Abstract
Stroke is a major neurologic disorder. Induced pluripotent stem (iPS) cells can be produced from basically any part of patients, with high reproduction ability and pluripotency to differentiate into various types of cells, suggesting that iPS cells can provide a hopeful therapy for cell transplantation. However, transplantation of iPS cells into ischemic brain has not been reported. In this study, we showed that the iPS cells fate in a mouse model of transient middle cerebral artery occlusion (MCAO). Undifferentiated iPS cells (5 × 105) were transplanted into ipsilateral striatum and cortex at 24 h after 30 mins of transient MCAO. Behavioral and histologic analyses were performed at 28 day after the cell transplantation. To our surprise, the transplanted iPS cells expanded and formed much larger tumors in mice postischemic brain than in sham-operated brain. The clinical recovery of the MCAO+iPS group was delayed as compared with the MCAO+PBS (phosphate-buffered saline) group. iPS cells formed tridermal teratoma, but could supply a great number of Dcx-positive neuroblasts and a few mature neurons in the ischemic lesion. iPS cells have a promising potential to provide neural cells after ischemic brain injury, if tumorigenesis is properly controlled.
Keywords: cell transplantation, cerebral ischemia, induced pluripotent stem cell, tumorigenesis
Introduction
Stroke is a major neurologic disorder and one of the leading causes of death in human. The treatment for cerebral infarction is only limited at present, and the development of new treatment is requested on clinical site. In previous experimental studies, bone marrow stromal cells (Shichinohe et al, 2007) and other stem cell transplantation therapy have been considered to be a hopeful strategy to supply neural cells repairing injury of ischemic brain (Abe, 2000; Chang et al, 2007; Chopp and Li, 2008; Miljan and Sinden, 2009). However, immunoreactive and ethical problems still remain to apply into the human stroke patients.
Mice-induced pluripotent stem (iPS) cells were first established by Yamanaka et al with introducing of four transcription factors (Oct3/4, Klf4, Sox2, and c-myc) into mouse fibroblasts (Takahashi and Yamanaka, 2006). iPS cells can be produced from basically any part of patients, with high reproduction ability and pluripotency to differentiate into various types of cells, suggesting that iPS cells can provide a hopeful therapy for cell transplantation (Amabile and Meissner, 2009; Koch et al, 2009; Ronaghi et al, 2009; Yu and Thomson, 2008). Although transplantations of embryonic stem (ES) cells into ischemic brain have been reported (Erdo et al, 2003; Takagi et al, 2005; Hayashi et al, 2006), such an examination with iPS cells has not been reported probably because of limited availability of iPS cells.
iPS cells do not have immunoreactive or ethical problems found in neurosphere, bone marrow cells, and ES cells, and therefore many stroke patients expect the feasibility and efficacy of iPS cells in the near future. Thus, we transplanted mice iPS cells into the ischemic mice brain to investigate the survival and possible differentiation of transplanted iPS cells in ischemic brain environment.
Materials and methods
Animals
Male C57BL/6N mice at the age of 8 to 10 weeks (body weight 20 to 25 g) were used in this study. All experimental procedures were approved by the Animal Committee of the Okayama University Graduate School of Medicine. We studied four experimental groups including the Sham+PBS (phosphate-buffered saline) group (n=6), the middle cerebral artery occlusion (MCAO)+PBS group (n=8), the Sham+iPS group (n=8), and the MCAO+iPS group (n=11). Each experimental group received intracerebral implantation of PBS or mice iPS cells at 24 h after sham operation or MCAO, as described below.
Focal Cerebral Ischemia
The mice were anesthetized with a nitrous oxide/oxygen/isoflurane mixture (69/30/1%) during surgery with the use of an inhalation mask. MCAO was induced by the intraluminal filament technique reported earlier (Yamashita et al, 2006). Briefly, the right carotid bifurcation was exposed, and the external carotid artery was clotted distal to the bifurcation. A 8-0 nylon filament with silicone coating was then inserted through the stump of the external carotid artery and gently advanced (9.0 to 10.0 mm) to occlude the origin of middle cerebral artery. After 30 mins of transient occlusion, the filament was gently removed to restore blood flow of middle cerebral artery, and the incision was closed. Rectal temperature was monitored and kept at 37.0°C using a heating bed (model BMT-100; Bio Research Center, Nagoya, Japan) during surgery and 30 mins of ischemic period. A laser Doppler flowmeter probe (MBF3D, Moor Instruments, Axminster, UK) was attached to the surface of the ipsilateral cortex to monitor regional cerebral blood flow. Mice were put in an incubater (V-80 Atom Infant Incubator; Atom Medical, Tokyo, Japan) at 28.5°C for 7 days after MCAO.
