Abstract
In human cortex it has been suggested that the tissue at risk is indicated by clusters of spreading depolarizations (SDs) with persistent depression of high-frequency electrocorticographic (ECoG) activity. We here characterized this zone in the ET-1 model in rats using direct current (DC)-ECoG recordings. Topical application of the vasoconstrictor endothelin-1 (ET-1) induces focal ischemia in a concentration-dependent manner restricted to a region exposed by a cranial window, while a healthy cortex can be studied at a second naïve window. SDs originate in the ET-1-exposed cortex and invade the surrounding tissue. Necrosis is restricted to the ET-1-exposed cortex. In this study, we discovered that persistent depression occurred in both ET-1-exposed and surrounding cortex during SD clusters. However, the ET-1-exposed cortex showed longer-lasting negative DC shifts and limited high-frequency ECoG recovery after the cluster. DC-ECoG recordings of SD clusters with persistent depression from patients with aneurysmal subarachnoid hemorrhage were then analyzed for comparison. Limited ECoG recovery was associated with significantly longer-lasting negative DC shifts in a similar manner to the experimental model. These preliminary results suggest that the ischemic zone in rat and human cortex is surrounded by a normally perfused belt with persistently reduced synaptic activity during the acute injury phase.
Keywords: neurogenesis, plasticity, spreading depression, stroke, vasospasm
Introduction
Spreading depolarization (SD) occurs when passive cation influx across the cellular membranes exceeds adenosine triphosphate-dependent sodium and calcium pump activity (Somjen, 2004). The hallmark of SD is the near-complete sustained depolarization of neurons above the inactivation threshold of the action potential-generating channels in contrast to that of epileptic ictaform activity in which sustained depolarization remains below this threshold. During SD, the cation influx is followed by water influx and shrinkage of the extracellular space by approximately 70%. This process is associated with marked alterations of the intra-/extracellular ion distribution. For example, the extracellular Ca2+ concentration falls from 1.2 to <0.1 mmol/L (Kraig and Nicholson, 1978; Hansen and Lauritzen, 1984; Windmüller et al, 2005). Accordingly, the intracellular Ca2+ concentration rises from approximately 60 nmol/L to 25 μmol/L (Dietz et al, 2009). If normal ion homeostasis is not restored through additional recruitment of sodium and calcium pump activity, the cell swelling is maintained—a process then termed ‘cytotoxic edema' because it potentially leads to cell death through the protracted intracellular Ca2+ surge (Somjen, 2004).
Sustained depolarization above the inactivation threshold of the action potential-generating channels results in spreading depression of high-frequency electrocorticographic (ECoG) activity unless ECoG activity is already suppressed by sedatives, preceding SDs or severe energy compromise (Fleidervish et al, 2001). Thus, the term spreading depolarization comprises the full spectrum of near-complete sustained neuronal depolarizations associated with either spreading depression or preceded by non-spreading depression of activity as described by the Brazilian physiologist Leão in 1944 and 1947. Spreading depression of ECoG activity was discovered by him in the rabbit cerebral cortex in 1944. Subsequently, he also described the large negative direct current (DC) shift of the extracellular space that accompanies ECoG depression (Leão, 1947). Moreover, he showed that a similar negative DC shift occurs in the cortex in response to ischemia induced by basilar and bilateral carotid artery occlusion (Leão, 1947), concluding that ‘in the spreading depression of activity, a change of the same nature as one resulting from prolonged interruption of circulation, occurs in the cerebral cortex' (authors' emphasis). Voltage inhomogeneities along neuronal membranes seem to be responsible for the large negative DC shift of SD (Canals et al, 2005).
Focal ischemia is a special condition because there are gradients of perfusion, oxygen, and glucose between the core ischemic zone and the normal tissue in which regional cerebral blood flow (rCBF) and energy substrates are unrestricted. Accordingly, direct evidence from microelectrode and intrinsic optical imaging studies and indirect evidence from laser speckle imaging indicate that SD starts as a long-lasting, pharmacoresistant, and harmful wave in the center of a low perfusion area and changes to a progressively shorter-lasting, pharmacosensitive, and more benign wave as it spreads through the metabolically compromised penumbra and traverses the healthy surrounding tissue (Nedergaard and Hansen, 1993; Busch et al, 1996; Petzold et al, 2005; Shin et al, 2006; Strong et al, 2007; Sakowitz et al, 2009).
On the basis of the animal experiments and human subdural recordings of high-pass filtered ECoG activity, it has been suggested that persistent depression of ECoG activity between recurrent SDs indicates tissue at risk (Hossmann, 1994; Fabricius et al, 2006; Dreier et al, 2006). Moreover, experimental evidence suggests that the same SD wave is associated with a prolonged negative DC shift in the tissue at risk, but with a short-lasting negative DC shift in surviving tissue (Hossmann, 1994). We here specifically analyzed clusters of recurrent SDs to determine whether persistent ECoG depression between recurrent SDs is also observed in normally perfused tissue surrounding the zone of injury and how ECoG depression periods relate to prolonged negative DC shifts. For this purpose, full-band DC-ECoG recordings were performed in a two-cranial window model in rats in which the potent vasoconstrictor endothelin-1 (ET-1) induces focal ischemia in one window and the surviving cortex can be studied in the second window. Using full-band DC-ECoG recordings in patients with aneurysmal subarachnoid hemorrhage (aSAH), we then identified two distinct patterns of clusters with persistent ECoG depression in a similar manner to that in rats: clusters with short-lasting, stereotypical negative DC shifts, and those with variable and prolonged negative DC shifts. On the basis of the experimental results, it is suggested that the latter indicate tissue at risk in the human brain, whereas the former could be involved in the early induction of repair, plasticity, and regeneration in the surrounding tissue.
Materials and methods
Animal Preparation
All animal experiments were performed in compliance with the Governmental Animal Care and Use Committee (Landesamt für Arbeitsschutz, Gesundheitsschutz und technische Sicherheit Berlin (LAGetSi), G 0346/98). The animals were housed in groups (two to four animals per cage) under a 12-hour light–dark cycle with food and tap water available ad libitum. For the surgical procedure, 42 male Wistar rats (250 to 380 g; Charles River Laboratories, Wilmington, MA, USA) were anesthetized with 100 mg/kg thiopental sodium intraperitoneally (Trapanal, BYK Pharmaceuticals, Konstanz, Germany), tracheotomized and artificially ventilated (Effenberger Rodent Respirator; Effenberger Med.-Techn. Gerätebau, Pfaffing/Attel, Germany) to maintain an arterial pCO2 between 35 to 45 mm Hg. The left femoral artery and vein were cannulated and saline solution was continuously infused to keep the vessels open (0.5 mL/h). Systemic arterial pressure (Pressure Monitor BP-1, World Precision Instruments, Berlin, Germany) and end-expiratory partial pressure of CO2 (Heyer CO2 Monitor EGM I, Bad Ems, Germany) were continuously monitored. Arterial pO2, pCO2, and pH were serially measured using a Compact 1 Blood Gas Analyzer (AVL Medizintechnik GmbH, Bad Homburg, Germany). Body temperature was maintained at 38.0±0.5°C using a heating pad (Temperature Control FHC, Bowdoinham, ME, USA). The level of anesthesia was assessed by testing motor responses and changes in blood pressure to foot-pinching. If necessary, additional doses of thiopental (25 mg/kg body weight) were applied. Animals were immediately killed after the experiment by intravenous administration of concentrated KCl solution. Another five male Wistar rats were anesthetized using isoflurane (1.5% to 2.0% in 30% O2 and 70% N2O) for the surgical procedure and breathed spontaneously. In these animals, the wounds were treated with lidocainehydrochloride gel (2% Astra GmBH, Wedel, Germany) and sutured after the experiment. The opioid buprenorphine (0.5 mg/kg body weight subcutaneously; Boehringer, Mannheim, Germany) was administered as a postoperative analgesic and rats were allowed to recover from anesthesia. Sixty hours after the experiment, cardiac perfusion fixation was performed with 4% paraformaldehyde under deep anesthesia with thiopental sodium. Then, 20-μm sagittal brain sections were collected serially at 800-μm intervals and stained with hematoxylin-eosin. The diameter of the caudal window as assessed by the brain's herniation, the largest rostral extent of the ET-1-induced lesion beyond the border of the caudal window, and the distance between the rostral border of the ET-1-induced necrosis and the rostral window were determined.
