined genotype including all five susceptible variants (assuming no LD exists between them, and assuming the frequency of each variant alone is 0.2 [20%]) is 0.2 × 0.2 × 0.2 × 0.2 × 0.2 = 0.00032 or 0.032% (Fig. 1). This is an extremely low frequency of the combined genotype and cannot explain the high frequency of AITD in the general population (up to 5%) (2). Therefore, our group has proposed two alternative mechanisms for the finding of very low ORs for most AITD genes identified: subset effect and gene–gene interactions.
FIG. 1.
This figure illustrates the inherent paradox with the dogma that numerous genes with small effect can cause an additive strong genetic effect on susceptibility to disease. We simulated five genetic variants predisposing to autoimmune thyroid diseases, each giving an odds ratio (OR) of 1.2 individually. Therefore, the combined OR if an individual inherited all five variants, assuming an additive effect is 1.2 × 1.2 × 1.2 × 1.2 × 1.2 = 2.5. However, the calculated frequency in the population of the combined genotype including all five susceptible variants (assuming no linkage disequilibrium exists between them), assuming the frequency of each variant alone is 0.2 (20%) would be 0.2 × 0.2 × 0.2 × 0.2 × 0.2 = 0.00032 or 0.032%. Such a low frequency of the combined genotype is not consistent with the strong genetic influence on highly prevalent complex diseases, such as autoimmune thyroid diseases.
Gene–gene interaction
According to this model two genes with weak effects (i.e., associated with low ORs) interact, biologically resulting in a combined OR that is significantly higher than the one expected with an additive effect alone. For example, two genes with ORs for disease of 1.2 when inherited together would give an OR of 1.44 (1.2 × 1.2) if there was only an additive effect. However, if there is an interaction between these two genes, the OR for disease will be significantly higher than 1.44. Indeed, we have shown suggestive evidence for interaction between the Tg gene and DRβ1-Arg74 when conferring susceptibility for GD (107).
Subset effect (also called genetic heterogeneity)
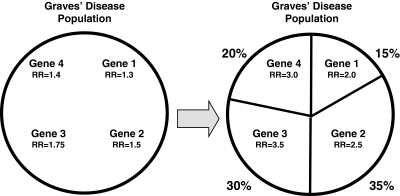
According to this model each of the genetic variants identified has a large effect resulting in a high OR in a subset of the AITD patients studied. However, when these variants are tested in the entire population of AITD patients, their effects are diluted, resulting in much smaller ORs. This model is illustrated in Figure 2. Indeed, we have shown that the CD40 gene was associated in all GD patients with an OR of 1.8, but when we divided the patients into the subset of those that had high antibody levels after treatment, the association became stronger and the OR was 2.5.
FIG. 2.
The model of subset effects or genetic heterogeneity. To simulate the effects of subsets we simulated a dataset of Graves' patients in which four genetic subsets exist: Subset 1 was assumed to comprise 15% of the total Graves' disease (GD) population; subset 2, 35%; subset 3, 30%; and subset 4, 20%. Each subset is simulated to be influenced by one gene. We simulated the relative risks of the subset-specific genes as follows: Gene 1 was assumed to have a relative risk (RR) of 2.0 for GD in subset 1; gene 2 was assumed to have an RR of 2.5 in subset 2; gene 3 was assumed to have an RR of 3.5 in subset 3; gene 4 was assumed to have an RR of 3.0 in subset 4. Using the assumed percent of the population belonging to each GD subset and the assumed relative risks contributed by each subset-specific gene, we calculated the relative risk contributed by the gene when testing the entire population of GD patients. The results showed that the relative risks decreased significantly when testing the gene in the entire dataset of GD patients; that is, the effect of the gene is diluted and the relative risk is reduced when the gene is tested in the entire GD population, most of which are not influenced by this gene.
Putative mechanisms for the development of AITD
So far, seven confirmed AITD susceptibility genes have been identified. We still do not know the mechanisms by which they confer susceptibility to disease, but several putative mechanisms are conceivable.
Immunological synapse genes
The genes involved in the immunological synapse can predispose to disease by enabling presentation of self-antigens. It is likely that the DRβ1-Arg74, which is associated with GD and HT, predisposes to AITD by creating a peptide binding pocket that can more easily accommodate pathogenic Tg and/or TSHR peptides that can trigger T-cell response to thyroid antigens. Studies by DeGroot (123) and by us (34) support this hypothesis. The costimulatory molecules can enhance the activation of cells participating in the immunological synapse. The CTLA-4 variants that are associated with autoimmunity are believed to reduce the inhibitory effect of CTLA-4 on T-cell activation, resulting in uncontrolled activation of T-cells, which can predispose to autoimmunity in general (43,57,59). On the other hand, the CD40 variant associated with disease increases its expression and function (73). Increased CD40 expression on B-cells can enhance their capacity to produce antibodies. Alternatively, it is possible that thyroid-specific CD40 expression can trigger thyroid autoimmunity by bystander mechanisms. The role of the PTPN22 gene in autoimmunity is unclear since the variant associated with autoimmunity activates the gene that normally suppresses T-cell activation.
Treg genes
Since Tregs play a major role in peripheral tolerance, it seems likely that variants in FOXP3 and/or CD25 that can reduce Treg function could predispose individuals to autoimmunity. Indeed, a complete knockout of FOXP3 results in a fatal lymphoproliferative disorder (93). Moreover, Tregs seem to play an important role in the mouse models of HT (90) and GD (92).
Thyroid-specific genes
Both the Tg and TSHR genes were found to be major susceptibility genes for AITD. It is conceivable that the variants in these genes associated with disease could influence the peptide profile generated by digestion of these proteins. Alternatively, the associated variants may influence Tg and TSH expression and splicing.
Conclusions
The AITD are complex diseases that are postulated to be caused by the combined effects of susceptibility genes and environmental triggers. Significant progress has been made in mapping the AITD susceptibility genes and understanding the mechanisms by which they confer risk for disease. The AITD susceptibility genes identified so far can be divided into three broad groups: (i) immunological synapse genes, (ii) Treg genes, and (iii) thyroid-specific genes. It is clear that additional genes contribute to the genetic susceptibility to AITD, as well as to the different phenotypes of AITD, disease severity, and, possibly, response to therapy. Ultimately, this interplay between susceptibility genes and environmental factors results in breakdown of self-tolerance leading to AITD. Understanding the mechanism of this interplay will be crucial for designing new, mechanism-based therapies for AITD.
Footnotes
Portions of this review were presented at the Spring 2010 Meeting of the American Thyroid Association, “Thyroid Disorders in the Era of Personalized Medicine,” Minneapolis, Minnesota, May 13–16, 2010.
