Abstract
No single animal model is able to encompass all of the variables known to affect human ischemic stroke. This review highlights the major strengths and weaknesses of the most commonly used animal models of acute ischemic stroke in the context of matching model and experimental aim. Particular emphasis is placed on the relationships between outcome and underlying vascular variability, physiologic control, and use of models of comorbidity. The aim is to provide, for novice and expert alike, an overview of the key controllable determinants of experimental stroke outcome to help ensure the most effective application of animal models to translational research.
Keywords: animal models, diabetes, hypertension, physiologic control, stroke, vascular variability
Introduction
Human stroke comes in many forms. We can classify them by cause, location, size, and by functional impact on the patient. Thus, there is no single universally appropriate model of stroke. The purpose of this review is to describe and critique the animal models most pertinent to the most common broad subtype of human stroke caused by occlusion of the middle cerebral artery (MCA). Even this apparently simple task is complicated by the need to consider questions such as the occlusive mechanism (thromboembolism from cardiac sources versus more proximal embolic processes, such as carotid atheroma), underlying vascular anatomy (the gross anatomy of the circle of Willis, the role of communicating arteries, collateralization, and anastomotic connections within the MCA and with adjacent vascular territories), and the affect of premorbid factors, such as hypertension, diabetes, obesity, and smoking habits on all of these. Within the models themselves, we also need to consider aspects of experimental design such as animal gender, temperature control, blood gas concentrations, and anesthesia that impinge directly on stroke pathophysiology.
Different Approaches to Induction of Focal Ischemia
In this section, we describe the critical characteristics of the most commonly used models rather than their precise methodology, which is beyond the scope of this review and has been described in detail by others (Wang-Fischer, 2008).
Broadly, two surgical approaches are used to give access to the cerebral vasculature to allow generation of focal ischemia.
The first group of methods requires opening of the skull to allow direct access to the cerebral arteries. In most instances, this has involved small craniotomies that allow distal branches of vessels such as the MCA to be ligated (Crowell et al, 1981), clipped (Tamura et al, 1979, 1981), or sealed by photothrombosis (Markgraf et al, 1993) or electrocoagulation (O'Brien and Waltz, 1973). Although occlusion of the vessel is usually permanent, ligatures can be released, pneumatic cuffs deflated, and even thrombotic lesions created by electrocoagulation, or photothrombosis can recanalize to permit transient occlusion.
As there are no species-specific constraints on the use of these techniques, they have been used not only in standard laboratory animals such as rabbits and rats but also in larger domesticated animals such as cats, dogs, and pigs (Corkill et al, 1978; Imai et al, 2006; Tamura et al, 1979), as well as in both small and large primates (Del Zoppo et al, 1986; Hudgins and Garcia, 1970). The larger of these species offer the significant advantages of large gyrencephalic brains with gray/white matter proportions closer to humans (Figure 1). A disadvantage of the variants that require large craniotomies to expose more proximal portions of vessels is the unavoidable damage to structures, such as the eye, temporalis muscle, and zygomatic arch. Moreover, with the recent demonstration that hemicraniectomy can have a profound beneficial effect on survival and function after space-occupying hemispheric infarction (Hofmeijer et al, 2009), the value of methods requiring large craniectomies to expose the vessels at the base of the brain is uncertain.
Figure 1.
Brain size and gyral complexity.
The vasoconstrictor endothelin can also be used to reversibly occlude an artery or vascular bed (Agnati et al, 1991). Although this also requires craniotomy, the opening in the skull needs to be just large enough to introduce a fine cannula, which can be left in situ and vasoconstriction initiated long after confounding anesthesia has been withdrawn (Callaway et al, 1999). It should be noted that endothelin is about four times more potent in conscious rats than in anesthetized rats (Bogaert et al, 2000), that control over ischemic intensity and duration are limited, and that stimulation of endothelin receptors may confound the study of stroke by directly modifying the expression of key molecules, such as matrix metalloproteinases and growth factors (Koyama et al, 2003, 2007).
To avoid opening the skull, a second group of methods has used intra-arterial access to occlude cerebral arteries. The most commonly used of these is thread occlusion of the MCA. Although this method has many variants, particularly with respect to the construction of the occluding thread and closure of additional vessels to manipulate collateral blood flow, the basic technique described originally by Koizumi et al (1986) and modified by Longa et al (1989) involves introducing an occluding thread into the extracranial internal carotid artery (ICA) and advancing it until its tip occludes the origin of the MCA. Although most frequently applied to rats and mice, the method has also been used in rabbits (Kong et al, 2004), gerbils, (Baskaya et al, 1999) and marmosets (Freret et al, 2008). In baboons, the concept has been extended to the use of a balloon catheter or wire coil introduced through either the carotid (Gao et al, 2006) or femoral arteries (Hamberg et al, 2002) to occlude the MCA.
The great advantage of these techniques is that the thread can either be left in place for permanent occlusion or withdrawn any time to permit controlled reperfusion, and the presence of a significant ischemic penumbra early after occlusion makes them particularly suitable for studies of neuroprotection. However, despite their utility, these are not simple methods. The surgery to access and manipulate the vasculature requires skilled and experienced hands, and in practice, the results are often highly variable. Moreover, the diameter and length of the occluding proportion of the thread combine to determine which vessels off the circle of Willis are blocked and to what degree. Importantly, thread dimensions need to be adjusted for specific animal strains. For example, using a fixed-size silicone-coated 4-0 nylon monofilament thread, blood flow reduction varied markedly by strain with Long–Evans showing greater reductions in flow than Sprague–Dawley (SD) or Wistar rats (Prieto et al, 2005).
Uncoated monofilament threads and the poly--lysine-coated threads, that were introduced to increase the proportion of successful surgeries (Belayev et al, 1996), are each prone to high rates of subarachnoid hemorrhage (Schmid-Elsaesser et al, 1998), confounding the physiologic basis of the model and leading to high mortality (Spratt et al, 2006). The use of silicone-coated threads is recommended because these reduce the problems of subarachnoid hemorrhage (Schmid-Elsaesser et al, 1998) and variability (Aspey et al, 1998), particularly in in-bred strains, such as the spontaneously hypertensive rat (SHR) (Spratt et al, 2006). Additional coating of the silicone with poly--lysine may further enhance their utility (Lourbopoulos et al, 2008). Providing guidelines for selecting silicone-coated thread dimensions suitable for all circumstances is difficult because there are too many variables likely to alter arterial dimensions. For example, in mice, a 15 g increase in body weight can result in a doubling of the required thread diameter from 100 to 200 μm (Hata et al, 1998). Eleven-week-old male SD rats are 100 g heavier than equivalent Fischer 344 rats, and in both strains, the males are >60 g heavier than the females (Seidel et al, 2006). A rational approach suitable for use across species and strains might be the first to establish the length of silicone coating desired by measuring the distance between the origin of the MCA and hypothalamic artery if occlusion of this vessel is to be avoided to minimize thermoregulatory disturbances (Li et al, 1999). This is easily carried out after perfusing the brain with Evans blue in gelatine (Crack et al, 2001). As the thread needs to pass through the carotid canal, starting with this measurement, the diameter is reduced to permit smooth passage into the skull without reducing the diameter beyond the point at which laser Doppler-measured MCA-territory flow starts to increase consistently.
Sprague–Dawley rats, the most widely used animals in stroke research (VO'C, personal communication), unfortunately give some of the most variable results (Spratt et al, 2006), most likely because of their highly variable MCA anatomy (Fox et al, 1993) and are thus not recommended as a starting point. Even the choice of vendor can alter outcome in the SD (Oliff et al, 1995). At present, the Wistar Kyoto (WKY) rat seems to be the best choice. It lacks the vascular variability of the SD, and does not display the extremes of inflammatory reactivity noted in the Lewis and Fischer 344 strains (Morand and Leech, 2001). Its genetic relationship to the SHR and stroke-prone SHR (spSHR) strains, which provide the most commonly used models of hypertension and spontaneous stroke, make it an ideal stepping stone for later preclinical evaluations.
In larger domestic animals (such as cats, dogs, sheep, goats, pigs, cows, and horses) that would otherwise offer significant advantages in size and cortical complexity, direct intravascular access to the MCA is prevented because blood is supplied to the cerebral hemispheres through a carotid rete (or rete mirabile), a plexus of fine freely anastomosing arteries. In dogs, this problem has recently been overcome by femoral artery catheterization and fluoroscopically controlled introduction of a platinum coil through the vertebrobasilar system to occlude the origin of the MCA (Rink et al, 2008).
Although thread occlusion and its variants effectively model induction of ischemia at the site most commonly occluded in humans, they do not model the mechanism of occlusion. Approximately 80% of human strokes are ischemic (Donnan et al, 2008), and most of the larger (nonlacunar) infarcts are caused by thromboembolism. Thus, the specific advantage of thromboembolic methods is that the mechanism of occlusion better matches that seen in a large proportion of human strokes, and that they permit the study of thrombolytic processes. However, success is highly dependent on the properties of the introduced clot and, as in humans, the timing of reperfusion can be uncertain.
Although the earliest embolic model of stroke was described in dogs (Hill et al, 1955), it was not until 1982 that an embolic model was described in rats (Kudo et al, 1982) using essentially the same surgical approach as used for intraluminal thread occlusion. The simplest embolic model injects a suspension of small clot fragments into the common carotid artery or ICA (Kudo et al, 1982). Reported mortality using this approach was low, but the foci of infarction were widely distributed and included significant numbers in the contralateral hemisphere (Kudo et al, 1982). With the aim of generating a more faithful model of human thromboembolic stroke in which the ‘obstructing emboli should be located in the proximal segment of a large feeder artery, the distal vascular bed should be open' (Busch et al, 1997), most methods now in routine use introduce a single larger clot of carefully controlled dimensions and consistency close to the origin of the MCA. When the clot is introduced into the ICA, there is little control over where it lodges, allowing infarcts that can include MCA, anterior, and posterior cerebral artery territories. By advancing the clot-introducing catheter into the MCA itself, using laser-Doppler flowmetry to verify the placement, and then withdrawing the catheter slightly, it is possible to obtain a high proportion of animals with only MCA occlusion (MCAo) (DiNapoli et al, 2006). As one of the main reasons for using embolic models is to be able to study thrombolysis, consistency of the clot has received much attention. Clots derived from unmodified arterial blood (DiNapoli et al, 2006), arterial blood mixed with thrombin (Wang et al, 2001), and whole blood mixed with CaCl2 and thrombin and subjected to ‘osmotic shock' (Toomey et al, 2002) have all been used. However, although both spontaneously formed and thrombin-induced clots seem to provide similar levels of occlusion, thrombin-induced clots appear more resistant to the effects of tissue plasminogen activator (tPA) (Niessen et al, 2003). Although more data are required to confirm this observation, it highlights an important choice for the experimenter. If the experimental aim is to provide a model system in which the beneficial effects of a new drug on infarct and behavioral outcome can be studied together with embolic blockade and tPA-mediated reperfusion, then the more readily thrombolyzed ‘red' spontaneously formed clots have the advantage. With these, reperfusion occurs within a time frame that permits tissue salvage. However, if the aim is to study the mechanics of ‘clot busting' and devise more effective ways of breaking up the embolus, the thrombin-induced and fibrin-rich ‘white' emboli (Kirchhof et al, 2002) which probably better represent the emboli that cause human stroke (Jorgensen and Torvik, 1969; Marder et al, 2006), have the advantage. Marder et al (2006) report that the thromboemboli retrieved from the MCA or the intracranial ICA of patients with acute ischemic stroke have similar histologic components, whether derived from presumptive cardiac or arterial sources. A disadvantage of thromboembolic methods is that varying the timing of occlusion and time to reperfusion is not a certain art (as indeed is true in humans). With spontaneously formed emboli, reperfusion can take more than an hour; with thrombin-enriched emboli, this can extend to 5 hours (Niessen et al, 2003). Thread occlusion models offer much greater flexibility and certainty.
Although these models offer researchers the opportunity to study a more realistic model of stroke, even in experienced hands, poststroke mortality can be as high as 30% to 40% within 24 hours (Toomey et al, 2002) with reports of up to 85% mortality if animals are maintained up to 72 hours (Alonso de Lecinana et al, 2006). Although early reperfusion and generally smaller infarcts in most thread occlusion experiments probably contribute to this difference in mortality between models, the persistence of high mortality after embolism when early tPA therapy successfully reduced infarct volume (Toomey et al, 2002) suggests this may not be the whole story. Although there are obvious reasons for concern about the practicality of using these methods, it should be remembered that our only effective acute therapy, tPA, shows similar profiles of activity in animal and human thromboembolic stroke (Perel et al, 2007) and that new agents will almost inevitably need to be tested in the presence of tPA. Differences in the efficacy of tPA in rodents and humans are also of concern, but may lead to new avenues for therapy (Zhu et al, 2010).
An alternative, although related, approach to occlusion is direct induction of thrombus formation at the origin of the MCA or its more distal branch points. To this end, thrombin has been infused at the origin of the MCA in rats (Zhang et al, 1997) and rabbits (Jahan et al, 2008), by drawing blood into a thrombin-filled catheter and then releasing the freshly formed clot (Beech et al, 2001) or by injecting thrombin directly into the distal MCA of mice (Orset et al, 2007). In small animals in whom the skull is thin and allows passage of sufficient light, noninvasive and highly reproducible and high-throughput photothrombotic methods are also available (Watson et al, 1985). However, thrombosis can occur in any illuminated vessel containing a high enough concentration of photo-activating agent. Although proximal MCAo with these techniques is similar to other occlusion methods (Watson et al, 1985), illumination through the parietal cortex (Sugimori et al, 2004) seems unlikely to allow study of penumbral involvement if blood vessels are completely congested with aggregated platelets (Haseldonckx et al, 2000). A perceived disadvantage of photothrombotic methods is early vasogenic edema and blood–brain barrier breakdown. However, recent examinations of these phenomena have suggested similar marked blood–brain barrier disruption within an hour in both thread occlusion and photothrombotic models (Chen et al, 2009; Stoll et al, 2009).