A craniotomy of 5 × 4 mm2 was performed over the somatosensory cortex using a saline-cooled drill as described previously (Dreier et al, 2002). The dura was cut to expose the cortex. In 36 animals, the cranial window was open (groups 1–3, 5). An open window allows the placement of ion-sensitive microelectrodes as described previously. In 11 animals, the window was closed to allow imaging of the pial arterioles using a CCD camera (Leica DFC300 FX Digital Color Camera, Leica Microsystems AG, CH-9435, Heerbrugg, Switzerland) connected to the microscope (Leica MZ16 FA, Leica Microsystems) and using Leica Application Multifocus module software (group 4). In contrast to groups 1–3, in groups 4 and 5 a second cranial window (3 × 3 mm2) was implanted over the frontal cortex of the same hemisphere to allow subdural recordings of SDs in naïve cortex. The cortical surface was continuously superfused with artificial cerebrospinal fluid containing (in mmol/L): 152 Na+, 3 K+, 1.5 Ca2+, 1.2 Mg2+, 24.5 HCO3−, 135 Cl−, 3.7 glucose and 6.7 urea. Artificial cerebrospinal fluid was equilibrated with a gas mixture containing 6% oxygen, 5.9% carbon dioxide, and 87.5% nitrogen. A pO2 of 90 to 130 mm Hg, pCO2 of 35 to 45 mm Hg, and pH of 7.35 to 7.45 were accepted as physiological. ET-1 (Sigma-Aldrich, Steinheim, Germany) was dissolved in artificial cerebrospinal fluid and topically applied to the cortical surface in groups 1, 2, 4, and 5. Group 3 served as a control group for groups 1 and 2.
Animal Recording Techniques
Regional cerebral blood flow was continuously monitored with two laser-Doppler flow probes (Periflux 4001, Perimed, Järfälla, Sweden). The subdural DC-ECoG (bandpass: 0–45 Hz) was measured with Ag/AgCl electrodes. Changes in the extracellular K+ concentration ([K+]o) (groups 1, 3), the extracellular pH (groups 2, 3), and the intracortical DC-ECoG (bandpass: 0 to 45 Hz) (groups 1–3) were recorded in a cortical depth of 300 μm with ion-sensitive microelectrodes that were prepared and tested as described previously from double-barreled thetaglass capillaries (Kugelstätter, Garching, Germany) (Windmüller et al, 2005). Potassium ionophore I-cocktail A and hydrogen ionophore I-cocktail A (Fluka/Sigma-Aldrich) ion exchangers were used for manufacturing K+- and H+-sensitive microelectrodes, respectively. The electrodes were connected to a differential amplifier (Jens Meyer, Munich, Germany). Analog-to-digital conversion was performed using a Power 1401 (Cambridge Electronic Design Limited, Cambridge, UK). Systemic arterial blood pressure as well as local cerebral ion, voltage and blood flow changes were continuously recorded using a personal computer and Spike 2 software (version 6, Cambridge Electronic Design Limited).
For the measurements of arteriolar diameter changes in the cranial window, we used Matlab to select and analyze the regions of interest representing lines across a given arteriole. A single region of interest was defined as an index profile of light absorbance in which the x axis represents length in pixels and the y axis brightness. Optically, the region of interest was the light absorbance of the tissue in the marked section. Typically, the arteriole led to a curve valley of absorbance in the green and blue channels because hemoglobin in the arteriole absorbs more green and blue light than the surrounding tissue. Only the green channel was used for analysis because signal-to-noise ratio was better. For detection of the two edges of the vessel, we used the second derivative of the curve representing the slope. The length between the two edges was the Euclidean distance, which was then corrected for the manually determined pixel length to give the real diameter in micrometers. Movement artifacts were compensated by the algorithm. Moreover, contrast information was extracted to compensate for changes in brightness because of changes in intrinsic optical signal or illumination artifacts. The lower diameter limit for detection was set to 5 μm.
Patient Recruitment and Clinical Care
Patients with major aSAH were recruited by two centers of the Co-Operative Study on Brain Injury Depolarizations (see www.cosbid.org). DC-ECoG recordings of six patients were taken for analysis, which showed a cluster of recurrent SDs with persistent depression of high-frequency ECoG activity between SDs (Campus Charité Virchow Berlin (n=4), Campus Benjamin Franklin Berlin (n=2)). The research protocol was approved by the local ethics committee. Clinical and research consents were obtained according to the Declaration of Helsinki after a clinical decision had been taken to offer surgical treatment. Aneurysmal subarachnoid hemorrhage was diagnosed by assessment of computed tomography scans. Hemorrhage was graded according to the Fisher scale, and clinical presentation according to the World Federation of Neurological Surgeons scale. The indications for neurosurgical treatment are given in Table 1. Surgery allowed the placement of a single, linear, six-contact (platinum) ECoG recording strip (Wyler, 5-mm diameter; Ad-Tech Medical, Racine, WI, USA) on cortex accessible through the craniotomy or via an extended burr-hole (Dreier et al, 2009). After surgery, patients were transferred to the intensive care unit. Intracranial pressure was monitored via a ventricular drainage catheter. Glasgow Coma Score, blood gases, glucose, and electrolytes were documented every 6 hours. A thorough neurological examination was performed at least daily. Serial computed tomography scans were performed postoperatively at the time of clinical deterioration and after the monitoring period to screen for delayed infarcts. Vasospasm was defined using digital subtraction angiography as >30% narrowing of the arterial luminal diameter in one of the following arterial segments: A1, A2, M1, M2, and C1–C2. Magnification errors were corrected by comparing the extradural segments of the internal carotid artery (C4–C5). Using transcranial Doppler sonography, significant vasospasm was defined by a mean velocity >200 cm/second in at least one middle cerebral artery and vasospasm was excluded if the middle cerebral artery mean velocities remained below 120 cm/second throughout the observation period. Patients with delayed ischemia were treated with triple-H therapy (hypertension, hypervolemia, and hemodilution) as described previously (Dreier et al, 2006).
Table 1. Summary of demographic, treatment, and SD-related data.
| No. | Age (years), sex | WFNS grade | Fisher grade | Location of aneurysm | Significant proximal vasospasm | Delayed CT- or MRI- proven infarct | Intervention | Location of electrode strip | Start of ECoG monitoring (day after insult) | Day of analyzed SD cluster | Number of SDs in cluster | Frequency of SDs in cluster (/h) | Average duration (min)/amplitude (mV) of negative DC shifts | Average propagation velocity (mm/min) | MRS on day 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 53, F | 5 | 3 | MCA | Y | Y | Aneurysm clipping, decompressive hemi-craniectomy | Fronto-parietal cortex | 2 | 5 | 27 | 1.5 | 4.4/6.2 | 5.1 | 5 |
| 2 | 51, F | 5 | 3 | MCA | Y | N | Aneurysm clipping | Adjacent to cortex over a 6 × 1 cm frontoparietal intracerebral hematoma | 0 | 0 | 6 | 2.1 | 21.5/12.4 | 2.9 | 5 |
| 3 | 71, M | 5 | 4 | ACoA | N | N | Aneurysm clipping | Adjacent to cortex over a 6 × 2 cm frontal intracerebral hematoma | 1 | 4 | 9 | 2.9 | 4.3/7.2 | 5.2 | 5 |
| 4 | 44, M | 5 | 3 | ACoA | N | Y | Aneurysm coiling, evacuation of intracerebral hematoma | Adjacent to cortex over a 5 × 2 cm frontoparietal intracerebral hematoma | 3 | 3 | 8 | 0.6 | 3.3/13.5 | 3.1 | 5 |
| 5 | 57, M | 1 | 3 | MCA | Y | N | Aneurysm clipping | Adjacent to cortex over 6 × 4 cm fronto-temporal intracerebral hematoma | 1 | 2 | 5 | 1.9 | 3.4/7.3 | 2.6 | 5 |
| 6 | 57, M | 5 | 3 | ACoA | N | Y | Aneurysm coiling, placement of oxygen sensor | Adjacent to cortex over a 5 × 5 cm frontal intracerebral hematoma | 3 | 7 | 6 | 3.2 | 12.3/7.6 | 4.9 | 6 |
ACoA, anterior communicating artery; CT, computed tomography; ECoG, electrocorticographic; F, female; M, male; MCA, middle cerebral artery; MRI, magnetic resonance imaging; MRS, modified Rankin scale; SD, spreading depolarization; WFNS, World Federation of Neurological Surgeons Scale.
Direct current-electrocorticographic recordings were acquired continuously in six active channels from the six-electrode (linear array) subdural strips. Electrodes 1 to 6 (interelectrode distance 1 cm) were connected in sequential unipolar manner to a GT205 amplifier (0.01 to 50 Hz) (ADInstruments, Bella Vista, New South Wales, Australia), each referenced to an ipsilateral subgaleal platinum electrode. The DC-ECoG (bandpass: 0 to 45 Hz) was additionally recorded using a BrainAmp amplifier (BrainProducts GmbH, Munich, Germany) as reported previously (Dreier et al, 2009). Data were sampled at 200 Hz and recorded and reviewed using a Powerlab 16/SP analog/digital converter, Chart-6 software (ADInstruments) and BrainVision Recorder software (BrainProducts GmbH).