Acknowledgment
This work was supported in part by grants DK061659, DK067555, and DK073681 from NIDDK.
Disclosure Statement
The author declares that no competing financial interests exist.
References
- 1.Huber A. Menconi F. Corathers S. Jacobson EM. Tomer Y. Joint genetic susceptibility to type 1 diabetes and autoimmune thyroiditis: from epidemiology to mechanisms. Endocr Rev. 2008;29:697–725. doi: 10.1210/er.2008-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson DL. Gange SJ. Rose NR. Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 3.Weetman AP. Chronic autoimmune thyroiditis. In: Braverman LE, editor; Utiger RD, editor. Werner and Ingbar's The Thyroid. Lippincott Williams and Wilkins; Philadelphia: 2000. pp. 721–732. [Google Scholar]
- 4.Menconi F. Oppenheim YL. Tomer Y. Graves' disease. In: Shoenfeld Y, editor; Cervera R, editor; Gershwin ME, editor. Diagnostic Criteria in Autoimmune Diseases. Humana Press; Totowa, NJ: 2008. pp. 231–235. [Google Scholar]
- 5.Ott J. Analysis of Human Genetic Linkage. third. Johns Hopkins University Press; Baltimore: 1999. [Google Scholar]
- 6.Lander E. Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- 7.Tomer Y. Ban Y. Concepcion E. Barbesino G. Villanueva R. Greenberg DA. Davies TF. Common and unique susceptibility loci in Graves and Hashimoto diseases: results of whole-genome screening in a data set of 102 multiplex families. Am J Hum Genet. 2003;73:736–747. doi: 10.1086/378588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glazier AM. Nadeau JH. Aitman TJ. Finding genes that underlie complex traits. Science. 2002;298:2345–2349. doi: 10.1126/science.1076641. [DOI] [PubMed] [Google Scholar]
- 9.Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955;19:251–253. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 10.Hodge SE. What association analysis can and cannot tell us about the genetics of complex disease. Am J Med Genet. 1994;54:318–323. doi: 10.1002/ajmg.1320540408. [DOI] [PubMed] [Google Scholar]
- 11.Altshuler D. Brooks LD. Chakravarti A. Collins FS. Daly MJ. Donnelly P. A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duerr RH. Taylor KD. Brant SR. Rioux JD. Silverberg MS. Daly MJ. Steinhart AH. Abraham C. Regueiro M. Griffiths A. Dassopoulos T. Bitton A. Yang H. Targan S. Datta LW. Kistner EO. Schumm LP. Lee AT. Gregersen PK. Barmada MM. Rotter JI. Nicolae DL. Cho JH. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burton PR. Clayton DG. Cardon LR. Craddock N. Deloukas P. Duncanson A. Kwiatkowski DP. McCarthy MI. Ouwehand WH. Samani NJ. Todd JA. Donnelly P. Barrett JC. Davison D. Easton D. Evans DM. Leung HT. Marchini JL. Morris AP. Spencer CC. Tobin MD. Attwood AP. Boorman JP. Cant B. Everson U. Hussey JM. Jolley JD. Knight AS. Koch K. Meech E. Nutland S. Prowse CV. Stevens HE. Taylor NC. Walters GR. Walker NM. Watkins NA. Winzer T. Jones RW. McArdle WL. Ring SM. Strachan DP. Pembrey M. Breen G. St. Clair D. Caesar S. Gordon-Smith K. Jones L. Fraser C. Green EK. Grozeva D. Hamshere ML. Holmans PA. Jones IR. Kirov G. Moskivina V. Nikolov I. O'Donovan MC. Owen MJ. Collier DA. Elkin A. Farmer A. Williamson R. McGuffin P. Young AH. Ferrier IN. Ball SG. Balmforth AJ. Barrett JH. Bishop TD. Iles MM. Maqbool A. Yuldasheva N. Hall AS. Braund PS. Dixon RJ. Mangino M. Stevens S. Thompson JR. Bredin F. Tremelling M. Parkes M. Drummond H. Lees CW. Nimmo ER. Satsangi J. Fisher SA. Forbes A. Lewis CM. Onnie CM. Prescott NJ. Sanderson J. Matthew CG. Barbour J. Mohiuddin MK. Todhunter CE. Mansfield JC. Ahmad T. Cummings FR. Jewell DP. Webster J. Brown MJ. Lathrop MG. Connell J. Dominiczak A. Marcano CA. Burke B. Dobson R. Gungadoo J. Lee KL. Munroe PB. Newhouse SJ. Onipinla A. Wallace C. Xue M. Caulfield M. Farrall M. Barton A. Bruce IN. Donovan H. Eyre S. Gilbert PD. Hilder SL. Hinks AM. John SL. Potter C. Silman AJ. Symmons DP. Thomson W. Worthington J. Dunger DB. Widmer B. Frayling TM. Freathy RM. Lango H. Perry JR. Shields BM. Weedon MN. Hattersley AT. Hitman GA. Walker M. Elliott KS. Groves CJ. Lindgren CM. Rayner NW. Timpson NJ. Zeggini E. Newport M. Sirugo G. Lyons E. Vannberg F. Hill AV. Bradbury LA. Farrar C. Pointon JJ. Wordsworth P. Brown MA. Franklyn JA. Heward JM. Simmonds MJ. Gough SC. Seal S. Stratton MR. Rahman N. Ban M. Goris A. Sawcer SJ. Compston A. Conway D. Jallow M. Newport M. Sirugo G. Rockett KA. Bumpstead SJ. Chaney A. Downes K. Ghori MJ. Gwilliam R. Hunt SE. Inouye M. Keniry A. King E. McGinnis R. Potter S. Ravindrarajah R. Whittaker P. Widden C. Withers D. Cardin NJ. Davison D. Ferreira T. Pereira-Gale J. Hallgrimsdo'ttir IB. Howie BN. Su Z. Teo YY. Vukcevic D. Bentley D. Brown MA. Compston A. Farrall M. Hall AS. Hattersley AT. Hill AV. Parkes M. Pembrey M. Stratton MR. Mitchell SL. Newby PR. Brand OJ. Carr-Smith J. Pearce SH. McGinnis R. Keniry A. Deloukas P. Reveille JD. Zhou X. Sims AM. Dowling A. Taylor J. Doan T. Davis JC. Savage L. Ward MM. Learch TL. Weisman MH. Brown M. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet. 2007;39:1329–1337. doi: 10.1038/ng.