Abstract
Obesity and type 2 diabetes have reached epidemic proportions; however, scarce information about how these metabolic syndromes influence brain energy and neurotransmitter homeostasis exist. The objective of this study was to elucidate how brain glycogen and neurotransmitter homeostasis are affected by these conditions. [1-13C]glucose was administered to Zucker obese (ZO) and Zucker diabetic fatty (ZDF) rats. Sprague–Dawley (SprD), Zucker lean (ZL), and ZDF lean rats were used as controls. Several brain regions were analyzed for glycogen levels along with 13C-labeling and content of glutamate, glutamine, GABA, aspartate, and alanine. Blood glucose concentrations and 13C enrichment were determined. 13C-labeling in glutamate was lower in ZO and ZDF rats in comparison with the controls. The molecular carbon labeling (MCL) ratio between alanine and glutamate was higher in the ZDF rats. The MCL ratios of glutamine and glutamate were decreased in the cerebellum of the ZO and the ZDF rats. Glycogen levels were also lower in this region. These results suggest that the obese and type 2 diabetic models were associated with lower brain glucose metabolism. Glucose metabolism through the TCA cycle was more decreased than glycolytic activity. Furthermore, reduced glutamate–glutamine cycling was also observed in the obese and type 2 diabetic states.
Keywords: brain metabolism, glutamate–glutamine cycle, glycogen, obesity, type 2 diabetes
Introduction
Most diabetics suffer from type 2 diabetes, which is a multifaceted metabolic disorder characterized by defects in insulin secretion, insulin action, or both (ADA, 2009). The resulting chronic hyperglycemia is associated with long-term dysfunction of several tissues such as the kidneys, eyes, and peripheral nerves (ADA, 2009). Overweight and physical inactivity are key risk factors for the development of type 2 diabetes, and today both obesity and type 2 diabetes have reached global epidemic proportions (WHO, 2009). Besides the undesirable pathologies in peripheral organs, diabetes is also associated with an increased risk of cognitive impairments and dementia (Biessels and Gispen, 2005). Several studies indicate that such devastating outcome on brain function may be associated with an impaired neurotransmitter homeostasis (Martinez-Tellez et al, 2005; Kamal et al, 2006; Galanopoulos et al, 1988). To date, effects of diabetes on neurotransmission have predominantly been observed in type 1 diabetic models, and accordingly there is a need to study this in the type 2 diabetic state. The most abundant neurotransmitters are glutamate and GABA. Glutamatergic and GABAergic neurotransmission is terminated by uptake which in the case of glutamate occurs predominantly into astrocytes (Danbolt, 2001), whereas GABA is mainly cleared into GABAergic neurons (Schousboe et al, 2004). The selective location of several enzymes pertinent to these processes in the astrocytic compartment (Norenberg and Martinez-Hernandez, 1979; Yu et al, 1983; Waagepetersen et al, 2001) necessitates a close metabolic interaction between neurons and astrocytes. Hence, neurons are unable to perform a net synthesis of glutamate from glucose. Instead, glutamine released from astrocytes that are capable of such synthesis serves as its precursor, and this process constitutes the so-called glutamate–glutamine cycle (Berl and Clarke, 1983; Hertz et al, 1999). In the type 1 diabetic state, it has been suggested that it is the astrocytes rather than the neurons that are particularly metabolically compromised affecting among other processes the glutamate–glutamine cycling (Garcia-Espinosa et al, 2003).
A small glucose depot in the form of glycogen exists in the brain (Kong et al, 2002; Cruz and Dienel, 2002), and recently it has been shown that glycogen may be essential for proper glutamatergic neurotransmission (Sickmann et al, 2009). Glycogen is selectively located in astrocytes (Cataldo and Broadwell, 1986), and in contrast to brain glucose levels, glycogen metabolism can be affected by changes in blood glucose concentrations (Choi et al, 2003; Oz et al, 2009; Seaquist et al, 2005). Accordingly, we wanted to establish whether glycogen metabolism may be affected by type 2 diabetes.
Limited knowledge regarding the effects of type 2 diabetes on brain energy and neurotransmitter homeostasis exists, and the aim of this study was to shed light on these aspects. Also, as type 2 diabetes is most often associated with obesity, we have explored brain metabolism in an obese animal model as well, representing the prediabetic state. Zucker obese (ZO) and Zucker diabetic fatty (ZDF) rats were used as animal models of obesity and type 2 diabetes. Sprague–Dawley (SprD), Zucker lean (ZL), and ZDF lean rats were used as control models. The neurotransmitters glutamate and GABA as well as glycogen and metabolites related to glycolysis (alanine) and tricarboxylic acid (TCA) cycle (aspartate) were in focus in this study.
Materials and methods
Animals
Male SprD rats (390±2 g, 14 weeks old) were purchased from Taconic (Ry, Denmark) and ZL (259±5 g, 10 weeks old), ZO (366±3 g, 10 weeks old), ZDF lean (329±5 g, 14 weeks old), and ZDF (359±7 g, 14 weeks old) rats were obtained from Charles River (Portage, MI, USA) (numbers in parentheses indicate the average weight±s.e.m. and age of the animals on the day of the experiment). The animals were housed group wise in a climate-controlled room under a 12:12 hours light/dark cycle, with ad libitum access to water and feed (Altromin 1324 (Brogaarden, Denmark) for the SprD, ZL, and ZO rats, and Purina 5008 (SDS, London, UK) for the ZDF lean and ZDF rats). The ZL and ZDF lean animals used in this study were genetically confirmed to be a purely heterozygous population. Glycosylated hemoglobin A1c for the ZDF rats was measured to be 7.8%±0.6% (average±s.e.m.), confirming that they were diabetic. Animal procedures were performed according to the Danish principles on Laboratory animal care, and approved by the Animal Experiments Inspectorate, Ministry of Justice, Denmark.
In Vivo Procedure
Animals were fasted overnight. At the beginning of the experiment, a 10-μL blood sample was taken from the tail tip for determination of blood glucose levels. [1-13C]glucose was administered i.p. (540 mg/kg; Sigma-Aldrich, St Louis, MO, USA), and after 28 minutes, three blood samples were taken from the tail tip, 10 μL for analysis of blood glucose levels and 2 × 80 μL for analysis of plasma glucose enrichment. At 30 minutes after the [1-13C]glucose injection, the animals were killed using microwave fixation (2.4 seconds, 4 kW, Model GA5013, Gerling Applied Engineering, Modesto, CA, USA). Cerebral cortex, hippocampus, and cerebellum were excised, freeze clamped in liquid nitrogen, and stored at −80 °C until extraction.