Preparation and Transplantation of Induced Pluripotent Stem Cells
Mice iPS cells, iPS-MEF-Ng-20D-17, were provided by RIKEN BRC through the National Bio-Resource Project of MEXT, Japan. The iPS cells were maintained on a feeder layer of mitomycin C-treated mouse embryonic fibroblasts (Millipore, Billerica, MA, USA) in Dulbecco's modified Eagle medium containing 15% fetal bovine serum, 0.1 mmol/L nonessential amino acids, 0.1 mmol/L 2-mercaptoethanol, and 1000 U/ml mouse leukemia-inhibiting factor (Takahashi and Yamanaka, 2006). For transplantation, the iPS cells were collected, suspended, and incubated with fresh medium on 0.1% gelatin-coated dishes for 30 mins to remove contaminated mouse embryonic fibroblasts. The iPS cells were collected, and washed three times with PBS (pH 7.2) to remove its medium and pelleted by centrifugation. The pellets were resuspended in 15 μl of PBS and placed on ice. Approximately 5 × 105 cells in 2 μl of PBS or only 2 μl of PBS were stereotaxically injected into the ipsilateral striatum and cortex (anterior, lateral, depth in mm: −0.5, 2.5, 2.5 to 3.5) (Hof et al, 2000). This position approximates the ischemic boundary zone in the striatum and cortex. The needle was retained in the striatum and cortex for an additional 5 mins interval to avoid donor backflow. The immunosuppressive compound cyclosporine A (Novartis, Basel, Switzerland) was applied intraperitoneally immediately after this implantation and every other day before animals were killed with a 10 mg/kg dose.
Histochemistry
The mice (Sham+PBS group, n=6; MCAO+PBS group, n=8; Sham+iPS group, n=8; MCAO+iPS group, n=7) were anesthetized by intraperitoneal injection of ketamine hydrochloride, and then perfused with chilled PBS, followed by 4% paraformaldehyde in 0.1 mol/L phosphate buffer. After postfixation overnight, 50 μm-thick coronal sections were cut by a vibrating blade microtome (VT1000S; Leica, Heidelberg, Germany). For immunohistochemistry, the following primary antibodies were used: goat antidoublecortin (Dcx) antibody, 1:100 (Santa Cruz Biotechnology, Santa Cruz, CA, USA); mouse antineuronal-specific nuclear protein (NeuN) antibody, 1:100 (Millipore); rabbit anti-c-myc antibody, 1:50 (Abcam, Cambridge, UK); biotinylated Lycopersicon esculentum (tomato) lectin, 1:200 (Vector Laboratories, Burlingame, CA, USA), which binds N-acetylglucosamine oligomer (NAGO). The antibodies against Dcx and NeuN were detected with secondary antibodies conjugated with Alexa Fluor (Molecular Probes, Eugene, OR, USA) (1:500). The sections were captured by a confocal laser microscope (LSM510; Carl Zeiss, Jena, Germany). To estimate the expression of c-myc and NAGO were incubated with the respective first antibody and with or without secondary antibody (1:500). After incubation with ABC Elite complex (Vector Laboratories), the signal was visualized with diaminobenzidine tetrahydrochloride. The sections were captured by a microscope (BX51; Olympus, Tokyo, Japan).