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lupski JR. Reid JG. Gonzaga-Jauregui C. Rio DD. Chen DC. Nazareth L. Bainbridge M. Dinh H. Jing C. Wheeler DA. McGuire AL. Zhang F. Stankiewicz P. Halperin JJ. Yang C. Gehman C. Guo D. Irikat RK. Tom W. Fantin NJ. Muzny DM. Gibbs RA. Whole-genome sequencing in a patient with Charcot-Marie-Tooth neuropathy. N Engl J Med. 2010;362:1181–1191. doi: 10.1056/NEJMoa0908094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mardis ER. Ding L. Dooling DJ. Larson DE. McLellan MD. Chen K. Koboldt DC. Fulton RS. Delehaunty KD. McGrath SD. Fulton LA. Locke DP. Magrini VJ. Abbott RM. Vickery TL. Reed JS. Robinson JS. Wylie T. Smith SM. Carmichael L. Eldred JM. Harris CC. Walker J. Peck JB. Du F. Dukes AF. Sanderson GE. Brummett AM. Clark E. McMichael JF. Meyer RJ. Schindler JK. Pohl CS. Wallis JW. Shi X. Lin L. Schmidt H. Tang Y. Haipek C. Wiechert ME. Ivy JV. Kalicki J. Elliott G. Ries RE. Payton JE. Westervelt P. Tomasson MH. Watson MA. Baty J. Heath S. Shannon WD. Nagarajan R. Link DC. Walter MJ. Graubert TA. Dipersio JF. Wilson RK. Ley TJ. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson EM. Huber A. Tomer Y. The HLA gene complex in thyroid autoimmunity: from epidemiology to etiology. J Autoimmun. 2008;30:58–62. doi: 10.1016/j.jaut.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobson EM. Tomer Y. The CD40, CTLA-4, thyroglobulin, TSH receptor, and PTPN22 gene quintet and its contribution to thyroid autoimmunity: back to the future. J Autoimmun. 2007;28:85–98. doi: 10.1016/j.jaut.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearce SH. Merriman TR. Genetics of type 1 diabetes and autoimmune thyroid disease. Endocrinol Metab Clin North Am. 2009;38:289–viii. doi: 10.1016/j.ecl.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Dustin ML. T-cell activation through immunological synapses and kinapses. Immunol Rev. 2008;221:77–89. doi: 10.1111/j.1600-065X.2008.00589.x. [DOI] [PubMed] [Google Scholar]
- 20.Farid NR. Graves' disease. In: Farid NR, editor. HLA in Endocrine and Metabolic Disorders. Academic Press; London: 1981. pp. 85–143. [Google Scholar]
- 21.Zamani M. Spaepen M. Bex M. Bouillon R. Cassiman JJ. Primary role of the HLA class II DRB1*0301 allele in Graves disease. Am J Med Genet. 2000;95:432–437. doi: 10.1002/1096-8628(20001218)95:5<432::aid-ajmg5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Farid NR. Sampson L. Moens H. Barnard JM. The association of goitrous autoimmune thyroiditis with HLA-DR5. Tissue Antigens. 1981;17:265–268. doi: 10.1111/j.1399-0039.1981.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 23.Moens H. Farid NR. Sampson L. Noel EP. Barnard JM. Hashimoto's thyroiditis is associated with HLA-DRw3. N Engl J Med. 1978;299:133–134. doi: 10.1056/NEJM197807202990306. [DOI] [PubMed] [Google Scholar]
- 24.Tandon N. Zhang L. Weetman AP. HLA associations with Hashimoto's thyroiditis. Clin Endocrinol (Oxf) 1991;34:383–386. doi: 10.1111/j.1365-2265.1991.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 25.Ban Y. Davies TF. Greenberg DA. Concepcion ES. Tomer Y. The influence of human leucocyte antigen (HLA) genes on autoimmune thyroid disease (AITD): results of studies in HLA-DR3 positive AITD families. Clin Endocrinol (Oxf) 2002;57:81–88. doi: 10.1046/j.1365-2265.2002.01566.x. [DOI] [PubMed] [Google Scholar]
- 26.Petrone A. Giorgi G. Mesturino CA. Capizzi M. Cascino I. Nistico L. Osborn J. Di Mario U. Buzzetti R. Association of DRB1*04-DQB1*0301 haplotype and lack of association of two polymorphic sites at CTLA-4 gene with Hashimoto's thyroiditis in an Italian population. Thyroid. 2001;11:171–175. doi: 10.1089/105072501300042901. [DOI] [PubMed] [Google Scholar]
- 27.Ban Y. Davies TF. Greenberg DA. Concepcion ES. Osman R. Oashi T. Tomer Y. Arginine at position 74 of the HLA-DRb1 chain is associated with Graves' disease. Genes Immun. 2004;5:203–208. doi: 10.1038/sj.gene.6364059. [DOI] [PubMed] [Google Scholar]
- 28.Menconi F. Monti MC. Greenberg DA. Oashi T. Osman R. Davies TF. Ban Y. Jacobson EM. Concepcion ES. Li CW. Tomer Y. Molecular amino acid signatures in the MHC class II peptide-binding pocket predispose to autoimmune thyroiditis in humans and in mice. Proc Natl Acad Sci U S A. 2008;105:14034–14039. doi: 10.1073/pnas.0806584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faas S. Trucco M. The genes influencing the susceptibility to IDDM in humans. J Endocrinol Invest. 1994;17:477–495. doi: 10.1007/BF03347743. [DOI] [PubMed] [Google Scholar]
- 30.Pugliese A. Genetics of type 1 diabetes. Endocrinol Metab Clin North Am. 2004;33:1–16. doi: 10.1016/S0889-8529(03)00082-3. vii. [DOI] [PubMed] [Google Scholar]
- 31.Aitman TJ. Todd JA. Molecular genetics of diabetes mellitus. Baillieres Clin Endocrinol Metab. 1995;9:631–656. doi: 10.1016/s0950-351x(95)80655-5. [DOI] [PubMed] [Google Scholar]
- 32.Morel PA. Dorman JS. Todd JA. McDevitt HO. Trucco M. Aspartic acid at position 57 of the HLA-DQ beta-chain protects against type I diabetes: a family study. Proc Natl Acad Sci U S A. 1988;85:8111–8115. doi: 10.1073/pnas.85.21.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simmonds MJ. Howson JM. Heward JM. Cordell HJ. Foxall H. Carr-Smith J. Gibson SM. Walker N. Tomer Y. Franklyn JA. Todd JA. Gough SC. Regression mapping of association between the human leukocyte antigen region and Graves disease. Am J Hum Genet. 2005;76:157–163. doi: 10.1086/426947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobson EM. Yang H. Menconi F. Wang R. Osman R. Skrabanek L. Li CW. Fadlalla M. Gandhi A. Chaturvedi V. Smith EP. Schwemberger S. Osterburg A. Babcock GF. Tomer Y. Employing a recombinant HLA-DR3 expression system to dissect MHC II-thyroglobulin peptide dynamism: a genetic, biochemical, and reverse immunological perspective. J Biol Chem. 2009;284:34231–34243. doi: 10.1074/jbc.M109.041574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Todd JA. Bell JI. McDevitt HO. HLA-DQbeta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987;329:599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- 36.Teft WA. Kirchhof MG. Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65–97. doi: 10.1146/annurev.immunol.24.021605.090535. [DOI] [PubMed] [Google Scholar]