Analysis of Blood Glucose Concentrations and [1-13C]Glucose Enrichment in Plasma
Blood glucose levels were analyzed using a Biosen analyzer (BIOSEN S line, EKF Diagnostic, Barleben, Germany). For glucose enrichment analysis, the blood samples were centrifuged at 8,000 g for 6 to 8 minutes, and plasma was transferred to a microtiter plate and stored at −80°C until further handling. Plasma was relocated to an Eppendorf tube and 200 μL 80% (v/v) ice-cold ethanol was added. The sample was centrifuged and the supernatant was frozen and lyophilized. Samples were reconstituted in PBS (137 mmol/L NaCl, 2.7 mmol/L KCl, 7.3 mmol/L Na2HPO4, 0.9 mmol/L CaCl2, 0.5 mmol/L MgCl2, pH=7.4) in which H2O had been exchanged with D2O (99.994% Sigma-Aldrich). [1-13C]glucose enrichment in plasma was determined using quantitative 1D 1H nuclear magnetic resonance spectroscopy (Mo et al, 2009). Spectra were acquired using a Bruker Avance III 700 MHz spectrometer (Bruker Biospin GmbH, Rheinstetten, Germany) equipped with both a TCI (HCN; hydrogen, carbon and nitrogen) triple resonance Cryoprobe (Bruker Biospin AG, Fällanden, Switzerland) with z axis pulsed field gradients, and a SampleJet cooled sample changer. All spectra were acquired on samples in 3 mm SampleJet tubes (Bruker Biospin AG, Fällanden, Switzerland) using Bruker's IconNMR (Bruker Biospin GmbH, Rheinstetten, Germany) automation software and in-house macros. The resulting spectra were analyzed using spectral intensity matching using in-house macros and Bruker's TopSpin and AMIX software (Bruker Biospin GmbH, Rheinstetten, Germany).
Tissue Extraction
Cerebral cortex, hippocampus, and cerebellum were extracted in 80% (v/v) ice-cold ethanol by sonication (Model VXC 400, Sonics and Material, Newtown, CT, USA). The suspension was subsequently centrifuged at 20,000 g for 20 minutes. The supernatant was collected, frozen, and lyophilized. The lyophilized samples and pellets were stored at −20°C until further analyses.
13C-Labeling in the Amino Acids and Calculation of Molecular Carbon Labeling
The lyophilized samples were reconstituted in milliQ water and centrifuged at 10,000 g for 10 minutes. 13C-labeling in glutamate, GABA, glutamine, aspartate, and alanine was determined using the Phenomenex EZ:faast amino-acid analysis kit for liquid chromatography-mass spectrometry (Phenomenex, Torrance, CA, USA), and mass spectrometric analysis was performed as described earlier (Bak et al, 2006). To achieve a measure of total incorporation of 13C label into each metabolite, the average percent of labeled carbon atoms for every metabolite was calculated, that is, molecular carbon labeling (MCL; Bak et al, 2006).
Glycogen Assay
Glycogen was quantitatively determined essentially as described earlier (Brown et al, 2003; Walls et al, 2009). Briefly, pellets were resuspended by ultrasound in milliQ water (pH∼2). Glycogen content was determined by degrading glycogen to glucose units using amyloglucosidase (0.87 U/mL), and subsequently measuring the increase of NADPH (reduced form of nicotinamide adenine dinucleotide phosphate) formed in the conversion of glucose-6-phosphate to 6-phosphogluconolactone. The reaction was initiated by adding glucose-6-phosphate dehydrogenase (0.54 U/mL) and hexokinase (1.52 U/mL). NADPH was measured fluorometrically after 40 minutes of incubation at room temperature, using 360 nm and 415 nm as, respectively, the excitation and emission wavelengths.
Data Analysis
Results are means±s.e.m. Statistical differences were analyzed by one-way analysis of variance with Bonferroni post hoc tests and results were considered statistically significantly different when P<0.05.
Results
Blood and Plasma Glucose Values
Blood glucose concentrations were determined in all animal models before the injection of [1-13C]glucose and 28 minutes later (i.e., before microwave fixation). In addition, [1-13C]glucose enrichment in plasma was analyzed, and the results are presented in Table 1. The average baseline blood glucose concentrations of the SprD, ZL, and ZDF lean rats (i.e., before the injection of [1-13C]glucose) were ∼4 mmol/L, showing that the three control models were similar in this regard. Baseline blood glucose in the ZO rat was 5.8±0.2 mmol/L, that is, slightly hyperglycemic (P=0.015), whereas average fasting blood glucose in the ZDF rats was 11.4±1.1 mmol/L (P<0.0001), demonstrating that this animal model was diabetic. Injection of [1-13C]glucose increased blood glucose concentrations in all strains. The absolute increase in blood glucose concentrations (t28 minutes−t0 minutes) was significantly higher in the ZDF lean (2.1±0.2 mmol/L, P=0.042) and ZDF (4.3±1.2 mmol/L, P=0.006) rats than in the SprD rats (1.4±0.2 mmol/L), but the relative increase was not significantly different between any of the strains. [1-13C]glucose enrichment in plasma was similar for all three control models and the ZO rat. The ZDF rat, however, was an exception. In this type 2 diabetic model, calculated blood [1-13C]glucose enrichment was ∼50% lower than in the other animal strains, and this is likely explained by the initial higher blood glucose concentration in this model. The concentration of [1-13C]glucose in blood (Figure 1) was calculated, and despite similar plasma [1-13C]glucose enrichment, the concentration of [1-13C]glucose in the blood was increased in the ZO model (P=0.019) compared with the SprD rats (but not in comparison to the ZL). Also, in the ZDF rat, the absolute concentration of blood [1-13C]glucose was higher than the SprD (P=0.022) but not the ZDF lean, despite lower plasma enrichment in this type 2 diabetic model than in either of the two control models.