Spreading depolarization was defined by the sequential onset of a propagating slow potential change in adjacent channels (Fabricius et al, 2006). The parallel high-frequency ECoG (bandpass: 0.5 to 45 Hz) depression was defined by a rapidly developing reduction of the power of the ECoG amplitude. The duration of the depression period of the high-frequency ECoG activity was measured as the interval between depression onset and onset of restoration of activity using the integral of power of the band-pass filtered activity (time constant decay, 60 seconds) as described previously (Dreier et al, 2006).
Results
Electrocorticographic Signatures of Spreading Depolarization in the Endothelin-1-Exposed Cortex in Rats
We first determined whether the patterns of negative DC shifts in the ET-1-exposed cortex depended on the dynamics of ET-1 application. Therefore, we compared an immediate brain topical application of ET-1 at 1 μmol/L for 1 hour (group 1, n=9, see Supplementary Table 1 online for vital signs) with stepwise increases from 10 to 100 nmol/L, and then to 1 μmol/L at 1-hour intervals (group 2, n=7). In group 1, ET-1 led to a decrease of rCBF to 68±18% of the baseline at the rostral and to 58±12% at the caudal laser probe before the first SD. A spreading depression of high-frequency ECoG activity to 26±13% (integral of power) rostrally and to 27±16% caudally occurred with the first negative DC shift and did not recover significantly after the SD cluster.
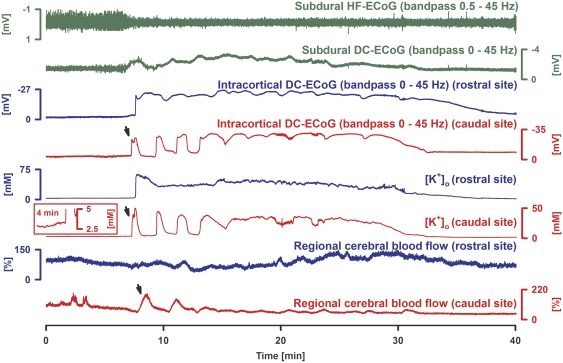
In eight of nine experiments, the SD cluster contained a very prolonged negative DC shift in at least one trace. This was frequently preceded by one or more isolated progressively longer-lasting SDs in at least one trace as shown in Figure 1. These isolated SDs seemed to melt into the prolonged negative DC shift (Figure 1). In one experiment, only shorter-lasting SDs were observed. The negative DC shift was characterized by an amplitude of −24.6±4.2 mV rostrally, −20.3±5.5 mV caudally, and −4.6±1.8 mV subdurally, and a duration of 29.0±8.8 minutes rostrally, 21.8±8.2 minutes caudally, and 26.6±8.8 minutes subdurally. The cluster was preceded by a gradual increase of [K+]o to 5.1±1.5 mmol/L at the rostral and 4.8±2.0 mmol/L at the caudal microelectrode. The sharp negative DC shift was accompanied by a sharp increase of [K+]o to 58.4±10.7 mmol/L rostrally and to 45.6±15.5 mmol/L caudally. The increase of [K+]o lasted as long as the prolonged negative DC shift. The onsets of the negative DC shifts at the two intracortical microelectrodes showed a delay of 40±33 seconds, indicating propagation. The rCBF response to the first SD was characterized by a short-lasting increase from 68% to 173±73% rostrally and from 58% to 116±38% caudally (Figure 1). Thereafter, rCBF decreased to 64±25% rostrally and to 67±15% caudally until it increased again when the second SD occurred.
Figure 1.
Cluster of spreading depolarizations (SDs) in response to immediate application of endothelin-1 (ET-1) at 1 μmol/L. Traces 1 and 2 (dark green) show depression of high-frequency (HF)-electrocorticographic (ECoG) activity and changes in the subdural direct current (DC)-ECoG. Traces 3 to 6: Simultaneous changes of intracortical DC-ECoG and [K+]o were recorded with two microelectrodes placed 2 mm apart in a cortical depth of 300 μm. Traces 7 and 8 show rCBF measured with two laser-Doppler flow probes placed over each microelectrode (blue: rostral; red: caudal recording site). Whereas a melting of shorter lasting, variable SDs into a very prolonged negative DC shift is observed at the caudal (red) recording site (arrow at onset of the cluster, compare a similar melting in human cortex in Figure 5), only one very prolonged negative DC shift occurs at the rostral (blue) recording site in the ET-1-exposed cortex. The inset shows the small rise of [K+]o before the SD at the caudal recording site.
In group 2, we increased ET-1 in a stepwise manner from 10 to 100 nmol/L and finally to 1 μmol/L at 1-hour intervals. The first SD was observed under 10 nmol/L in one, 100 nmol/L in three, and 1 μmol/L in three animals. Regional cerebral blood flow was only measured caudally and did not change significantly before the first ET-1-induced SD (97±33% of baseline). This was significantly different from group 1 (t-test, P=0.007). During SD, rCBF increased to 213±73%, which was significantly higher compared with group 1 (t-test, P=0.009). Spreading depolarization was accompanied by spreading depression of high-frequency ECoG activity to 38±14% (integral of power), which did not recover in four of seven experiments during the observation period of at least 1 hour.
A very prolonged negative DC shift was only observed in one of seven experiments in group 2, while a cluster of 4±4 shorter-lasting SDs occurred in the other experiments. Thus, very prolonged negative DC shifts were significantly more frequent when the exposure to ET-1 started with the high concentration (group 1) and was not preceded by lower concentrations as in group 2 (two-tailed Fisher's exact test, P=0.009).
The negative DC shift showed an amplitude of −24.1±10.3 mV rostrally and −16.2±8.3 mV caudally in group 2. Whereas the shortest SD in such a cluster lasted for 76±43 seconds rostrally and 133±110 seconds caudally, the longest SD lasted for 113±64 seconds rostrally and 157±122 seconds caudally. We then compared the difference between the durations of the shortest and longest negative DC shifts with the same difference between SDs in naive cortex that were remotely triggered by electrical stimulation (group 3, n=15). We found that short-lasting SDs in the ET-1-exposed cortex showed a significantly higher variability of durations than SDs in a healthy cortex (variability in group 2: 44±34 seconds versus variability in group 3: 8±8 secs, Mann–Whitney rank sum test, P=0.003).
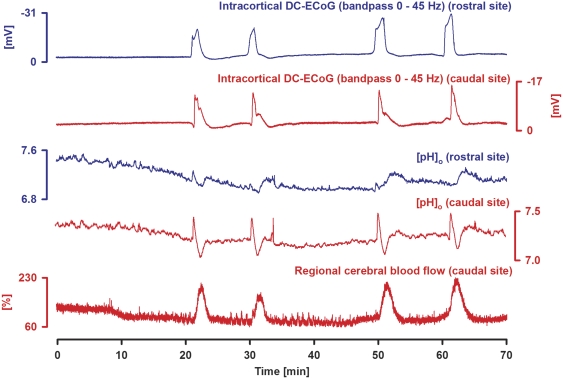
The onsets of the negative DC shifts at the two intracortical microelectrodes in group 2 showed a delay of 18±11 seconds, indicating propagation. We also determined the pH response using pH-sensitive microelectrodes in group 2. The SD cluster was preceded by a significant gradual acidification from 7.36±0.05 to 7.24±0.05 at the caudal microelectrode (Figure 2). The onset of the negative DC shift was accompanied by a fast, short-lasting alkaline shift to 7.44±0.11, followed by another slow acidification to 6.89±0.12 lasting for 196±155 seconds. This was succeeded by a slow transient alkalinization to 7.29±0.11 lasting for 186±84 seconds. At some recording sites this slow alkalinization occurred instead of the slow acidification. Both patterns are illustrated in Figure 2, in which SDs 3 and 4 show the fast alkaline shift followed first by slow acidification and then by slow alkalinization at the caudal recording site and only the fast alkaline shift followed by slow alkalinization at the rostral recording site. The delayed slow alkalinization in the ET-1-exposed cortex represents an unusual pH response pattern to SD and was not observed in six experiments in the naïve cortex of control group 3 in which the pH response consisted of the typical fast, short-lasting alkaline shift from 7.36±0.02 to 7.53±0.08 followed by slow acidification to 7.10±0.08 lasting for 84±65 seconds.
Figure 2.