- 37.Yanagawa T. Hidaka Y. Guimaraes V. Soliman M. DeGroot LJ. CTLA-4 gene polymorphism associated with Graves' disease in a Caucasian population. J Clin Endocrinol Metab. 1995;80:41–45. doi: 10.1210/jcem.80.1.7829637. [DOI] [PubMed] [Google Scholar]
- 38.Nistico L. Buzzetti R. Pritchard LE. Van der Auwera B. Giovannini C. Bosi E. Larrad MT. Rios MS. Chow CC. Cockram CS. Jacobs K. Mijovic C. Bain SC. Barnett AH. Vandewalle CL. Schuit F. Gorus FK. Tosi R. Pozzilli P. Todd JA. The CTLA-4 gene region of chromosome 2q33 is linked to, and associated with, type 1 diabetes. Begian Diabetes Registry. Hum Mol Genet. 1996;5:1075–1080. doi: 10.1093/hmg/5.7.1075. [DOI] [PubMed] [Google Scholar]
- 39.Donner H. Rau H. Walfish PG. Braun J. Siegmund T. Finke R. Herwig J. Usadel KH. Badenhoop K. CTLA4 alanine-17 confers genetic susceptibility to Graves' disease and to type 1 diabetes mellitus. J Clin Endocrinol Metab. 1997;82:143–146. doi: 10.1210/jcem.82.1.3699. [DOI] [PubMed] [Google Scholar]
- 40.Kotsa K. Watson PF. Weetman AP. A CTLA-4 gene polymorphism is associated with both Graves' disease autoimmune hypothyroidism. Clin Endocrinol. 1997;46:551–554. doi: 10.1046/j.1365-2265.1997.1710996.x. [DOI] [PubMed] [Google Scholar]
- 41.Kouki T. Gardine CA. Yanagawa T. DeGroot LJ. Relation of three polymorphisms of the CTLA-4 gene in patients with Graves' disease. J Endocrinol Invest. 2002;25:208–213. doi: 10.1007/BF03343992. [DOI] [PubMed] [Google Scholar]
- 42.Ueda H. Howson JM. Esposito L. Heward J. Snook H. Chamberlain G. Rainbow DB. Hunter KM. Smith AN. Di Genova G. Herr MH. Dahlman I. Payne F. Smyth D. Lowe C. Twells RC. Howlett S. Healy B. Nutland S. Rance HE. Everett V. Smink LJ. Lam AC. Cordell HJ. Walker NM. Bordin C. Hulme J. Motzo C. Cucca F. Hess JF. Metzker ML. Rogers J. Gregory S. Allahabadia A. Nithiyananthan R. Tuomilehto-Wolf E. Tuomilehto J. Bingley P. Gillespie KM. Undlien DE. Ronningen KS. Guja C. Ionescu-Tirgoviste C. Savage DA. Maxwell AP. Carson DJ. Patterson CC. Franklyn JA. Clayton DG. Peterson LB. Wicker LS. Todd JA. Gough SC. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 43.Ban Y. Davies TF. Greenberg DA. Kissin A. Marder B. Murphy B. Concepcion ES. Villanueva RB. Barbesino G. Ling V. Tomer Y. Analysis of the CTLA-4, CD28 and inducible co-stimulator (ICOS) genes in autoimmune thyroid disease. Genes Immun. 2003;4:586–593. doi: 10.1038/sj.gene.6364018. [DOI] [PubMed] [Google Scholar]
- 44.Tomer Y. Greenberg DA. Barbesino G. Concepcion ES. Davies TF. CTLA-4 and not CD28 is a susceptibility gene for thyroid autoantibody production. J Clin Endocrinol Metab. 2001;86:1687–1693. doi: 10.1210/jcem.86.4.7372. [DOI] [PubMed] [Google Scholar]
- 45.Zaletel K. Krhin B. Gaberscek S. Pirnat E. Hojker S. The influence of the exon 1 polymorphism of the cytotoxic T lymphocyte antigen 4 gene on thyroid antibody production in patients with newly diagnosed graves' disease. Thyroid. 2002;12:373–376. doi: 10.1089/105072502760043431. [DOI] [PubMed] [Google Scholar]
- 46.Zaletel K. Krhin B. Gaberscek S. Hojker S. Thyroid autoantibody production is influenced by exon 1 and promoter CTLA-4 polymorphisms in patients with Hashimoto's thyroiditis. Int J Immunogenet. 2006;33:87–91. doi: 10.1111/j.1744-313X.2006.00574.x. [DOI] [PubMed] [Google Scholar]
- 47.Heward JM. Allahabadia A. Armitage M. Hattersley A. Dodson PM. Macleod K. Carr-Smith J. Daykin J. Daly A. Sheppard MC. Holder RL. Barnett AH. Franklyn JA. Gough SC. The development of Graves' disease and the CTLA-4 gene on chromosome 2q33. J Clin Endocrinol Metab. 1999;84:2398–2401. doi: 10.1210/jcem.84.7.5820. [DOI] [PubMed] [Google Scholar]
- 48.Akamizu T. Sale MM. Rich SS. Hiratani H. Noh JY. Kanamoto N. Saijo M. Miyamoto Y. Saito Y. Nakao K. Bowden DW. Association of autoimmune thyroid disease with microsatellite markers for the thyrotropin receptor gene and CTLA-4 in Japanese patients. Thyroid. 2000;10:851–858. doi: 10.1089/thy.2000.10.851. [DOI] [PubMed] [Google Scholar]
- 49.Park YJ. Chung HK. Park DJ. Kim WB. Kim SW. Koh JJ. Cho BY. Polymorphism in the promoter and exon 1 of the cytotoxic T lymphocyte antigen-4 gene associated with autoimmune thyroid disease in Koreans. Thyroid. 2000;10:453–459. doi: 10.1089/thy.2000.10.453. [DOI] [PubMed] [Google Scholar]
- 50.Donner H. Braun J. Seidl C. Rau H. Finke R. Ventz M. Walfish PG. Usadel KH. Badenhoop K. Codon 17 polymorphism of the cytotoxic T lymphocyte antigen 4 gene in Hashimoto's thyroiditis and Addison's disease. J Clin Endocrinol Metab. 1997;82:4130–4132. doi: 10.1210/jcem.82.12.4406. [DOI] [PubMed] [Google Scholar]