Behavioral Analysis
At 24 h after transplantation of iPS cells, the surviving mice were tested every 7 days for behavioral changes and scored, as described by Bederson et al (1986) with minor modifications, as follows: 0, no observable neurologic deficits; 1, failure to extend the right forepaw; 2, circling to the contralateral side; 3, falling to the right; 4, unable to walk spontaneously. In addition, the mice were evaluated by rotarod test on the same day. To get habituated, the mice were exposed to a rotating rod 3 days before MCAO. Initially, mice were placed on a rod that was rotated at 0 r.p.m. The speed was slowly accelerated to 45 r.p.m. over a period of 5 mins. The mice were allowed maximum of three trials to remain on the rotarod for 5 mins and the evaluator was terminated when the animal fell from the rotarod or reached criterion level. The maximum time that the animal remained on the rod was recorded and used as an indicator of integrity of motor coordination.
Statistical Analysis
Values are expressed as mean±s.d. The differences in tumor volume were evaluated for statistical significance by nonrepeated measures analysis of variance (ANOVA) and Student-Newman-Keuls (SNK) test. The intensity of NAGO staining was evaluated for statistical significance by nonrepeated measures ANOVA and Bonferroni correction. In all statistical analyses, significance was accepted at **P<0.01. The clinical score was evaluated for statistical significance by the Student's t-test.
Results
In this study, we used a mouse model of MCAO (Yamashita et al, 2006). Pluripotency of the iPS cells derived from Nanog-green fluorescent protein (GFP) transgenic mice (Okita et al, 2007), iPS-mouse embryonic fibroblasts (MEF)-Ng-20D-17, was confirmed beforehand by staining with SSEA-1, Nestin, Sox-2, and Oct-3/4, which are recognized as pluripotential markers (data not shown). Nanog-GFP was also confirmed in the iPS cells to be under the undifferentiated condition.
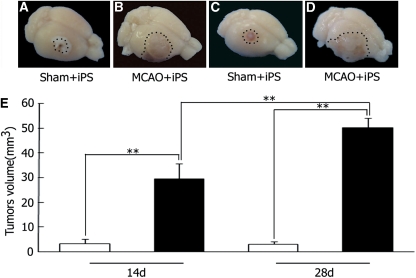
Ipsilateral transplantation of undifferentiated iPS cells into sham-operated brains formed only a small tumor within the limited area at 14 and 28 days after the transplantation (Figures 1A and 1C). However, iPS cells formed much larger tumors at 14 day (Figure 1B), which invaded over the infarcted area and formed strikingly big tumors at 28 day occupying hemisphere (Figure 1D). Tumor volume of the MCAO+iPS group was significantly larger than the Sham+iPS group (**P<0.01) both at 14 and 28 days, where that on 28 day is again larger than that on 14 day (Figure 1E).
Figure 1.
Tumorigenesis and tumor volume after intracerebral transplantation of mice iPS cells. Transplanted-induced pluripotent stem (iPS) cells formed only a small tumor within the sham-operated brain at 14 and 28 days (A, C), whereas iPS cells formed much larger tumors in the postischemic brain at 14 and 28 days (B, D). **P<0.01, nonrepeated measures analysis of variance (ANOVA) and SNK test (E). Values are mean±s.d. SNK, Student-Newman-Keuls.
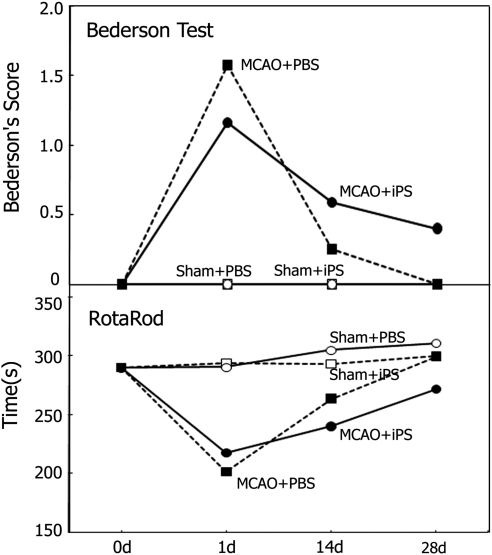
Behavioral examination of the mice showed that motor function described by Bederson's score was not statistically significantly different between the Sham+PBS and the Sham+iPS groups (Figure 2, upper panel). MCAO groups showed marked worsening of Bederson's score at 1day, whereas the score returned to the normal level at 28 day in the MCAO+PBS group, which was delayed in the MCAO+iPS group (Figure 2, upper panel), but did not reach a statistically significant difference. The clinical score described by Rotarod time was not significantly different between the Sham+PBS and the Sham+iPS groups (Figure 2, lower panel). MCAO groups showed marked worsening of Rotarod time at 1 day, whereas the time returned to the nomal level at 28 day in the MCAO+PBS group, which was delayed in the MCAO+iPS group (Figure 2, lower panel), but did not reach a statistically significant difference.