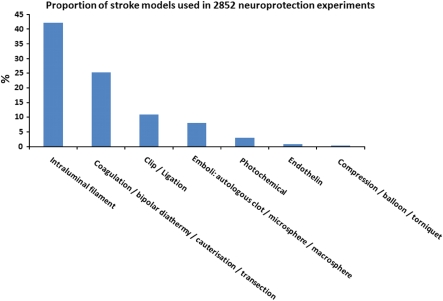
Table 1 provides an overview of the advantages and disadvantages of the most commonly used models of stroke, whereas Figure 2 shows the frequency with which different model types were used in a recent analysis of neuroprotection in stroke (O'Collins et al, 2006). Of experiments using intraluminal sutures, 51.4% used heat-blunted or mechanically formed sutures, 42.2% use silicone coated sutures, and 6.4% used poly--lysine-coated sutures.
Table 1. Overview of advantages and disadvantages of commonly used models of stroke.
| Model | Advantages | Disadvantages |
|---|---|---|
| Intraluminal thread occlusion of the MCA | Avoids opening the skull and surgical injury to the brain Suitable for both permanent and transient MCAo Recannalization can be timed precisely High proportion of successful procedures in experienced hands | Does not model thromboembolism or thrombolysis Requires significant neck surgery with peri-surgical morbidity Lesion volume/variability highly dependent on the anatomy of the circle of Willis and degree/duration of occlusion achieved Contribution of transient and permanent vessel occlusion in the neck to overall outcome unclear Not feasible in animals with rete mirabile |
| Heat-treated suture Heat/poly--lysine treated Silicone coated | Rarely used today, essentially of historical interest Effective occlusion, high reproducibility for permanent MCAo Very effective occlusion, low mortality, good reproducibility | Inconsistent occlusion Firm anchoring to the endothelium leading to bleeding on withdrawal Coating can become detached and cause secondary occlusion |
| Transcranial surgical occlusion of MCA (e.g., ligation, clip, cautery) | Possible in any species, particularly suited to large animals High proportion of successful procedures | Does not model thromboembolism or thrombolysis Significant surgical trauma can accompany the stroke Skull opened, dura breached, and CSF released |
| Proximal MCAoa Distal MCAob | Approaching 100% successful induction of infarction Highly reproducible lesion size and behavioral outcomes Relatively little surgical comorbidity as skull opening can be small | Requires significant surgical skill Significant surgical comorbidity Recannalization possible but not usual |
| Thromboembolic MCA territory occlusion | Models most common cause of human stroke Allows study of thrombolysis | Low rate of successful induction of stroke Highly variable histologic and behavioral outcome Timing of reperfusion dependent on thrombolysis Rodent thrombolysis requires ∼10 times more tPA than in humans |
| Spontaneous clotting Thrombin-enhanced clotting Distal introduction Proximal introduction | Easy to show effect as thrombolysis occurs early Better model of usual consistency of human emboli More certain occlusion of the origin of the MCA | Probably higher incidence of TIA-like events Certainty of occlusion counterbalanced by difficulty of reperfusion Little control over site of clot lodgment and infarction |
| Others: Nonclot embolism Endothelin Photothrombosis Balloon catheters | Simple to manufacture and introduce Can be induced in the absence of anesthesia Reproducible, possible without opening skull, high throughput Minimal surgery, occlusion assured, timed recannalization | Little control over occlusion site, not amenable to thrombolysis Duration of occlusion uncertain, additional direct effects on brain function Thrombosis generally distributed over all vessels illuminated Expensive materials and currently confined to large animals, requires fluoroscopy for catheter guidance |
CSF, cerebrospinal fluid; MCA, middle cerebral artery; MCAo, MCA occlusion; TIA, transient ischemic attack; tPA, tissue plasminogen activator.
Occlusion at the circle of Willis, or between the origin of MCA and the M1 branch of MCA.
Occlusion after the M1 branch of MCA.
Figure 2.
Proportion of stroke models used in 2,852 neuroprotection experiments.
Vascular Anatomy and Concordance with Human Disease
Stroke incidence and subtype proportion vary considerably between communities (Feigin et al, 2006), but overall, occlusion of (a branch of) the MCA is the most commonly identified type of human ischemic stroke (Olsen et al, 1985) and thus the most common target for animal models.
Although the human brain sits at one end of the spectrum of mammalian brain complexity, it still adheres to the basic mammalian pattern of neural and vascular organization. Thus, strokes induced in laboratory animals look very similar to those in humans. Blockage of the origin of the MCA in most mammals studied results in infarcts, which incorporate the gray matter of the motor and somatosensory cortex, the underlying white matter tracts, and the basal ganglia (caudate-putamen and thalamus), which have blood supplied by the small perforating arteries that branch from the MCA or adjacent segments of the circle of Willis.
Despite the broad similarities, there are important differences between species and strains of animals that can affect the experimental outcome. In humans, a pronounced anterior communicating artery usually completes the circle of Willis providing some capacity to redistribute blood between hemispheres. Although relatively uncommon (Kapoor et al, 2008), when the anterior communicating artery is absent or of reduced bore, ischemic stroke outcome may be worse (Jaramillo et al, 2006). Similarly, patients (∼30%) who have absent or hypoplastic posterior communicating arteries appear to be at a greater risk of stroke (Chuang et al, 2008). Cross-sectional studies in humans also suggest that an incomplete circle of Willis distinguishes patients with symptomatic and asymptomatic ICA stenosis (Waaijer et al, 2007).
Similar anatomic variation is seen in most species that have been used to model stroke. A recent examination of the incidence of anatomic variation in the circle of Willis in humans, cows, sheep, goats, and pigs illustrates that although variation is greatest in humans (probably because a deeper genetic pool was sampled), it is also present in most other species (Ashwini et al, 2008). Rats and mice, the animals most commonly used to model stroke, exhibit similar variation. In rats, differences in the posterior communicating artery bore influences of the outcome of white matter injury induced by chronic cerebral hypoperfusion, with Wistar rats with small-diameter vessels more susceptible to ischemic damage than SD rats (Kim et al, 2008). In mice, which are becoming increasingly important because of the availability of transgenic animals, it has been reported that only 10% of C57Black/6 mice have a complete circle of Willis (McColl et al, 2004). In CD1 mice, patency of the posterior communicating artery is not only highly variable but also correlated with the extent of ischemic injury (Zhen and Dore, 2007). Similarly, the increasing sensitivity of BDF (F(1) hybrids of C57BL/6 and DBA/2 normal strains)<CFW (Swiss Webster)<BALB/C mice to MCAo seems to be dependent on a decreasing frequency of patent posterior communicating arteries (Barone et al, 1993). It seems likely that possession of a patent posterior communicating artery permits maintenance of residual cortical perfusion above the ischemic threshold in territories that would otherwise die after MCAo (Kitagawa et al, 1998). Restricting collateral blood supply by occlusion of additional vessels can have a profound effect on the regions infarcted and on experimental variability (Chen et al, 2008). Indeed the gerbil has been promoted as a model species for stroke studies, specifically because their lack of posterior communicating arteries and an absence of an anterior communicating artery in ∼20% of the gerbil population leads to more consistent infarct volumes (Oostveen et al, 1992).
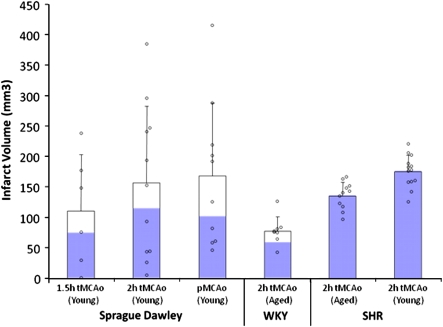
Vascular variability and plasticity in more distal parts of the cerebral circulation also has the potential to alter human stroke outcome and animal modeling. Sprague–Dawley rats (∼60% of all neuroprotection data come from this strain (VO'C, personal communication) display a much greater range of infarct sizes in thread occlusion models than strains, such as the SHR and WKY (Figure 3), probably because of the highly variable origin and branching pattern of the MCA in this strain (Fox et al, 1993). Similarly, although changes in vascular contractility and blood–brain barrier permeability undoubtedly contribute to the propensity to stroke in SHR and spSHR strains (Knox et al, 1980), the observation that anastomotic vessels linking the anterior cerebral artery and MCA territories are narrower in spSHR than in normotensive rats is probably a major determinant of blood flow to the threatened territory and of the amount of tissue that can be protected from infarction by collateral circulation (Coyle and Heistad, 1991).
Figure 3.
Not all rats were created equal: infarct volume variability after thread occlusion in Sprague–Dawley (SD), Wistar Kyoto (WKY), and spontaneously hypertensive rat (SHR) under a range of experimental circumstances. Blue bars represent the proportion of animals with cortical infarction. Mean and s.d. plus individual data points.
Although it is possible to exploit these species/strain differences to reduce experimental variability in infarct volume, the relevance to human stroke subtypes must always be considered if the aim is the evaluation of a drug's potential to treat human stroke.
Experimentally Controllable Physiologic Variables that Affect Outcome
In addition to differences in model construction and the underlying vascular anatomy, controllable variables such as regulation of blood flow, temperature, and blood gas concentration all have the potential to affect experimental outcome.
Blood Flow
One of the most important variables is the reduction of blood flow achieved by thread or embolus occlusion. In most species studied, including humans, the evidence suggests that unless blood flow is reduced to below a flow of ∼0.12 ml/g per min for a significant period, infarction is not inevitable. Above this threshold, electrical activity and normal function may be suppressed, but there is sufficient metabolic reserve to preserve cellular integrity (Astrup et al, 1981; Sakoh et al, 2000). It is not uncommon for animals to have acute, but transient, functional deficits upon waking from anesthesia, which do not progress to frank infarction (Sicard et al, 2006).
In laboratories without access to high-resolution computed tomography or magnetic resonance imaging, laser-Doppler flowmetry is widely used to judge whether blood flow reduction has been sufficient to induce infarction and reveal when spontaneous reperfusion is a cause of failed experiments (DiNapoli et al, 2006; Schmid-Elsaesser et al, 1998), emphasizing the need for frequent and long-term monitoring. Moreover, it has been reported that excessive and sustained reduction of cortical blood flow (93.6%±5%) after thread occlusion suggests subarachnoid hemorrhage (Woitzik and Schilling, 2002). When occlusion is successful, both thread and embolic methodologies produce similar reductions (70% to 85%) in blood flow detected over the parietal cortex (Chen et al, 2008; DiNapoli et al, 2006; Schmid-Elsaesser et al, 1998; Woitzik and Schilling, 2002). Endothelin-1-induced occlusion of the MCA produces a similar reduction in blood flow (Bogaert et al, 2000), but local induction of thrombosis by thrombin seems to be less effective, producing deficits of only 40% to 50% of baseline (Orset et al, 2007). In most laboratories, laser-Doppler flowmetry probes are placed over the parietal cortex of rodents because the lack of musculature and a relatively flat skull make probe attachment easy. However, these sites sample a varying mixture of MCA and anterior cerebral artery territory flow. Harada et al (2005) have reported that sampling MCA flow specifically by placing a flat probe between the temporalis muscle and the lateral aspect of the skull allows more successful induction of stroke with smaller variation. Although the numbers of animals studied were small (12 per cohort), the suggestion warrants further attention.
Temperature
Temperature is an important determinant of mammalian cell function and survival. Our biochemistry has evolved to function most effectively within narrow temperature ranges, and we have evolved specific mechanisms to help maintain an optimal body temperature and to limit damage to our proteins if we overheat.
In small mammals, a precipitous decrease in body temperature is common during anesthesia because their high-surface-area-to-mass ratio makes thermoregulation difficult, a phenomenon compounded by the use of unwarmed gases during inhalational anesthesia (Haskins and Patz, 1980). With agents such as sodium pentobarbital, the core temperature can decrease by 3.5°C to 4.5°C within an hour and brain temperature can be 0.3°C to 0.4°C lower (Kiyatkin and Brown, 2005). As cooling can be profoundly neuroprotective (van der Worp et al, 2007), preclinical evaluation of neuroprotectants should at some stage incorporate an evaluation of the impact of body or brain temperature. Although some recommend measuring both brain and body temperatures (Busto et al, 1989), the observation that for isoflurane, the differential between the two measurements is constant (R2=0.9996) (Zhu et al, 2009) suggests that less-invasive measures of core temperature may suffice. It would be foolish to simply constrain animal temperature to the normal range during experiments as the induction of hypothermia, or other changes that lead to it, might be the mechanism of action of a new drug. A number of candidate pharmacological neuroprotectants such as the AMPA (α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate) receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione) (NBQX) (Nurse and Corbett, 1996) and the sedative clomethiazole (Visser et al, 2005) probably involve hypothermic effects in their mechanism of action, whereas others such as Mg2+ (Campbell et al, 2008) and tacrolimus (FK506) (Nito et al, 2004) are reported to have their effects enhanced by even mild hypothermia. Even the prototypical antiexcitotoxic dizocilpine (MK-801) alters body temperature and can have its effects masked by concomitant hyperthermia (Memezawa et al, 1995).
