Abstract
Hypercholesterolemia is associated with decreased nitric oxide (NO) bioavailability and endothelial dysfunction, a phenomenon thought to have a major role in the altered cerebral blood flow evident in stroke. Therefore, strategies that increase endothelial NO production have potential utility. Vascular reactivity of the middle cerebral artery (MCA) from C57BL/6J wild-type (WT) mice, apolipoprotein-E knockout (ApoE−/−) mice, and mice treated with the phosphodiesterase inhibitor cilostazol (100 mg/kg) was analyzed using the tension myograph. Contractile responses to endothelin-1 were significantly enhanced in MCA from ApoE−/− mice compared with WT mice (P<0.01), an effect absent in cilostazol-treated ApoE−/− mice. Acetylcholine-induced relaxation (which is entirely NO-dependent) was significantly impaired in MCA of ApoE−/− mice compared with WT mice (P<0.05), again an effect prevented by cilostazol treatment. Endothelial NOS phosphorylation at Ser1179 was decreased in the aorta of ApoE−/− mice compared with WT mice (P<0.05), an effect normalized by cilostazol. Taken together, our data suggest that the endothelial dysfunction observed in MCA associated with hypercholesterolemia is prevented by cilostazol, an effect likely due to the increase in eNOS phosphorylation and, therefore, activity.
Keywords: cerebral arteries, endothelium, hypercholesterolemia, nitric oxide, vasomotor function
Introduction
Atherosclerosis is a major underlying cause for ischemic stroke, and therapuetics targeting atherogenesis (particularly statin therapy) decrease the risk of stroke in high-risk individuals or in patients with stroke or transient ischemic attack (Amarenco and Labreuche, 2009). In part, this improved outcome has been attributed to a slowed progression of intracranial (carotid) atherosclerosis (Konishi et al, 1993) consequent to a decrease in hypercholesterolemia (Amarenco and Labreuche, 2009). However, there is also evidence that the beneficial effects of such interventions potentially relate to effects independent of lipid modification. Alterations in vascular function precede, and are thought to be pathogenic, in cardiovascular disease, including stroke (Knottnerus et al, 2009). Altered vascular reactivity associated with atherosclerotic disease in patients has been attributed to hypercholesterolemia-induced ‘endothelial dysfunction,' which is characterized specifically by a decrease in the bioavailability of the vasodilator endothelium-derived nitric oxide (NO) (Casino et al, 1993). This view, that endothelial vasodilator NO production is reduced in stroke, is supported by observations that patients with ischemic stroke have impaired cerebral blood flow (Maeda et al, 1993), an effect that is predictive of lacunar infarction (Molina et al, 1999) and is associated with a higher risk of stroke (Yonas et al, 1993). In addition, recent work suggests that reduced capacity to generate NO, reflected by decreased levels of the metabolites of NO in plasma, correlates with risk of stroke (Rashid et al, 2003) and may be due to the presence of specific genetic polymorphisms in the endothelial form of the NO synthase enzyme, eNOS, gene (Tao and Chen, 2009).
Conceptually, therapeutic targeting of vascular dysfunction in stroke has become an attractive proposition with the findings that a component of the beneficial effects of statin therapy in patients at risk of stroke is the result of improvements in cerebral blood flow. Moreover, this effect is secondary to a reversal of endothelial dysfunction (Sterzer et al, 2001; Pretnar-Oblak et al, 2006) resulting in improved levels of NO (Endres, 2005). This phenomenon of decreased bioactive NO in disease is recapitulated in animal models of atherosclerosis, particularly in the apolipoprotein-E (ApoE−/−) knockout mice that develop spontaneous hypercholesterolemia and atherosclerotic lesions resembling those observed in humans (Zhang et al, 1992; Breslow, 1996). Studies show that NO-mediated endothelium-dependent relaxation is impaired in the systemic and the cerebral vasculature of ApoE−/− mice (d'Uscio et al, 2001; Kitayama et al, 2007) and that the effects of ischemic stroke are substantially enhanced in these animals compared with wild-type (WT) controls (Sheng et al, 1999). Such observations suggest that these mice provide a useful model not only to investigate mechanisms of cerebrovascular dysfunction but also for testing the potential of novel therapeutics.
Although there has been some recent advance in the treatment of ischemic stroke, therapeutics that limit cerebral infarct and decrease further risk of stroke after transient ischemic attacks are limited. Cilostazol is a selective inhibitor of phosphodiesterase-3 (PDE3) and as such inhibits hydrolysis of cyclic AMP (cAMP) by PDE3 resulting in increases in intracellular cAMP levels in platelets (Kimura et al, 1985) and vascular smooth muscle cells (Takahashi et al, 1992). Moreover, cilostazol reduces infarct volume in animal models of ischemic stroke (Yuzawa et al, 2008) and reduces the recurrence of cerebral infarction (Gotoh et al, 2000) and prevents the progression of intracranial arterial stenosis in stroke patients (Kwon et al, 2005). These findings underlie the approval for use of cilostazol for stroke in Japan.
The beneficial effects of cilostazol appear to extend beyond the obvious antiplatelet effects and have, in part, been attributed to its vasodilator actions (Tanaka et al, 1998). In line with such an effect is the demonstration of improvement in cerebrovascular blood flow in patients with stroke (Kobayashi et al, 1985; Mochizuki et al, 2001). However, the mechanisms regarding its beneficial effect remain unclear. In addition to its direct effect on smooth muscle cells (Tanaka et al, 1998), a recent study has shown that cilostazol causes vasodilatation through an endothelial-NO-dependent pathway in rat aorta (Nakamura et al, 2001). In addition, in human aortic endothelial cells (HAECs) in culture (Hashimoto et al, 2006), cilostazol increases eNOS activity by stimulating phosphorylation, an important pathway regulating vascular NO synthesis (Dimmeler et al, 1999; Fulton et al, 1999).
The aim of this study was to investigate whether the vasomotor function in the middle cerebral artery (MCA) of ApoE−/− mice is altered compared with WT animals. In addition, we have examined the possibility that cilostazol might improve vascular function in ApoE−/− mice and dissected the mechanisms involved in any beneficial effects seen.