Figure 2.
Behavioral analysis after the induced pluripotent stem (iPS) cell transplantation. Motor functional analysis performed every 7 days after the iPS cell transplantation. The clinical scores shown as Bederson's score (upper panel) and Rotarod time (lower panel) were not significantly different between the Sham+PBS (open circles) and the Sham+iPS (open squares) groups. After transient decrease in the MCAO grpups (filled circles and squares), the recovery of clinical scores delayed in the MCAO+iPS group (filled circles) as compared with the MCAO+PBS group (filled squares), but did not reach a statistically significant difference. MCAO, middle cerebral artery occlusion; PBS, phosphate-buffered saline.
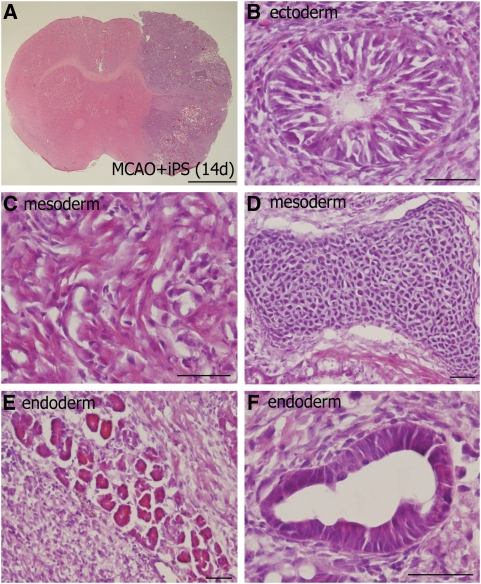
Hematoxylin–eosin staining showed tridermal transformation of iPS cells transplanted in MCAO at 14 (Figure 3A) and 28 days, where neural tube-like cells (Figure 3B), striated muscle fiber (Figure 3C), chondrocyte (Figure 3D), and immature or cylinder-like epithelium (Figures 3E and 3F), and endodermal cells formed cylinder-like epithelium (Figure 3B), and mesodermal cells striated muscle fiber (Figure 3C). A small tumor formed in the Sham+iPS group at 14 day showed only immature stage of cells.
Figure 3.
Undifferentiated induced pluripotent stem (iPS) cells formed tridermal teratoma in ischemic brain. Coronal brain sections obtained from the MCAO+iPS group (A to F) at 14 day after the transplantation, and stained with hematoxylin–eosin. The iPS cells expanded over the infacted area (A). The iPS-derived tumors consisted of ectodermal cells with neural tube-like cells (B), mesodermal cells with striated muscle fiber (C) and chondrocyte (D), and endodermal cells with immature (E) or cylinder-like (F) epithelium, scale bar=2 mm (A), 50 μm (B to F).
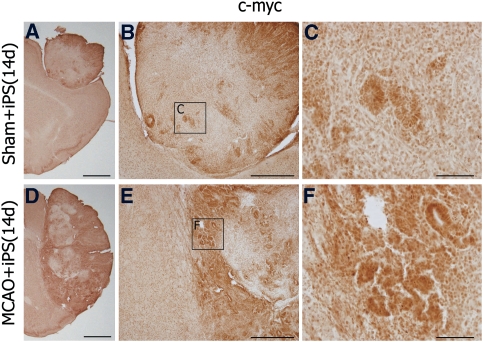
Single immunohistochemistry showed a prominent c-myc expression in cells of the tumors especially at the interface of iPS tumor and ischemic host brain (Figure 4) where c-myc expression was observed especially nuclear in the cylinder-like epithelial cells (Figures 4A and 4F).