Hyperthermia is a common and significant complication in stroke. In humans, a core temperature of >37.5°C on the first day after stroke onset has been reported in up to a third of patients and is a strong predictor of poor outcome. Conversely, low body temperature on admission is associated with good short-term outcome (den Hertog et al, 2007). In rats, increasing the body temperature to 40°C 24 h after stroke has been reported to cause up to a three-fold increase in infarct volume (Kim et al, 1996). Furthermore, there are fluctuations in endogenous temperature of up to 1.3°C which have been found to correlate with differences in stroke size found when surgery is conducted at different times of the day (Vinall et al, 2000). In experimental stroke, hyperthermia is a particular problem when the thermoregulatory centers of the hypothalamus are damaged by infarction. The body temperature can increase quickly (within 15 minutes of the onset of ischemia), reach 39°C to 40°C within an hour, and can be sustained for >24 hours (Reglodi et al, 2000). Because of the mechanics of occlusion, this problem is usually only seen when thread occlusion blocks the multiple vessels which contribute to hypothalamic perfusion (Li et al, 1999).
Anesthesia
Anesthesia is required at some stage in all models of stroke, which require surgery for access to the vasculature or the brain. For the most part, anesthetics act through two principal mechanisms: an increase in inhibition through GABA A receptors (such as barbiturates, benzodiazepines, propofol, isoflurane, etomidate, enflurane, and halothane) or a decreased excitation through NMDA (N-methyl--aspartic acid) receptors (such as nitrous oxide, ketamine, and xenon) (Traystman, 2010). However, anesthesia itself seems to have both neuroprotective and preconditioning effects mediated by the inhibition of spontaneous depolarization (Patel et al, 1998), activity as antioxidants (Wilson and Gelb, 2002), antagonism of NMDA receptors (Harada et al, 1999), GABA potentiation (Harris et al, 1994), and alteration of cerebral blood flow redistribution (Warner et al, 1989). Early clinical observations that patients under general anesthesia were more tolerant of ischemia than were unanesthetized patients (Wells et al, 1963) support this view.
Data obtained from animals are however difficult to interpret. The impractibility of unanesthetized surgery makes experimental control difficult, and the precise mechanism is difficult to ascertain when effects on the cerebrovasculature, brain metabolism, brain electrophysiology, temperature, and blood pressure can all interact (Traystman, 2010). Observations that some agents induce neuronal apoptosis which can potentially make these agents neurotoxic (Ikonomidou et al, 1999) confuse the picture further. Importantly, anesthesia can interact with neuroprotectants to increase apparent efficacy (Macleod et al, 2005a, 2005b). Whether this is caused by enhanced induction of hypothermia, suppression of metabolism, modulation of blood flow, or specific neurochemical interactions is not always clear.
A practical approach is to avoid using anesthetics with marked intrinsic neuroprotective properties (Anderson and Sundt, 1983; Macleod et al, 2009) such as barbiturates and ketamine, which also make the depth and duration of anesthesia difficult to control. Instead, inhalational anesthetics such as isoflurane are recommended because of the ease with which the depth of anesthesia can be controlled and animals recovered, even though they also have neuroprotective properties (Warner et al, 1993). If mechanical ventilation and if possible continuous pCO2 monitoring for dose adjustment are available, this approach is recommended (Zausinger et al, 2002). Although spontaneous breathing of inhalational anesthetics gives less experimental control and larger infarcts (Zausinger et al, 2002), it provides a practical solution for smaller laboratories. Whatever the route of administration, overuse is to be avoided, and with inhalational agents, the staff must be protected from inadvertent exposure. As human stroke patients are not routinely anesthetized, developing methods that avoid anesthesia during stroke induction, as is possible with thromboembolic (Zivin et al, 1985) and endothelin (Callaway et al, 1999) models, should perhaps receive more attention. To date, there does not seem to have been a formal comparison of different drugs with or without anesthesia at the time of stroke induction in the thromboembolic or endothelin models.
Blood Gases, Blood Pressure, and pH
In an ideal world, changes in partial pressure of O2 and CO2, pH, as well as blood pressure and anesthetic concentration would be monitored constantly during stroke modeling and adjusted minute by minute to ensure that blood and oxygen supply to the tissue beds only changed because of the stroke, and not by some inadvertent vasoconstriction or dilation of collateral blood vessels caused by our experimental machinery. We know that blood gas concentrations influence experimental stroke outcome (Browning et al, 1997; Zausinger et al, 2002), that increasing blood pressure slightly improves blood flow and oxygen metabolism (Shin et al, 2008), and that pH also influences outcome (Anderson and Meyer, 2002). However, we currently lack the data and sophistication required to allow us to effectively control all of these parameters. Monitoring them is a starting point and many believe this should be mandatory. However, to be of value, the frequency of monitoring needs to be high enough to detect a number of relatively short periods of decompensation during occlusion. A similar scenario is seen when laser Doppler measurements of cortical flow velocity reveal transient periods of premature reperfusion, which keep an animal above the threshold for infarction (Schmid-Elsaesser et al, 1998).
Effects of Comorbidities on Outcome
Perhaps the most powerful strategy in animal modeling is to identify and analyze the key subcomponents of a problem and thus reduce the complexity of human disease to manageable proportions. Conversely, to evaluate the therapeutic potential of a new therapy, we may need, at some point, to model those complexities. In the development of stroke drugs, we have often failed to consider the impact of risk factors such as hypertension and diabetes that are present in a large proportion of patients with ischemic stroke (Fisher et al, 2009a).
Co-morbidities in the Clinic
Hypertension can account for 30% to 40% of the risk of stroke (Lawes et al, 2004). A 10 mm Hg increase in arterial blood pressure increases stroke risk by 20% to 30% (Alberts and Atkinson, 2004) and a blood pressure above 120/80 mm Hg doubles the lifetime risk of stroke (Kelly et al, 2007). Similarly, hyperglycemia and diabetes are common in stroke patients. Approximately one-fourth of stroke patients have a history of diabetes (Kaarisalo et al, 2005), whereas hyperglycemia is detected in up to 40% of stroke patients on admission (Williams et al, 2002). Both type 1 and type 2 diabetes are associated with an increased risk of stroke (Jeerakathil et al, 2007; Sundquist and Li, 2006) with newly treated type 2 diabetes doubling the short-term risk of stroke in one study (Jeerakathil et al, 2007). In the United States, it has been estimated that 37% to 42% of all ischemic strokes may be attributable to the effects of diabetes alone or in combination with hypertension (Kissela et al, 2005). Moreover, although hypertension and type 2 diabetes increase stroke risk independently, their combination appears to increase the risk drastically (Hu et al, 2005). As for hypertension, evidence is beginning to emerge which suggests that better diabetic control reduces stroke risk (Boden-Albala et al, 2008).
Although perhaps not strictly comorbidities, age and gender have a profound influence on stroke biology. Between 19 and 77 years of age, each additional year of age increases stroke risk by 9% in women and 10% in men (Asplund et al, 2009). Although the risk is greater in men, because they live longer, women are more likely to experience a stroke and to have a more disabling stroke (Reeves et al, 2008).
Clearly, it is important to know whether candidate stroke drugs retain efficacy in the face of these comorbidities and how they influence the pathophysiology of stroke. However, of 3,142 animal experiments on neuroprotection abstracted from the literature (O'Collins et al, 2006), only 11% involved testing in hypertensive and only 1% in diabetic animals.
Hypertension
At least 20 models of hypertension have been reported. These range from surgical ligation of arteries supplying a kidney, through pharmacological or genetic manipulation of vascular reactivity, to selective breeding of hypertensive rabbits and rats (Lerman et al, 2005). Despite the range of available methodologies, only a few have been used in stroke modeling.
The Dahl salt-sensitive rat develops hypertension dependent on the salt content of their diet (Meneely and Ball, 1958). On a high-salt diet (8.7% NaCl2), marked hypertension (∼200 mm Hg systolic) develops in ∼4 weeks and blood–brain barrier disruption, stroke, and death can quickly follow (Payne and Smeda, 2002). At lower salt concentrations, the same end is reached but over a longer time frame, with animals fed a 1% NaCl2 diet starting to die at ∼5 months of age (Rapp and Dene, 1985). Arterial lesions characterized by histiocytic, eosinophilic, and neutrophilic infiltration and frank coagulation are prominent in the mesentery, pancreas, intestine, testis, heart, and kidney but absent from the brain and lung (Rapp and Dene, 1985). When thread occlusion for 120 minutes was used to occlude the MCA of Dahl salt-sensitive rats after 5 weeks of high-salt diet (8%), 80% died or experienced intracranial hemorrhage within 24 hours. Reducing occlusion time to 90 minutes still left 40% of the animals with hemorrhage (Bright et al, 2007). These losses are substantially higher than reported with similar occlusion times in SHRs (21 or 3% using poly--lysine- or silicone-coated filaments, respectively) (Spratt et al, 2006).
The SHR and related spSHR, which were both selectively bred from the WKY strain, are the most widely used hypertensive animals in stroke research. Although normotensive at birth, SHRs start to develop hypertension in the first 2 to 4 months of life and usually reach a stable systolic blood pressure plateau of ∼200 mm Hg by 6 months. The phenotype of the SHR and spSHR is complex. In addition to hypertension, these animals have smaller brains (Tajima et al, 1993), enlarged ventricles (Bendel and Eilam, 1992; Tajima et al, 1993), hypertrophy (due to increased smooth muscle cell number) of the large cerebral arteries (Mangiarua and Lee, 1992), increased circulating monocyte number, increased endothelial macrophage infiltration, and inflammatory marker expression (Liu et al, 1996).
The spSHR was derived by inbreeding of the offspring of SHRs that died of stroke until >80% of the population developed stroke characterized by multifocal microvascular and spongy-cystic parenchymal lesions (Fredriksson et al, 1988). Similar to the Dahl rat, spSHRs are prone to salt-sensitive renal injury which precipitates hypertension (>240 mm Hg) and rapid onset of hemorrhagic stroke (Lee et al, 2007). Approximately 70% of strokes occur in the gray matter of the cortex (Yamori et al, 1976). Magnetic resonance imaging studies confirming vasogenic edema and blood–brain barrier breakdown without metabolic impairment have confirmed the absence of ischemia as a precipitating event (Guerrini et al, 2002). The cortical focus for the lesions is believed to reflect abnormal vascular structure and vascular reactivity (Baumbach et al, 1989; Coyle, 1987). After MCAo, nitric oxide production is also impaired and correlates with increased infarct size (Kidd et al, 2000).
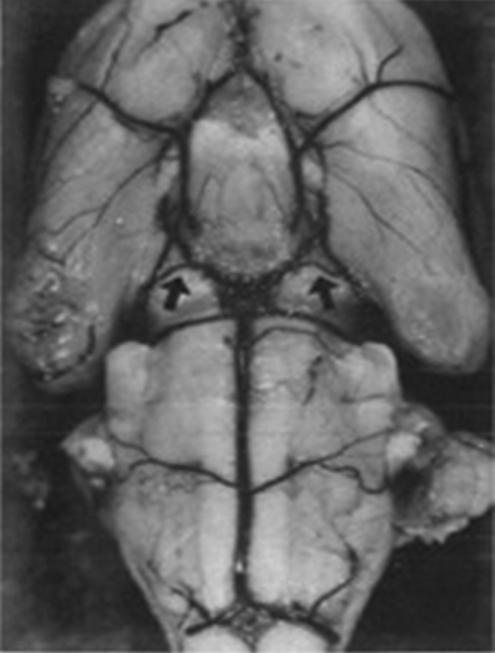
After induction of focal ischemia, blood flow reductions are more pronounced and brain injury as measured by infarct volume and behavior after focal stroke in SHR and spSHR is significantly larger and more reproducible than in normotensive rat strains (Barone et al, 1992; Spratt et al, 2006). Unlike the gerbil (Baskaya et al, 1999), this reproducibility is not due to the absence of the posterior communicating arteries (Figure 4) (Ogata et al, 1976) and the constraints on collateral blood flow this imposes. However, larger than normal infarcts in the spSHR are independent of blood pressure, age, or sex and appear to result from inadequate cortical collateral blood flow (Gratton et al, 1998). Moreover, because SHRs have smaller body and brain weights than the WKY strain throughout life and successful thread occlusion is highly dependent on the relationship between thread and vessel size, using the WKY as a normotensive control for the SHR or spSHR presents a significant challenge in its own right.
Figure 4.
Circle of Willis and posterior communicating arteries of the spontaneously hypertensive rat (Ogata et al, 1976).
Of 493 drugs tested in 45,512 animals with focal cerebral ischemia (VO'C, personal communication), 409 were tested only in normotensive animals, just 56 were tested in both normotensive and hypertensive animals, whereas 28 appear to have been tested only in hypertensive animals. The majority of this testing was performed in the SHR. For six drugs reviewed systematically, only 10% of publications included the modeling of efficacy in animals with high blood pressure or diabetes. Where efficacy was reported in the context of comorbidity, it was generally substantially lower. Disufenton sodium (NXY-059) was less effective in SHRs than in healthy animals (17.6% versus 47.8% P<0.001) (Macleod et al, 2008). Tissue plasminogen activator had no beneficial effect on either infarct volume or neurobehavioral score but did increase the observed odds of hemorrhage in SHRs (ESS, personal communication). Nicotinamide was less effective in animals with diabetes or hypertension (21.8% versus 30% P<0.01) (Macleod et al, 2004) as was FK506 (17% versus 33.3% P<10−10) (Macleod et al, 2005b). In contrast, hypothermia was slightly more effective in SHR than in normotensive SD and Wister rats (van der Worp et al, 2007). Melatonin was not tested in hypertensive animals (Macleod et al, 2005a).