Materials and methods
Experimental Animals
All experiments were conducted according to the Animals (Scientific Procedures) Act of 1986 (United Kingdom). Male C57BL/6J WT (Charles River, UK) or ApoE−/− mice (Jackson Lab, Bar Harbor, ME, USA) at 16 weeks of age were used in this study. All mice were maintained on normal chow diet. In some experiments, ApoE−/− mice were given cilostazol (100 mg/kg/day orally) (Takase et al, 2007) or 0.5% w/v carboxylmethylcellulose in dH2O vehicle by gavage for 2 weeks from 14 weeks of age.
Plasma Lipid, Nitrite and Nitrate, and Cyclic Guanosine Monophosphate Measurement
Mice were anesthetized (sodium pentobarbital, 60 mg/kg, intraperitoneally) and blood samples collected by intracardiac puncture into heparin (12 U/ml). Blood was centrifuged at 13,000 r.p.m. and plasma collected. High-density lipoprotein (HDL) and low-density lipoprotein (LDL)/very-low-density lipoprotein (VLDL) levels were measured using a commercially available ELISA Kit (Abcam, Cambridge, UK) and the triglyceride level was measured with the Serum Triglyceride Determination ELISA Kit (Sigma, Poole, UK) according to the manufacturer's instructions. Plasma NOx (nitrite and nitrate) was measured using chemiluminescence as previously described (Ignarro et al, 1993). Cyclic guanosine monophosphate (cGMP) levels were determined using an enzyme immunoassay (cGMP EIA Biotrak System, GE Healthcare UK Ltd, Chalfont St. Giles, UK) according to the manufacturer's instructions.
Tension Myograph
After sacrifice of mice, the brain was carefully removed and the MCA cleared of extraneous tissue. Segments (∼2 mm lengths) of MCA were mounted in an automated tension myograph (Danish Myotechnology, Aarhus, Denmark) containing Krebs solution gassed with 95% O2 and 5% CO2 at 37°C. After an equilibration period of 60 mins, vessels were normalized to give approximately 1 to 1.5 mN basal tension. In brief, vessels were stretched in 0.5 mN steps until a tension of ⩾2.5 mN was achieved. Using the automated myograph software (to construct a diameter tension graph), the vessel was set at a diameter 90% of that when under 2.5 mN, approximating to the tension these vessels usually sustain in vivo (Mulvany and Halpern, 1977). Vessel viability was assessed by the addition of KCL (75 mmol/L), and only vessels developing tension ⩾1 mN were used. Vessel tension was recorded with an isometric force transducer and Powerlab software (ADInstruments, Chalgrove, UK). In all experiments, only a single concentration–response curve was conducted in any vessel.
Cumulative concentration–response curves to the vasoconstrictors endothelin-1 (ET-1; 0.01 to 30 nmol/L), thromboxane A2 mimetic U-46619 (1 to 3000 nmol/L), or phenylephrine (0.001 to 300 μmol/L) were constructed. As these studies showed that the vascular reactivity to ET-1 was the most altered in MCA from ApoE−/− mice compared with WT, further experiments testing the effect of cilostazol treatment focused on measuring alterations in ET-1 reactivity.
Cumulative concentration–response curves to ET-1 were constructed in arteries of ApoE−/− mice and those treated with cilostazol. To examine the relationship between vascular reactivity to ET-1 and NO production, vessels were preincubated with NOS inhibitor L-NAME (300 μmol/L for 15 mins) and cumulative concentration–response curves to ET-1 constructed.
To investigate changes in basal endothelial NO production in MCA, contractile concentration–response curves to L-NAME (1 to 300 μmol/L) were constructed in arteries of WT, ApoE−/−, and ApoE−/− + cilostazol-treated mice. Vessels were pretreated with U-46619 (1 to 30 nmol/L) to produce a small level of tone (EC10) and then L-NAME curves constructed. To investigate endothelium-dependent relaxant responses, in a separate series of experiments, vessels were precontracted with U-46619 at a concentration designed to induce 80% of the maximum contraction to KCL (75 mmol/L), after which cumulative relaxation concentration–response curves were constructed for acetylcholine (ACh; 1 to 30 μmol/L) or the NO donor spermine-NO (SPER-NO; 1 to 30 μmol/L) in MCA of WT, ApoE−/−, and ApoE−/− + cilostazol-treated mice. All relaxations were expressed as % reversal of the U-46619-induced tone.
Western Blot Analysis
Owing to the limitation in sample size of cerebral arteries, thoracic aortae of mice were collected for western blotting. Aortae of ApoE−/− and ApoE−/− + cilostazol-treated mice were individually homogenized, on ice, in lysis buffer containing 10 mmol/L Tris (pH 7.5), 50 mmol/L NaCl, 30 mmol/L NaPPi, 2 mmol/L ethylenediaminetetraacetic acid, 50 mmol/L NaF, 1% Triton X-100, 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L Na3VO4, 1 μg/ml protease inhibitor mix (aprotinin, antipan hydrochloride, benzamidine hydrochloride hydrate, and leupeptin hydrochloride; Sigma). Equal amounts of protein (20 μg/lane) were subjected to electrophoresis in a 7.5% Tris-HCL polyacrylamide gel and transferred to nitrocellulose membrane. The membrane was incubated overnight at 4°C with primary antibodies against phospho-eNOS (Ser1179) (1:1000 dilution; Cell Signalling, Danvers, MA, USA), eNOS (1:2000 dilution; Santa Cruz, Santa Cruz, CA, USA), or α-actin (1:2000 dilution; Serotec, Oxford, UK). Nitrocellulose was then incubated with horseradish peroxide-conjugated antirabbit or antimouse IgG secondary antibodies (1:2000 dilution; Dako, Glostrup, Denmark). Bands were visualized by a chemiluminescence reagent (Cell Signaling) and quantified using Scan Image software (Scion Corporation, Frederick, MD, USA).