Table 1. Blood glucose concentrations (mmol/L) and [1-13C]glucose enrichments (%) in plasma.
| Blood glucose concentration | Plasma glucose | |||
|---|---|---|---|---|
| Animal strain | t=0 minute (mmol/L) | t=28 minutes (mmol/L) | Increase (%) | [1-13C]glucose enrichment, t=28 minutes (%) |
| SprD | 3.9±0.1 | 5.3±0.2* | 36.2±4.3 | 33.3±2.3 |
| Zucker lean | 3.5±0.1 | 5.2±0.2* | 55.5±7.5 | 35.5±4.6 |
| ZO | 5.8±0.2# | 7.9±0.3* | 37.0±4.2 | 34.3±1.7 |
| ZDF lean | 3.8±0.1 | 5.9±0.2* | 55.6±5.7 | 38.7±1.6 |
| ZDF | 11.4±1.1$ | 15.1±1.2* | 41.1±13.3 | 18.1±1.4$ |
NMR, nuclear magnetic resonance; SprD, Sprague–Dawley; ZDF, Zucker diabetic fatty; ZL, Zucker lean; ZO, Zucker obese.
Blood samples were taken from the tail tip and glucose concentrations determined using a BIOSEN analyzer as detailed in Materials and methods. Plasma [1-13C]glucose enrichment was determined by 1H NMR. For NMR analysis, blood samples in EDTA-coated capillary tubes were centrifuged and the plasma fraction was collected. Plasma proteins were denatured in ethanol, and analyzed by NMR as described in the Materials and methods section. Results are means±s.e.m. values, n=4–8. The statistical significance of the increase in glucose concentration was determined using a paired Student's t-test within a strain, and a one-way analysis of variance followed by a Bonferroni post hoc test between strains. In both cases, P<0.05 was used as significance level.
*Different from the blood glucose concentration at t=0 minute.
#Significantly different from SprD and ZL.
$Significantly different from all other strains.
Figure 1.
Concentration of [1-13C]glucose (mmol/L) in the blood of SprD, ZL, ZO, ZDF lean, and ZDF rats before microwave fixation. For each animal, the concentration of [1-13C]glucose in blood was calculated by multiplying blood glucose concentration at t=28 minutes with plasma enrichment, see Table 1. The specific activity of isotopically enriched glucose between plasma and blood is 0.85 at 30 minutes after the injection (Heath and Rose, 1969), and the present values have been corrected for this. Values are averages±s.e.m., n=4 to 8. Statistically significant differences were determined by one-way analysis of variance followed by Bonferroni post hoc test. An asterisk (*) indicates statistically significant difference from SprD (P<0.05). SprD, Sprague–Dawley; ZDF, Zucker diabetic fatty; ZL, Zucker lean; ZO, Zucker obese.
Brain Glycogen
Glycogen was determined in cerebral cortex, hippocampus, and cerebellum. In cortex of the SprD, the brain glycogen level was 5.6±0.3 μmol/g wet weight (Figure 2). The ZL and ZDF lean had markedly higher glycogen levels in comparison to the SprD model in all three brain regions, highlighting the necessity of including all three control models to obtain a more detailed metabolic picture of the pathologies. Interestingly, in the ZO model, the amount of glycogen compared with the ZL (which is its genetically most proper control) was higher in cerebral cortex but lower in the hippocampal and cerebellar brain regions. The ZDF rat had a tendency for a lower glycogen level in comparison to its ZDF lean littermate in all brain regions, and in cerebellum, this difference was significant (P<0.0001).
Figure 2.
Glycogen levels (μmol/g wet weight) in the cerebral cortex (A), hippocampus (B), and cerebellum (C) of SprD, ZL, ZO, ZDF lean, and ZDF rats. Brain tissue was extracted and the pellet fraction was used for glycogen analysis. Glycogen was degraded to glucose, and subsequently glucose concentrations were determined as outlined in Materials and methods. Results are averages±s.e.m., n=8. Statistically significant differences within each brain region were determined by one-way analysis of variance followed by Bonferroni post hoc test, where P<0.05 was taken to indicate statistically significant differences. $ indicates statistically significant difference from all other strains, * indicates statistically significant difference from SprD, # indicates statistically significant difference from ZL, and  indicates statistically significant difference from ZO. SprD, Sprague–Dawley; ZDF, Zucker diabetic fatty; ZL, Zucker lean; ZO, Zucker obese.
indicates statistically significant difference from ZO. SprD, Sprague–Dawley; ZDF, Zucker diabetic fatty; ZL, Zucker lean; ZO, Zucker obese.
Molecular Carbon Labeling for Glutamate, Glutamine, Aspartate, GABA, and Alanine
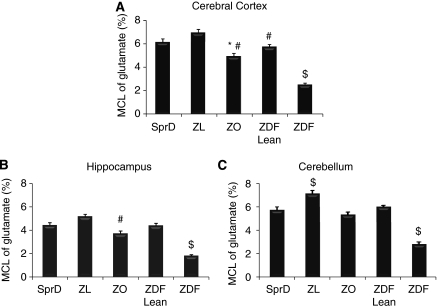
The interrelationship between neurons and astrocytes with regard to energy and neurotransmitter homeostasis is illustrated in Figure 3. The MCL was determined for glutamate, glutamine, aspartate, GABA, and alanine (Bak et al, 2006). It should be kept in mind that the precursor enrichment, that is, blood glucose enrichment, is different for the ZDF rat compared with the other strains, which has a potential impact on the MCL values. In the cerebral cortex of the SprD, MCL of glutamate was 6.1±0.3%, and a similar MCL for glutamate was observed in the two lean controls (Figure 4). The ZO and the ZDF rats, however, had lower MCL for glutamate. In the hippocampal region, all three control models exhibit similar MCL for glutamate. In this brain region, the obesity and type 2 diabetes models also had lower MCL for glutamate in comparison to their respective lean littermates. The cerebellum was different in that the MCL for glutamate in the ZL control was 7.11%±0.28% and higher than both of the other two controls (SprD and ZDF lean). Once more it was observed that the ZDF rat had the most reduced MCL for glutamate in comparison to the other strains, which is likely related to the fact that the ZDF rat had far the lowest blood glucose enrichment.
Figure 3.