Of the many other models of hypertension, renovascular models (induced by various combinations of renal artery clipping and kidney removal) are the next most commonly used. By keeping both kidneys in place and clipping one renal artery (two-kidney one-clip, 2K1C) only mild and relatively unstable hypertension is achieved, yet this model provided one of the earliest reports that hypertension exacerbates ischemic injury (Fujishima et al, 1978). When a kidney is removed and the other renal artery clipped (one-kidney one-clip, 1K1C) animals often die of acute renal failure accompanied by diffuse edematous lesions in the brain (Nag, 1984). To avoid these problems, Zeng et al described clipping both renal arteries without kidney removal (two-kidney two-clip) to model stroke in hypertension. All SD rats developed stable hypertension without acute renal failure or diffuse cerebral lesions. Within 40 weeks, 62% had developed spontaneous stroke, significantly more than in 2K1C or 1K1C models. The strokes, which were a mixture of small infarcts with clear evidence of thrombotic occlusion and hemorrhagic lesions caused by bleeding from the arteriolar wall of fibrinoid necrosis or ruptured microaneurysms, correlated with the presence of vascular pathology in small arteries or arterioles (Zeng et al, 1998). Using serial magnetic resonance imaging to assess the consequences of inducing hypertension by partial occlusion of both renal arteries, showed a tight relationship between the degree of hypertension and development of cerebral lesions. Below a mean systolic pressure of 210 mm Hg, rats never had brain lesions, but when pressure exceeded 276 mm Hg, rats consistently developed brain lesions (Del Bigio et al, 1999).
Similar to the spSHR and renovascular models, rats made hypertensive by treatment with deoxycorticosterone (a mineralocorticoid receptor agonist) and salt can also have spontaneous strokes when blood pressure is high (Sukamoto et al, 1980), but they are more readily protected by the antihypertensive captopril against the effects of acute MCAo than spSHR (Coyle, 1984). Experiments in young Wistar rats suggest that these effects may be mediated in part by remodeling of the cerebrovasculature as treatment with deoxycorticosterone acetate alone (without salt) for 6 weeks stiffens and narrows the MCA, induces mild hypertension, and renders the rats more sensitive to MCAo (Dorrance et al, 2006).
Little is known about the impact of most other methods of inducing hypertension on stroke. For example, the New Zealand, Milan hypertensive, and Lyon hypertensive rats do not die due to strokes or cardiovascular disease like the spSHR and do not appear to have been used in the study of MCAo (Bianchi et al, 1984; Phelan, 1968; Vincent et al, 1984). To date, no stroke studies seem to have been undertaken in hypertensive transgenic rats expressing an extra copy of the renin gene (Wagner et al, 1997). Ischemic stroke has been induced in Cynomolgus monkeys made hypertensive by surgical coarctation of the aorta in animals fed an atherogenic diet (Prusty et al, 1988), but this would not seem to be a practical model for widespread use.
Diabetes and Hyperglycemia
As for hypertension, there are numerous models of diabetes. Although surgical removal of the pancreas has been used since the 1880s, diabetes is most commonly induced by selectively poisoning pancreatic β-cells. This can be achieved using the uric acid derivative alloxan, but streptozotocin which is isolated from the soil bacterium Streptomyces achromogenes is more widely used and mimics most of the major hallmarks of clinical type 1 diabetes, including hyperglycemia, elevated HbA1c concentration, weight loss, polydipsia, and polyurea. Other models including the Non-Obese Diabetic mouse, the BioBreeding rat, and the Zucker Diabetic Fatty rat have been generated by selective inbreeding (Rees and Alcolado, 2005). Defects in the db gene on mouse chromosome 4 and the fa gene on rat chromosome 5 both lead to leptin receptor defects (Chen et al, 1996; Takaya et al, 1996). The db/db mouse develops severe diabetes by 6 weeks of age, characterized by hyperglycemia, hyperinsulinemia, and obesity (Vannucci et al, 2001). Rats homozygous for an amino-acid substitution in the fa gene become obese (reaching ∼500 g at 6 months of age), hyperlipidemic, and develop insulin-resistant hyperglycemia (400 to 500 mg/dL) when 7 to 10 weeks old. The Goto-Kakizaki rat offers a model of spontaneous type 2 diabetes generated by inbreeding glucose-intolerant Wistar rats (Ergul et al, 2007).
Both diabetes as a metabolic condition and hyperglycemia independently have been used in conjunction with animal models of stroke. Intraperitoneal dextrose to increase blood glucose to >15 mmol/L accelerates and extends infarct development after transient (thread occlusion) and permanent (distal MCA cautery) MCAo (Liu et al, 2007). Others have found that hyperglycemia enlarged infarcts but only in the cortex (Martin et al, 2006). In cats, hyperglycemia led to a three- to four-fold increase in infarct size after permanent MCAo and increased death due to edema upon reperfusion (de Courten-Myers et al, 1989). Similar observations have been made in dogs (Palmon et al, 1995), rabbits (Kraft et al, 1990), and rats (Dietrich et al, 1993). Damage to the blood–brain barrier may be relevant to reports that hyperglycemia exacerbates injury after tPA therapy (Ribo et al, 2007). Damage to the insular cortex and hyperglycemia as a result of endogenous stress responses further complicates our understanding of the impact of hyperglycemia (Allport et al, 2004).
Although early attention focused on acidosis as the mechanism of hyperglycemia-enhanced neuronal injury, other targets have also gained favor. After thread occlusion of the MCA in rats, hyperglycemia was reported to lead to a progressive reduction in cerebral blood flow and enhanced blood–brain barrier permeability (Kawai et al, 1998). After chemical poisoning of pancreatic β-cells with alloxan or streptozotocin, the results are similar with increased edematous change (Kamada et al, 2007), exacerbation of transient and permanent ischemic lesions with increased speed of lesion development, and continued growth upon reperfusion (Huang et al, 1996; Kittaka et al, 1996). Studies of the mechanism of damage also implicate altered inflammatory responses with exaggerated leukocyte-endothelial cell adhesion (Panes et al, 1996), and increased interlukin-1 and intercellular adhesion molecule-1 expression (Ding et al, 2005). Blood–brain barrier dysfunction has also been attributed to increased oxidative stress and matrix metalloproteinase-9 activation (Kamada et al, 2007). Interestingly, it has been reported that although acute hyperglycemia has no effect on endogenous tPA expression, a similar but persistent elevation of blood glucose (∼15 mmol/L) in streptozotocin-treated rats led to a complete depletion of tPA protein and more than six-fold loss of tPA mRNA expression (Kittaka et al, 1996).
In genetically determined models of diabetes, such as the BioBreeding Rat and db/db mouse, the effects of hyperglycemia on ischemic injury are gender specific. In the BioBreeding rat, cortical injury is the same in diabetic and control animals, but males had larger and females smaller subcortical infarction (Toung et al, 2000). Similar observations have been made after unilateral common carotid artery ligation combined with systemic hypoxia in the db/db mouse wherein even though female diabetic mice were more hyperglycemic and acidotic than the males, they were more resistant to damage (Vannucci et al, 2001).
The recently described Goto-Kakizaki rat, generated by inbreeding of glucose-intolerant Wistar rats, develops mild hyperglycemia at 6 weeks of age, but is also unusual in producing significantly smaller infarcts after extended (3-hour) thread occlusion of the MCA (but high rates of subcortical hemorrhagic transformation) than nondiabetic controls, which is interpreted as the result of diabetes-induced vascular remodeling (Ergul et al, 2007). However, the shape of the infarcts is reminiscent of the hypothalamic lesions that can be produced when only hypothalamic-perforating arteries but not the MCA are occluded (He et al, 1999), and suggests that reduced effectiveness of thread occlusion in the more tortuous vessels of the Goto-Kakizaki rat (Ergul et al, 2007) might provide an alternative explanation.
Using insulin to reduce blood glucose is reported to induce marked neuroprotection (Hamilton et al, 1995). Similar protective effects on infarct volume were reported when insulin was used together with tPA in normoglycemic animals to treat thromboembolic strokes. Others have reported that tight glycemic control does not improve infarct size in male BioBreeding rats (Toung et al, 2000). Moreover, despite reduced infarct volumes, others have reported that mortality was as high after insulin treatment alone (47%) as it was when combined with tPA (38%) (Meden et al, 2002).
Age and Gender
It is surprising that we perform almost all of our testing in male animals and have little representation of half the human population. The main reason for this choice seems to be simply that lack of an estrus cycle (in male rats) might reduce the overall experimental variability. However, even in male rats, the influence of testosterone seems to be age dependent, with castration conferring protection in the young and supplementation conferring protection in the middle aged (Cheng et al, 2009) and the effects of estrogens, which are far from clear (Strom et al, 2009), may be dependent on interactions with specific elements of models of hypertension (Carswell et al, 2000, 2005) and diabetes (Vannucci et al, 2001), which may possibly be linked by differences in vascular reactivity (Miller et al, 2007). Moreover, as most strokes in women occur after menopause (average age of menopause and average age of incident stroke in women have been reported as 49 and 80 years, respectively) (Lisabeth et al, 2009), ignoring half of our species because of a possibly spurious advantage in the laboratory seems very unwise.
Our understanding of the influence of animal age in the laboratory is also limited. Although age-dependent increases in infarct size are most often reported (Davis et al, 1995; Driscoll et al, 2008; Hachinski et al, 1992), others report reductions in behavioral deficits in aged rats (Shapira et al, 2002). Whether this reflects our lack of understanding of stroke biology or of the models we use is unclear. Differential responses to candidate therapeutics with aging (Won et al, 2006) suggest that we would be wise to learn more. Why has aging been studied so little when the majority of stroke patients are old? The answer would seem to be just the cost of maintaining animals for longer periods. It is certainly feasible to induce consistent strokes in aged animals even when they are also diabetic and hypertensive (Rewell et al, 2010).
Conclusions
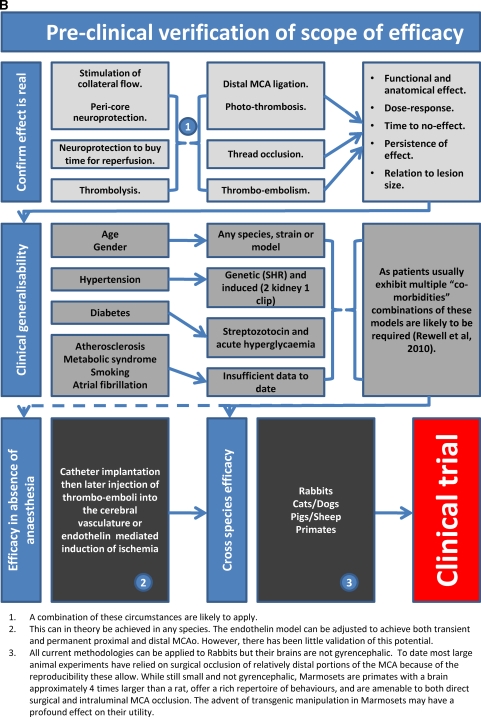
The utility of animal models of stroke is governed by many factors, and it is clear that no single model can encompass all of the variables known to affect human stroke. Which model we choose is determined by a series of compromises and questions we have to ask about the aims of our experiments. Do we wish to occlude single or multiple vessels, do we need control of the timing of reperfusion, or do we need to investigate how a drug interacts with the natural process? Are we trying to determine whether a drug has an effect or trying to define the limits of its efficacy. If the later, we need to consider the common comorbidities of age, atherosclerosis, hypertension, and diabetes. Is limiting experimental variability more important than demonstrating robust effects across a range of genetic backgrounds? Does variability in vascular anatomy matter to your experiment? Brain temperature can dramatically alter infarct size but should we control it or monitor it, do we lose valuable insight by performing all experiments at a fixed temperature that might not be relevant in the clinic? Owing to space limitations, this article could not discuss all the possible variables. For example, blood gas concentrations are often measured and used as part of the physiologic work-up to show equivalence of experimental cohorts or to exclude animals with hypoxia from further analysis. However, how do the subtleties of blood gas concentration encountered during different forms of ventilation and their interaction with anesthesia impact on outcome? Hypertension, hyperglycemia, and diabetes have been considered where specific stroke modeling has been performed, but the broader implications of metabolic syndrome and atherosclerosis remain largely unexplored in animal stroke modeling. There is still much to learn and every experimenter faces many choices. Figure 5 provides a guide to matching model characteristics to experimental aim and an outline of the experiments likely to be needed to move from identifying a candidate drug through to clinical trial. These figures provide only a framework for the questions that will be encountered and decisions that will need to be made as we learn more about stroke and move closer to introducing new and more effective therapies. The deliberations of the Stroke Therapy Academic Industry Roundtable (Fisher et al, 2009b; STAIR, 1999) and those of Macleod et al (2009) are recommended for their insight into the problems of stroke translational medicine and avoidance of bias at the bench.
Figure 5.
Guidelines for stroke modeling. (A) A guide to matching model characteristics to experimental aim; (B) a guide to preclinical stroke modeling required to move from a hypothesis to a clinical trial.
In conclusion, ‘The lack of translation between the animal work and clinical benefits does not lie in the animal models, but in how we use the models and how we apply this knowledge to design of clinical trials' (Willing, 2009).
The authors declare no conflict of interest.