Acidification preceding a cluster of spreading depolarizations (SDs) in the presence of endothelin-1 (ET-1) (1 μmol/L) after stepwise increases to 10 and 100 nmol/L. Rostral (blue) and caudal (red) intracortical direct current (DC)-electrocorticographic (ECoG) activity (traces 1 and 2) and extracellular pH (traces 3 and 4) were recorded with pH-sensitive microelectrodes. Trace 5 shows caudal regional cerebral blood flow (rCBF). Note initial rCBF decrease before the first SD because ET-1 induced vasoconstriction and then hyperemic responses to the recurrent SDs. The initial acidification before the first SD is typical of ischemia-induced SD. SDs themselves are characterized by an alkaline shift followed by acidification. Note that the negative DC shifts vary in duration between recurring SDs whereas SDs in metabolically uncompromised tissue would be more stereotypical. In contrast to an immediate increase of ET-1 from 0 to 1 μmol/L (see Figure 1), in the present figure, it is observed that stepwise increases of ET-1 from 10 nmol/L to 1 μmol/L led to only mildly prolonged but not very prolonged negative DC shifts at the two recording sites. Note that SDs three and four show a fast alkaline shift followed first by slow acidification and then by slow alkalinization at the caudal recording site and only the fast alkaline shift followed by slow alkalinization at the rostral recording site. This delayed slow alkalinization in the ET-1-exposed cortex represents an unusual pH response pattern to SD, which is possibly related to the hyperemic flow response. The transient rise in rCBF may transiently increase supply with oxidative substrates and, thus, transiently decrease overproduction of lactic acid in the ischemic zone.
Significant Constriction of Pial Arterioles Precedes Endothelin-1-Induced Spreading Depolarization
In group 4 (n=11), two cranial windows were implanted. ET-1 was brain topically applied at the caudal window at increasing concentrations of 100 nmol/L and 1 μmol/L at 1-hour intervals. The rostral window served as control. The distance between the two windows was ∼5 mm (Figure 4A). DC-ECoG with a silver/silver chloride electrode and rCBF with laser-Doppler flowmetry were recorded at each window. The pial arterioles were imaged onto a camera to assess whether significant vasoconstriction preceded ET-1-induced SD.
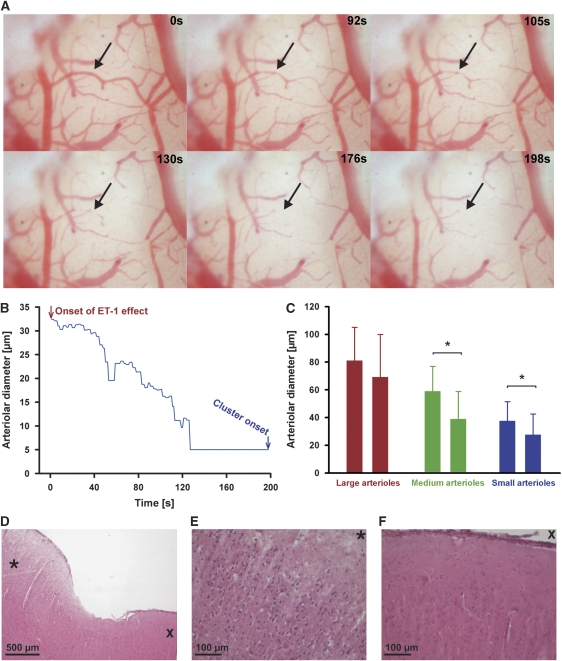
Arteriolar diameters of 102 arterioles of eight experiments were analyzed using the image series as shown in Figure 3. In each experiment, the median diameter of large, medium, and small arterioles was determined before wash-in of ET-1 and during the last 60 seconds before the first SD. Large arteriole diameters changed insignificantly from 81±24 to 69±31 μm, whereas medium and small arteriole diameters changed significantly from 58±18 to 38±20 μm and from 37±14 to 27±15 μm, respectively (paired t-test, P=0.013 and P=0.012, respectively). A marked variability of diameter changes was observed between individual arterioles. In total, 1 of 22 large, 1 of 25 medium, and 9 of 55 small arterioles totally collapsed before the first SD. During this period, rCBF significantly decreased to 63±26% in the ET-1-exposed cortex whereas it remained unchanged in the control window (98±13%, P=0.004, paired t-test).
Figure 3.
Pial arteriolar constriction in response to endothelin-1 (ET-1) before the first spreading depolarization (SD). (A) Note the virtual disappearance of pial arterioles (arrow) in presence of ET-1 at 100 nmol/L before the first SD. (B) Time course of pial arteriolar constriction (corresponding to the arteriole in (A) marked by the arrow) before the first SD. (C) In each experiment, the median diameter of large, medium, and small arterioles was determined before wash-in of ET-1 and during the last 60 seconds before the first SD. Large arteriole diameters changed insignificantly whereas medium and small arteriole diameters showed significant constriction. (D) Histologic changes at the site of ET-1 application to the cortical surface, 60 hours after treatment. *Indicates the area shown in (E). (E) Shrunken, hyperchromatic, acidophilic neurons with perineuronal halo indicating necrosis after exposure to ET-1. (F) Neurons surrounding the area of damage were morphologically intact. Staining was performed with hematoxylin-eosin.
Persistent Depression of High-Frequency Electrocorticographic Activity both in the Endothelin-1-Exposed and Surrounding Cortex
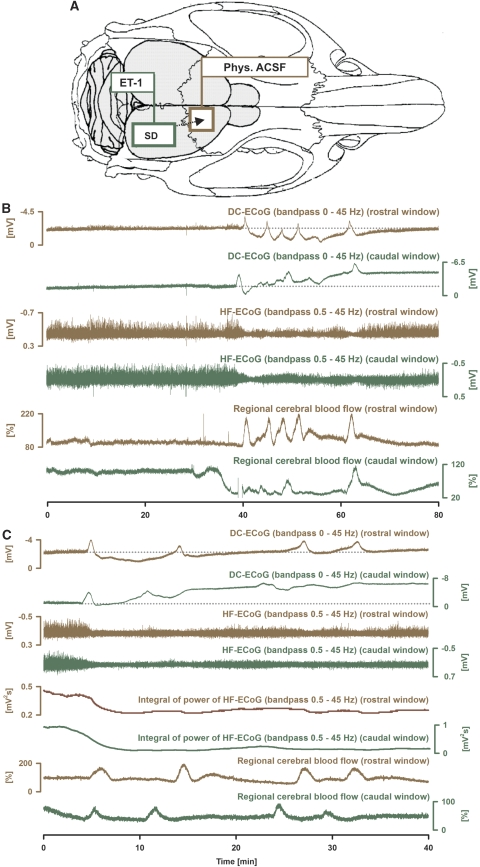
Endothelin-1 induced a cluster of 5.5±3.1 SDs at a concentration of 100 nmol/L and 4.4±3.1 SDs at 1 μmol/L in group 4 (SD frequency within cluster: 11.9±5.0 per hour). In the ET-1-exposed cortex, the cluster was associated with a subdural DC negativity of −2.6±2.2 mV, on which sharp transient negative DC shifts typical of SDs were riding (amplitude: −4.0±1.9 mV) (Figure 4). This was accompanied by a shallow DC positivity of 0.9±0.9 mV superimposed with SDs (amplitude: −1.2±1.0 mV) in the control window. The durations of the negative DC shifts of the first and second SDs were significantly longer in the ET-1-exposed than in the remote cortex (72±12 versus 52±19 seconds, P=0.044, and 120±36 versus 59±23 seconds, P=0.016, paired t-tests). The SD propagation velocity was 4.8±4.0 mm/minute. The mean integral of power of the high-frequency ECoG activity was similar in the ET-1-exposed and remote cortex before the cluster of recurrent SDs (0.52±0.22 versus 0.46±0.18 mV2 s (bandpass: 0.5–45 Hz)). The cluster of recurrent SDs was associated with a persistent depression of high-frequency ECoG activity in both the ET-1-exposed and remote cortex, but the depression was significantly more pronounced in the ET-1-exposed cortex (mean reduction to 27±13% under ET-1 (100 nmol/L) versus 55±15% in the remote cortex (P<0.001) and to 16±7% under ET-1 (1 μmol/L) versus 55±18% in the remote cortex (P<0.001, paired t-tests)). After wash-out of ET-1, the integral of high-frequency power returned to 71±19% in the remote cortex, whereas it only returned to 39±16% in the ET-1-exposed cortex (P=0.004, paired t-test).
Figure 4.
The zone of persistent depression of high-frequency (HF) electrocorticographic (ECoG) activity exceeds the zone with prolonged negative direct current (DC) shifts during an endothelin-1 (ET-1)-induced cluster in the rat. The figure shows an experiment with two cranial windows (A). At the caudal window (dark green), cortex was exposed to ET-1 whereas the rostral window (brown) was placed over naïve cortex. (B) The figure shows the evolution of a cluster of spreading depolarizations (SDs) in response to ET-1 (100 nmol/L). Traces 1 and 2 give the subdural DC-ECoG potentials. Note the shallow negative DC shift on which SDs are riding at the caudal window in contrast to the shallow positivity observed between SDs at the rostral window. Traces 3 and 4 show marked depressions of the HF-ECoG activity that is somewhat more pronounced in the ET-1-exposed cortex. The lowest traces give regional cerebral blood flow (rCBF). Note the significant decline of rCBF before the cluster onset in the caudal window whereas it remained unchanged in the rostral window. (C) Another experiment is shown at higher temporal resolution that shows the onset of a cluster of SDs in response to ET-1 (100 nmol/L). Traces 5 and 6 show the integral of power of the HF ECoG activity that can be used to calculate the duration of the depression periods and shows the persistence of depression between the recurrent SDs in both windows.