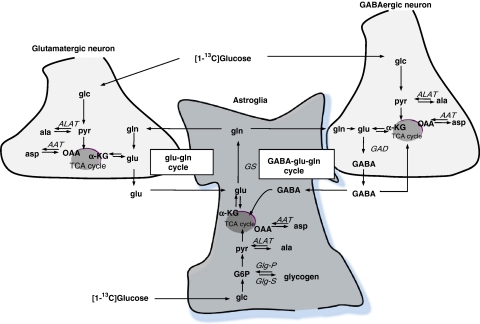
Simplified illustration of energy and neurotransmitter homeostasis in glutamatergic and GABAergic neurons and astrocytes, indicating the interdependence between the cell types. [1-13C]glucose (glc) is taken up by neurons and astrocytes and glycolytically metabolized to pyruvate (pyr). In astrocytes, where glycogen is located, some glucose is used for the synthesis of glycogen incorporating 13C-glycosyl units into this carbohydrate reserve. Glycogen is a dynamic molecule, and 13C labeled as well as unlabeled glycosyl units may be released from glycogen, catalyzed by glycogen phosphorylase (Glg-P) the rate-limiting enzyme in this degradation process. In both types of neurons and the astrocytes, pyruvate may be transaminated to alanine (ala). This transamination process is rapid and hence 13C-labeling in alanine may reflect glycolytic activity. A major part of pyruvate will be transported into mitochondria, where it will be oxidatively decarboxylated to acetyl-CoA. Oxaloacetate (OAA) will condense with acetyl-CoA in the TCA cycle and consecutive reactions will yield α-ketoglutarate (α-KG), a TCA cycle intermediate. α-KG may be transaminated to glutamate, and 13C-labeling in glutamate may mirror TCA cycle activity due to the high activity of aspartate aminotransferase. Alternatively, α-KG may remain in the TCA cycle where successive reactions will yield OAA, transamination of which will generate aspartate (asp). In astrocytes, glutamine (gln) may be formed from glutamate, a reaction catalyzed by the astrocyte-specific enzyme glutamine synthetase (GS). During glutamatergic neurotransmission, glutamate is released from neurons and subsequently predominantly taken up into astrocytes. To prevent drainage of glutamate in neurons, they are highly dependent on glutamine transfer from astrocytes as a precursor for glutamate synthesis, and a glutamate–glutamine cycle exists. Glutamate decarboxylase is exclusively located in GABAergic neurons, and thus GABA is formed from glutamate only in this compartment. After GABAergic signaling, GABA is primarily cleared into neurons. However, a part is taken up by astrocytes, and eventually glutamine may be formed and transferred to GABAergic neurons, constituting the so-called GABA–glutamate–glutamine cycle. Neurotransmitter glutamate is in equilibrium with aspartate and alanine through aspartate and alanine aminotransferase, respectively, and as indicated it serves as the precursor for the synthesis of glutamine and GABA. Thus, labeling from [1-13C]glucose into glutamine, GABA, aspartate, and alanine is highly affected by the turnover in the distinct pools of glutamate, which are available as precursor for each of these amino acids, respectively. Glg-S, glycogen synthase; G6P, glucose-6-phosphate.
Figure 4.
Molecular carbon labeling (MCL) of glutamate in cerebral cortex (A), hippocampus (B), and cerebellum (C) of SprD, ZL, ZO, ZDF lean, and ZDF rats 30 minutes after i.p. injection of [1-13C]glucose (540 mg/kg), as detailed in the Materials and methods section. Extracts of the excised brain regions were purified, and the amino acids were derivatized and analyzed by LC-MS. Results are means±s.e.m., n=4 to 8. Statistically significant differences between the strains were calculated by one-way analysis of variance followed by Bonferroni post hoc test, where P<0.05 was taken to indicate statistically significant differences. $ indicates statistically significant difference from all other strains, * statistically significant difference from SprD, and # statistically significant difference from ZL. LC-MS, liquid chromatography-mass spectrometry; SprD, Sprague–Dawley; ZDF, Zucker diabetic fatty; ZL, Zucker lean; ZO, Zucker obese.
As mentioned earlier, the animal models were different regarding plasma enrichment and blood concentration of [1-13C]glucose, which ultimately may affect the availability of [1-13C]glucose for brain metabolism. Therefore, and due to the central function of glutamate in sustaining homeostasis of the other amino acids in question, we calculated the MCL values of glutamine, GABA, aspartate, and alanine relative to that of glutamate (Figure 5). The glutamine/glutamate and GABA/glutamate MCL ratios indicate the extent to which glutamate is converted to glutamine and GABA, respectively. The alanine/glutamate and aspartate/glutamate MCL ratios are used as an indication of glycolytic activity and TCA cycle activity, respectively (Figure 3). These ratios enable a comparison of brain metabolism between the different animal models irrespective of the differences in [1-13C]glucose plasma enrichment and/or differences in glucose transport into the brain.
Figure 5.
13C-labeling, calculated as molecular carbon labeling (MCL, see Materials and methods), for glutamine, aspartate, GABA, and alanine was determined and expressed relative to that for glutamate. These ratios were determined for cerebral cortex (A), hippocampus (B), and cerebellum (C) of SprD, ZL, ZO, ZDF lean, and ZDF rats. [1-13C]glucose was injected (540 mg/kg, i.p.), and the animals were killed 30 minutes later. Tissue extracts were purified and the amino acids were derivatized after which they were analyzed by LC-MS as detailed in the Materials and methods section. Results are means±s.e.m., n=4 to 8. Statistically significant differences between the animal models were determined by one-way analysis of variance followed by Bonferroni post hoc test, where P<0.05 was taken to indicate statistically significant differences. $ marks statistically significant difference from all other strains, * from SprD, # from ZL,  from ZO, and £ indicates statistically significant difference from ZDF lean. LC-MS, liquid chromatography-mass spectrometry; SprD, Sprague–Dawley; ZDF, Zucker diabetic fatty; ZL, Zucker lean; ZO, Zucker obese.
from ZO, and £ indicates statistically significant difference from ZDF lean. LC-MS, liquid chromatography-mass spectrometry; SprD, Sprague–Dawley; ZDF, Zucker diabetic fatty; ZL, Zucker lean; ZO, Zucker obese.