References
- Agnati LF, Zoli M, Kurosawa M, Benfenati F, Biagini G, Zini I, Hallstrom A, Ungerstedt U, Toffano G, Fuxe K. A new model of focal brain ischemia based on the intracerebral injection of endothelin-1. Italian J Neurol Sci. 1991;12:49–53. [PubMed] [Google Scholar]
- Alberts MJ, Atkinson R. Risk reduction strategies in ischaemic stroke: the role of antiplatelet therapy. Clin Drug Invest. 2004;24:245–254. doi: 10.2165/00044011-200424050-00001. [DOI] [PubMed] [Google Scholar]
- Allport LE, Butcher KS, Baird TA, MacGregor L, Desmond PM, Tress BM, Colman P, Davis SM. Insular cortical ischemia is independently associated with acute stress hyperglycemia. Stroke. 2004;35:1886–1891. doi: 10.1161/01.STR.0000133687.33868.71. [DOI] [PubMed] [Google Scholar]
- Alonso de Lecinana M, Gutierrez M, Roda JM, Carceller F, Diez-Tejedor E. Effect of combined therapy with thrombolysis and citicoline in a rat model of embolic stroke. J Neurol Sci. 2006;247:121–129. doi: 10.1016/j.jns.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Anderson RE, Sundt TM., Jr Brain pH in focal cerebral ischemia and the protective effects of barbiturate anesthesia. J Cereb Blood Flow Metab. 1983;3:493–497. doi: 10.1038/jcbfm.1983.76. [DOI] [PubMed] [Google Scholar]
- Anderson RE, Meyer FB.2002Protection of focal cerebral ischemia by alkalinization of systemic pH Neurosurgery 511256–1265.discussion 65–6 [DOI] [PubMed] [Google Scholar]
- Ashwini C, Shubha R, Jayanthi K. Comparative anatomy of the circle of Willis in man, cow, sheep, goat, and pig. Neuroanatomy. 2008;7:54–85. [Google Scholar]
- Aspey BS, Cohen S, Patel Y, Terruli M, Harrison MJ. Middle cerebral artery occlusion in the rat: consistent protocol for a model of stroke. Neuropathol Appl Neurobiol. 1998;24:487–497. doi: 10.1046/j.1365-2990.1998.00146.x. [DOI] [PubMed] [Google Scholar]
- Asplund K, Karvanen J, Giampaoli S, Jousilahti P, Niemela M, Broda G, Cesana G, Dallongeville J, Ducimetriere P, Evans A, Ferrieres J, Haas B, Jorgensen T, Tamosiunas A, Vanuzzo D, Wiklund PG, Yarnell J, Kuulasmaa K, Kulathinal S. Relative risks for stroke by age, sex, and population based on follow-up of 18 European populations in the MORGAM Project. Stroke. 2009;40:2319–2326. doi: 10.1161/STROKEAHA.109.547869. [DOI] [PubMed] [Google Scholar]
- Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia—the ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- Barone FC, Price WJ, White RF, Willette RN, Feuerstein GZ. Genetic hypertension and increased susceptibility to cerebral ischemia. Neurosci Biobehav Rev. 1992;16:219–233. doi: 10.1016/s0149-7634(05)80182-4. [DOI] [PubMed] [Google Scholar]
- Barone FC, Knudsen DJ, Nelson AH, Feuerstein GZ, Willette RN. Mouse strain differences in susceptibility to cerebral ischemia are related to cerebral vascular anatomy. J Cereb Blood Flow Metab. 1993;13:683–692. doi: 10.1038/jcbfm.1993.87. [DOI] [PubMed] [Google Scholar]
- Baskaya MK, Dogan A, Dempsey RJ. Application of endovascular suture occlusion of middle cerebral artery in gerbils to obtain consistent infarction. Neurol Res. 1999;21:574–578. doi: 10.1080/01616412.1999.11740979. [DOI] [PubMed] [Google Scholar]
- Baumbach GL, Heistad DD, Siems JE. Effect of sympathetic nerves on composition and distensibility of cerebral arterioles in rats. J Physiol. 1989;416:123–140. doi: 10.1113/jphysiol.1989.sp017753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech JS, Williams SC, Campbell CA, Bath PM, Parsons AA, Hunter AJ, Menon DK. Further characterisation of a thromboembolic model of stroke in the rat. Brain Res. 2001;895:18–24. doi: 10.1016/s0006-8993(00)03331-x. [DOI] [PubMed] [Google Scholar]
- Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD.1996Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model Stroke 271616–1622.discussion 23 [DOI] [PubMed] [Google Scholar]
- Bendel P, Eilam R. Quantitation of ventricular size in normal and spontaneously hypertensive rats by magnetic resonance imaging. Brain Res. 1992;574:224–228. doi: 10.1016/0006-8993(92)90820-y. [DOI] [PubMed] [Google Scholar]
- Bianchi G, Ferrari P, Barber BR.1984The Milan hypertensive strain Handbook of Hypertension: Experimental and Genetic Models of Hypertension(de Jong W, ed), vol. 4Amsterdam: Elsevier Science Publishers; 328–349. [Google Scholar]
- Boden-Albala B, Cammack S, Chong J, Wang C, Wright C, Rundek T, Elkind MS, Paik MC, Sacco RL. Diabetes, fasting glucose levels, and risk of ischemic stroke and vascular events: findings from the Northern Manhattan Study (NOMAS) Diabetes Care. 2008;31:1132–1137. doi: 10.2337/dc07-0797. [DOI] [PubMed] [Google Scholar]
- Bogaert L, Scheller D, Moonen J, Sarre S, Smolders I, Ebinger G, Michotte Y. Neurochemical changes and laser Doppler flowmetry in the endothelin-1 rat model for focal cerebral ischemia. Brain Res. 2000;887:266–275. doi: 10.1016/s0006-8993(00)02959-0. [DOI] [PubMed] [Google Scholar]
- Bright R, Steinberg GK, Mochly-Rosen D. DeltaPKC mediates microcerebrovascular dysfunction in acute ischemia and in chronic hypertensive stress in vivo. Brain Res. 2007;1144:146–155. doi: 10.1016/j.brainres.2007.01.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning JL, Heizer ML, Widmayer MA, Baskin DS. Effects of halothane, alpha-chloralose, and pCO2 on injury volume and CSF beta-endorphin levels in focal cerebral ischemia. Mol Chem Neuropathol. 1997;31:29–42. doi: 10.1007/BF02815158. [DOI] [PubMed] [Google Scholar]
- Busch E, Kruger K, Hossmann KA. Improved model of thromboembolic stroke and rt-PA induced reperfusion in the rat. Brain Res. 1997;778:16–24. doi: 10.1016/s0006-8993(97)01008-1. [DOI] [PubMed] [Google Scholar]
- Busto R, Dietrich WD, Globus MY, Ginsberg MD. The importance of brain temperature in cerebral ischemic injury. Stroke. 1989;20:1113–1114. doi: 10.1161/01.str.20.8.1113. [DOI] [PubMed] [Google Scholar]
- Callaway JK, Knight MJ, Watkins DJ, Beart PM, Jarrott B.1999Delayed treatment with AM-36, a novel neuroprotective agent, reduces neuronal damage after endothelin-1-induced middle cerebral artery occlusion in conscious rats Stroke 302704–2712;.discussion 12 [DOI] [PubMed] [Google Scholar]
- Campbell K, Meloni BP, Knuckey NW. Combined magnesium and mild hypothermia (35 degrees C) treatment reduces infarct volumes after permanent middle cerebral artery occlusion in the rat at 2 and 4, but not 6 h. Brain Res. 2008;1230:258–264. doi: 10.1016/j.brainres.2008.06.110. [DOI] [PubMed] [Google Scholar]
- Carswell HV, Anderson NH, Morton JJ, McCulloch J, Dominiczak AF, Macrae IM. Investigation of estrogen status and increased stroke sensitivity on cerebral blood flow after a focal ischemic insult. J Cereb Blood Flow Metab. 2000;20:931–936. doi: 10.1097/00004647-200006000-00005. [DOI] [PubMed] [Google Scholar]
- Carswell HV, McBride MW, Graham D, Dominiczak AF, Macrae IM. Mutant animal models of stroke and gene expression: the stroke-prone spontaneously hypertensive rat. Methods Mol Med. 2005;104:49–74. doi: 10.1385/1-59259-836-6:049. [DOI] [PubMed] [Google Scholar]
- Chen B, Friedman B, Cheng Q, Tsai P, Schim E, Kleinfeld D, Lyden PD. Severe blood-brain barrier disruption and surrounding tissue injury. Stroke. 2009;40:e666–e674. doi: 10.1161/STROKEAHA.109.551341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ito A, Takai K, Saito N. Blocking pterygopalatine arterial blood flow decreases infarct volume variability in a mouse model of intraluminal suture middle cerebral artery occlusion. J Neurosci Methods. 2008;174:18–24. doi: 10.1016/j.jneumeth.2008.06.021. [DOI] [PubMed] [Google Scholar]
- Cheng J, Hu W, Toung TJ, Zhang Z, Parker SM, Roselli CE, Hurn PD. Age-dependent effects of testosterone in experimental stroke. J Cereb Blood Flow Metab. 2009;29:486–494. doi: 10.1038/jcbfm.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang YM, Liu CY, Pan PJ, Lin CP. Posterior communicating artery hypoplasia as a risk factor for acute ischemic stroke in the absence of carotid artery occlusion. J Clin Neurosci. 2008;15:1376–1381. doi: 10.1016/j.jocn.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Corkill G, Sivalingam S, Reitan JA, Gilroy BA, Helphrey MG. Dose dependency of the post-insult protective effect of pentobarbital in the canine experimental stroke model. Stroke. 1978;9:10–12. doi: 10.1161/01.str.9.1.10. [DOI] [PubMed] [Google Scholar]
- Coyle P. Outcomes to middle cerebral artery occlusion in hypertensive and normotensive rats. Hypertension. 1984;6:I69–I74. doi: 10.1161/01.hyp.6.2_pt_2.i69. [DOI] [PubMed] [Google Scholar]
- Coyle P. Dorsal cerebral collaterals of stroke-prone spontaneously hypertensive rats (SHRSP) and Wistar Kyoto rats (WKY) Anat Rec. 1987;218:40–44. doi: 10.1002/ar.1092180108. [DOI] [PubMed] [Google Scholar]
- Coyle P, Heistad DD. Development of collaterals in the cerebral circulation. Blood Vessels. 1991;28:183–189. doi: 10.1159/000158860. [DOI] [PubMed] [Google Scholar]
- Crack PJ, Taylor JM, Flentjar NJ, de Haan J, Hertzog P, Iannello RC, Kola I. Increased infarct size and exacerbated apoptosis in the glutathione peroxidase-1 (Gpx-1) knockout mouse brain in response to ischemia/reperfusion injury. J Neurochem. 2001;78:1389–1399. doi: 10.1046/j.1471-4159.2001.00535.x. [DOI] [PubMed] [Google Scholar]
- Crowell RM, Marcoux FW, DeGirolami U. Variability and reversibility of focal cerebral ischemia in unanesthetized monkeys. Neurology. 1981;31:1295–1302. doi: 10.1212/wnl.31.10.1295. [DOI] [PubMed] [Google Scholar]
- Davis M, Mendelow AD, Perry RH, Chambers IR, James OF. Experimental stroke and neuroprotection in the aging rat brain. Stroke. 1995;26:1072–1078. doi: 10.1161/01.str.26.6.1072. [DOI] [PubMed] [Google Scholar]
- de Courten-Myers GM, Kleinholz M, Wagner KR, Myers RE. Fatal strokes in hyperglycemic cats. Stroke. 1989;20:1707–1715. doi: 10.1161/01.str.20.12.1707. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR, Yan HJ, Kozlowski P, Sutherland GR, Peeling J. Serial magnetic resonance imaging of rat brain after induction of renal hypertension. Stroke. 1999;30:2440–2447. doi: 10.1161/01.str.30.11.2440. [DOI] [PubMed] [Google Scholar]
- Del Zoppo GJ, Copeland BR, Harker LA, Waltz TA, Zyroff J, Hanson SR, Battenberg E. Experimental acute thrombotic stroke in baboons. Stroke. 1986;17:1254–1265. doi: 10.1161/01.str.17.6.1254. [DOI] [PubMed] [Google Scholar]
- den Hertog H, van der Worp B, van Gemert M, Dippel D. Therapeutic hypothermia in acute ischemic stroke. Expert Rev Neurother. 2007;7:155–164. doi: 10.1586/14737175.7.2.155. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Alonso O, Busto R. Moderate hyperglycemia worsens acute blood-brain barrier injury after forebrain ischemia in rats. Stroke. 1993;24:111–116. doi: 10.1161/01.str.24.1.111. [DOI] [PubMed] [Google Scholar]
- DiNapoli VA, Rosen CL, Nagamine T, Crocco T. Selective MCA occlusion: a precise embolic stroke model. J Neurosci Methods. 2006;154:233–238. doi: 10.1016/j.jneumeth.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Ding C, He Q, Li PA. Diabetes increases expression of ICAM after a brief period of cerebral ischemia. J Neuroimmunol. 2005;161:61–67. doi: 10.1016/j.jneuroim.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- Dorrance AM, Rupp NC, Nogueira EF. Mineralocorticoid receptor activation causes cerebral vessel remodeling and exacerbates the damage caused by cerebral ischemia. Hypertension. 2006;47:590–595. doi: 10.1161/01.HYP.0000196945.73586.0d. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Hong NS, Craig LA, Sutherland RJ, McDonald RJ. Enhanced cell death and learning deficits after a mini-stroke in aged hippocampus. Neurobiol Aging. 2008;29:1847–1858. doi: 10.1016/j.neurobiolaging.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Ergul A, Elgebaly MM, Middlemore ML, Li W, Elewa H, Switzer JA, Hall C, Kozak A, Fagan SC. Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. BMC Neurol. 2007;7:33. doi: 10.1186/1471-2377-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin V, Carter K, Hackett M, Barber PA, McNaughton H, Dyall L, Chen MH, Anderson C. Ethnic disparities in incidence of stroke subtypes: Auckland Regional Community Stroke Study, 2002–2003. Lancet Neurol. 2006;5:130–139. doi: 10.1016/S1474-4422(05)70325-2. [DOI] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH. Update of the Stroke Therapy Academic Industry Roundtable Preclinical Recommendations. Stroke. 2009a;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH. Update of the Stroke Therapy Academic Industry Roundtable Preclinical Recommendations. Stroke. 2009b;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G, Gallacher D, Shevde S, Loftus J, Swayne G.1993Anatomic variation of the middle cerebral artery in the Sprague-Dawley rat Stroke 242087–2092.discussion 92–3 [DOI] [PubMed] [Google Scholar]