During the first SD, rCBF only increased to 125±56% in the ET-1-exposed cortex, whereas it increased to 194±43% in the remote cortex (P=0.036, paired t-test). Thereafter, it decreased again to 59±19% in the ET-1-exposed cortex, whereas it decreased to 90±14% in the remote cortex (P=0.005, paired t-test). Similar values were observed for the second SD of the cluster.
The Endothelin-1-Induced Lesion Does Not Include the Rostral Recording Site
In group 5 (n=5), ET-1 at 1 μmol/L led to an rCBF decrease of 76±29% at the caudal window, whereas rCBF slightly increased to 121±28% at the rostral control window under isoflurane anesthesia (P=0.044, paired t-test). Endothelin-1 induced 2.6 SDs that propagated from the caudal to the rostral window at a rate of 2.6 mm/minute. During SD, rCBF rose to 120±56% at the caudal window, while it showed an increase to 211±32% at the rostral window (P=0.035, paired t-test). Thereafter, rCBF decreased to 57±19% caudally and 72±24% rostrally (P=0.035, paired t-test). The amplitude of the negative DC shift was significantly larger and the duration significantly longer at the caudal compared with the rostral window (−5.4±1.5 mV caudally versus −2.3±0.7 mV rostrally, P=0.002; 80±17 seconds caudally versus 44±14 seconds rostrally, P=0.019, paired t-tests). Similar to group 4, the depression of high-frequency ECoG activity was more pronounced in the ET-1-exposed cortex (mean reduction to 30±10% versus 52±25% in the remote cortex). After wash-out of ET-1, the integral of high-frequency power returned to 82±22% in the remote cortex, whereas it only returned to 52±10% in the ET-1-exposed cortex.
As assessed by the brain's herniation, the caudal window had a diameter of 4310±960 μm. The sagittal diameter of the lesion of the ET-1-exposed cortex was 5140±1060 μm. The distance between the rostral border of the ET-1-induced lesion and the caudal border of the rostral control window was 4570±840 μm (Figure 3D–3F). The ET-1-induced lesion did not include the rostral control window in any of the experiments.
Evidence of Two Distinct Zones Associated with Persistent Electrocorticographic Depression in Patients with Aneurysmal Subarachnoid Hemorrhage
We analyzed six clusters of six different patients with recurrent SDs and persistent depression of high-frequency ECoG activity between SDs. The patient characteristics are given in Table 1. On average, four of six electrode positions participated in the cluster (24 electrode positions). The mean propagation velocity was 4.0±1.2 mm/minute, assuming an ideal linear spread of SDs along the strip. The clusters consisted of 10.2±8.4 SDs with a frequency of 2.0±0.9 SDs per hour and an amplitude of 9.0±3.1 mV. In 43 SDs, the average duration of the negative DC shift was shorter than 5 minutes; in 14 SDs it lasted between 5 and 10 minutes and in 4 SDs longer than 10 minutes (range: 1.7 to 88.1 minutes). Clusters were accompanied by significant depression of high-frequency ECoG activity from 0.162±0.141 to 0.016±0.012 mV2 s (integral of power, P<0.05, one-way repeated-measures analysis of variance) with a partial recovery to 0.095±0.081 mV2 s after the cluster. The depression lasted for 18.0±21.0 hours.
We then calculated a cut-off value from the animal experiments to separate the group of recordings with less recovery of high-frequency ECoG activity after the cluster (n=9 recordings) from those with more recovery (n=15 recordings) and compared the longest recorded negative DC shift between those two groups. To determine the cut-off value, a receiver-operating characteristic analysis was performed using the empirical data of group 4 and a model-based approach. The area under the receiver operating characteristic curve was determined for both approaches and differed by <1%. The calculation of cut-off points was performed according to the model-based approach. The best cut-off value for the recovery of high-frequency ECoG activity after the cluster was 55%, with a sensitivity of 80% and a specificity of 85%. Consistently with the animal experiments, longer-lasting negative DC shifts were found in the recording group with <55% recovery of high-frequency ECoG activity after the cluster (duration of negative DC shift: 36.1±32.5 versus 6.3±5.3 minutes, P<0.001, Mann–Whitney rank sum test). Whereas the integral of power was similar between the two groups before the cluster, the recording group with <55% recovery showed a significantly more pronounced depression during the cluster (mean reduction to 11±14% versus 35±26%, P=0.019, t-test). Thus, similar to the animal experiments a central area seems to exist in the human brain during acute injury that is characterized by prolonged negative DC shifts, almost complete depression of high-frequency ECoG activity during and only little recovery after the cluster (Figure 5). This central area seems to be surrounded by a belt with less pronounced but still persistent depression of high-frequency ECoG activity and only short-lasting, stereotypical negative DC shifts. Beyond this there seems to be another belt with faster recovery of high-frequency ECoG activity between the SDs (Figure 6).
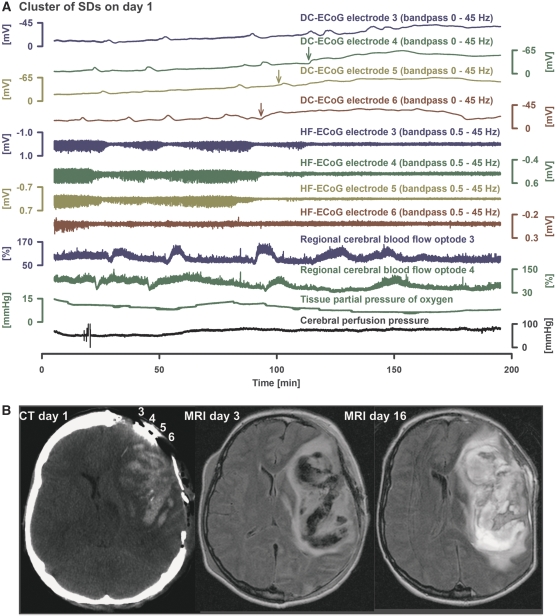
Figure 5.
Development of a very prolonged negative direct current (DC) shift during a cluster of recurrent spreading depolarizations (SDs) in a patient with aneurysmal subarachnoid hemorrhage (aSAH) (case 2 in Table 1). (A) Traces 1 to 4 show the DC-electrocorticographic activity (ECoG) (bandpass 0 to 45 Hz) of electrodes 3 (dark blue), 4 (dark green), 5 (light green), and 6 (brown) (interelectrode distance: 1 cm). Note the long-lasting negative DC shift propagating from electrodes 6 to 4 (arrows indicate the onset in each trace). Traces 5 to 8 give the corresponding changes of high-frequency ECoG activity, which represents synaptic activity (recovery of this activity was <55% after this event). Note that the depression periods become progressively longer with each SD until a persistent depression of high-frequency ECoG activity develops together with the very prolonged negative DC shifts. Traces 9 and 10 show recordings of regional cerebral blood flow (rCBF) using optodes close to electrodes 3 and 4, and laser-Doppler flowmetry. In trace 10, note that rCBF shows a prolonged decrease followed by a delayed hyperemia in response to the long-lasting SD at electrode 4, as indicated by the arrow in trace 2. This rCBF response fulfils the criteria of spreading ischemia as described previously (Dreier et al, 2009). In contrast, in trace 9, the rCBF responses to the last SDs are characterized by increases, and the corresponding SDs in trace 1 (electrode 3) are shorter lasting. Consistent with the decrease of rCBF at optode 4, tissue partial pressure of oxygen (Licox, Integra Lifesciences Corporation, Plainsboro, NJ, USA) showed a simultaneous decrease, as measured in the vicinity of optoelectrode 4. The earlier decrease of tissue partial pressure of oxygen seems to follow a decrease of cerebral perfusion pressure (trace 12), which indicates a disturbance of autoregulation. (B) The SDs originate closer to electrode 6 and the prolongation of the negative DC shifts is most pronounced at this electrode. Consistently, the computed tomography (CT) of day 1 indicates that electrode 6 is closer to a large intracerebral hemorrhage than the other electrodes. The cluster developed on day 1 after the CT. An exact comparison between the CT of day 1 and the fluid attenuated inversion recovery (FLAIR) magnetic resonance image (MRI) on day 3 is limited. However, there seems to be a progression of the lesion. The FLAIR MRI on day 16 shows further progression of the perilesional edema.
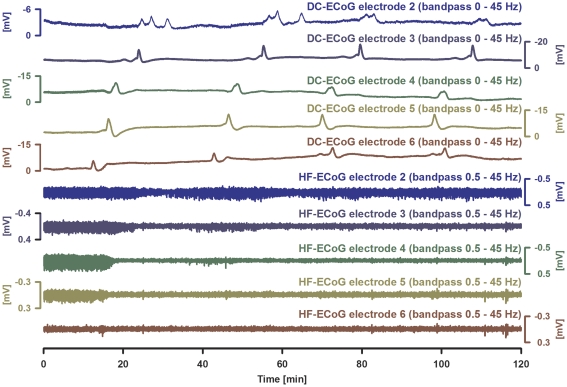
Figure 6.