- 51.Nithiyananthan R. Heward JM. Allahabadia A. Franklyn JA. Gough SC. Polymorphism of the CTLA-4 gene is associated with autoimmune hypothyroidism in the United Kingdom. Thyroid. 2002;12:3–6. doi: 10.1089/105072502753451896. [DOI] [PubMed] [Google Scholar]
- 52.Sale MM. Akamizu T. Howard TD. Yokota T. Nakao K. Mori T. Iwasaki H. Rich SS. Jennings-Gee JE. Yamada M. Bowden DW. Association of autoimmune thyroid disease with a microsatellite marker for the thyrotropin receptor gene and CTLA-4 in a Japanese population. Proc Assoc Am Physicians. 1997;109:453–461. [PubMed] [Google Scholar]
- 53.Vieland VJ. Huang Y. Bartlett C. Davies TF. Tomer Y 2008 A multilocus model of the genetic architecture of autoimmune thyroid disorder, with clinical implications. Am J Hum Genet. 82:1349–1356. doi: 10.1016/j.ajhg.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villanueva RB. Inzerillo AM. Tomer Y. Barbesino G. Meltzer M. Concepcion ES. Greenberg DA. Maclaren N. Sun ZS. Zhang DM. Tucci S. Davies TF. Limited genetic susceptibility to severe graves' ophthalmopathy: no role for ctla-4 and evidence for an environmental etiology. Thyroid. 2000;10:791–798. doi: 10.1089/thy.2000.10.791. [DOI] [PubMed] [Google Scholar]
- 55.Braun J. Donner H. Siegmund T. Walfish PG. Usadel KH. Badenhoop K. CTLA-4 promoter variants in patients with Graves' disease and Hashimoto's thyroiditis. Tissue Antigens. 1998;51:563–566. doi: 10.1111/j.1399-0039.1998.tb02993.x. [DOI] [PubMed] [Google Scholar]
- 56.Yanagawa T. Taniyama M. Enomoto S. Gomi K. Maruyama H. Ban Y. Saruta T. CTLA4 gene polymorphism confers susceptibility to Graves' disease in Japanese. Thyroid. 1997;7:843–846. doi: 10.1089/thy.1997.7.843. [DOI] [PubMed] [Google Scholar]
- 57.Kouki T. Sawai Y. Gardine CA. Fisfalen ME. Alegre ML. DeGroot LJ. CTLA-4 gene polymorphism at position 49 in exon 1 reduces the inhibitory function of CTLA-4 and contributes to the pathogenesis of Graves' disease. J Immunol. 2000;165:6606–6611. doi: 10.4049/jimmunol.165.11.6606. [DOI] [PubMed] [Google Scholar]
- 58.Xu Y. Graves P. Tomer Y. Davies T. CTLA-4 and autoimmune thyroid disease: lack of influence of the A49G signal peptide polymorphism on functional recombinant human CTLA-4. Cell Immunol. 2002;215:133–140. doi: 10.1016/s0008-8749(02)00018-7. [DOI] [PubMed] [Google Scholar]
- 59.Takara M. Kouki T. DeGroot LJ. CTLA-4 AT-repeat polymorphism reduces the inhibitory function of CTLA-4 in Graves' disease. Thyroid. 2003;13:1083–1089. doi: 10.1089/10507250360731479. [DOI] [PubMed] [Google Scholar]
- 60.Wang XB. Kakoulidou M. Giscombe R. Qiu Q. Huang D. Pirskanen R. Lefvert AK. Abnormal expression of CTLA-4 by T cells from patients with myasthenia gravis: effect of an AT-rich gene sequence. J Neuroimmunol. 2002;130:224–232. doi: 10.1016/s0165-5728(02)00228-x. [DOI] [PubMed] [Google Scholar]
- 61.Banchereau J. Bazan F. Blanchard D. Briere F. Galizzi JP. van Kooten C. Liu YJ. Rousset F. Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 62.Armitage RJ. Macduff BM. Spriggs MK. Fanslow WC. Human B cell proliferation and Ig secretion induced by recombinant CD40 ligand are modulated by soluble cytokines. J Immunol. 1993;150:3671–3680. [PubMed] [Google Scholar]
- 63.Arpin C. Dechanet J. van Kooten C. Merville P. Grouard G. Briere F. Banchereau J. Liu YJ. Generation of memory B cells and plasma cells in vitro. Science. 1995;268:720–722. doi: 10.1126/science.7537388. [DOI] [PubMed] [Google Scholar]
- 64.Tomer Y. Barbesino G. Greenberg DA. Concepcion ES. Davies TF. A new Graves disease-susceptibility locus maps to chromosome 20q11.2. Am J Hum Genet. 1998;63:1749–1756. doi: 10.1086/302146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomer Y. Concepcion E. Greenberg DA. A C/T single nucleotide polymorphism in the region of the CD40 gene is associated with Graves' disease. Thyroid. 2002;12:1129–1135. doi: 10.1089/105072502321085234. [DOI] [PubMed] [Google Scholar]
- 66.Pearce SH. Vaidya B. Imrie H. Perros P. Kelly WF. Toft AD. McCarthy MI. Young ET. Kendall-Taylor P. Further evidence for a susceptibility locus on chromosome 20q13.11 in families with dominant transmission of Graves disease [letter] Am J Hum Genet. 1999;65:1462–1465. doi: 10.1086/302610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim TY. Park YJ. Hwang JK. Song JY. Park KS. Cho BY. Park DJ. A C/T polymorphism in the 5′-untranslated region of the CD40 gene is associated with Graves' disease in Koreans. Thyroid. 2003;13:919–926. doi: 10.1089/105072503322511319. [DOI] [PubMed] [Google Scholar]
- 68.Ban Y. Tozaki T. Taniyama M. Tomita M. Ban Y. Association of a C/T single-nucleotide polymorphism in the 5′ untranslated region of the CD40 gene with Graves' disease in Japanese. Thyroid. 2006;16:443–446. doi: 10.1089/thy.2006.16.443. [DOI] [PubMed] [Google Scholar]
- 69.Kurylowicz A. Kula D. Ploski R. Skorka A. Jurecka-Lubieniecka B. Zebracka J. Steinhof-Radwanska K. Hasse-Lazar K. Hiromatsu Y. Jarzab B. Bednarczuk T. Association of CD40 gene polymorphism (C-1T) with susceptibility and phenotype of Graves' disease. Thyroid. 2005;15:1119–1124. doi: 10.1089/thy.2005.15.1119. [DOI] [PubMed] [Google Scholar]
- 70.Mukai T. Hiromatsu Y. Fukutani T. Ichimura M. Kaku H. Miyake I. Yamada K. A C/T polymorphism in the 5′ untranslated region of the CD40 gene is associated with later onset of Graves' disease in Japanese. Endocr J. 2005;52:471–477. doi: 10.1507/endocrj.52.471. [DOI] [PubMed] [Google Scholar]