Figure 4.
c-myc was prominently expressed in the tumors. c-myc was expressed in cells of the tumors at 14 day after the induced pluripotent stem (iPS) transplantation in the Sham+iPS (A) and the MCAO+PBS groups (D). c-myc expression was prominent at the interface of iPS tumor and ischemic host brain (D), as compared with sham brain (A), and c-myc stain was observed in the cylinder-like epithelial cells (B, C, E, F). (C) and (F) represent magnification of the boxed areas (c) and (f), respectively. Scale bar =2 mm (A, D), 500 μm (B, E), 100 μm (C, F). MCAO, middle cerebral artery occlusion; PBS, phosphate-buffered saline.
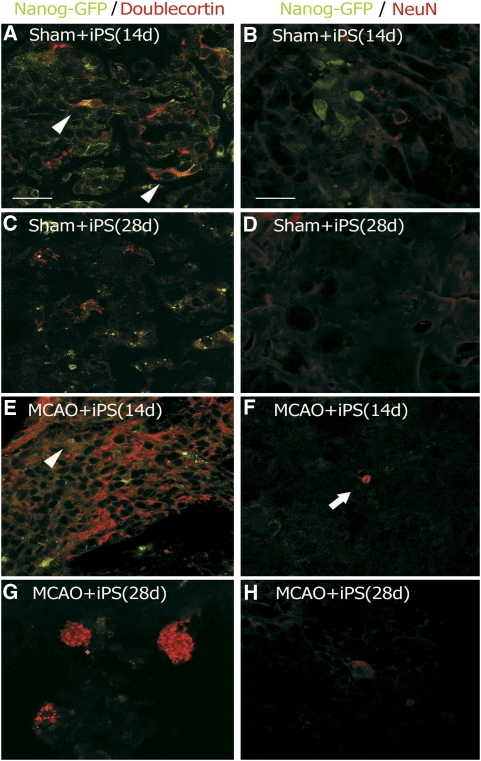
Double immunofluorescent analysis detected a lot of Nanog-GFP-positive cells with some Dcx-positive cells in the tumors of the Sham+iPS group at 14 day (Figure 5A), both of which then decreased at 28 day (Figure 5C). In contrast, a lot of Dcx-positive cells with only a small number of Nanog-GFP-positive cells were found in the tumors of the MCAO+iPS group at 14 day (Figure 5E), whereas most Nanog-GFP-positive cells disappeared with some cluster formation of Dcx-positive cells at 28 day (Figure 5G). Small number of Nanog-GFP and Dcx double-positive cells were found in the tumors of the Sham+iPS group and the MCAO+iPS group at 14 day (Figures 5A and 5E, arrowheads). Although a lot of Nanog-GFP-positive cells were found in the Sham+iPS group at 14 day, there was no double-positive cells with NeuN (Figure 5B). Both Nanog-GFP and NeuN showed a weak immunofluorescence without double positivity at 28 day in the Sham+iPS group (Figure 5D). In contrast, an early disappearance of Nanog-GFP fluorescence and a few NeuN-positive cells were found within the tumors of the MCAO+iPS group at 14 day (Figure 5F, arrow), but no more such NeuN-positive cells at 28 day (Figure 5H).
Figure 5.
Induced pluripotent stem (iPS) cells have a potential to produce both neuroblasts and mature neurons in normal and postischemic brains. iPS cells were originally labeled with Nanog-GFP before transplantation, and Dcx (a neuroblast marker) or NeuN (a mature neuronal marker) was stained in sections of the infracted area of the Sham+iPS (A to D) and the MCAO+iPS (E to H) groups. Small number of Nanog-GFP and Dcx double-positive cells were found in the tumors of the Sham+iPS group and the MCAO+iPS group at 14 day (A and E, arrowheads). A few NeuN-positive cells were found in the tumors of the MCAO+iPS group at 14 day (F, arrow). Scale bar =20 μm. GFP, green fluorescent protein; MCAO, middle cerebral artery occlusion; PBS, phosphate-buffered saline.