A zone of persistent depression of high-frequency (HF) electrocorticographic (ECoG) activity can be associated with short-lasting, very stereotypical spreading depolarizations (SDs) in humans (case 5 in Table 1). Traces 1 to 5 give the direct current (DC)-ECoG of electrodes 2 (blue), 3 (dark blue), 4 (dark green), 5 (light green), and 6 (brown) showing the propagation of stereotypical negative DC potential shifts typical of SD across the cortex (interelectrode distance: 1 cm). Traces 6 to 10 show the HF-ECoG activities showing persistent depression between recurrent SDs in all electrodes apart from electrode 2 that is most distant from the origin of the SDs. Note that electrode 6 is significant for a shallow negative DC shift between the recurrent SDs. It is possible that the mechanism of this shallow DC negativity corresponds to that underlying similar negativities in endothelin-1 (ET-1)-exposed cortex in rats as shown in Figure 4. Such shallow DC negativities between recurrent SDs possibly indicate some local neuronal damage. However, a drift artifact of the platinum electrodes cannot be excluded (see Discussion).
Discussion
Using high-pass filtered recordings with a lower frequency limit of 0.02 Hz, it has been found previously that SDs can occur in the human cortex after traumatic brain injury, while ECoG activity above ∼0.2 Hz is already depressed (Fabricius et al, 2006). Moreover, it was shown in patients with aSAH that such SDs typically occur in clusters and that such clusters coincide with the development of delayed ischemic stroke after aSAH as assessed by serial neuroimaging (Dreier et al, 2006). Such clusters were also observed in patients with malignant hemispheric stroke (Dohmen et al, 2008). On the basis of the animal experiments (Hossmann, 1994), it was speculated that such clusters with persistent depression of high-frequency ECoG activity indicate tissue at risk. In this study, the ET-1 model in rats helped to refine this speculation. According to this model, two distinct zones with persistent depression of high-frequency ECoG activity between recurrent SDs are distinguished: an outer zone of surviving tissue characterized by clusters with short-lasting and stereotypical SDs and good recovery of the high-frequency ECoG activity after the cluster, and an inner zone at risk of progressive damage with more variable and longer-lasting negative DC shifts, even more pronounced depression of high-frequency ECoG activity and limited recovery. The inner zone may correspond to the ischemic core and penumbra, whereas the outer zone may have importance for repair, plasticity, and regeneration, which have to be investigated in future studies. Both patterns were then identified in DC-ECoG recordings of clusters of recurrent SDs in patients with aSAH.
The Endothelin-1 Model of Focal Ischemia
Endothelin-1 is a potent cerebrovascular constrictor (Yanagisawa et al, 1988). A concentration of 1 μmol/L was sufficient to produce severe arterial spasm and ischemic damage in the vascular territory when ET-1 was directly applied to the middle cerebral artery (Macrae et al, 1993).
As shown previously and confirmed in group 2 of this study, brain topical application of ET-1 induces only mildly prolonged SDs at low concentrations before a marked reduction of rCBF can be detected by laser-Doppler flowmetry (Dreier et al, 2002). As ET-1-induced rCBF decline can be subtle, doubts remained whether those SDs are the consequence of ischemia or are related to the direct effects of ET-1 on neurons or astrocytes (Dreier et al, 2002; Venance et al, 1997). However, experimental evidence has increasingly supported their ischemic origin. Thus, it was found previously that the receptor profile of ET-1-induced SD is consistent with vasoconstriction, as ETA receptors only mediated ET-1-induced SD (Kleeberg et al, 2004). Furthermore, it was shown using K+-sensitive microelectrodes that a gradual rise of [K+]o typical of ischemia preceded SDs under ET-1 in a similar manner to group 1 of this study, and ET-1 failed to elicit SD in brain slices that are devoid of a blood circulation (Dreier et al, 2002). It has been asked previously whether significant pial arteriolar constriction precedes ET-1-induced SDs. In this study, we quantified the vasoconstriction in response to increasing concentrations of ET-1 and found a marked decrease of medium and small arteriolar diameters in the ET-1-exposed cortex before the first SD. Moreover, in this study, the pattern of pH changes further supported the ischemic origin of the ET-1-induced SDs. Thus, a significant acidification preceded the alkaline shift at SD onset, which contrasts with the lack of preceding acidification in a healthy cortex (Hansen and Lauritzen, 1984; Taylor et al, 1996). We also found a peculiar delayed, slow, relative alkalinization in response to SD in the ET-1-exposed cortex, which remained, however, below baseline pH. Such a delayed slow alkalinization was not present in the naïve normoxic/normoglycemic cortex. It is neither typical of severe ischemia (Hansen and Lauritzen, 1984). Hypothetically, it results from spreading hyperemia in the ET-1-exposed cortex, which transiently increases supply with oxidative substrates and, thus, transiently decreases overproduction of lactic acid in the ischemic zone.
Laser-Doppler flowmetry does not allow absolute measurements of rCBF. Therefore, only a rough estimation of absolute rCBF is possible to relate the biophysical penumbra concept in this paper with the traditional definition as a region of constrained blood supply in which the energy metabolism is preserved (Hossmann, 1994). According to Hossmann (1994), rCBF has to decrease to a level between 6 and 15 mL per 100 g per minute to induce SD. Under barbiturate anesthesia, rCBF ranges between 50 and 70 mL per 100 g per minute, which roughly corresponds to a 40% reduction compared with normal (Todd and Weeks, 1996; Linde et al, 1999). In this study, the mean rCBF level immediately before SD ranged between 58% in group 1 and 97% in group 2 in the ET-1-exposed cortex. Thus, starting from a reduced rCBF level under thiopental, the mean rCBF immediately before the first SD roughly ranged between 35 and 58 mL per 100 g per minute. This range of rCBF would only correspond to the upper range of the ischemic penumbra definition given by Hossmann. The discrepancy between measured rCBF and occurrence of SD is possibly explained by inhomogeneities of flow in the window area. Possibly, very low flow areas are underestimated because of neighboring higher flow areas, both of which influence laser-Doppler flowmetry as it measures rCBF in a tissue volume of approximately 1 mm3. The occurrence of flow inhomogeneities is supported here by the observation that pial arteriolar diameter alterations showed marked variability in response to ET-1. The reasons for this variability are not clear. Possibly, heterogeneous diffusion of ET-1 in the window or differences in arteriolar ETA receptor densities are involved.
Brain Topical Endothelin-1 Application Provides a Model of Subtle, Gradually Developing Focal Ischemia
A majority of the animal models of focal ischemia are designed to replicate severe sudden-onset ischemia in humans, which, for example, occurs in embolic stroke. In contrast, the ET-1 model is interesting because it allows to grade a focal ischemic condition according to the applied concentrations. Thus, the neuronal response to gradually developing ischemia, initially restricted to a small spot in the tissue, can be analyzed. Gradual development of ischemia also occurs in the human brain, for example, in patients with aSAH suffering from cerebral vasospasm (Macdonald et al, 2007). The ET-1 model is attractive for the principal understanding of such conditions. Moreover, ET-1 may be involved in the induction of cerebral vasospasm after aSAH (Vatter et al, 2007). Consistently, the ETA receptor antagonist clazosentan robustly reduced anigographically detectable proximal vasospasm in patients with aSAH (Macdonald et al, 2008).
Synthesis of ET-1 is stimulated at the level of gene transcription by a number of factors released from blood clot after aSAH, including hemoglobin, thrombin, and prostaglandin F2α (Vatter et al, 2007). However, cerebrospinal concentrations of ET-1 are lower after aSAH than the concentrations necessary for induction of ischemia in this study. On the other hand, the sensitivity of vascular smooth muscle to ET-1 significantly increases after aSAH (Vatter et al, 2007; Xie et al, 2007) and ET-1 concentrations in the relevant abluminal compartment for vasoconstriction are possibly higher than those in cerebrospinal fluid (Kastner et al, 2005). In fact, cerebrospinal ET-1 may not reflect the increased abluminal ET-1 release of endothelium stimulated by subarachnoid clot, but release by astrocytes in response to oxidative substrate depletion (Pluta et al, 1997). Thus, elevation of cerebrospinal ET-1 occurred time-locked to the neuronal damage after aSAH rather than to the development of angiographic vasospasm (Mascia et al, 2001).