- 71.Houston FA. Wilson V. Jennings CE. Owen CJ. Donaldson P. Perros P. Pearce SH. Role of the CD40 locus in Graves' disease. Thyroid. 2004;14:506–509. doi: 10.1089/1050725041517039. [DOI] [PubMed] [Google Scholar]
- 72.Jacobson EM. Huber AK. Akeno N. Sivak M. Li CW. Concepcion E. Ho K. Tomer Y. A CD40 Kozak sequence polymorphism and susceptibility to antibody-mediated autoimmune conditions: the role of CD40 tissue-specific expression. Genes Immun. 2007;8:205–214. doi: 10.1038/sj.gene.6364375. [DOI] [PubMed] [Google Scholar]
- 73.Jacobson EM. Concepcion E. Oashi T. Tomer Y. A Graves' disease-associated Kozak sequence single-nucleotide polymorphism enhances the efficiency of CD40 gene translation: a case for translational pathophysiology. Endocrinology. 2005;146:2684–2691. doi: 10.1210/en.2004-1617. [DOI] [PubMed] [Google Scholar]
- 74.Park JH. Chang HS. Park CS. Jang AS. Park BL. Rhim TY. Uh ST. Kim YH. Chung IY. Shin HD. Association analysis of CD40 polymorphisms with asthma and the level of serum total IgE. Am J Respir Crit Care Med. 2007;175:775–782. doi: 10.1164/rccm.200609-1286OC. [DOI] [PubMed] [Google Scholar]
- 75.Metcalfe RA. McIntosh RS. Marelli-Berg F. Lombardi G. Lechler R. Weetman AP. Detection of CD40 on human thyroid follicular cells: analysis of expression and function. J Clin Endocrinol Metab. 1998;83:1268–1274. doi: 10.1210/jcem.83.4.4732. [DOI] [PubMed] [Google Scholar]
- 76.Smith TJ. Sciaky D. Phipps RP. Jennings TA. CD40 expression in human thyroid tissue: evidence for involvement of multiple cell types in autoimmune and neoplastic diseases. Thyroid. 1999;9:749–755. doi: 10.1089/thy.1999.9.749. [DOI] [PubMed] [Google Scholar]
- 77.Raychaudhuri S. Remmers EF. Lee AT. Hackett R. Guiducci C. Burtt NP. Gianniny L. Korman BD. Padyukov L. Kurreeman FA. Chang M. Catanese JJ. Ding B. Wong S. van der Helm-Van Mil AH. Neale BM. Coblyn J. Cui J. Tak PP. Wolbink GJ. Crusius JB. van der Horst-Bruinsma IE. Criswell LA. Amos CI. Seldin MF. Kastner DL. Ardlie KG. Alfredsson L. Costenbader KH. Altshuler D. Huizinga TW. Shadick NA. Weinblatt ME. de Vries N. Worthington J. Seielstad M. Toes RE. Karlson EW. Begovich AB. Klareskog L. Gregersen PK. Daly MJ. Plenge RM. Common variants at CD40 and other loci confer risk of rheumatoid arthritis. Nat Genet. 2008;40:1216–1223. doi: 10.1038/ng.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van der Linden MP. Feitsma AL. le Cessie S. Kern M. Olsson LM. Raychaudhuri S. Begovich AB. Chang M. Catanese JJ. Kurreeman FA. van Nies J. van der Heijde DM. Gregersen PK. Huizinga TW. Toes RE. van der Helm-Van Mil AH. Association of a single-nucleotide polymorphism in CD40 with the rate of joint destruction in rheumatoid arthritis. Arthritis Rheum. 2009;60:2242–2247. doi: 10.1002/art.24721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gaffney PM. Langefeld CD. Graham RR. Ortmann WA. Williams AH. Rodine PR. Moser KL. Behrens TW. Fine-mapping chromosome 20 in 230 systemic lupus erythematosus sib pair and multiplex families: evidence for genetic epistasis with chromosome 16q12. Am J Hum Genet. 2006;78:747–758. doi: 10.1086/503686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Australia and New Zealand Multiple Sclerosis Genetics Consortium (ANZgene) Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat Genet. 2009;41:824–828. doi: 10.1038/ng.396. [DOI] [PubMed] [Google Scholar]
- 81.Cloutier JF. Veillette A. Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase. J Exp Med. 1999;189:111–121. doi: 10.1084/jem.189.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Velaga MR. Wilson V. Jennings CE. Owen CJ. Herington S. Donaldson PT. Ball SG. James RA. Quinton R. Perros P. Pearce SH. The codon 620 tryptophan allele of the lymphoid tyrosine phosphatase (LYP) gene is a major determinant of Graves' disease. J Clin Endocrinol Metab. 2004;89:5862–5865. doi: 10.1210/jc.2004-1108. [DOI] [PubMed] [Google Scholar]
- 83.Criswell LA. Pfeiffer KA. Lum RF. Gonzales B. Novitzke J. Kern M. Moser KL. Begovich AB. Carlton VE. Li W. Lee AT. Ortmann W. Behrens TW. Gregersen PK. Analysis of families in the multiple autoimmune disease genetics consortium (MADGC) collection: the PTPN22 620W allele associates with multiple autoimmune phenotypes. Am J Hum Genet. 2005;76:561–571. doi: 10.1086/429096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Begovich AB. Carlton VE. Honigberg LA. Schrodi SJ. Chokkalingam AP. Alexander HC. Ardlie KG. Huang Q. Smith AM. Spoerke JM. Conn MT. Chang M. Chang SY. Saiki RK. Catanese JJ. Leong DU. Garcia VE. McAllister LB. Jeffery DA. Lee AT. Batliwalla F. Remmers E. Criswell LA. Seldin MF. Kastner DL. Amos CI. Sninsky JJ. Gregersen PK. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet. 2004;75:330–337. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kyogoku C. Langefeld CD. Ortmann WA. Lee A. Selby S. Carlton VE. Chang M. Ramos P. Baechler EC. Batliwalla FM. Novitzke J. Williams AH. Gillett C. Rodine P. Graham RR. Ardlie KG. Gaffney PM. Moser KL. Petri M. Begovich AB. Gregersen PK. Behrens TW. Genetic association of the R620W polymorphism of protein tyrosine phosphatase PTPN22 with human SLE. Am J Hum Genet. 2004;75:504–507. doi: 10.1086/423790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bottini N. Musumeci L. Alonso A. Rahmouni S. Nika K. Rostamkhani M. MacMurray J. Meloni GF. Lucarelli P. Pellecchia M. Eisenbarth GS. Comings D. Mustelin T. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004;36:337–338. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- 87.Smyth D. Cooper JD. Collins JE. Heward JM. Franklyn JA. Howson JM. Vella A. Nutland S. Rance HE. Maier L. Barratt BJ. Guja C. Ionescu-Tirgoviste C. Savage DA. Dunger DB. Widmer B. Strachan DP. Ring SM. Walker N. Clayton DG. Twells RC. Gough SC. Todd JA. Replication of an association between the lymphoid tyrosine phosphatase locus (LYP/PTPN22) with type 1 diabetes, and evidence for its role as a general autoimmunity locus. Diabetes. 2004;53:3020–3023. doi: 10.2337/diabetes.53.11.3020. [DOI] [PubMed] [Google Scholar]