Human Aortic Endothelial Cells
Human aortic endothelial cells were cultured in 5% CO2 at 37°C in endothelial cell growth medium (Lonza, Slough, UK) and used at passage 4. For the study, HAECs were incubated in the presence or absence of 100 μmol/L isobutylmethylxanthine, 30 μmol/L cilostazol, and 10 μmol/L SPER-NO for 1 h before the medium was frozen for measurement of nitrite (believed to be a more accurate indicator of acute changes in eNOS activity (Lauer et al, 2001)) using chemiluminesence as previously described (Ignarro et al, 1993). Cells were lysed and cGMP levels were determined using an enzyme immunoassay (cGMP EIA Biotrak System, GE Healthcare UK Ltd) according to the manufacturer's instructions.
Drugs
Cilostazol was supplied by Otsuka Pharmaceutical Co Ltd. Acetylcholine hydrochloride, phenylephrine, L-NAME, and isobutylmethylxanthine were obtained from Sigma. U-46619 was from Biomol (Exeter, UK). Endothelin-1 was purchased from Peptide Institute, Osaka, Japan. Spermine NONOate (SPER-NO) was from Cayman Chemical (Ann Arbor, MI, USA).
Statistical Analysis
Results are expressed as the mean±s.d. Relaxation to ACh and SPER-NO is expressed as a percent relaxation of U-46619-induced contraction. The n-values refer to the number of mice from which tissues were obtained or, for cell culture studies, the number of times the experiment was repeated. Statistical comparisons between curves were made using two-way analysis of variance followed by Bonferroni's post hoc test for individual concentration comparisons or one-way analysis of variance followed by Bonferroni post-tests for individual values and for cell culture studies using Graph Pad Prism software (La Jolla, CA, USA). A value of P<0.05 was considered to be significant.
Results
The internal diameter, basal tension, and contractile response to KCL (75 mmol/L) of MCA were similar between WT, ApoE−/−, and ApoE−/− + cilostazol-treated mice (Table 1). HDL level was decreased, whereas LDL/VLDL and triglyceride levels were significantly increased in ApoE−/− mice compared with WT mice. Cilostazol treatment did not significantly alter these lipid levels (Table 2).
Table 1. The internal diameters and mean responses to KCL (75 mmol/L) of middle cerebral artery.
| WT | ApoE−/− | ApoE−/− +Cilostazol | |
|---|---|---|---|
| Diameter (μm) | 121.1±9.1 | 124.0±9.0 | 125.4±1.0 |
| Response to KCL (mN) | 1.79±0.36 | 1.81±0.38 | 1.86±0.28 |
Data are expressed as mean±s.e.m. of n=23 for wild-type (WT), n=28 for apolipoprotein-E (ApoE−/−) mice, and n=23 for ApoE−/− mice treated with cilostazol.
Table 2. Plasma lipid profile.
| WT | ApoE−/− | ApoE−/− +Cilostazol | |
|---|---|---|---|
| HDL (mg/dL) | 65.7±5.7 | 13.6±1.5‡ | 13.8±1.4‡ |
| LDL/VLDL (mg/dL) | 39.6±4.1 | 378.0±204.2* | 369.0±236.8* |
| Triglyceride (mg/dL) | 206.2±27.9 | 286.0±18.5‡ | 269.4±33.1† |
Apo-E, apolipoprotein-E; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein; WT, wild type.
Data are expressed as mean±s.e.m. of at least five mice per group. Statistical significance shown as *P<0.05, †P<0.01, ‡P<0.001 versus WT mice.
Vasoconstrictor Responses
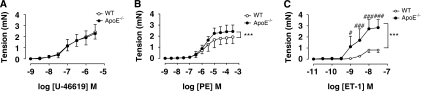
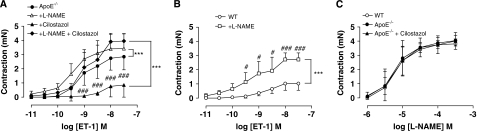
Contractile responses to U-46619 were not different between WT and ApoE−/− mice (Figure 1). Analysis of the response curve profiles to phenylephrine using two-way analysis of variance showed a significant (P<0.001, Figure 1) enhancement of the responses in ApoE−/− mice. Similarly, the vascular reactivity to ET-1 was markedly increased in ApoE−/− mice compared with WT mice with both an increase in the potency and the maximum response (Figure 1, Table 3). Cilostazol treatment of ApoE−/− mice suppressed ET-1 responses back to levels observed in WT mice (Figure 2A, Table 3). This effect of cilostazol was reversed by L-NAME treatment (Figure 2A, Table 3). Treatment of vessels from WT mice with L-NAME substantially increased both the potency and the maximum response to ET-1 (Figure 2B).
Figure 1.
Concentration–response curves to (A) U-46619 (n=6), (B) phenylephrine (PE; n=6), and (C) endothelin-1 (ET-1; n=4) in middle cerebral artery (MCA) from wild-type (WT) and ApoE−/− mice. Data are expressed as mean±s.d. and statistical significance is shown as ***P<0.001 for comparison of curves followed by Bonferroni post-tests shown as #P=0.05 and ###P=0.001.
Table 3. Reactivity parameters of ET-1 in MCA from WT and ApoE−/− mice.
| WT | WT + L-NAME | ApoE−/− | ApoE KO + L-NAME | ApoE−/−+Cilostazol | |
|---|---|---|---|---|---|
| pEC50 | 8.5±0.1 | 9.1±0.4 | 9.0±0.1 | 9.4±0.1 | 8.3±0.2## |
| Max (mN) | 0.9±0.1 | 2.7±0.2*** | 2.8±0.3*** | 3.4 ±0.5 | 0.8±0.3### |
| n | 8 | 4 | 8 | 4 | 6 |
Apo-E, apolipoprotein-E; ET-1, endothelin-1; KO, knockout; MCA, middle cerebral artery; WT, wild-type.
Data are expressed as mean±s.e.m. The maximum response (Max) is expressed in mN. Animals were treated in vivo with cilostazol (100 mg/kg orally daily for 2 weeks), for L-NAME (300 μmol/L) experiments arteries were pretreated for 30 mins. Statistical significance shown as ***P<0.001 versus WT, #P<0.05, ###P<0.01 versus ApoE−/−.
Figure 2.