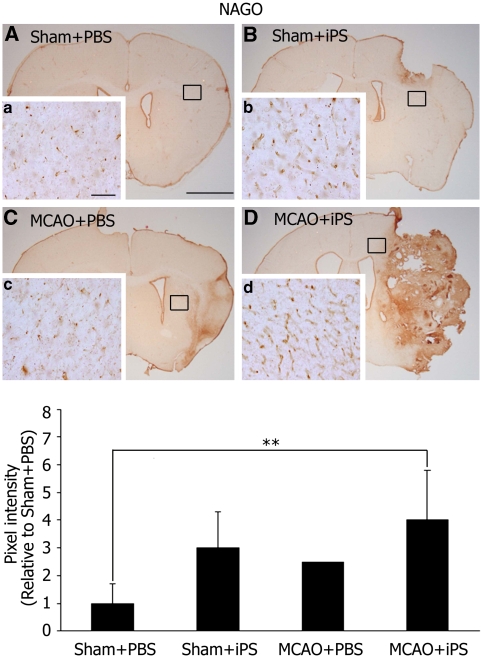
A protein marker of vascular endothelial cells, NAGO, was expressed at basal level in the Sham+PBS group (Figures 6A, 6a). An increase of NAGO staining was observed in the periphery of tumors in the Sham+iPS group at 28 day (Figures 6B, 6b). After tMCAO, NAGO staining of peri-infarct area slight increases in the MCAO+PBS group at 28 day (Figures 6C, 6c), but such staining was much more striking in the MCAO+iPS group (**P<0.01, Figures 6D, 6d).
Figure 6.
NAGO was abundantly expressed outside of tumors. NAGO, a protein marker of vascular endothelial cells, was observed at basal level at 28 day after the iPS cell transplantation in the Sham+PBS group (A), which was highly induced in the periphery of tumors in the Sham+iPS (B), and slightly induced in the MCAO+PBS groups (C). Such NAGO induction was more striking in the MCAO+iPS group (D); (a to d) represent magnification of the boxed areas in (A, B, C, D), respectively. Scale bar=2 mm (A), 100 μm (a). The intensity of NAGO staining was significantly higher in the MCAO+iPS group compared with Sham+PBS group (**P<0.01). Statistical analysis: nonrepeated measures analysis of variance (ANOVA) and Bonferroni correction. Data represent mean±s.d. iPS, induced pluripotent stem; MCAO, middle cerebral artery occlusion; NAGO, N-acetylglucosamine oligomer; PBS, phosphate-buffered saline.
Discussion
In this study, we first discovered mice iPS cells expanded and formed much larger tumors in mice postischemic brain than in sham-operated brain until 28 day after the transplantation (Figure 1). The clinical score described by Bederson's score and Rotarod time was not significantly different between the Sham+PBS and the Sham+iPS groups (Figure 2). However, MCAO groups showed a marked worsening of clinical score at 1 day, and the clinical recovery of the MCAO+iPS group was delayed as compared with the MCAO+PBS group (Figure 2). In addition, tridermal transformation of iPS cells was formed only in postischemic brains (Figure 3), where a part of iPS cells differentiated into neuroblasts and neurons at 14 or 28 day after the transplantation (Figure 5). In the tumors, c-myc expression was increased significantly (Figure 4), and in the periphery of the tumors, NAGO staining was observed at 28 day after the transplantation (Figure 6).
Although transplantation of undifferentiated iPS cells surprisingly developed quantitatively (Figure 1) and qualitatively (Figure 3) in the ischemic brain as compared with sham control brain, the fate of the transplanted cells in the ischemic brain may be determined based on the balances of the cells and the host interaction (Takagi et al, 2005). In another experiment, we also confirmed that matrix metalloproteinases 9 was remarkably expressed in the MCAO+iPS group in comparison with the Sham+iPS group (data not shown), which may allow the transplanted iPS cells to explosively expand. A previous report also showed a tridermal transformation of ES cells transplanted in postischemic brains (Erdo et al, 2003). In the cerebral ischemia, various cytokines, such as epidermal growth factor (Ninomiya et al, 2006) and leukemia-inhibiting factor (Suzuki et al, 2000), chemokines, and trophic factors are known to be produced and secreted mainly by reactive microglias and astrocytes surrounding the ischemic lesion. ES cell-derived neuronal progenitor cells were transplanted into the brain at 24 h after ischemia, and the transplanted cells expressed interleukin 6, which is considered to protect neurons from ischemic injury (Hayashi et al, 2006).