- 88.Vang T. Congia M. Macis MD. Musumeci L. Orru V. Zavattari P. Nika K. Tautz L. Tasken K. Cucca F. Mustelin T. Bottini N. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat Genet. 2005;37:1317–1319. doi: 10.1038/ng1673. [DOI] [PubMed] [Google Scholar]
- 89.Paust S. Cantor H. Regulatory T cells and autoimmune disease. Immunol Rev. 2005;204:195–207. doi: 10.1111/j.0105-2896.2005.00247.x. [DOI] [PubMed] [Google Scholar]
- 90.Gangi E. Vasu C. Cheatem D. Prabhakar BS. IL-10-producing CD4 + CD25 + regulatory T cells play a critical role in granulocyte-macrophage colony-stimulating factor-induced suppression of experimental autoimmune thyroiditis. J Immunol. 2005;174:7006–7013. doi: 10.4049/jimmunol.174.11.7006. [DOI] [PubMed] [Google Scholar]
- 91.Saitoh O. Nagayama Y. Regulation of Graves' hyperthyroidism with naturally occurring CD4 + CD25 + regulatory T cells in a mouse model. Endocrinology. 2006;147:2417–2422. doi: 10.1210/en.2005-1024. [DOI] [PubMed] [Google Scholar]
- 92.McLachlan SM. Nagayama Y. Pichurin PN. Mizutori Y. Chen CR. Misharin A. Aliesky HA. Rapoport B. The link between Graves' disease and Hashimoto's thyroiditis: a role for regulatory T cells. Endocrinology. 2007;148:5724–5733. doi: 10.1210/en.2007-1024. [DOI] [PubMed] [Google Scholar]
- 93.Brunkow ME. Jeffery EW. Hjerrild KA. Paeper B. Clark LB. Yasayko SA. Wilkinson JE. Galas D. Ziegler SF. Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 94.Ban Y. Tozaki T. Tobe T. Ban Y. Jacobson EM. Concepcion ES. Tomer Y. The regulatory T cell gene FOXP3 and genetic susceptibility to thyroid autoimmunity: an association analysis in Caucasian and Japanese cohorts. J Autoimmun. 2007;28:201–207. doi: 10.1016/j.jaut.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 95.Tomer Y. Menconi F. Davies TF. Barbesino G. Rocchi R. Pinchera A. Concepcion E. Greenberg DA. Dissecting genetic heterogeneity in autoimmune thyroid diseases by subset analysis. J Autoimmun. 2007;29:69–77. doi: 10.1016/j.jaut.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 96.Vella A. Cooper JD. Lowe CE. Walker N. Nutland S. Widmer B. Jones R. Ring SM. McArdle W. Pembrey ME. Strachan DP. Dunger DB. Twells RC. Clayton DG. Todd JA. Localization of a type 1 diabetes locus in the IL2RA/CD25 region by use of tag single-nucleotide polymorphisms. Am J Hum Genet. 2005;76:773–779. doi: 10.1086/429843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brand OJ. Lowe CE. Heward JM. Franklyn JA. Cooper JD. Todd JA. Gough SC. Association of the interleukin-2 receptor alpha (IL-2Ralpha)/CD25 gene region with Graves' disease using a multilocus test and tag SNPs. Clin Endocrinol (Oxf) 2007;66:508–512. doi: 10.1111/j.1365-2265.2007.02762.x. [DOI] [PubMed] [Google Scholar]
- 98.Takahashi T. Kuniyasu Y. Toda M. Sakaguchi N. Itoh M. Iwata M. Shimizu J. Sakaguchi S. Immunologic self-tolerance maintained by CD25 + CD4 + naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 99.Tomer Y. Anti-thyroglobulin autoantibodies in autoimmune thyroid diseases: cross-reactive or pathogenic? Clin Immunol Immunopathol. 1997;82:3–11. doi: 10.1006/clin.1996.4243. [DOI] [PubMed] [Google Scholar]
- 100.Tomer Y. Greenberg DA. Concepcion E. Ban Y. Davies TF. Thyroglobulin is a thyroid specific gene for the familial autoimmune thyroid diseases. J Clin Endocrinol Metab. 2002;87:404–407. doi: 10.1210/jcem.87.1.8291. [DOI] [PubMed] [Google Scholar]
- 101.Collins JE. Heward JM. Carr-Smith J. Daykin J. Franklyn JA. Gough SCL. Association of a rare thyroglobulin gene microsatellite variant with autoimmune thyroid disease. J Clin Endocrinol Metab. 2003;88:5039–5042. doi: 10.1210/jc.2003-030093. [DOI] [PubMed] [Google Scholar]
- 102.Tomer Y. Greenberg D. The thyroglobulin gene as the first thyroid-specific susceptibility gene for autoimmune thyroid disease. Trends Mol Med. 2004;10:306–308. doi: 10.1016/j.molmed.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 103.Ban Y. Tozaki T. Taniyama M. Tomita M. Ban Y. Association of a thyroglobulin gene polymorphism with Hashimoto's thyroiditis in the Japanese population. Clin Endocrinol (Oxf) 2004;61:263–268. doi: 10.1111/j.1365-2265.2004.02096.x. [DOI] [PubMed] [Google Scholar]
- 104.Hsiao JY. Hsieh MC. Tien KJ. Hsu SC. Shin SJ. Lin SR. Association between a C/T polymorphism in exon 33 of the thyroglobulin gene is associated with relapse of Graves' hyperthyroidism after antithyroid withdrawal in Taiwanese. J Clin Endocrinol Metab. 2007;92:3197–3201. doi: 10.1210/jc.2007-0675. [DOI] [PubMed] [Google Scholar]