In cerebral cortex, the glutamine/glutamate MCL ratios were ∼0.65 in the control models (SprD, ZL, and ZDF lean), meaning that glutamine was labeled to a lesser extent than glutamate, and that the extent to which glutamate was converted to glutamine was similar in the three control models. There was a tendency that the ZO (0.60±0.02) animal model had a lower glutamine/glutamate MCL ratio than its lean littermate (0.66±0.01, P=0.07). This ratio was reduced in the ZDF (0.58±0.03) animal model when compared with the ZDF lean (0.64±0.01, P=0.029 using Student's paired t-test). The aspartate/glutamate MCL ratios were above 1 in all strains, and were significantly higher in the ZDF lean (1.28±0.01) and the ZDF (1.30±0.01) rats than in the SprD (1.22±0.01) rats. The GABA/glutamate MCL ratio was 1.00±0.04 in the SprD rat, whereas it was almost 1.20 in the ZO, ZDF lean, and the ZDF animal models. Accordingly, GABA turnover was increased compared with glutamate turnover in the latter three strains. Finally, the alanine/glutamate MCL ratio was 0.81±0.01 in cerebral cortex of the SprD, and it was not significantly different in the ZL (0.84±0.01) and ZO (0.89±0.03) rats. The alanine/glutamate MCL ratio in the cerebral cortex was higher in the ZDF (0.98±0.02) rat than all other strains, indicating increased glycolysis relative to glucose metabolism through the TCA cycle in these type 2 diabetic animals.
In the hippocampus, the glutamine/glutamate MCL ratio was 0.72±0.02 in the SprD rat, and as in cortex, there was a tendency for the models of obesity and type 2 diabetes to have reduced glutamine/glutamate MCL ratios when compared with their lean littermates. The ZDF rat was shown to have higher aspartate/glutamate, GABA/glutamate, and alanine/glutamate MCL ratios in hippocampus compared with the other strains, indicating that glucose metabolism and amino-acid homeostasis in the type 2 diabetic state were highly affected. The ZO rat had a tendency toward a higher alanine/glutamate MCL ratio in comparison to the ZL control, whereas this was not observed for the aspartate/glutamate and GABA/glutamate MCL ratios. Finally, it was generally observed that the amino acid/glutamate MCL ratios within each strain were higher in the hippocampus compared with cerebral cortex. For instance, in the ZDF rat, the alanine/glutamate MCL ratio was 0.98±0.02 and 1.31±0.04 in cerebral cortex and hippocampus, respectively (P<0.001).
In the cerebellar region, the SprD control had a glutamine/glutamate MCL ratio of 0.63±0.01. This MCL ratio was decreased to 0.55±0.01 (P<0.001) and 0.48±0.03 (P<0.001) in the ZO and the ZDF rats, respectively. As in the hippocampus, there was a tendency for the ZO model to have a higher alanine/glutamate MCL ratio, and in the ZDF model both the aspartate/glutamate and alanine/glutamate MCL ratios were elevated, indicating alterations in glucose metabolism through glycolysis as well as the TCA cycle. All animal models showed the same GABA/glutamate MCL ratios in the cerebellum, which was in contrast to the other brain areas.
Discussion
Obesity and type 2 diabetes are complex metabolic syndromes. Limited knowledge exists about the effects of these metabolic states on brain energy and amino-acid metabolism, and this study was designed to elucidate this. The ZO and ZDF rats were used as obese and type 2 diabetes models, respectively, and SprD, ZL, and ZDF lean rats were used as controls. Our study indicates that glycolysis and TCA cycle activity were reduced to a similar extent in the obese model, whereas the TCA cycle activity was decreased relatively more than glycolytic activity in the type 2 diabetes model. Brain glycogen levels were generally more affected in the ZO rats in comparison to the ZDF rats; however, in the cerebellum, both models showed reduced glycogen contents. Finally, glutamate–glutamine cycling was also reduced in the cerebellar region.
Blood Glucose and the Effects of Obesity and Type 2 Diabetes
Diabetes is characterized by hyperglycemia and hence plasma [1-13C]glucose enrichments were expected to vary between the models. All three control models (SprD, ZL, and ZDF lean) exhibited similar blood glucose concentrations before and after the injection of [1-13C]glucose. The obese (ZO) and type 2 diabetes (ZDF) models had higher blood glucose levels as expected. The increase in blood glucose concentration after injection of [1-13C]glucose was greater in both the ZDF lean and the ZDF rats compared with the other strains. This may imply (1) that endogenous glucose, most likely derived from glycogen degradation in the liver, contributed to the increase in blood glucose and (2) that the contribution of endogenously produced glucose varies between the non-diabetic, obese and type 2 diabetic models. The ZDF rats are insulin resistant, and indeed it has been shown that the rate of disappearance of glucose is 50% slower in this model compared with SprD rats (Pold et al, 2005; Li et al, 2006). In agreement with this, we found that the concentration of [1-13C]glucose in blood was higher in the obese and type 2 diabetic states, supporting the suggestion that less glucose was used. It is debated whether hyperglycemia and diabetes affect glucose transport into the brain (Gjedde and Crone, 1981; McCall et al, 1982; Garcia-Espinosa et al, 2003; Seaquist et al, 2005). This uncertainty underlines the importance of studying MCL ratios rather than its absolute values, because this circumvents the differences between the animal models with regard to the amount of glucose reaching the brain. In so doing, 13C-labeled metabolites derived from the degradation of [1-13C]glucose reaching the brain are compared with one central metabolite, in this case glutamate.
Brain Glycogen in the Obese Animal Model
It is well known that glycogen concentrations vary between different brain regions (Kong et al, 2002; Sagar et al, 1987), and the brain regional variations observed in the SprD rats are consistent with an earlier study, in which cerebellum was found to have the highest concentration of glycogen (Sagar et al, 1987). To our knowledge, this is the first study to report glycogen levels in an obese animal model. Our results indicate that obesity may be associated with alterations in the glucose buffer system, that is, brain glycogen. Furthermore, these alterations are distinct within different brain regions. In cortex, the augmented glycogen level may be a consequence of mild hyperglycemia, and astrocytes might simply store more glucose as glycogen under these conditions. In line with this, low doses of streptozotocin inducing modest hyperglycemia and partial diabetes in rats have been shown to increase cortical glycogen concentrations (Plaschke and Hoyer, 1993). However, in the hippocampus and cerebellum, the obese animal model had lower glycogen levels compared with its lean littermates, which illustrates that such a straightforward relation between blood glucose concentration and glycogen levels is an oversimplification. It remains to be established why the obese state is associated with such brain regional diversities in glycogen levels and regulation of glycogen metabolism. The observation that the obesity control (ZL) had augmented glycogen levels in all three brain regions in comparison to the standard control (SprD) also highlights the importance of using appropriate controls when studying animal disease models.