(A) Concentration–response curves for endothelin (ET)-1 with or without L-NAME (300 μmol/L) in middle cerebral artery (MCA) from ApoE−/− mice, and those treated with cilostazol, n=4 to 6. (B) Concentration–response curves to ET-1 with or without L-NAME (300 μmol/L) in MCA from wild-type (WT) mice, n=4. (C) Concentration–response curves to L-NAME in MCA from WT, ApoE−/− mice, and those treated with cilostazol (n=4). All data are expressed as mean±s.d. Statistical significance shown as ***P<0.001 for comparison of curves as indicated on a graph followed by Bonferroni post-tests shown as #P<0.05 and ###P<0.001.
In contrast, there were no differences in the contractile concentration–response curves to L-NAME between the MCA of WT, ApoE−/−, or ApoE−/− + cilostazol-treated mice (Figure 2C).
Endothelium-Dependent and -Independent Relaxation
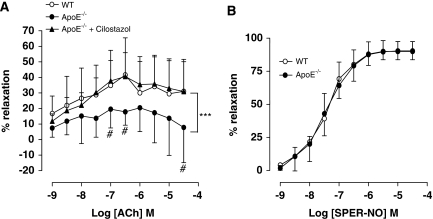
Endothelium-dependent relaxation to ACh was significantly impaired in MCA of ApoE−/− mice. Cilostazol treatment of mice restored responses to ACh to levels observed in arteries of WT mice (Figure 3A). Endothelium-independent relaxation in response to SPER-NO was not different between the MCA of WT and ApoE−/− mice (Figure 3B).
Figure 3.
Relaxation responses to the endothelium-dependent vasodilator (A) acetylcholine (ACh) and (B) the endothelium-independent vasodilator, the nitric oxide (NO) donor spermine-NO (SPER-NO), in middle cerebral artery (MCA) from wild-type (WT) and ApoE−/− mice and those mice treated with cilostazol. Data are expressed as mean±s.d. of five to nine mice for ACh and four to six for SPER-NO. Statistical significance shown as ***P<0.001 for comparison of curves followed by Bonferroni post-tests shown as #P<0.05 for ApoE−/− versus ApoE−/− + cilostazol.
Plasma Nitrite and Nitrate and Cyclic Guanosine Monophosphate Levels
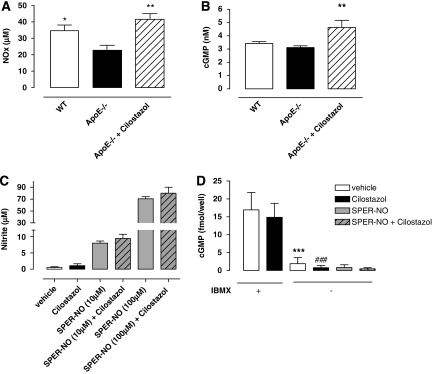
Plasma NOx and cGMP levels were significantly decreased in ApoE−/− mice compared with WT (P<0.05). However, treatment with cilostazol reversed this depression (P<0.01; Figures 4A and 4B).
Figure 4.
Plasma nitrite and nitrate (Nox) (A) and cyclic guanosine monophosphate (cGMP) (B) levels in wild-type (WT), ApoE−/− mice, and cilostazol-treated ApoE−/−. Extracellular nitrite (C) and intracellular cGMP (D) in human aortic endothelial cells (HAECs) incubated with and without isobutylmethylxanthine (IBMX) (100 μmol/L), cilostazol (30 μmol/L), and spermine-nitric oxide (SPER-NO) (10 to 100 μmol/L for nitrite and 100 μmol/L for cGMP) for 1 h at 37°C. Data are expressed as mean±s.d. of n=6 to 8 mice for in vivo experimentation or n=4 for cell culture analyses. Statistical significance shown as *P< 0.05, **P<0.01 versus ApoE−/− mice or versus vehicle control and ***P<0.001 and ###P<0.001 versus respective control in the presence of IBMX.
Acute treatment of HAECs with cilostazol did not alter nitrite levels in untreated cells or cells incubated with SPER-NO (Figure 4C). In addition, this treatment with cilostazol did not increase cGMP levels in HAECs per se. In contrast, in the presence of isobutylmethylxanthine, cGMP levels were equally elevated in both control or cilostazol-treated cells (Figure 4D).
Endothelial Nitric Oxide Synthase Phosphorylation at Ser1179 and Endothelial Nitric Oxide Synthase Expression
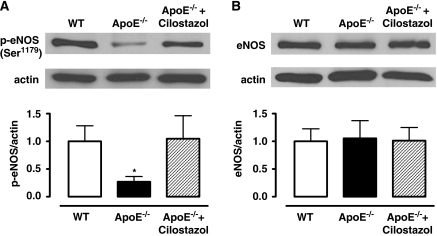
Western blot analysis showed that phosphorylation of eNOS at Ser1179 was significantly decreased in ApoE−/− mice compared with WT mice (P<0.05). However, levels of eNOS phosphorylation were restored in arteries of cilostazol-treated ApoE−/− mice (P<0.05). Total eNOS expression was similar between all groups (Figure 5).
Figure 5.
Western blot analysis for (A) endothelial nitric oxide synthase (eNOS) phosphorylation at Ser1179 and (B) eNOS in aorta from wild-type (WT), ApoE−/− mice, and those treated with cilostazol. Data are expressed as mean±s.d. of n=4. Statistical significance shown as *P<0.05 versus WT, ApoE−/− + cilostazol.
Discussion
Cerebrovascular dysfunction precedes and is believed to be pathogenic in ischemic stroke. Recent evidence suggests that the targeting of the cerebral vasculature to improve endothelial function, in addition to strategies that limit atherosclerotic plaque formation, is likely to provide significantly improved outcome in disease. Therefore, a greater understanding of the pathways involved in cerebrovascular dysfunction is warranted. In this study, using the hypercholesterolemic ApoE−/− mouse, we show that there is a selective enhancement of responses of cerebral arteries to the vasoconstrictor ET-1 in addition to suppressed endothelial vasodilator activity. In addition, we show that the mechanisms involved in both of these effects relates to a decrease in endothelial NO generation that is likely due to a suppression of eNOS activity, as a consequence of reduced eNOS phosphorylation. Moreover, we show that treatment in vivo of mice with cilostazol, a drug relatively recently introduced as treatment for stroke, restores vascular reactivity to both endothelium-dependent vasodilators and ET-1, an effect due to enhancement of eNOS phosphorylation. We suggest that drugs such as cilostazol, which improve endothelial function, also decrease the sensitivity to the potent vasoconstrictor ET-1, and that this effect likely has a role in mediating the beneficial effects of such strategies in cerebrovascular disease, specifically ischemic stroke.