- 105.Hsiao JY. Hsieh MC. Tien KJ. Hsu SC. Lin SR. Ke DS. Exon 33 T/T genotype of the thyroglobulin gene is a susceptibility gene for Graves' disease in Taiwanese and exon 12 C/C genotype protects against it. Clin Exp Med. 2008;8:17–21. doi: 10.1007/s10238-008-0151-5. [DOI] [PubMed] [Google Scholar]
- 106.Ban Y. Greenberg DA. Concepcion E. Skrabanek L. Villanueva R. Tomer Y. Amino acid substitutions in the thyroglobulin gene are associated with susceptibility to human and murine autoimmune thyroid disease. Proc Natl Acad Sci USA. 2003;100:15119–15124. doi: 10.1073/pnas.2434175100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hodge SE. Ban Y. Strug LJ. Greenberg DA. Davies TF. Concepcion ES. Villanueva R. Tomer Y. Possible interaction between HLA-DRbeta1 and thyroglobulin variants in Graves' disease. Thyroid. 2006;16:351–355. doi: 10.1089/thy.2006.16.351. [DOI] [PubMed] [Google Scholar]
- 108.Menconi F. Huber A. Osman R. Concepcion E. Jacobson EM. Stefan M. David CS. Tomer Y. Tg.2098 is a major human thyroglobulin T-cell epitope. J Autoimmun. 2010 2010 Mar 18; doi: 10.1016/j.jaut.2010.01.004. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cuddihy RM. Dutton CM. Bahn RS. A polymorphism in the extracellular domain of the thyrotropin receptor is highly associated with autoimmune thyroid disease in females. Thyroid. 1995;5:89–95. doi: 10.1089/thy.1995.5.89. [DOI] [PubMed] [Google Scholar]
- 110.Chistyakov DA. Savost'anov KV. Turakulov RI. Petunina NA. Trukhina LV. Kudinova AV. Balabolkin MI. Nosikov VV. Complex association analysis of graves disease using a set of polymorphic markers. Mol Genet Metab. 2000;70:214–218. doi: 10.1006/mgme.2000.3007. [DOI] [PubMed] [Google Scholar]
- 111.Allahabadia A. Heward JM. Mijovic C. Carr-Smith J. Daykin J. Cockram C. Barnett AH. Sheppard MC. Franklyn JA. Gough SC. Lack of association between polymorphism of the thyrotropin receptor gene and Graves' disease in United Kingdom and Hong Kong Chinese patients: case control and family-based studies. Thyroid. 1998;8:777–780. doi: 10.1089/thy.1998.8.777. [DOI] [PubMed] [Google Scholar]
- 112.Kotsa KD. Watson PF. Weetman AP. No association between a thyrotropin receptor gene polymorphism and Graves' disease in the female population. Thyroid. 1997;7:31–33. doi: 10.1089/thy.1997.7.31. [DOI] [PubMed] [Google Scholar]
- 113.Simanainen J. Kinch A. Westermark K. Winsa B. Bengtsson M. Schuppert F. Westermark B. Heldin NE. Analysis of mutations in exon 1 of the human thyrotropin receptor gene: high frequency of the D36H and P52T polymorphic variants. Thyroid. 1999;9:7–11. doi: 10.1089/thy.1999.9.7. [DOI] [PubMed] [Google Scholar]
- 114.Kaczur V. Takacs M. Szalai C. Falus A. Nagy Z. Berencsi G. Balazs C. Analysis of the genetic variability of the 1st (CCC/ACC, P52T) and the 10th exons (bp 1012–1704) of the TSH receptor gene in Graves' disease. Eur J Immunogenet. 2000;27:17–23. doi: 10.1046/j.1365-2370.2000.00187.x. [DOI] [PubMed] [Google Scholar]
- 115.Hiratani H. Bowden DW. Ikegami S. Shirasawa S. Shimizu A. Iwatani Y. Akamizu T. Multiple SNPs in intron 7 of thyrotropin receptor are associated with Graves' disease. J Clin Endocrinol Metab. 2005;90:2898–2903. doi: 10.1210/jc.2004-2148. [DOI] [PubMed] [Google Scholar]
- 116.Ho SC. Goh SS. Khoo DH. Association of Graves' disease with intragenic polymorphism of the thyrotropin receptor gene in a cohort of Singapore patients of multi-ethnic origins. Thyroid. 2003;13:523–528. doi: 10.1089/105072503322238773. [DOI] [PubMed] [Google Scholar]
- 117.Dechairo BM. Zabaneh D. Collins J. Brand O. Dawson GJ. Green AP. Mackay I. Franklyn JA. Connell JM. Wass JA. Wiersinga WM. Hegedus L. Brix T. Robinson BG. Hunt PJ. Weetman AP. Carey AH. Gough SC. Association of the TSHR gene with Graves' disease: the first disease specific locus. Eur J Hum Genet. 2005;13:1223–1230. doi: 10.1038/sj.ejhg.5201485. [DOI] [PubMed] [Google Scholar]
- 118.Yin X. Latif R. Bahn R. Tomer Y. Davies TF. Influence of the TSH receptor gene on susceptibility to Graves' disease and Graves' ophthalmopathy. Thyroid. 2008;18:1201–1206. doi: 10.1089/thy.2008.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Graves PN. Tomer Y. Davies TF. Cloning and sequencing of a 1.3 kb variant of human thyrotropin receptor mRNA lacking the transmembrane domain. Biochem Biophys Res Commun. 1992;187:1135–1143. doi: 10.1016/0006-291x(92)91315-h. [DOI] [PubMed] [Google Scholar]
- 120.Kakinuma A. Nagayama Y. Multiple messenger ribonucleic acid transcripts and revised gene organization of the human TSH receptor. Endocr J. 2002;49:175–180. doi: 10.1507/endocrj.49.175. [DOI] [PubMed] [Google Scholar]
- 121.Ku CS. Loy EY. Pawitan Y. Chia KS. The pursuit of genome-wide association studies: where are we now? J Hum Genet. 2010;55:195–206. doi: 10.1038/jhg.2010.19. [DOI] [PubMed] [Google Scholar]
- 122.Brix TH. Kyvik KO. Christensen K. Hegedus L. Evidence for a major role of heredity in Graves' disease: a population-based study of two Danish twin cohorts. J Clin Endocrinol Metab. 2001;86:930–934. doi: 10.1210/jcem.86.2.7242. [DOI] [PubMed] [Google Scholar]
- 123.Sawai Y. DeGroot LJ. Binding of human thyrotropin receptor peptides to a Graves' disease-predisposing human leukocyte antigen class II molecule. J Clin Endocrinol Metab. 2000;85:1176–1179. doi: 10.1210/jcem.85.3.6376. [DOI] [PubMed] [Google Scholar]