Decreased Glucose Metabolism Through both Glycolysis and the TCA Cycle in the Obese Model
Molecular carbon labeling, that is, a measure of total 13C incorporation into a metabolite, for glutamate was reduced in all brain regions in the obese model, despite similar plasma enrichment. This suggests that metabolism of glucose through glycolysis and/or the TCA cycle was decreased in the obese state, leading to reduced 13C-labeling in glutamate from the TCA cycle intermediate α-ketoglutarate. In line with this, decreased glucose utilization in different brain areas of the ZO rat has been reported (Marfaing-Jallat et al, 1992; Tsujii et al, 1988). Our results indicate that metabolism of glucose through glycolysis and the TCA cycle was reduced to a similar extent. As illustrated by lower glutamate MCL (Figure 4), that is, decreased entry into the TCA cycle, combined with no significant difference between the control and the obese model in the alanine/glutamate MCL ratio (Figure 5). Thus, the labeling of alanine (i.e., glycolysis) follows that of glutamate.
In general, all animal strains exhibited regional variations in brain energy metabolism as entry of glucose-derived acetyl-CoA into the TCA cycle was lower in the hippocampus than in cortex. This interpretation was based on the lower MCL for glutamate in the hippocampus combined with a significantly higher alanine/glutamate MCL ratio in the hippocampus of all strains.
GABA–Glutamate–Glutamine Cycle and Its Link to Glycogen Metabolism in the Obese Model
Other in vivo studies have shown that 80% to 90% of astrocytic glutamine labeling is derived from vesicularly released glutamate from neurons (Kanamori et al, 2002). Hence, a reduction in glutamatergic activity will likely affect the activity of the glutamate–glutamine cycle. Indeed, the glutamine/glutamate MCL ratio was decreased in the cerebellum of the obese animal model, and similar tendencies were observed in the other brain regions. Recently, a link between glycogen utilization and glutamatergic neurotransmission and consequently the glutamate–glutamine cycle was established (Sickmann et al, 2009). Also, studies in chickens have indicated that glycogen is important for the synthesis of glutamate and glutamine (Gibbs et al, 2007). Intriguingly, this study may support that an association between glycogen metabolism and glutamate–glutamine cycle activity exists in the obese model as well, as indicated by a concomitant reduction in glycogen levels and the glutamine/glutamate MCL ratio. However, it should be mentioned that data regarding 13C-labeling in the glycogen pool would strengthen this observation. Nevertheless, this study suggests that obesity may be associated with reduced glutamatergic neurotransmission and glutamate–glutamine cycle activity as well as hampered glycogen metabolism in various parts of the brain.
Despite reduced glutamate–glutamine cycle activity in the obese model, the MCL ratio between GABA and glutamate was not impaired. Keeping in mind that MCL for glutamate (Figure 4) was lower in the obese model, this shows that the MCL of GABA follows that of glutamate (Figure 5). Accordingly, it may indicate that glutamate decarboxylase activity and GABA–glutamate–glutamine cycling are not impaired as a consequence of the obese condition. These observations may rather be explained by a reduction in glucose metabolism leading to lower labeling in glutamate (and subsequently GABA).
Metabolism of Glucose Through the TCA Cycle is Decreased Comparatively more than Glycolytic Activity in the Type 2 Diabetic Model
In the type 2 diabetes model, MCL of glutamate was decreased (Figure 4), even when considering the differences in [1-13C]glucose enrichment in plasma, implying that type 2 diabetes may also be associated with a general reduction in brain glucose metabolism. Such reduced metabolism of glucose could be explained by a downregulation of glucose transport from blood to brain as a consequence of hyperglycemia (Gjedde and Crone, 1981; McCall et al, 1982). To specify whether glycolysis and TCA cycle activity were affected to a similar extent, one has to look at MCL for glutamate (Figure 4) and alanine/glutamate MCL ratios (Figure 5) combined. The alanine/glutamate MCL ratios were higher in all brain regions of the ZDF rats, indicating that labeling in alanine (i.e., glycolysis) does not follow that of glutamate (i.e., TCA cycle). This suggests that, although glucose metabolism is generally reduced (Figure 4), oxidative glucose metabolism was probably more affected than glycolytic activity in the type 2 diabetic state was linked to a clear decrease in oxidative glucose metabolism relative to glycolytic activity. On the basis of the understandings that acetyl-CoA originating from glucose is metabolized in neurons to a large extent (Zielke et al, 2007), and that glutamate is predominantly located in neurons (Ottersen, 1989), it may be suggested that neurons are metabolically compromised in the type 2 diabetic state. One explanation for these changes in metabolism of glucose through the TCA cycle could be an increased turnover of lipids, leading to competition with glucose with regards to supply of acetyl-CoA to the TCA cycle. In contrast to our study, it has been reported that in a type 1 diabetes animal model, TCA cycle activity was not reduced (Garcia-Espinosa et al, 2003). Does this mean that type 1 and type 2 diabetes have distinct effects on brain energy and neurotransmitter homeostasis? This may be one explanation; however, it is important to be cautious when making such a statement. A variety of different diabetic models exists, and in addition to this, different laboratories use animals at different ages with diverse degrees of diabetes and hyperglycemia, and finally genetic variations may also partly explain these differences.
Brain Glycogen and the Glutamate–Glutamine Cycle in the Type 2 Diabetic Animals
This study indicates that type 2 diabetes is linked to altered glycogen levels in the cerebellum, but not in the other brain areas analyzed. Glycogen levels in the cortex have been shown not to be affected in a type 1 diabetes model (Sanchez-Chavez et al, 2008), and our study confirms that this applies to type 2 diabetes as well. Brain glycogen synthase and phosphorylase activities, that is, the rate-limiting enzymes in glycogen synthesis and degradation, respectively, was shown to be affected in diabetes with distinct differences among brain regions (Plaschke and Hoyer, 1993). Accordingly, based on these results, it may be suggested that glycogen synthase and phosphorylase in the cerebellum are particularly affected by the type 2 diabetic state.