- Fredriksson K, Kalimo H, Nordborg C, Johansson BB, Olsson Y. Nerve cell injury in the brain of stroke-prone spontaneously hypertensive rats. Acta Neuropathologica. 1988;76:227–237. doi: 10.1007/BF00687769. [DOI] [PubMed] [Google Scholar]
- Freret T, Bouet V, Toutain J, Saulnier R, Pro-Sistiaga P, Bihel E, Mackenzie ET, Roussel S, Schumann-Bard P, Touzani O. Intraluminal thread model of focal stroke in the non-human primate. J Cereb Blood Flow Metab. 2008;28:786–796. doi: 10.1038/sj.jcbfm.9600575. [DOI] [PubMed] [Google Scholar]
- Fujishima M, Onoyama K, Oniki H, Ogata J, Omae T. Effects of acute hypertension on brain metabolism in normotensive, renovascular hypertensive and spontaneously hypertensive rats. Stroke. 1978;9:349–353. doi: 10.1161/01.str.9.4.349. [DOI] [PubMed] [Google Scholar]
- Gao H, Liu Y, Lu S, Xiang B, Wang C. A reversible middle cerebral artery occlusion model using intraluminal balloon technique in monkeys. J Stroke Cerebrovasc Dis. 2006;15:202–208. doi: 10.1016/j.jstrokecerebrovasdis.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Gratton JA, Sauter A, Rudin M, Lees KR, McColl J, Reid JL, Dominiczak AF, Macrae IM. Susceptibility to cerebral infarction in the stroke-prone spontaneously hypertensive rat is inherited as a dominant trait. Stroke. 1998;29:690–694. doi: 10.1161/01.str.29.3.690. [DOI] [PubMed] [Google Scholar]
- Guerrini U, Sironi L, Tremoli E, Cimino M, Pollo B, Calvio AM, Paoletti R, Asdente M. New insights into brain damage in stroke-prone rats: a nuclear magnetic imaging study. Stroke. 2002;33:825–830. doi: 10.1161/hs0302.104111. [DOI] [PubMed] [Google Scholar]
- Hachinski VC, Wilson JX, Smith KE, Cechetto DF. Effect of age on autonomic and cardiac responses in a rat stroke model. Arch Neurol. 1992;49:690–696. doi: 10.1001/archneur.1992.00530310032009. [DOI] [PubMed] [Google Scholar]
- Hamberg LM, Hunter GJ, Maynard KI, Owen C, Morris PP, Putman CM, Ogilvy C, Gonzalez RG. Functional CT perfusion imaging in predicting the extent of cerebral infarction from a 3-hour middle cerebral arterial occlusion in a primate stroke model. AJNR. 2002;23:1013–1021. [PMC free article] [PubMed] [Google Scholar]
- Hamilton MG, Tranmer BI, Auer RN. Insulin reduction of cerebral infarction due to transient focal ischemia. J Neurosurg. 1995;82:262–268. doi: 10.3171/jns.1995.82.2.0262. [DOI] [PubMed] [Google Scholar]
- Harada H, Kelly PJ, Cole DJ, Drummond JC, Patel PM. Isoflurane reduces N-methyl-D-aspartate toxicity in vivo in the rat cerebral cortex. Anesth Analg. 1999;89:1442–1447. doi: 10.1097/00000539-199912000-00022. [DOI] [PubMed] [Google Scholar]
- Harada H, Wang Y, Mishima Y, Uehara N, Makaya T, Kano T. A novel method of detecting rCBF with laser-Doppler flowmetry without cranial window through the skull for a MCAO rat model. Brain Res Brain Res Protoc. 2005;14:165–170. doi: 10.1016/j.brainresprot.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Harris BD, Moody EJ, Basile AS, Skolnick P. Volatile anesthetics bidirectionally and stereospecifically modulate ligand binding to GABA receptors. Eur J Pharmacol. 1994;267:269–274. doi: 10.1016/0922-4106(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Haseldonckx M, van Bedaf D, van de Ven M, van Reempts J, Borgers M. Vasogenic oedema and brain infarction in an experimental penumbra model. Acta Neurochir Suppl. 2000;76:105–109. doi: 10.1007/978-3-7091-6346-7_22. [DOI] [PubMed] [Google Scholar]
- Haskins SC, Patz JD. Effect of inspired-air warming and humidification in the prevention of hypothermia during general anesthesia in cats. Am J Vet Res. 1980;41:1669–1673. [PubMed] [Google Scholar]
- Hata R, Mies G, Wiessner C, Fritze K, Hesselbarth D, Brinker G, Hossmann KA. A reproducible model of middle cerebral artery occlusion in mice: hemodynamic, biochemical, and magnetic resonance imaging. J Cereb Blood Flow Metab. 1998;18:367–375. doi: 10.1097/00004647-199804000-00004. [DOI] [PubMed] [Google Scholar]
- He Z, Yamawaki T, Yang S, Day AL, Simpkins JW, Naritomi H.1999Experimental model of small deep infarcts involving the hypothalamus in rats: changes in body temperature and postural reflex Stroke 302743–2751.discussion 51 [DOI] [PubMed] [Google Scholar]
- Hill NC, Millikan CH, Wakim KG, Sayre GP. Studies in cerebrovascular disease. VII. Experimental production of cerebral infarction by intracarotid injection of homologous blood clot; preliminary report. Proc Staff Meet. 1955;30:625–633. [PubMed] [Google Scholar]
- Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol. 2009;8:326–333. doi: 10.1016/S1474-4422(09)70047-X. [DOI] [PubMed] [Google Scholar]
- Hu G, Sarti C, Jousilahti P, Peltonen M, Qiao Q, Antikainen R, Tuomilehto J. The impact of history of hypertension and type 2 diabetes at baseline on the incidence of stroke and stroke mortality. Stroke. 2005;36:2538–2543. doi: 10.1161/01.STR.0000190894.30964.75. [DOI] [PubMed] [Google Scholar]
- Huang NC, Wei J, Quast MJ. A comparison of the early development of ischemic brain damage in normoglycemic and hyperglycemic rats using magnetic resonance imaging. Exp Brain Res Experimentelle Hirnforschung. 1996;109:33–42. doi: 10.1007/BF00228624. [DOI] [PubMed] [Google Scholar]
- Hudgins WR, Garcia JH. Transorbital approach to the middle cerebral artery of the squirrel monkey: a technique for experimental cerebral infarction applicable to ultrastructural studies. Stroke. 1970;1:107–111. doi: 10.1161/01.str.1.2.107. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Imai H, Konno K, Nakamura M, Shimizu T, Kubota C, Seki K, Honda F, Tomizawa S, Tanaka Y, Hata H, Saito N. A new model of focal cerebral ischemia in the miniature pig. J Neurosurg. 2006;104:123–132. doi: 10.3171/ped.2006.104.2.123. [DOI] [PubMed] [Google Scholar]
- Jahan R, Stewart D, Vinters HV, Yong W, Vinuela F, Vandeberg P, Marder VJ. Middle cerebral artery occlusion in the rabbit using selective angiography: application for assessment of thrombolysis. Stroke. 2008;39:1613–1615. doi: 10.1161/STROKEAHA.107.507376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo A, Gongora-Rivera F, Labreuche J, Hauw JJ, Amarenco P. Predictors for malignant middle cerebral artery infarctions: a postmortem analysis. Neurology. 2006;66:815–820. doi: 10.1212/01.wnl.0000203649.60211.0e. [DOI] [PubMed] [Google Scholar]
- Jeerakathil T, Johnson JA, Simpson SH, Majumdar SR. Short-term risk for stroke is doubled in persons with newly treated type 2 diabetes compared with persons without diabetes: a population-based cohort study. Stroke. 2007;38:1739–1743. doi: 10.1161/STROKEAHA.106.481390. [DOI] [PubMed] [Google Scholar]
- Jorgensen L, Torvik A. Ischaemic cerebrovascular diseases in an autopsy series. 2. Prevalence, location, pathogenesis, and clinical course of cerebral infarcts. J Neurol Sci. 1969;9:285–320. doi: 10.1016/0022-510x(69)90078-1. [DOI] [PubMed] [Google Scholar]
- Kaarisalo MM, Raiha I, Sivenius J, Immonen-Raiha P, Lehtonen A, Sarti C, Mahonen M, Torppa J, Tuomilehto J, Salomaa V. Diabetes worsens the outcome of acute ischemic stroke. Diabetes Res Clin Pract. 2005;69:293–298. doi: 10.1016/j.diabres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke. 2007;38:1044–1049. doi: 10.1161/01.STR.0000258041.75739.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor K, Singh B, Dewan LI. Variations in the configuration of the circle of Willis. Anat Sci Int. 2008;83:96–106. doi: 10.1111/j.1447-073X.2007.00216.x. [DOI] [PubMed] [Google Scholar]
- Kawai N, Keep RF, Betz AL, Nagao S. Hyperglycemia induces progressive changes in the cerebral microvasculature and blood-brain barrier transport during focal cerebral ischemia. Acta Neurochir Suppl. 1998;71:219–221. doi: 10.1007/978-3-7091-6475-4_63. [DOI] [PubMed] [Google Scholar]
- Kelly BM, Pangilinan PH, Jr, Rodriguez GM.2007The stroke rehabilitation paradigm Phys Med Rehabil Clin N Am 18631–650.v [DOI] [PubMed] [Google Scholar]
- Kidd GA, Dobrucki LW, Brovkovych V, Bohr DF, Malinski T. Nitric oxide deficiency contributes to large cerebral infarct size. Hypertension. 2000;35:1111–1118. doi: 10.1161/01.hyp.35.5.1111. [DOI] [PubMed] [Google Scholar]
- Kim SK, Cho KO, Kim SY. The plasticity of posterior communicating artery influences on the outcome of white matter injury induced by chronic cerebral hypoperfusion in rats. Neurol Res. 2008;31:245–250. doi: 10.1179/174313209X382278. [DOI] [PubMed] [Google Scholar]
- Kim Y, Busto R, Dietrich WD, Kraydieh S, Ginsberg MD.1996Delayed postischemic hyperthermia in awake rats worsens the histopathological outcome of transient focal cerebral ischemia Stroke 272274–2280.discussion 81 [DOI] [PubMed] [Google Scholar]
- Kirchhof K, Welzel T, Zoubaa S, Lichy C, Sikinger M, de Ruiz HL, Sartor K. New method of embolus preparation for standardized embolic stroke in rabbits. Stroke. 2002;33:2329–2333. doi: 10.1161/01.str.0000027436.82700.73. [DOI] [PubMed] [Google Scholar]
- Kissela BM, Khoury J, Kleindorfer D, Woo D, Schneider A, Alwell K, Miller R, Ewing I, Moomaw CJ, Szaflarski JP, Gebel J, Shukla R, Broderick JP. Epidemiology of ischemic stroke in patients with diabetes: the greater Cincinnati/Northern Kentucky Stroke Study. Diabetes Care. 2005;28:355–359. doi: 10.2337/diacare.28.2.355. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Yang G, Mabuchi T, Yagita Y, Hori M, Yanagihara T. Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: evaluation of the patency of the posterior communicating artery. J Cereb Blood Flow Metab. 1998;18:570–579. doi: 10.1097/00004647-199805000-00012. [DOI] [PubMed] [Google Scholar]
- Kittaka M, Wang L, Sun N, Schreiber SS, Seeds NW, Fisher M, Zlokovic BV. Brain capillary tissue plasminogen activator in a diabetes stroke model. Stroke. 1996;27:712–719. doi: 10.1161/01.str.27.4.712. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL. Brain and body temperature homeostasis during sodium pentobarbital anesthesia with and without body warming in rats. Physiol Behav. 2005;84:563–570. doi: 10.1016/j.physbeh.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Knox CA, Yates RD, Chen I, Klara PM. Effects of aging on the structural and permeability characteristics of cerebrovasculature in normotensive and hypertensive strains of rats. Acta Neuropathologica. 1980;51:1–13. doi: 10.1007/BF00688844. [DOI] [PubMed] [Google Scholar]
- Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema. 1. A new experimental model of experimental embolism in rats in which recirculation can be reintroduced in the ischemic area. Jpn J Stroke. 1986;8:1–8. [Google Scholar]
- Kong LQ, Xie JX, Han HB, Liu HD. Improvements in the intraluminal thread technique to induce focal cerebral ischaemia in rabbits. J Neurosci Methods. 2004;137:315–319. doi: 10.1016/j.jneumeth.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Tsujikawa K, Matsuda T, Baba A. Endothelin-1 stimulates glial cell line-derived neurotrophic factor expression in cultured rat astrocytes. Biochem Biophys Res Commun. 2003;303:1101–1105. doi: 10.1016/s0006-291x(03)00491-1. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Baba A, Matsuda T. Intracerebroventricular administration of an endothelin ETB receptor agonist increases expression of tissue inhibitor of matrix metalloproteinase-1 and -3 in rat brain. Neuroscience. 2007;147:620–630. doi: 10.1016/j.neuroscience.2007.04.047. [DOI] [PubMed] [Google Scholar]