Our study first showed iPS cells have a potential to produce neuroblasts in normal and postischemic brain (Figure 5), as was the case of ES cells that also produced neuronal and grial cells in postischemic brain (Erdo et al, 2003). In the previous study, we showed that neuroblasts derived from subventricular zone migrated into ischemic region, but the amount of such endogenous neuroblasts was only a little (Yamashita et al, 2006). In contrast, our this study showed that exogenous iPS cells could supply a great number of Dcx-positive neuroblasts into the ischemic lesion (Figure 5E), indicating that iPS cell could be a promising therapeutic approach to provide sufficient neuronal cells for various neurologic disorders such as cerebral ischemia or other acute brain injury.
We showed the expression of c-myc increased significantly in the cylinder-like epithelial cells of the tumors (Figures 4C and 4F), and NAGO expression was observed in cells of the edge around the tumors (Figure 6). Proto-oncogene c-myc showed a strong nuclear staining in the epithelial cells (Zong et al, 2009), and c-myc induced in cells that have the morphologic characteristics of neurons after cerebral ischemia (Huang et al, 2001) and is essential not only to propagate and grow neural cells but also to promote vasculogenesis and angiogenesis (Baudino et al, 2002). To continuously grow, tumor usually requires nutritional and oxygen supply from surrounding vessels with angiogenesis (Lopes, 2003). The prominent expression of c-myc at the interface of iPS tumor and ischemic host brain (Figure 4D) supports a strong tumorigenicity of iPS cells in ischemic brain as compared with sham host brain (Figure 1), which also suggest that c-myc promoted angiogenesis in the tumors of the Sham+iPS and the MCAO+iPS groups (Figure 4), resulting in new vessel formation in the region around the tumors confirmed by NAGO staining (Figure 6).
In summary, our present data show that mice iPS cell transplanted into the normal and ischemic mice brains survived for 28 days and differentiated into neuroblasts especially in cases after tMCAO (Figure 5F). As iPS cells provided a great number of neuroblasts within transplanted tissue in the ischemic brain after MCAO (Figure 5), they have a promising potential to replace neural cells after ischemic brain injury, if tumorigenesis is properly controlled. In fact, a clinical trial reported that transplantation of differentiated murine iPS cells into the brain of Parkinson's disease model rat improved behavioral function (Wernig et al, 2007). A recent study reported that the teratoma formation depends on source of iPS cell (Miura et al, 2009). The reason why behavioral scores did not reach a significant difference between iPS and PBS-treated mice after MCAO may be that those tumorigenic iPS cells were not cytotoxic enough to make a great difference in clinical scores. Further study with iPS cells of another cell source, or with progenitor cells from iPS cells could clarify detailed effect of iPS cell transplantation for ischemic brain.
The authors declare no conflict of interest.
References
- Abe K. Therapeutic potential of neurotrophic factors and neural stem cells against ischemic brain injury. J Cereb Blood Flow Metab. 2000;20:1393–1408. doi: 10.1097/00004647-200010000-00001. [DOI] [PubMed] [Google Scholar]
- Amabile G, Meissner A. Induced pluripotent stem cells: current progress and potential for regenerative medicine. Trends Mol Med. 2009;15:59–68. doi: 10.1016/j.molmed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Baudino TA, McKay C, Pendeville-Samain H, Nilsson JA, Maclean KH, White EL, Davis AC, Ihle JN, Cleveland JL. c-Myc is essential for vasculogenesis and angiogenesis during development and tumor progression. Genes Dev. 2002;16:2530–2543. doi: 10.1101/gad.1024602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Chang YC, Shyu WC, Lin SZ, Li H. Regenerative therapy for stroke. Cell Transplant. 2007;16:171–181. [PubMed] [Google Scholar]