In this study, hypercholesterolemia was associated with substantial vascular dysfunction in MCA of ApoE−/− mice as evidenced by an enhancement of the contractile response to ET-1 (3.5-fold increase in the maximum response) with a relatively more moderate, albeit significant, increase in reactivity to phenylephrine (1.2-fold) in comparison with WT controls. In contrast, there was no alteration in the sensitivity to the TXA2-mimetic U-46619. This apparent selectivity, particularly for ET-1, implies that the enhancement in contractile reactivity was not due to a generalized alteration in function of the underlying smooth muscle. This view is supported by the observation that the contractile response to the depolarizing stimulus, KCL, was almost identical in cerebral arteries of WT and ApoE−/− mice.
In cerebral arteries, as in the peripheral vasculature, ET-1 primarily acts on ETA receptors on the vascular smooth muscle to promote vasoconstriction but causes NO-mediated dilatation through activation of ETB receptors expressed on the endothelium (Szok et al, 2001). However, following cerebral ischemia, responses to ET-1 are enhanced and relate to increased ETB receptor expression on smooth muscle cells (Stenman et al, 2002), or enhanced signaling mediated by protein kinase-C and Rho-kinase (Barman, 2007). It is unlikely that these latter mechanisms underlie the enhanced activity in this study since animals were not subjected to an ischemic insult. However, previous studies have also shown that ApoE−/− mice have elevated ETB receptor expression and elevated ET-1 in the aorta. Likewise, ET-1 levels have been reported to be elevated in the plasma, coronary artery, and aorta of patients with hypercholesterolemia (Lerman et al, 1991; Kobayashi et al, 2000). In addition, ET-1-induced contractile responses are enhanced in the aorta of ApoE−/− mice (Maguire et al, 2006). Our studies show, for the first time, that in addition to the aorta, ET-1 sensitivity is enhanced in the cerebral vasculature of ApoE−/− mice compared with WT controls. Although whether ET receptor expression is also altered in the cerebral artery in the current study is unknown.
An alternative explanation for the altered sensitivity to both ET-1 and phenylephrine is that although the contraction of smooth muscle in response to these agonists is the consequence of activating smooth muscle receptors, these agonists also simultaneously result in endothelial NO generation that limits the magnitude of the contractile response, whereas this is not the case for thromboxane A2 (Chauhan et al, 2003). Therefore, in situations where endothelial dysfunction exists, this buffering mechanism will be diminished and contraction enhanced. In accord with the hypothesis, the relaxant responses to the endothelium-dependent vasodilator, ACh, were substantially attenuated in the MCA of ApoE−/− mice in comparison with WT animals. This decreased response to ACh is similarly observed in the aorta, carotid artery, and cerebral arterioles of ApoE−/− mice (d'Uscio et al, 2001; Kitayama et al, 2007), and also in the forearm and coronary arteries of hypercholesterolemic patients (Casino et al, 1993). In agreement with the thesis that stimulus-induced NO production was altered in ApoE−/− animals, treatment of arteries in vitro, with L-NAME, also enhanced ET-1-induced contraction in MCA of WT animals but had no further enhancing effect in the arteries of ApoE−/− mice. That this effect is due to loss of NO synthesis by the endothelium, rather than a depressed sensitivity of vascular smooth muscle to NO, is shown by the fact that the relaxant effects of the NO donor, SPER-NO, were no different between the two genotypes.
Our experiments investigating the effect of L-NAME in uncontracted arteries suggest that although stimulus-induced NO production is altered in the MCA of ApoE−/− mice, basal NO synthesis is not. This is shown by the fact that the contractile concentration–response curve to L-NAME was identical between the genotypes. This finding contrasts with recent observations in aortic tissue, where the responses to L-NAME (300 μmol/L) were substantially attenuated in aortic rings of ApoE−/− mice compared with WT controls (Fransen et al, 2008). The reason for this difference is uncertain but may simply be a reflection of the functional differences between a large conduit vessel, with little role in regulating blood flow, and an artery such as the MCA that has a major role in regulating organ perfusion pressure.
Treatment of ApoE−/− animals with the PDE3 inhibitor, cilostazol, at a dose associated with decreased atheroma formation in ApoE−/− mice (Takase et al, 2007) suppressed ET-1-induced contraction and restored ACh-induced relaxation to levels evident in the WT controls. In contrast, cilostazol had no effect on the relaxation response to SPER-NO and did not alter the contractile response curve to L-NAME. These findings suggest that cilostazol exerts a selective enhancing effect on stimulus-evoked NO production, rather than suppressing basal NO synthesis or by causing a generalized alteration in vascular smooth muscle reactivity. This apparent improvement in NO synthesis was reflected in the plasma measures of NOx showing a recovery of vascular NO synthesis to levels observed in WT animals.
Impairment of NO-mediated vasomotor function in hypercholesterolemia is caused by several mechanisms including alterations of substrate and/or cofactor availability for eNOS, as well as alterations of NOS expression and/or activation and enhanced degradation of NO by superoxide anions. In addition, studies show that eNOS phosphorylation at Ser1179 (Ser1177 in humans) has an important role in regulating eNOS activity and NO production, as well as vascular reactivity, in response to normal physiological stimuli, notably physiological shear stress, circulating hormones, and female sex hormones (Dimmeler et al, 1999; Fulton et al, 1999). Of particular relevance to this study, phosphorylation of eNOS to enhance NO generation has been implicated as an endogenous protective mechanism limiting the damage caused by cerebral ischemia (Atochin et al, 2007). In this study, we showed that treatment of ApoE−/− mice with cilostazol restored the levels of phosphorylated eNOS in blood vessels to those evident in the blood vessels of WT animals without changes in total eNOS expression. These results suggest that the beneficial effects of cilostazol likely relate to enhanced NO provision through upregulation of the post-translational phosphorylation of eNOS. Furthermore, as cilostazol is a selective PDE3 inhibitor, elevation of cAMP is implicated. Indeed, it has been reported in endothelial cells in culture that cilostazol increases eNOS activity and NO production indirectly through cAMP/protein kinase-A (PKA)-dependent phosphorylation of the Ser1177 residue, as the PKA selective inhibitor PKAI (cell-permeable inhibitor peptide sequence (14 to 22)) amide completely blocks eNOS phosphorylation (Hashimoto et al, 2006).