Interestingly, in this study, we found that both the rCBF decrease and the neuronal response to the ischemic condition depended not only on the absolute ET-1 concentration but also on the rate of concentration increase. Thus, in contrast to a slow increase of ET-1 from 10 to 100 nmol/L to 1 μmol/L in group 2, an immediate application of 1 μmol/L in group 1 induced a more pronounced decline of rCBF before the first SD. Furthermore, very prolonged negative DC shifts of approximately 20 minutes duration were only observed with immediate application of 1 μmol/L, but not when lower concentrations preceded the application of 1 μmol/L. This is probably explained by tachyphylaxis to the vasoconstrictor effect of ET-1 (Hollenberg et al, 1993) and possible counterregulatory mechanisms such as release of vasodilators like NO (Pluta and Oldfield, 2007). These results indicate that a comparison between the effects of ET-1 in different models and pathological conditions should consider not only absolute concentrations but also the time course of exposure to ET-1. Moreover, rCBF decrease and the neuronal response to the ischemic condition were less pronounced in the presence of isoflurane compared with thiopental anesthesia. This corresponds well with previous observations under halothane, a similar volatile anesthetic (Dreier et al, 2007).
Distinct Electrocorticographic Signatures in Endothelin-1 Exposed and Control Cortex
Brain topical application of the potent vasoconstrictor ET-1 in rats allows to induce focal ischemia in a concentration-dependent manner restricted to a small region exposed by a cranial window, while a healthy surrounding cortex can be studied at a second untreated window over the same hemisphere as in group 4 of this study. Consistently, a cortical area of selective neuronal necrosis was previously found at the ET-1-exposed cranial window whenever ET-1 elicited SD, but not when ET-1 failed to trigger SD (Dreier et al, 2007). Furthermore, a similar area of necrosis as after ET-1-induced SD was also found in the same region when SD did not originate in the ET-1-exposed cortex but invaded into this area from outside. If no SD propagated through the ET-1-exposed cortex no neuronal damage occurred. These findings provided further evidence that SD initiates cellular necrosis under energy depletion, no matter where the SD originates, and corresponded well with earlier work in models of middle cerebral artery occlusion in which artificially triggered SDs invaded the ischemic zone from outside and each SD recruited further tissue into necrosis (Busch et al, 1996).
It has remained puzzling in the previous study that only mildly prolonged SDs were associated with neuronal necrosis (Dreier et al, 2007). However, here we found in group 4 that mildly prolonged SDs typically occur superimposed on a more shallow subdural DC negativity in the ET-1-exposed cortex while shorter-lasting SDs rode on a shallow DC positivity in the healthy surrounding cortex. The subdural DC potential results from summation of all potential changes in the window area while the intracortical recordings only reflect potential changes in a small cortical volume of several cubic micrometers. Thus, it is possible that the shallow subdural negativity between SDs reflects the small fraction of dying neurons that fail to repolarize while the persistent positivity in the healthy cortex represents the current source of the current circuit between ET-1-exposed and healthy cortex. Consistently, the shallow subdural negativity between SDs grew to almost the same magnitude as that of single SDs when the microelectrodes in group 1 showed very long-lasting negative intracortical DC shifts at both recording positions—separated by approximately 2 mm—indicating that a large volume of cortex failed to repolarize for a prolonged period. Thus, such shallow DC negativities between recurrent SDs may have importance for determining in surface recordings whether the local effect of a given SD is partially detrimental to the tissue or more benign even if the SD itself is relatively short lasting. It deserves further study whether the polarizable platinum electrodes for human recordings are sufficient to measure subtle DC shifts between recurrent SDs (Tallgren et al, 2005).
From Bench to Bedside
The experimental evidence suggested that the ischemic center in the ET-1 model is characterized by both persistent depression of high-frequency ECoG activity and prolonged negative DC shifts of variable duration that can occur either in a cluster or as only one very long-lasting negative DC shift. Frequently, a melting of shorter-lasting, variable SDs into a very prolonged negative DC shift was observed in a similar manner to clusters in aSAH patients with <55% recovery of ECoG activity. In contrast to the zone of neuronal injury, the surrounding cortex showed negative DC shifts that were all short lasting and of stereotypical configuration. However, also in the surrounding tissue, spreading depression of high-frequency ECoG activity could persist between recurrent SDs. Thus, persistent depression between SDs seems to indicate neuronal injury (Fabricius et al, 2006; Dreier et al, 2006), but the actual neuronal injury may occur in a remote region. It deserves further study whether the prolonged suppression of synaptic activity in a belt around the ischemic penumbra has importance for the induction of repair and regeneration in this area (Yanamoto et al, 2005; Urbach et al, 2008; Brown et al, 2008; Lo, 2008). Possibly, prolonged synaptic depression between short-lasting SDs provides a stimulus in this zone to establish novel synaptic contacts. Moreover, acutely, it may save energy that could be beneficial for the survival of the neighboring penumbra (Attwell and Laughlin, 2001; Alle et al, 2009). In contrast to this, the actual SDs would increase the energy demand because they activate Na, K-ATPases for the recovery of ion homeostasis (Somjen, 2004). The exact cut-off value between detrimental and more benign SDs is not so simple as explained above. Whereas it is increasingly recognized that long-lasting negative DC shifts produce cellular death because of prolonged calcium overload (Dietz et al, 2009), short-lasting SDs may have indirect effects that render them beneficial. Although the latter should be taken with a grain of salt (Busch et al, 1996; Hartings et al, 2006; Dreier et al, 2007, 2009; Takano et al, 2007), it is intriguing to relate the role of SDs to the very recent concept that the early death signals confer repair and regeneration later (Lo, 2008).
Diagnostic assessment of SDs in the ECoG has become a novel field of clinical neurophysiology. Correlations between ECoG findings and neuroimaging, clinical outcome, and other neuromonitoring methods such as microdialysis may guide us how to interpret ECoG findings in patients correctly and how to base clinical decisions on these findings in the future (Dreier et al, 2006; Hartings et al, 2009). This study shows how comparative studies between animal models and patients can contribute to the development of an ECoG classification of SD that can be further validated using clinical outcome studies. Thus, a large prospective multicenter study using ECoG and serial magnetic resonance imaging in patients with aSAH has been started to determine whether and how distinct patterns of clusters of recurrent SDs correlate with the evolution of local tissue damage (DISCHARGE-1, http://www.controlled-trials.com/ISCRTN05667702; http://www.strokecenter.org/trials/ trialDetail.aspx?tid=1014).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Alle H, Roth A, Geiger JR. Energy-efficient action potentials in hippocampal mossy fibers. Science. 2009;325:1405–1408. doi: 10.1126/science.1174331. [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Brown CE, Wong C, Murphy TH. Rapid morphologic plasticity of peri-infarct dendritic spines after focal ischemic stroke. Stroke. 2008;39:1286–1291. doi: 10.1161/STROKEAHA.107.498238. [DOI] [PubMed] [Google Scholar]
- Busch E, Gyngell ML, Eis M, Hoehn-Berlage M, Hossmann KA. Potassium-induced cortical spreading depressions during focal cerebral ischemia in rats: contribution to lesion growth assessed by diffusion-weighted NMR and biochemical imaging. J Cereb Blood Flow Metab. 1996;16:1090–1099. doi: 10.1097/00004647-199611000-00002. [DOI] [PubMed] [Google Scholar]
- Canals S, Makarova I, Lopez-Aguado L, Largo C, Ibarz JM, Herreras O. Longitudinal depolarization gradients along the somatodendritic axis of CA1 pyramidal cells: a novel feature of spreading depression. J Neurophysiol. 2005;94:943–951. doi: 10.1152/jn.01145.2004. [DOI] [PubMed] [Google Scholar]