- Kraft SA, Larson CP, Jr, Shuer LM, Steinberg GK, Benson GV, Pearl RG. Effect of hyperglycemia on neuronal changes in a rabbit model of focal cerebral ischemia. Stroke. 1990;21:447–450. doi: 10.1161/01.str.21.3.447. [DOI] [PubMed] [Google Scholar]
- Kudo M, Aoyama A, Ichimori S, Fukunaga N. An animal model of cerebral infarction. Homologous blood clot emboli in rats. Stroke. 1982;13:505–508. doi: 10.1161/01.str.13.4.505. [DOI] [PubMed] [Google Scholar]
- Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke. 2004;35:1024. [PubMed] [Google Scholar]
- Lee JM, Zhai G, Liu Q, Gonzales ER, Yin K, Yan P, Hsu CY, Vo KD, Lin W. Vascular permeability precedes spontaneous intracerebral hemorrhage in stroke-prone spontaneously hypertensive rats. Stroke. 2007;38:3289–3291. doi: 10.1161/STROKEAHA.107.491621. [DOI] [PubMed] [Google Scholar]
- Lerman LO, Chade AR, Sica V, Napoli C. Animal models of hypertension: an overview. J Lab Clin Med. 2005;146:160–173. doi: 10.1016/j.lab.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Li F, Omae T, Fisher M.1999Spontaneous hyperthermia and its mechanism in the intraluminal suture middle cerebral artery occlusion model of rats Stroke 302464–2470.discussion 70–1 [DOI] [PubMed] [Google Scholar]
- Lisabeth LD, Beiser AS, Brown DL, Murabito JM, Kelly-Hayes M, Wolf PA. Age at natural menopause and risk of ischemic stroke: the Framingham heart study. Stroke. 2009;40:1044–1049. doi: 10.1161/STROKEAHA.108.542993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wang Z, Wang X, Song L, Chen H, Bemeur C, Ste-Marie L, Montgomery J. Comparison of two rat models of cerebral ischemia under hyperglycemic conditions. Microsurgery. 2007;27:258–262. doi: 10.1002/micr.20351. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu T, McCarron RM, Spatz M, Feuerstein G, Hallenbeck JM, Siren AL. Evidence for activation of endothelium and monocytes in hypertensive rats. Am J Physiol. 1996;270:H2125–H2131. doi: 10.1152/ajpheart.1996.270.6.H2125. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Lourbopoulos A, Karacostas D, Artemis N, Milonas I, Grigoriadis N. Effectiveness of a new modified intraluminal suture for temporary middle cerebral artery occlusion in rats of various weight. J Neurosci Methods. 2008;173:225–234. doi: 10.1016/j.jneumeth.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Macleod MR, O'Collins T, Howells DW, Donnan GA. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004;35:1203–1208. doi: 10.1161/01.STR.0000125719.25853.20. [DOI] [PubMed] [Google Scholar]
- Macleod MR, O′Collins T, Horky LL, Howells DW, Donnan GA. Systematic review and meta-analysis of the efficacy of melatonin in experimental stroke. J Pineal Res. 2005a;38:35–41. doi: 10.1111/j.1600-079X.2004.00172.x. [DOI] [PubMed] [Google Scholar]
- Macleod MR, O'Collins T, Horky LL, Howells DW, Donnan GA. Systematic review and metaanalysis of the efficacy of FK506 in experimental stroke. J Cereb Blood Flow Metab. 2005b;25:713–721. doi: 10.1038/sj.jcbfm.9600064. [DOI] [PubMed] [Google Scholar]
- Macleod MR, van der Worp HB, Sena ES, Howells DW, Dirnagl U, Donnan GA. Evidence for the efficacy of NXY-059 in experimental focal cerebral ischaemia is confounded by study quality. Stroke. 2008;39:2824–2829. doi: 10.1161/STROKEAHA.108.515957. [DOI] [PubMed] [Google Scholar]
- Macleod MR, Fisher M, O′Collins V, Sena ES, Dirnagl U, Bath PM, Buchan A, van der Worp HB, Traystman R, Minematsu K, Donnan GA, Howells DW. Good laboratory practice: preventing introduction of bias at the bench. Stroke. 2009;40:e50–e52. doi: 10.1161/STROKEAHA.108.525386. [DOI] [PubMed] [Google Scholar]
- Mangiarua EI, Lee RM. Morphometric study of cerebral arteries from spontaneously hypertensive and stroke-prone spontaneously hypertensive rats. J Hypertens. 1992;10:1183–1190. doi: 10.1097/00004872-199210000-00011. [DOI] [PubMed] [Google Scholar]
- Marder VJ, Chute DJ, Starkman S, Abolian AM, Kidwell C, Liebeskind D, Ovbiagele B, Vinuela F, Duckwiler G, Jahan R, Vespa PM, Selco S, Rajajee V, Kim D, Sanossian N, Saver JL. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke. 2006;37:2086–2093. doi: 10.1161/01.STR.0000230307.03438.94. [DOI] [PubMed] [Google Scholar]
- Markgraf CG, Kraydieh S, Prado R, Watson BD, Dietrich WD, Ginsberg MD.1993Comparative histopathologic consequences of photothrombotic occlusion of the distal middle cerebral artery in Sprague-Dawley and Wistar rats Stroke 24286–292.discussion 92–3 [DOI] [PubMed] [Google Scholar]
- Martin A, Rojas S, Chamorro A, Falcon C, Bargallo N, Planas AM. Why does acute hyperglycemia worsen the outcome of transient focal cerebral ischemia? Role of corticosteroids, inflammation, and protein O-glycosylation. Stroke. 2006;37:1288–1295. doi: 10.1161/01.STR.0000217389.55009.f8. [DOI] [PubMed] [Google Scholar]
- McColl BW, Carswell HV, McCulloch J, Horsburgh K. Extension of cerebral hypoperfusion and ischaemic pathology beyond MCA territory after intraluminal filament occlusion in C57Bl/6J mice. Brain Res. 2004;997:15–23. doi: 10.1016/j.brainres.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Meden P, Andersen M, Overgaard K, Rasmussen RS, Boysen G. The effects of early insulin treatment combined with thrombolysis in rat embolic stroke. Neurol Res. 2002;24:399–404. doi: 10.1179/016164102101200096. [DOI] [PubMed] [Google Scholar]
- Memezawa H, Zhao Q, Smith ML, Siesjo BK. Hyperthermia nullifies the ameliorating effect of dizocilpine maleate (MK-801) in focal cerebral ischemia. Brain Res. 1995;670:48–52. doi: 10.1016/0006-8993(94)01251-c. [DOI] [PubMed] [Google Scholar]
- Meneely GR, Ball CO. Experimental epidemiology of chronic sodium chloride toxicity and the protective effect of potassium chloride. Am J Med. 1958;25:713–725. doi: 10.1016/0002-9343(58)90009-3. [DOI] [PubMed] [Google Scholar]
- Miller AA, Drummond GR, Mast AE, Schmidt HH, Sobey CG. Effect of gender on NADPH-oxidase activity, expression, and function in the cerebral circulation: role of estrogen. Stroke. 2007;38:2142–2149. doi: 10.1161/STROKEAHA.106.477406. [DOI] [PubMed] [Google Scholar]
- Morand EF, Leech M. Hypothalamic-pituitary-adrenal axis regulation of inflammation in rheumatoid arthritis. Immunol Cell Biol. 2001;79:395–399. doi: 10.1046/j.1440-1711.2001.01028.x. [DOI] [PubMed] [Google Scholar]
- Nag S. Cerebral changes in chronic hypertension: combined permeability and immunohistochemical studies. Acta Neuropathologica. 1984;62:178–184. doi: 10.1007/BF00691850. [DOI] [PubMed] [Google Scholar]
- Niessen F, Hilger T, Hoehn M, Hossmann KA. Differences in clot preparation determine outcome of recombinant tissue plasminogen activator treatment in experimental thromboembolic stroke. Stroke. 2003;34:2019–2024. doi: 10.1161/01.STR.0000080941.73934.30. [DOI] [PubMed] [Google Scholar]
- Nito C, Kamiya T, Ueda M, Arii T, Katayama Y. Mild hypothermia enhances the neuroprotective effects of FK506 and expands its therapeutic window following transient focal ischemia in rats. Brain Res. 2004;1008:179–185. doi: 10.1016/j.brainres.2004.02.031. [DOI] [PubMed] [Google Scholar]
- Nurse S, Corbett D. Neuroprotection after several days of mild, drug-induced hypothermia. J Cereb Blood Flow Metab. 1996;16:474–480. doi: 10.1097/00004647-199605000-00014. [DOI] [PubMed] [Google Scholar]
- O'Brien MD, Waltz AG. Transorbital approach for occluding the middle cerebral artery without craniectomy. Stroke. 1973;4:201–206. doi: 10.1161/01.str.4.2.201. [DOI] [PubMed] [Google Scholar]
- O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- Ogata J, Fujishima M, Morotomi Y, Omae T. Cerebral infarction following bilateral carotid artery ligation in normotensive and spontaneously hypertensive rats: a pathological study. Stroke. 1976;7:54–60. doi: 10.1161/01.str.7.1.54. [DOI] [PubMed] [Google Scholar]
- Oliff HS, Weber E, Eilon G, Marek P. The role of strain/vendor differences on the outcome of focal ischemia induced by intraluminal middle cerebral artery occlusion in the rat. Brain Res. 1995;675:20–26. doi: 10.1016/0006-8993(95)00033-m. [DOI] [PubMed] [Google Scholar]
- Olsen TS, Skriver EB, Herning M. Cause of cerebral infarction in the carotid territory. Its relation to the size and the location of the infarct and to the underlying vascular lesion. Stroke. 1985;16:459–466. doi: 10.1161/01.str.16.3.459. [DOI] [PubMed] [Google Scholar]
- Oostveen JA, Timby K, Williams LR.1992Prediction of cerebral ischemia by ophthalmoscopy after carotid occlusion in gerbils Stroke 231588–1593.discussion 94 [DOI] [PubMed] [Google Scholar]
- Orset C, Macrez R, Young AR, Panthou D, Angles-Cano E, Maubert E, Agin V, Vivien D. Mouse model of in situ thromboembolic stroke and reperfusion. Stroke. 2007;38:2771–2778. doi: 10.1161/STROKEAHA.107.487520. [DOI] [PubMed] [Google Scholar]
- Palmon SC, Sieber FE, Brown PR, Koehler RC, Eleff SM, Traystman RJ. Poor hemodynamic and metabolic recovery after global incomplete cerebral ischemia associated with short-term diabetes in dogs. J Cereb Blood Flow Metab. 1995;15:673–680. doi: 10.1038/jcbfm.1995.83. [DOI] [PubMed] [Google Scholar]
- Panes J, Kurose I, Rodriguez-Vaca D, Anderson DC, Miyasaka M, Tso P, Granger DN. Diabetes exacerbates inflammatory responses to ischemia-reperfusion. Circulation. 1996;93:161–167. doi: 10.1161/01.cir.93.1.161. [DOI] [PubMed] [Google Scholar]
- Patel PM, Drummond JC, Cole DJ, Kelly PJ, Watson M. Isoflurane and pentobarbital reduce the frequency of transient ischemic depolarizations during focal ischemia in rats. Anesth Analg. 1998;86:773–780. doi: 10.1097/00000539-199804000-00018. [DOI] [PubMed] [Google Scholar]
- Payne GW, Smeda JS. Cerebrovascular alterations in pressure and protein kinase C-mediated constriction in Dahl salt-sensitive rats. J Hypertens. 2002;20:1355–1363. doi: 10.1097/00004872-200207000-00022. [DOI] [PubMed] [Google Scholar]
- Perel P, Roberts I, Sena E, Wheble P, Briscoe C, Sandercock P, Macleod M, Mignini LE, Jayaram P, Khan KS. Comparison of treatment effects between animal experiments and clinical trials: systematic review. BMJ. 2007;334:197. doi: 10.1136/bmj.39048.407928.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan EL. The New Zealand strain of rats with genetic hypertension. NZ Med J. 1968;67:334–344. [PubMed] [Google Scholar]
- Prieto R, Carceller F, Roda JM, Avendano C. The intraluminal thread model revisited: rat strain differences in local cerebral blood flow. Neurol Res. 2005;27:47–52. doi: 10.1179/016164105X18214. [DOI] [PubMed] [Google Scholar]
- Prusty S, Kemper T, Moss MB, Hollander W. Occurrence of stroke in a nonhuman primate model of cerebrovascular disease. Stroke. 1988;19:84–90. doi: 10.1161/01.str.19.1.84. [DOI] [PubMed] [Google Scholar]
- Rapp JP, Dene H. Development and characteristics of inbred strains of Dahl salt-sensitive and salt-resistant rats. Hypertension. 1985;7:340–349. [PubMed] [Google Scholar]
- Rees DA, Alcolado JC. Animal models of diabetes mellitus. Diabet Med. 2005;22:359–370. doi: 10.1111/j.1464-5491.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reglodi D, Somogyvari-Vigh A, Maderdrut JL, Vigh S, Arimura A. Postischemic spontaneous hyperthermia and its effects in middle cerebral artery occlusion in the rat. Exp Neurol. 2000;163:399–407. doi: 10.1006/exnr.2000.7367. [DOI] [PubMed] [Google Scholar]
- Rewell SS, Fernandez JA, Cox SF, Spratt NJ, Hogan L, Aleksoska E, van Raay L, Liberatore GT, Batchelor PE, Howells DW. Inducing stroke in aged, hypertensive, diabetic rats. J Cereb Blood Flow Metab. 2010;30:729–733. doi: 10.1038/jcbfm.2009.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribo M, Molina CA, Delgado P, Rubiera M, Delgado-Mederos R, Rovira A, Munuera J, Alvarez-Sabin J. Hyperglycemia during ischemia rapidly accelerates brain damage in stroke patients treated with tPA. J Cereb Blood Flow Metab. 2007;27:1616–1622. doi: 10.1038/sj.jcbfm.9600460. [DOI] [PubMed] [Google Scholar]