The predominant cAMP PDE in endothelial cells is PDE4 (Maurice et al, 2003), although there is also evidence that PDE3 variants are expressed in arterial, venous, and microvascular endothelial cells (Netherton and Maurice, 2005). Moreover, in these cells, another selective PDE3 inhibitor, cilostamide, in addition to cilostazol, elevates cAMP levels (Netherton and Maurice, 2005), supporting the view that the effects of cilostazol likely relate to block of PDE3 activity. In terms of selectivity in studies using recombinant enzymes, cilostazol is approximately 200 to 400 times more potent at PDE3 (IC50∼0.2 μmol/L) than at PDE4 and 20 times more potent at PDE5 (Sudo et al, 2000). Our experiments with HAECs suggest that it is unlikely that the elevations in cGMP, evident in vivo, are a consequence of a direct inhibition of PDE5 because acute treatment of cells with cilostazol (1 h) did not alter cGMP levels in response to the NO donor SPER-NO. These findings suggest that the effects of cilostazol are not due to a direct effect of the drug on the sGC/cGMP/PDE5 pathway and that our findings in vivo showing elevated NO generation and cGMP levels relate to the downstream effects of cilostazol on the cAMP/PDE pathway. Whether the effect of cilostazol relates to an action at PDE3 or PDE4, in vivo, is uncertain as both PDEs are found in the endothelium. To address this possibility, studies in vivo using selective PKA inhibitors might be useful; however, these studies are compromised by the fact that PKA has a major role in many other pathways activated by the atherosclerotic process, and therefore separating the effects of a cilostazol-driven PKA pathway from other pathways would be complicated.
It is unlikely that cilostazol caused an elevation of NO levels by directly altering the metabolism of NO because the levels of NOx measured in HAECs treated with the NO donor SPER-NO were similar in the absence or presence of the drug. This scenario is the opposite of the findings in vivo where the levels of nitrite and cGMP were elevated by cilostazol. One could speculate that although under unstimulated (as in the cell culture) conditions the effects of cilostazol on the endothelial cell are negligible, once the endothelial cells are stimulated, as would occur in vivo by shear stress, cilostazol acts to enhance the effects on the cAMP/PKA pathway.
A limitation of our findings, however, is that our measurements of eNOS phosphorylation were conducted using aortic tissue rather than cerebral arteries. This was due to the insufficient sample size available to adequately estimate eNOS protein expression using western blotting techniques. It is possible that there may be regional differences in the effect of cilostazol on eNOS phosphorylation that our studies cannot account for. In addition, we cannot exclude the possibility that the alterations in reactivity, in part, relate to changes in systemic blood pressure. However, long-term cilostazol treatment is not associated with changes in mean arterial blood pressure, either in preclinical studies (rats or mice: Woo et al, 2002; Yuzawa et al, 2008; Nonaka et al, 2009) or in humans (Woo et al, 2002), although changes in heart rate and, consequently, diastolic blood pressure were evident.
In conclusion, we have shown that ET-1-induced contraction is enhanced and endothelium-dependent relaxation to ACh is impaired in MCA from ApoE−/− mice. We suggest that alterations in sensitivity of the cerebral vasculature to ET-1 likely has a major role in the depressed cerebral blood flow that both precedes and is pathogenic in ischemic stroke. We also show that the abnormalities in vascular reactivity are prevented by cilostazol treatment. Cilostazol reduces infarct volume in animal models of ischemic stroke (Yuzawa et al, 2008), prevents the progression of intracranial arterial stenosis (Kwon et al, 2005), and protects against recurrent stroke in at-risk patients (Gotoh et al, 2000). In this study, we show that cilostazol treatment reverses the vascular dysfunction evident in the MCA of ApoE−/− mice and that this effect is directly due to an improvement in eNOS activity as a consequence of enhanced phosphorylation.
The authors declare no conflict of interest.