Glutamate–glutamine cycling was also affected in the type 2 diabetes model, especially in cerebellum, and it was more compromised than in the obese model. This may suggest that in this model, the pathology of diabetes has a more devastating outcome on glutamatergic neurotransmission than in the obese state. Studies have concluded that the activity of glutamine synthetase converting glutamate to glutamine was increased in type 1 diabetes (Garcia-Espinosa et al, 2003; Bhardwaj et al, 1998) possibly contradicting these results. [1-13C]glucose was also used as the precursor in the study by Garcia-Espinosa et al (2003), and to compare with our study, we estimated the ratio between 13C enrichment in glutamine and glutamate in that study to be ∼80% and 45% in control and type 1 diabetic animals, respectively. Accordingly, our study and that of Garcia-Espinosa et al (2003) indicate that both types of diabetes may be associated with reduced glutamate–glutamine cycle activity. In brain areas where glycogen levels were not affected, there was no significant reduction in glutamate–glutamine cycling either. In contrast, in the cerebellum, having decreased levels of glycogen, a decrease was also observed in glutamate–glutamine cycling supporting the hypothesis that glycogen metabolism is connected to the maintenance of glutamatergic activity (Sickmann et al, 2009; Gibbs et al, 2007).
GABAergic Neurons and Type 2 Diabetes
13C-labeling in GABA relative to glutamate showed brain regional variations in the type 2 diabetic state. GABA–glutamate–glutamine cycling in cortex and cerebellum appear to be less affected by diabetes, as no changes in the GABA/glutamate MCL ratio were observed. It has been indicated earlier that at least type 1 diabetes is associated with reduced glutamate decarboxylase activity (Galanopoulos et al, 1988) unfortunately no such information exists for a type 2 diabetes model. Interestingly, our results imply that the GABA–glutamate–glutamine cycle in the hippocampus may be disturbed in diabetic animals, as observed by a marked increase in the GABA/glutamate MCL ratio, and accordingly indirectly suggests that glutamate decarboxylase activity is increased in this brain region. The study by Galanopoulos et al (1988) was not performed on hippocampal tissue, and brain regional variations could explain these different observations as well as distinct metabolic differences between the type 1 and type 2 diabetic state.
Conclusions and Perspective
To summarize, brain glucose metabolism was altered in both the obese and type 2 diabetes models. Glycogen levels were lower, and glycolysis and TCA cycle activity were reduced to a similar extent in the obese model. In the type 2 diabetes model, entry of glucose-derived acetyl-CoA into the TCA cycle was decreased relatively more than glycolytic activity. Furthermore, decreased glutamate–glutamine cycling is suggested in both models, although brain regional variations existed. The type 2 diabetic state showed more marked alterations in the amino-acid homeostasis than the obese model. Finally, this study shows that brain metabolism in the three control models differed and thus underlines the importance of using the appropriate lean controls.
Acknowledgments
Merete Achen, Maibritt C Pedersen, Tobias S Bruun, Lene Vigh, Heidi Nielsen, and Katrine Brunstedt are acknowledged for technical assistance.
The authors declare no conflict of interest.
References
- American Diabetes Association, all about diabetes 2009Type 2 diabetesAvailable from http://www.diabetes.org/type-2-diabetes.jsp accessed 14 September 2009
- Bak LK, Schousboe A, Sonnewald U, Waagepetersen HS. Glucose is necessary to maintain neurotransmitter homeostasis during synaptic activity in cultured glutamatergic neurons. J Cereb Blood Flow Metab. 2006;26:1285–1297. doi: 10.1038/sj.jcbfm.9600281. [DOI] [PubMed] [Google Scholar]
- Berl S, Clarke DD.1983The metabolic compartmentation concept Glutamine, Glutamate and GABA in the Central Nervous System(Hertz L, Kvamme E, McGeer EG, Schousboe A, eds),New York: Alan R Liss Inc; 205–217. [Google Scholar]
- Bhardwaj SK, Sharma P, Kaur G. Alterations in free radical scavenger system profile of type I diabetic rat brain. Mol Chem Neuropathol. 1998;35:187–202. doi: 10.1007/BF02815124. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Gispen WH. The impact of diabetes on cognition: what can be learned from rodent models. Neurobiol Aging. 2005;26 (Suppl 1:36–41. doi: 10.1016/j.neurobiolaging.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Brown AM, Tekkok SB, Ransom BR. Glycogen regulation and functional role in mouse white matter. J Physiol. 2003;549:501–512. doi: 10.1113/jphysiol.2003.042416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo AM, Broadwell RD. Cytochemical identification of cerebral glycogen and glucose-6-phosphatase activity under normal and experimental conditions. II. Choroid plexus and ependymal epithelia, endothelia and pericytes. J Neurocytol. 1986;15:511–524. doi: 10.1007/BF01611733. [DOI] [PubMed] [Google Scholar]
- Choi IY, Seaquist ER, Gruetter R. Effect of hypoglycemia on brain glycogen metabolism in vivo. J Neurosci Res. 2003;72:25–32. doi: 10.1002/jnr.10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz NF, Dienel GA. High glycogen levels in brains of rats with minimal environmental stimuli: implications for metabolic contributions of working astrocytes. J Cereb Blood Flow Metab. 2002;22:1476–1489. doi: 10.1097/01.WCB.0000034362.37277.C0. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Galanopoulos E, Lellos V, Papadakis M, Philippidis H, Palaiologos G. Effects of fasting and diabetes on some enzymes and transport of glutamate in cortex slices or synaptosomes from rat brain. Neurochem Res. 1988;13:243–248. doi: 10.1007/BF00971540. [DOI] [PubMed] [Google Scholar]
- Garcia-Espinosa MA, Garcia-Martin ML, Cerdan S. Role of glial metabolism in diabetic encephalopathy as detected by high resolution 13C NMR. NMR Biomed. 2003;16:440–449. doi: 10.1002/nbm.843. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Crone C. Blood-brain glucose transfer: repression in chronic hyperglycemia. Science. 1981;214:456–457. doi: 10.1126/science.7027439. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Lloyd HG, Santa T, Hertz L. Glycogen is a preferred glutamate precursor during learning in 1-day-old chick: biochemical and behavioral evidence. J Neurosci Res. 2007;85:3326–3333. doi: 10.1002/jnr.21307. [DOI] [PubMed] [Google Scholar]