- Chopp M, Li Y. Treatment of stroke and intracerebral hemorrhage with cellular and pharmacological restorative therapies. Acta Neurochir Suppl. 2008;105:79–83. doi: 10.1007/978-3-211-09469-3_16. [DOI] [PubMed] [Google Scholar]
- Erdo F, Buhrle C, Blunk J, Hoehn M, Xia Y, Fleischmann B, Focking M, Kustermann E, Kolossov E, Hescheler J, Hossmann KA, Trapp T. Host-dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J Cereb Blood Flow Metab. 2003;23:780–785. doi: 10.1097/01.WCB.0000071886.63724.FB. [DOI] [PubMed] [Google Scholar]
- Hayashi J, Takagi Y, Fukuda H, Imazato T, Nishimura M, Fujimoto M, Takahashi J, Hashimoto N, Nozaki K. Primate embryonic stem cell-derived neuronal progenitors transplanted into ischemic brain. J Cereb Blood Flow Metab. 2006;26:906–914. doi: 10.1038/sj.jcbfm.9600247. [DOI] [PubMed] [Google Scholar]
- Hof P, Young WG, Bloom FE, Belichenko PV, Celio MR. Mouse brains: comparative cytoarchiteftonic atras of the C57BL/6 and 129/Sv.228. Amsterdam, The Netherlands: Elsevier; 2000. [Google Scholar]
- Huang CY, Fujimura M, Noshita N, Chang YY, Chan PH. SOD1 down-regulates NF-kappaB and c-Myc expression in mice after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:163–173. doi: 10.1097/00004647-200102000-00008. [DOI] [PubMed] [Google Scholar]
- Koch P, Kokaia Z, Lindvall O, Brustle O. Emerging concepts in neural stem cell research: autologous repair and cell-based disease modelling. Lancet Neurol. 2009;8:819–829. doi: 10.1016/S1474-4422(09)70202-9. [DOI] [PubMed] [Google Scholar]
- Lopes MB. Angiogenesis in brain tumors. Microsc Res Tech. 2003;60:225–230. doi: 10.1002/jemt.10260. [DOI] [PubMed] [Google Scholar]
- Miljan EA, Sinden JD. Stem cell treatment of ischemic brain injury. Curr Opin Mol Ther. 2009;11:394–403. [PubMed] [Google Scholar]
- Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, Nakagawa M, Koyanagi M, Tanabe K, Ohnuki M, Ogawa D, Ikeda E, Okano H, Yamanaka S. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- Ninomiya M, Yamashita T, Araki N, Okano H, Sawamoto K. Enhanced neurogenesis in the ischemic striatum followingEGF-induced expansion of transit-amplifying cells in the subventricular zone. Neurosci Lett. 2006;403:63–67. doi: 10.1016/j.neulet.2006.04.039. [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Ronaghi M, Erceg S, Moreno-Manzano V, Stojkovic M. Challenges of stem cell therapy for spinal cord injury: human embryonic stem cells, endogenous neural stem cells or induced pluripotent stem cells. Stem Cells. 2009;28:93–99. doi: 10.1002/stem.253. [DOI] [PubMed] [Google Scholar]
- Shichinohe H, Kuroda S, Yano S, Hida K, Iwasaki Y. Role of SDF-1/CXCR4 system in survival and migration of bone marrow stromal cells after transplantation into mice cerebral infarct. Brain Res. 2007;1183:138–147. doi: 10.1016/j.brainres.2007.08.091. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Tanaka K, Nogawa S, Ito D, Dembo T, Kosakai A, Fukuuchi Y. Immunohistochemical detection of leukemia inhibitory factor after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2000;20:661–668. doi: 10.1097/00004647-200004000-00003. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Nishimura M, Morizane A, Takahashi J, Nozaki K, Hayashi J, Hashimoto N. Survival and differentiation of neural progenitor cells derived from embryonic stem cells and transplanted into ischemic brain. J Neurosurg. 2005;103:304–310. doi: 10.3171/jns.2005.103.2.0304. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Thomson JA. Pluripotent stem cell lines. Genes Dev. 2008;22:1987–1997. doi: 10.1101/gad.1689808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong Y, Xin L, Goldstein, Lawson DA, Teitell MA, Witte ON. ETS family transcription factors collaborate with alternative signaling pathways to induce carcinoma from adult murine prostate cells. Proc Natl Acad Sci USA. 2009;106:12465–12470. doi: 10.1073/pnas.0905931106. [DOI] [PMC free article] [PubMed] [Google Scholar]