References
- Amarenco P, Labreuche J. Lipid management in the prevention of stroke: review and updated meta-analysis of statins for stroke prevention. Lancet Neurol. 2009;8:453–463. doi: 10.1016/S1474-4422(09)70058-4. [DOI] [PubMed] [Google Scholar]
- Atochin DN, Wang A, Liu VW, Critchlow JD, Dantas AP, Looft-Wilson R, Murata T, Salomone S, Shin HK, Ayata C, Moskowitz MA, Michel T, Sessa WC, Huang PL. The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. J Clin Invest. 2007;117:1961–1967. doi: 10.1172/JCI29877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman SA. Vasoconstrictor effect of endothelin-1 on hypertensive pulmonary arterial smooth muscle involves Rho-kinase and protein kinase C. Am J Physiol Lung Cell Mol Physiol. 2007;293:L472–L479. doi: 10.1152/ajplung.00101.2006. [DOI] [PubMed] [Google Scholar]
- Breslow JL. Mouse models of atherosclerosis. Science. 1996;272:685–688. doi: 10.1126/science.272.5262.685. [DOI] [PubMed] [Google Scholar]
- Casino PR, Kilcoyne CM, Quyyumi AA, Hoeg JM, Panza JA. The role of nitric oxide in endothelium-dependent vasodilation of hypercholesterolemic patients. Circulation. 1993;88:2541–2547. doi: 10.1161/01.cir.88.6.2541. [DOI] [PubMed] [Google Scholar]
- Chauhan SD, Seggara G, Vo PA, MacAllister RJ, Hobbs AJ, Ahluwalia A. Protection against lipopolysaccharide-induced endothelial dysfunction in resistance and conduit vasculature of iNOS knockout mice. FASEB J. 2003;17:773–775. doi: 10.1096/fj.02-0668fje. [DOI] [PubMed] [Google Scholar]
- d'Uscio LV, Smith LA, Katusic ZS. Hypercholesterolemia impairs endothelium-dependent relaxations in common carotid arteries of apolipoprotein E-deficient mice. Stroke. 2001;32:2658–2664. doi: 10.1161/hs1101.097393. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Endres M. Statins and stroke. J Cereb Blood Flow Metab. 2005;25:1093–1110. doi: 10.1038/sj.jcbfm.9600116. [DOI] [PubMed] [Google Scholar]
- Fransen P, Van Assche T, Guns PJ, Van Hove CE, De Keulenaer GW, Herman AG, Bult H. Endothelial function in aorta segments of apolipoprotein E-deficient mice before development of atherosclerotic lesions. Pflugers Arch. 2008;455:811–818. doi: 10.1007/s00424-007-0337-9. [DOI] [PubMed] [Google Scholar]
- Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh F, Tohgi H, Hirai S, Terashi A, Fukuuchi Y, Otomo E, Shinohara Y, Itoh E, Matsuda T, Sawada T, Yamaguchi T, Nishimaru K, Ohashi Y. Cilostazol Stroke Prevention Study: a placebo-controlled double-blind trial for secondary prevention of cerebral infarction. J Stroke Cerebrovasc Dis. 2000;9:147–157. doi: 10.1053/jscd.2000.7216. [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Miyakoda G, Hirose Y, Mori T. Activation of endothelial nitric oxide synthase by cilostazol via a cAMP/protein kinase A- and phosphatidylinositol 3-kinase/Akt-dependent mechanism. Atherosclerosis. 2006;189:350–357. doi: 10.1016/j.atherosclerosis.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ, Fukuto JM, Griscavage JM, Rogers NE, Byrns RE. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from L-arginine. Proc Natl Acad Sci USA. 1993;90:8103–8107. doi: 10.1073/pnas.90.17.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Tani T, Kanbe T, Watanabe K. Effect of cilostazol on platelet aggregation and experimental thrombosis. Arzneimittelforschung. 1985;35:1144–1149. [PubMed] [Google Scholar]
- Kitayama J, Faraci FM, Lentz SR, Heistad DD. Cerebral vascular dysfunction during hypercholesterolemia. Stroke. 2007;38:2136–2141. doi: 10.1161/STROKEAHA.107.481879. [DOI] [PubMed] [Google Scholar]
- Knottnerus IL, Ten Cate H, Lodder J, Kessels F, van Oostenbrugge RJ. Endothelial dysfunction in lacunar stroke: a systematic review. Cerebrovasc Dis. 2009;27:519–526. doi: 10.1159/000212672. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Yamaguchi S, Katsube T, Kitani M, Okada K, Tsunematsu T. Long-term effect of cilostazol on cerebral blood flow in chronic cerebral infarction. Arzneimittelforschung. 1985;35:1193–1197. [PubMed] [Google Scholar]
- Kobayashi T, Miyauchi T, Iwasa S, Sakai S, Fan J, Nagata M, Goto K, Watanabe T. Corresponding distributions of increased endothelin-B receptor expression and increased endothelin-1 expression in the aorta of apolipoprotein E-deficient mice with advanced atherosclerosis. Pathol Int. 2000;50:929–936. doi: 10.1046/j.1440-1827.2000.01152.x. [DOI] [PubMed] [Google Scholar]
- Konishi M, Iso H, Komachi Y, Iida M, Shimamoto T, Jacobs DR, Jr, Terao A, Baba S, Sankai T, Ito M. Associations of serum total cholesterol, different types of stroke, and stenosis distribution of cerebral arteries. The Akita Pathology Study. Stroke. 1993;24:954–964. doi: 10.1161/01.str.24.7.954. [DOI] [PubMed] [Google Scholar]
- Kwon SU, Cho YJ, Koo JS, Bae HJ, Lee YS, Hong KS, Lee JH, Kim JS. Cilostazol prevents the progression of the symptomatic intracranial arterial stenosis: the multicenter double-blind placebo-controlled trial of cilostazol in symptomatic intracranial arterial stenosis. Stroke. 2005;36:782–786. doi: 10.1161/01.STR.0000157667.06542.b7. [DOI] [PubMed] [Google Scholar]
- Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci USA. 2001;98:12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman A, Edwards BS, Hallett JW, Heublein DM, Sandberg SM, Burnett JC., Jr Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N Engl J Med. 1991;325:997–1001. doi: 10.1056/NEJM199110033251404. [DOI] [PubMed] [Google Scholar]
- Maeda H, Matsumoto M, Handa N, Hougaku H, Ogawa S, Itoh T, Tsukamoto Y, Kamada T. Reactivity of cerebral blood flow to carbon dioxide in various types of ischemic cerebrovascular disease: evaluation by the transcranial Doppler method. Stroke. 1993;24:670–675. doi: 10.1161/01.str.24.5.670. [DOI] [PubMed] [Google Scholar]