- Heath DF, Rose JG. The distribution of glucose and [14C]glucose between erythrocytes and plasma in the rat. Biochem J. 1969;112:373–377. doi: 10.1042/bj1120373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Dringen R, Schousboe A, Robinson SR. Astrocytes: glutamate producers for neurons. J Neurosci Res. 1999;57:417–428. [PubMed] [Google Scholar]
- Kamal A, Biessels GJ, Gispen WH, Ramakers GM. Synaptic transmission changes in the pyramidal cells of the hippocampus in streptozotocin-induced diabetes mellitus in rats. Brain Res. 2006;1073–1074:276–280. doi: 10.1016/j.brainres.2005.12.070. [DOI] [PubMed] [Google Scholar]
- Kanamori K, Ross BD, Kondrat RW. Glial uptake of neurotransmitter glutamate from the extracellular fluid studied in vivo by microdialysis and (13)C NMR. J Neurochem. 2002;83:682–695. doi: 10.1046/j.1471-4159.2002.01161.x. [DOI] [PubMed] [Google Scholar]
- Kong J, Shepel PN, Holden CP, Mackiewicz M, Pack AI, Geiger JD. Brain glycogen decreases with increased periods of wakefulness: implications for homeostatic drive to sleep. J Neurosci. 2002;22:5581–5587. doi: 10.1523/JNEUROSCI.22-13-05581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yang G, Li Q, Tang Y, Li K. High-fat- and lipid-induced insulin resistance in rats: the comparison of glucose metabolism, plasma resistin and adiponectin levels. Ann Nutr Metab. 2006;50:499–505. doi: 10.1159/000098141. [DOI] [PubMed] [Google Scholar]
- Martinez-Tellez R, Gomez-Villalobos MJ, Flores G. Alteration in dendritic morphology of cortical neurons in rats with diabetes mellitus induced by streptozotocin. Brain Res. 2005;1048:108–115. doi: 10.1016/j.brainres.2005.04.048. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Martinez-Hernandez A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 1979;161:303–310. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Marfaing-Jallat P, Levacher C, Calando Y, Picon L, Penicaud L. Glucose utilization and insulin binding in discrete brain areas of obese rats. Physiol Behav. 1992;52:713–716. doi: 10.1016/0031-9384(92)90402-n. [DOI] [PubMed] [Google Scholar]
- McCall AL, Millington WR, Wurtman RJ. Metabolic fuel and amino acid transport into the brain in experimental diabetes mellitus. Proc Natl Acad Sci USA. 1982;79:5406–5410. doi: 10.1073/pnas.79.17.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo H, Harwood J, Zhang S, Xue Y, Santini R, Raftery D. R: A quantitative measure of NMR signal receiving efficiency. J Magn Reson. 2009;200:239–244. doi: 10.1016/j.jmr.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen OP. Quantitative electron microscopic immunocytochemistry of neuroactive amino acids. Anat Embryol. 1989;180:1–15. doi: 10.1007/BF00321895. [DOI] [PubMed] [Google Scholar]
- Oz G, Kumar A, Rao JP, Kodl CT, Chow L, Eberly LE, Seaquist ER. Human brain glycogen metabolism during and following hypoglycemia. Diabetes. 2009;58:1978–1985. doi: 10.2337/db09-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaschke K, Hoyer S. Action of the diabetogenic drug streptozotocin on glycolytic and glycogenolytic metabolism in adult rat brain cortex and hippocampus. Int J Dev Neurosci. 1993;11:477–483. doi: 10.1016/0736-5748(93)90021-5. [DOI] [PubMed] [Google Scholar]
- Pold R, Jensen LS, Jessen N, Buhl ES, Schmitz O, Flyvbjerg A, Fujii N, Goodyear LJ, Gotfredsen CF, Brand CL, Lund S. Long-term AICAR administration and exercise prevents diabetes in ZDF rats. Diabetes. 2005;54:928–934. doi: 10.2337/diabetes.54.4.928. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, Swanson RA. The regional distribution of glycogen in rat brain fixed by microwave irradiation. Brain Res. 1987;417:172–174. doi: 10.1016/0006-8993(87)90195-8. [DOI] [PubMed] [Google Scholar]
- Sanchez-Chavez G, Hernandez-Berrones J, Luna-Ulloa LB, Coffe V, Salceda R. Effect of diabetes on glycogen metabolism in rat retina. Neurochem Res. 2008;33:1301–1308. doi: 10.1007/s11064-007-9583-7. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Sarup A, Bak LK, Waagepetersen HS, Larsson OM. Role of astrocytic transport processes in glutamatergic and GABAergic neurotransmission. Neurochem Int. 2004;45:521–527. doi: 10.1016/j.neuint.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Seaquist ER, Tkac I, Damberg G, Thomas W, Gruetter R. Brain glucose concentrations in poorly controlled diabetes mellitus as measured by high-field magnetic resonance spectroscopy. Metabolism. 2005;54:1008–1013. doi: 10.1016/j.metabol.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Sickmann HM, Walls AB, Schousboe A, Bouman SD, Waagepetersen HS. Functional significance of brain glycogen in sustaining glutamatergic neurotransmission. J Neurochem. 2009;109 (Suppl 1:80–86. doi: 10.1111/j.1471-4159.2009.05915.x. [DOI] [PubMed] [Google Scholar]
- Tsujii S, Nakai Y, Takahashi H, Usui T, Koh T, Yonekura Y, Konishi J, Imura H. Effect of food deprivation on regional brain glucose utilization in lean and fatty Zucker rats. Brain Res. 1988;475:371–375. doi: 10.1016/0006-8993(88)90628-2. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Qu H, Schousboe A, Sonnewald U. Elucidation of the quantitative significance of pyruvate carboxylation in cultured cerebellar neurons and astrocytes. J Neurosci Res. 2001;66:763–770. doi: 10.1002/jnr.10061. [DOI] [PubMed] [Google Scholar]
- Walls AB, Heimburger CM, Bouman SD, Schousboe A, Waagepetersen HS. Robust glycogen shunt activity in astrocytes: effects of glutamatergic and adrenergic agents. Neuroscience. 2009;158:284–292. doi: 10.1016/j.neuroscience.2008.09.058. [DOI] [PubMed] [Google Scholar]
- World Health Organisation, Programmes and projects, Media Center 2009Obesity and overweightAvailable from http://www.who.int/mediacentre/factsheets/fs311/en/index.html , accessed 15 July 2009
- Yu AC, Drejer J, Hertz L, Schousboe A. Pyruvate carboxylase activity in primary cultures of astrocytes and neurons. J Neurochem. 1983;41:1484–1487. doi: 10.1111/j.1471-4159.1983.tb00849.x. [DOI] [PubMed] [Google Scholar]
- Zielke HR, Zielke CL, Baab PJ, Tildon JT. Effect of fluorocitrate on cerebral oxidation of lactate and glucose in freely moving rats. J Neurochem. 2007;101:9–16. doi: 10.1111/j.1471-4159.2006.04335.x. [DOI] [PubMed] [Google Scholar]