- Rink C, Christoforidis G, Abduljalil A, Kontzialis M, Bergdall V, Roy S, Khanna S, Slivka A, Knopp M, Sen CK. Minimally invasive neuroradiologic model of preclinical transient middle cerebral artery occlusion in canines. Proc Natl Acad Sci USA. 2008;105:14100–14105. doi: 10.1073/pnas.0806678105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoh M, Ostergaard L, Rohl L, Smith DF, Simonsen CZ, Sorensen JC, Poulsen PV, Gyldensted C, Sakaki S, Gjedde A. Relationship between residual cerebral blood flow and oxygen metabolism as predictive of ischemic tissue viability: sequential multitracer positron emission tomography scanning of middle cerebral artery occlusion during the critical first 6 hours after stroke in pigs. J Neurosurg. 2000;93:647–657. doi: 10.3171/jns.2000.93.4.0647. [DOI] [PubMed] [Google Scholar]
- Schmid-Elsaesser R, Zausinger S, Hungerhuber E, Baethmann A, Reulen HJ. A critical reevaluation of the intraluminal thread model of focal cerebral ischemia: evidence of inadvertent premature reperfusion and subarachnoid hemorrhage in rats by laser-Doppler flowmetry. Stroke. 1998;29:2162–2170. doi: 10.1161/01.str.29.10.2162. [DOI] [PubMed] [Google Scholar]
- Seidel SD, Hung SC, Lynn Kan H, Bhaskar Gollapudi B. Background gene expression in rat kidney: influence of strain, gender, and diet. Toxicol Sci. 2006;94:226–233. doi: 10.1093/toxsci/kfl082. [DOI] [PubMed] [Google Scholar]
- Shapira S, Sapir M, Wengier A, Grauer E, Kadar T. Aging has a complex effect on a rat model of ischemic stroke. Brain Res. 2002;925:148–158. doi: 10.1016/s0006-8993(01)03270-x. [DOI] [PubMed] [Google Scholar]
- Shin HK, Nishimura M, Jones PB, Ay H, Boas DA, Moskowitz MA, Ayata C. Mild induced hypertension improves blood flow and oxygen metabolism in transient focal cerebral ischemia. Stroke. 2008;39:1548–1555. doi: 10.1161/STROKEAHA.107.499483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard KM, Henninger N, Fisher M, Duong TQ, Ferris CF. Differential recovery of multimodal MRI and behavior after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2006;26:1451–1462. doi: 10.1038/sj.jcbfm.9600299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt NJ, Fernandez J, Chen M, Rewell S, Cox S, van Raay L, Hogan L, Howells DW. Modification of the method of thread manufacture improves stroke induction rate and reduces mortality after thread-occlusion of the middle cerebral artery in young or aged rats. J Neurosci Methods. 2006;155:285–290. doi: 10.1016/j.jneumeth.2006.01.020. [DOI] [PubMed] [Google Scholar]
- STAIR Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Stoll G, Kleinschnitz C, Meuth SG, Braeuninger S, Ip CW, Wessig C, Nolte I, Bendszus M. Transient widespread blood-brain barrier alterations after cerebral photothrombosis as revealed by gadofluorine M-enhanced magnetic resonance imaging. J Cereb Blood Flow Metab. 2009;29:331–341. doi: 10.1038/jcbfm.2008.129. [DOI] [PubMed] [Google Scholar]
- Strom JO, Theodorsson A, Theodorsson E. Dose-related neuroprotective versus neurodamaging effects of estrogens in rat cerebral ischemia: a systematic analysis. J Cereb Blood Flow Metab. 2009;29:1359–1372. doi: 10.1038/jcbfm.2009.66. [DOI] [PubMed] [Google Scholar]
- Sugimori H, Yao H, Ooboshi H, Ibayashi S, Iida M. Krypton laser-induced photothrombotic distal middle cerebral artery occlusion without craniectomy in mice. Brain Res Brain Res Protoc. 2004;13:189–196. doi: 10.1016/j.brainresprot.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Sukamoto T, Shiono K, Watanabe TX, Sokabe H. Effects of beta-adrenergic blocking drugs in hypertensive rats. J Pharmacobiodynamics. 1980;3:1–10. doi: 10.1248/bpb1978.3.1. [DOI] [PubMed] [Google Scholar]
- Sundquist K, Li X. Type 1 diabetes as a risk factor for stroke in men and women aged 15-49: a nationwide study from Sweden. Diabet Med. 2006;23:1261–1267. doi: 10.1111/j.1464-5491.2006.01959.x. [DOI] [PubMed] [Google Scholar]
- Tajima A, Hans FJ, Livingstone D, Wei L, Finnegan W, DeMaro J, Fenstermacher J. Smaller local brain volumes and cerebral atrophy in spontaneously hypertensive rats. Hypertension. 1993;21:105–111. doi: 10.1161/01.hyp.21.1.105. [DOI] [PubMed] [Google Scholar]
- Takaya K, Ogawa Y, Isse N, Okazaki T, Satoh N, Masuzaki H, Mori K, Tamura N, Hosoda K, Nakao K. Molecular cloning of rat leptin receptor isoform complementary DNAs—identification of a missense mutation in Zucker fatty (fa/fa) rats. Biochem Biophys Res Commun. 1996;225:75–83. doi: 10.1006/bbrc.1996.1133. [DOI] [PubMed] [Google Scholar]
- Tamura A, Asano T, Sano K, Tsumagari T, Nakajima A. Protection from cerebral ischemia by a new imidazole derivative (Y-9179) and pentobarbital. A comparative study in chronic middle cerebral artery occlusion in cats. Stroke. 1979;10:126–134. doi: 10.1161/01.str.10.2.126. [DOI] [PubMed] [Google Scholar]
- Tamura A, Graham DI, McCulloch J, Teasdale GM. Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1981;1:53–60. doi: 10.1038/jcbfm.1981.6. [DOI] [PubMed] [Google Scholar]
- Toomey JR, Valocik RE, Koster PF, Gabriel MA, McVey M, Hart TK, Ohlstein EH, Parsons AA, Barone FC. Inhibition of factor IX(a) is protective in a rat model of thromboembolic stroke. Stroke. 2002;33:578–585. doi: 10.1161/hs0202.102950. [DOI] [PubMed] [Google Scholar]
- Toung TK, Hurn PD, Traystman RJ, Sieber FE. Estrogen decreases infarct size after temporary focal ischemia in a genetic model of type 1 diabetes mellitus. Stroke. 2000;31:2701–2706. doi: 10.1161/01.str.31.11.2701. [DOI] [PubMed] [Google Scholar]
- Traystman RJ.2010Effect of anesthesia in stroke models Rodent Models of Stroke(Dirnagl U, ed), vol. 47. New York: Humana Press; (in press) [Google Scholar]
- van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain. 2007;130:3063–3074. doi: 10.1093/brain/awm083. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Willing LB, Goto S, Alkayed NJ, Brucklacher RM, Wood TL, Towfighi J, Hurn PD, Simpson IA. Experimental stroke in the female diabetic, db/db, mouse. J Cereb Blood Flow Metab. 2001;21:52–60. doi: 10.1097/00004647-200101000-00007. [DOI] [PubMed] [Google Scholar]
- Vinall PE, Kramer MS, Heinel LA, Rosenwasser RH. Temporal changes in sensitivity of rats to cerebral ischemic insult. J Neurosurg. 2000;93:82–89. doi: 10.3171/jns.2000.93.1.0082. [DOI] [PubMed] [Google Scholar]
- Vincent M, Sacquet J, Sassard J.1984The Lyon strains of hypertensive, normotensive and low-blood-pressure rats Handbook of Hypertension: Experimental and Genetic Models of Hypertension(de Jong W, ed), vol. 4Amsterdam: Elsevier Science Publishers; 314–327. [Google Scholar]
- Visser SA, Pozarek S, Martinsson S, Forsberg T, Ross SB, Gabrielsson J. Rapid and long-lasting tolerance to clomethiazole-induced hypothermia in the rat. Eur J Pharmacol. 2005;512:139–151. doi: 10.1016/j.ejphar.2005.02.036. [DOI] [PubMed] [Google Scholar]
- Waaijer A, van Leeuwen MS, van der Worp HB, Verhagen HJ, Mali WP, Velthuis BK. Anatomic variations in the circle of Willis in patients with symptomatic carotid artery stenosis assessed with multidetector row CT angiography. Cerebrovasc Dis (Basel, Switzerland) 2007;23:267–274. doi: 10.1159/000098326. [DOI] [PubMed] [Google Scholar]
- Wagner J, Klotz S, Haufe CC, Danser JA, Amann K, Ganten D, Ritz E. Progression of renal failure after subtotal nephrectomy in transgenic rats carrying an additional renin gene [TGR(mREN2)27] J Hypertens. 1997;15:441–449. doi: 10.1097/00004872-199715040-00015. [DOI] [PubMed] [Google Scholar]
- Wang-Fischer Y.(ed) (2008Manual of Stroke Models in Rats Boca Raton: CRC Press: [Google Scholar]
- Wang CX, Yang T, Shuaib A. An improved version of embolic model of brain ischemic injury in the rat. J Neurosci Methods. 2001;109:147–151. doi: 10.1016/s0165-0270(01)00408-3. [DOI] [PubMed] [Google Scholar]
- Warner DS, Hansen TD, Vust L, Todd MM. Distribution of cerebral blood flow during deep isoflurane vs. pentobarbital anesthesia in rats with middle cerebral artery occlusion. J Neurosurg Anesthesiol. 1989;1:219–226. doi: 10.1097/00008506-198909000-00003. [DOI] [PubMed] [Google Scholar]
- Warner DS, McFarlane C, Todd MM, Ludwig P, McAllister AM. Sevoflurane and halothane reduce focal ischemic brain damage in the rat. Possible influence on thermoregulation. Anesthesiology. 1993;79:985–992. doi: 10.1097/00000542-199311000-00017. [DOI] [PubMed] [Google Scholar]
- Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol. 1985;17:497–504. doi: 10.1002/ana.410170513. [DOI] [PubMed] [Google Scholar]
- Wells BA, Keats AS, Cooley DA. Increased tolerance to cerebral ischemia produced by general anesthesia during temporary carotid occlusion. Surgery. 1963;54:216–223. [PubMed] [Google Scholar]
- Williams LS, Rotich J, Qi R, Fineberg N, Espay A, Bruno A, Fineberg SE, Tierney WR. Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke. Neurology. 2002;59:67–71. doi: 10.1212/wnl.59.1.67. [DOI] [PubMed] [Google Scholar]
- Willing AE. Experimental models: help or hindrance. Stroke. 2009;40:S152–S154. doi: 10.1161/STROKEAHA.108.533505. [DOI] [PubMed] [Google Scholar]
- Wilson JX, Gelb AW. Free radicals, antioxidants, and neurologic injury: possible relationship to cerebral protection by anesthetics. J Neurosurg Anesthesiol. 2002;14:66–79. doi: 10.1097/00008506-200201000-00014. [DOI] [PubMed] [Google Scholar]
- Woitzik J, Schilling L. Control of completeness and immediate detection of bleeding by a single laser-Doppler flow probe during intravascular middle cerebral artery occlusion in rats. J Neurosci Methods. 2002;122:75–78. doi: 10.1016/s0165-0270(02)00277-7. [DOI] [PubMed] [Google Scholar]
- Won SJ, Xie L, Kim SH, Tang H, Wang Y, Mao X, Banwait S, Jin K. Influence of age on the response to fibroblast growth factor-2 treatment in a rat model of stroke. Brain Res. 2006;1123:237–244. doi: 10.1016/j.brainres.2006.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamori Y, Horie R, Handa H, Sato M, Fukase M. Pathogenetic similarity of strokes in stroke-prone spontaneously hypertensive rats and humans. Stroke. 1976;7:46–53. doi: 10.1161/01.str.7.1.46. [DOI] [PubMed] [Google Scholar]
- Zausinger S, Baethmann A, Schmid-Elsaesser R. Anesthetic methods in rats determine outcome after experimental focal cerebral ischemia: mechanical ventilation is required to obtain controlled experimental conditions. Brain Res Brain Res Protoc. 2002;9:112–121. doi: 10.1016/s1385-299x(02)00138-1. [DOI] [PubMed] [Google Scholar]
- Zeng J, Zhang Y, Mo J, Su Z, Huang R.1998Two-kidney, two clip renovascular hypertensive rats can be used as stroke-prone rats Stroke 291708–1713.discussion 13–4 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang RL, Jiang Q, Raman SB, Cantwell L, Chopp M. A new rat model of thrombotic focal cerebral ischemia. J Cereb Blood Flow Metab. 1997;17:123–135. doi: 10.1097/00004647-199702000-00001. [DOI] [PubMed] [Google Scholar]
- Zhen G, Dore S. Optimized protocol to reduce variable outcomes for the bilateral common carotid artery occlusion model in mice. J Neurosci Methods. 2007;166:73–80. doi: 10.1016/j.jneumeth.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Fan X, Yu Z, Liu J, Murata Y, Lu J, Zhao S, Hajjar KA, Lo EH, Wang X. Annexin A2 combined with low-dose tPA improves thrombolytic therapy in a rat model of focal embolic stroke. J Cereb Blood Flow Metab. 2010;30:1137–1146. doi: 10.1038/jcbfm.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Ackerman JJ, Yablonskiy DA. Body and brain temperature coupling: the critical role of cerebral blood flow. J Comp Physiol B. 2009;179:701–710. doi: 10.1007/s00360-009-0352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivin JA, Fisher M, DeGirolami U, Hemenway CC, Stashak JA. Tissue plasminogen activator reduces neurological damage after cerebral embolism. Science. 1985;230:1289–1292. doi: 10.1126/science.3934754. [DOI] [PubMed] [Google Scholar]