- Maguire JJ, Wiley KE, Kuc RE, Stoneman VE, Bennett MR, Davenport AP. Endothelin-mediated vasoconstriction in early atherosclerosis is markedly increased in ApoE−/− mouse but prevented by atorvastatin. Exp Biol Med (Maywood) 2006;231:806–812. [PubMed] [Google Scholar]
- Maurice DH, Palmer D, Tilley DG, Dunkerley HA, Netherton SJ, Raymond DR, Elbatarny HS, Jimmo SL. Cyclic nucleotide phosphodiesterase activity, expression, and targeting in cells of the cardiovascular system. Mol Pharmacol. 2003;64:533–546. doi: 10.1124/mol.64.3.533. [DOI] [PubMed] [Google Scholar]
- Mochizuki Y, Oishi M, Mizutani T. Effects of cilostazol on cerebral blood flow, P300, and serum lipid levels in the chronic stage of cerebral infarction. J Stroke Cerebrovasc Dis. 2001;10:63–69. doi: 10.1053/jscd.2001.24657. [DOI] [PubMed] [Google Scholar]
- Molina C, Sabin JA, Montaner J, Rovira A, Abilleira S, Codina A. Impaired cerebrovascular reactivity as a risk marker for first-ever lacunar infarction: a Case-Control Study. Stroke. 1999;30:2296–2301. doi: 10.1161/01.str.30.11.2296. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Houchi H, Minami A, Sakamoto S, Tsuchiya K, Niwa Y, Minakuchi K, Nakaya Y. Endothelium-dependent relaxation by cilostazol, a phosphodiesteras III inhibitor, on rat thoracic aorta. Life Sci. 2001;69:1709–1715. doi: 10.1016/s0024-3205(01)01258-9. [DOI] [PubMed] [Google Scholar]
- Netherton SJ, Maurice DH. Vascular endothelial cell cyclic nucleotide phosphodiesterases and regulated cell migration: implications in angiogenesis. Mol Pharmacol. 2005;67:263–272. doi: 10.1124/mol.104.004853. [DOI] [PubMed] [Google Scholar]
- Nonaka Y, Tsuruma K, Shimazawa M, Yoshimura S, Iwama T, Hara H. Cilostazol protects against hemorrhagic transformation in mice transient focal cerebral ischemia-induced brain damage. Neurosci Lett. 2009;452:156–161. doi: 10.1016/j.neulet.2009.01.039. [DOI] [PubMed] [Google Scholar]
- Pretnar-Oblak J, Sabovic M, Sebestjen M, Pogacnik T, Zaletel M. Influence of atorvastatin treatment on -arginine cerebrovascular reactivity and flow-mediated dilatation in patients with lacunar infarctions. Stroke. 2006;37:2540–2545. doi: 10.1161/01.STR.0000239659.99112.fb. [DOI] [PubMed] [Google Scholar]
- Rashid PA, Whitehurst A, Lawson N, Bath PMW. Plasma nitric oxide (nitrate/nitrite) levels in acute stroke and their relationship with severity and outcome. J Stroke Cerebrovasc Dis. 2003;12:82–87. doi: 10.1053/jscd.2003.9. [DOI] [PubMed] [Google Scholar]
- Sheng H, Laskowitz DT, Mackensen GB, Kudo M, Pearlstein RD, Warner DS, Iadecola C. Apolipoprotein E deficiency worsens outcome from global cerebral Ischemia in the mouse ò Editorial comment. Stroke. 1999;30:1118–1124. doi: 10.1161/01.str.30.5.1118. [DOI] [PubMed] [Google Scholar]
- Stenman E, Malmsjo M, Uddman E, Gido G, Wieloch T, Edvinsson L. Cerebral ischemia upregulates vascular endothelin ETB receptors in rat. Stroke. 2002;33:2311–2316. doi: 10.1161/01.str.0000028183.04277.32. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Meintzschel F, Rosler A, Lanfermann H, Steinmetz H, Sitzer M. Pravastatin improves cerebral vasomotor reactivity in patients with subcortical small-vessel disease. Stroke. 2001;32:2817–2820. doi: 10.1161/hs1201.099663. [DOI] [PubMed] [Google Scholar]
- Sudo T, Tachibana K, Toga K, Tochizawa S, Inoue Y, Kimura Y, Hidaka H. Potent effects of novel anti-platelet aggregatory cilostamide analogues on recombinant cyclic nucleotide phosphodiesterase isozyme activity. Biochem Pharmacol. 2000;59:347–356. doi: 10.1016/s0006-2952(99)00346-9. [DOI] [PubMed] [Google Scholar]
- Szok D, Hansen-Schwartz J, Edvinsson L. In depth pharmacological characterization of endothelin B receptors in the rat middle cerebral artery. Neurosci Lett. 2001;314:69–72. doi: 10.1016/s0304-3940(01)02293-5. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Oida K, Fujiwara R, Maeda H, Hayashi S, Takai H, Tamai T, Nakai T, Miyabo S. Effect of cilostazol, a cyclic AMP phosphodiesterase inhibitor, on the proliferation of rat aortic smooth muscle cells in culture. J Cardiovasc Pharmacol. 1992;20:900–906. doi: 10.1097/00005344-199212000-00009. [DOI] [PubMed] [Google Scholar]
- Takase H, Hashimoto A, Okutsu R, Hirose Y, Ito H, Imaizumi T, Miyakoda G, Mori T. Anti-atherosclerotic effect of cilostazol in apolipoprotein-E knockout mice. Arzneimittelforschung. 2007;57:185–191. doi: 10.1055/s-0031-1296604. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Ishikawa T, Hagiwara M, Onoda K, Itoh H, Hidaka H. Effects of cilostazol, a selective cAMP phosphodiesterase inhibitor on the contraction of vascular smooth muscle. Pharmacology. 1998;36:313–320. doi: 10.1159/000138400. [DOI] [PubMed] [Google Scholar]
- Tao HM, Chen GZ. Endothelial NO synthase gene polymorphisms and risk of ischemic stroke: a meta-analysis. Neurosci Res. 2009;64:311–316. doi: 10.1016/j.neures.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Woo SK, Kang WK, Kwon KI. Pharmacokinetic and pharmacodynamic modeling of the antiplatelet and cardiovascular effects of cilostazol in healthy humans. Clin Pharmacol Ther. 2002;71:246–252. doi: 10.1067/mcp.2002.122474. [DOI] [PubMed] [Google Scholar]
- Yonas H, Smith HA, Durham SR, Pentheny SL, Johnson DW. Increased stroke risk predicted by compromised cerebral blood flow reactivity. J Neurosurgery. 1993;79:483–489. doi: 10.3171/jns.1993.79.4.0483. [DOI] [PubMed] [Google Scholar]
- Yuzawa I, Yamada M, Fujii K. An oral administration of cilostazol before focal ischemia reduces the infarct volume with delayed cerebral blood flow increase in rats. J Stroke Cerebrovasc Dis. 2008;17:281–286. doi: 10.1016/j.jstrokecerebrovasdis.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Zhang SH, Reddick SH, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]