- Dietz RM, Weiss JH, Shuttleworth CW. Contributions of Ca2+ and Zn2+ to spreading depression-like events and neuronal injury. J Neurochem. 2009;109 (Suppl 1:145–152. doi: 10.1111/j.1471-4159.2009.05853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmen C, Sakowitz OW, Fabricius M, Bosche B, Reithmeier T, Ernestus RI, Brinker G, Dreier JP, Woitzik J, Strong AJ, Graf R. Spreading depolarizations occur in human ischemic stroke with high incidence. Ann Neurol. 2008;63:720–728. doi: 10.1002/ana.21390. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Kleeberg J, Alam M, Major S, Kohl-Bareis M, Petzold GC, Victorov I, Dirnagl U, Obrenovitch TP, Priller J. Endothelin-1-induced spreading depression in rats is associated with a microarea of selective neuronal necrosis. Exp Biol Med (Maywood) 2007;232:204–213. [PubMed] [Google Scholar]
- Dreier JP, Kleeberg J, Petzold GC, Priller J, Windmuller O, Orzechowski HD, Lindauer U, Heinemann U, Einhaupl KM, Dirnagl U. Endothelin-1 potently induces Leão's cortical spreading depression in vivo in the rat: a model for an endothelial trigger of migrainous aura. Brain. 2002;125:102–112. doi: 10.1093/brain/awf007. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Major S, Manning A, Woitzik J, Drenckhahn C, Steinbrink J, Tolias C, Oliveira-Ferreira AI, Fabricius M, Hartings JA, Vajkoczy P, Lauritzen M, Dirnagl U, Bohner G, Strong AJ. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 2009;132:1866–1881. doi: 10.1093/brain/awp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier JP, Woitzik J, Fabricius M, Bhatia R, Major S, Drenckhahn C, Lehmann TN, Sarrafzadeh A, Willumsen L, Hartings JA, Sakowitz OW, Seemann JH, Thieme A, Lauritzen M, Strong AJ. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129:3224–3237. doi: 10.1093/brain/awl297. [DOI] [PubMed] [Google Scholar]
- Fabricius M, Fuhr S, Bhatia R, Boutelle M, Hashemi P, Strong AJ, Lauritzen M. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain. 2006;129:778–790. doi: 10.1093/brain/awh716. [DOI] [PubMed] [Google Scholar]
- Fleidervish IA, Gebhardt C, Astman N, Gutnick MJ, Heinemann U. Enhanced spontaneous transmitter release is the earliest consequence of neocortical hypoxia that can explain the disruption of normal circuit function. J Neurosci. 2001;21:4600–4608. doi: 10.1523/JNEUROSCI.21-13-04600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AJ, Lauritzen M. The role of spreading depression in acute brain disorders. An Acad Bras Cienc. 1984;56:457–479. [PubMed] [Google Scholar]
- Hartings JA, Strong AJ, Fabricius M, Manning A, Bhatia R, Dreier JP, Mazzeo TA, Tortella CF, Bullock R, Co-Operative Study of Brain Injury Depolarizations Spreading depolarizations and late secondary insults after traumatic brain injury. J Neurotrauma. 2009;26:1857–1866. doi: 10.1089/neu.2009.0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartings JA, Tortella FC, Rolli ML. AC electrocorticographic correlates of peri-infarct depolarizations during transient focal ischemia and reperfusion. J Cereb Blood Flow Metab. 2006;26:696–707. doi: 10.1038/sj.jcbfm.9600223. [DOI] [PubMed] [Google Scholar]
- Hollenberg SM, Shelhamer JH, Cunnion RE. Tachyphylaxis to the vasopressor effects of endothelin in rat aortic rings. Am J Physiol. 1993;264:H352–H356. doi: 10.1152/ajpheart.1993.264.2.H352. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36:557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- Kastner S, Oertel MF, Scharbrodt W, Krause M, Boker DK, Deinsberger W. Endothelin-1 in plasma, cisternal CSF and microdialysate following aneurysmal SAH. Acta Neurochir (Wien) 2005;147:1271–1279. doi: 10.1007/s00701-005-0633-0. [DOI] [PubMed] [Google Scholar]
- Kleeberg J, Petzold GC, Major S, Dirnagl U, Dreier JP. ET-1 induces cortical spreading depression via activation of the ETA receptor/phospholipase C pathway in vivo. Am J Physiol Heart Circ Physiol. 2004;286:H1339–H1346. doi: 10.1152/ajpheart.00227.2003. [DOI] [PubMed] [Google Scholar]
- Kraig RP, Nicholson C. Extracellular ionic variations during spreading depression. Neuroscience. 1978;3:1045–1059. doi: 10.1016/0306-4522(78)90122-7. [DOI] [PubMed] [Google Scholar]
- Leão AAP. Spreading depression of activity in the cerebral cortex. J Neurophysiol. 1944;7:359–390. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- Leão AAP. Further observations on the spreading depression of activity in the cerebral cortex. J Neurophysiol. 1947;10:409–414. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- Linde R, Schmalbruch IK, Paulson OB, Madesen PL. The Kety-Schmidt technique for repeated measurements of global cerebral blood flow and metabolism in the conscious rat. Acta Physiol Scand. 1999;165:395–401. doi: 10.1046/j.1365-201x.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Kassell NF, Mayer S, Ruefenacht D, Schmiedek P, Weidauer S, Frey A, Roux S, Pasqualin A. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008;39:3015–3021. doi: 10.1161/STROKEAHA.108.519942. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Pluta RM, Zhang JH. Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. Nat Clin Pract Neurol. 2007;3:256–263. doi: 10.1038/ncpneuro0490. [DOI] [PubMed] [Google Scholar]
- Macrae IM, Robinson MJ, Graham DI, Reid JL, McCulloch J. Endothelin-1-induced reductions in cerebral blood flow: dose dependency, time course, and neuropathological consequences. J Cereb Blood Flow Metab. 1993;13:276–284. doi: 10.1038/jcbfm.1993.34. [DOI] [PubMed] [Google Scholar]
- Mascia L, Fedorko L, Stewart DJ, Mohamed F, terBrugge K, Ranieri VM, Wallace MC. Temporal relationship between endothelin-1 concentrations and cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2001;32:1185–1190. doi: 10.1161/01.str.32.5.1185. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Hansen AJ. Characterization of cortical depolarizations evoked in focal cerebral ischemia. J Cereb Blood Flow Metab. 1993;13:568–574. doi: 10.1038/jcbfm.1993.74. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Windmuller O, Haack S, Major S, Buchheim K, Megow D, Gabriel S, Lehmann TN, Drenckhahn C, Peters O, Meierkord H, Heinemann U, Dirnagl U, Dreier JP. Increased extracellular K+ concentration reduces the efficacy of N-methyl-D-aspartate receptor antagonists to block spreading depression-like depolarizations and spreading ischemia. Stroke. 2005;36:1270–1277. doi: 10.1161/01.STR.0000166023.51307.e0. [DOI] [PubMed] [Google Scholar]
- Pluta RM, Boock RJ, Afshar JK, Clouse K, Bacic M, Ehrenreich H, Oldfield EH. Source and causeof endothelin-1 release into cerebrospinal fluid after subarachnoid hemorrhage. J Neurosurg. 1997;87:287–293. doi: 10.3171/jns.1997.87.2.0287. [DOI] [PubMed] [Google Scholar]
- Pluta RM, Oldfield EH. Analysis of nitric oxide (NO) in cerebral vasospasm after aneursymal bleeding. Rev Recent Clin Trials. 2007;2:59–67. doi: 10.2174/157488707779318062. [DOI] [PubMed] [Google Scholar]
- Sakowitz OW, Kiening KL, Krajewski KL, Sarrafzadeh AS, Fabricius M, Strong AJ, Unterberg AW, Dreier JP. Preliminary evidence thatketamine inhibits spreading depolarizations in acute human brain injury. Stroke. 2009;40:e519–e522. doi: 10.1161/STROKEAHA.109.549303. [DOI] [PubMed] [Google Scholar]
- Shin HK, Dunn AK, Jones PB, Boas DA, Moskowitz MA, Ayata C. Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J Cereb Blood Flow Metab. 2006;26:1018–1030. doi: 10.1038/sj.jcbfm.9600252. [DOI] [PubMed] [Google Scholar]
- Somjen GG.(ed). (2004Ions in the Brain. Normal Function, Seizures and Stroke New York, NY: Oxford University Press [Google Scholar]
- Strong AJ, Anderson PJ, Watts HR, Virley DJ, Lloyd A, Irving EA, Nagafuji T, Ninomiya M, Nakamura H, Dunn AK, Graf R. Peri-infarct depolarizations lead to loss of perfusion in ischaemic gyrencephalic cerebral cortex. Brain. 2007;130:995–1008. doi: 10.1093/brain/awl392. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Lovatt D, Hansen AJ, Kasischke KA, Nedergaard M. Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci. 2007;10:754–762. doi: 10.1038/nn1902. [DOI] [PubMed] [Google Scholar]
- Tallgren P, Vanhatalo S, Kaila K, Voipio J. Evaluation of commercially available electrodes and gels for recording of slow EEG potentials. Clin Neurophysiol. 2005;116:799–806. doi: 10.1016/j.clinph.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Taylor DL, Obrenovitch TP, Symon L. Changes in extracellular acid-base homeostasis in cerebral ischemia. Neurochem Res. 1996;21:1013–1021. doi: 10.1007/BF02532411. [DOI] [PubMed] [Google Scholar]
- Todd MM, Weeks J. Comparative effects of propofol, pentobarbital and isoflurane on cerebral blood flow volume. J Neurosurg Anesthesiol. 1996;8:296–303. doi: 10.1097/00008506-199610000-00007. [DOI] [PubMed] [Google Scholar]
- Urbach A, Redecker C, Witte OW. Induction of neurogenesis in the adult dentate gyrus by cortical spreading depression. Stroke. 2008;39:3064–3072. doi: 10.1161/STROKEAHA.108.518076. [DOI] [PubMed] [Google Scholar]
- Vatter H, Konczalla J, Weidauer S, Preibisch C, Zimmermann M, Raabe A, Seifert V. Effect of delayed cerebral vasospasm on cerebrovascular endothelin A receptor expression and function. J Neurosurg. 2007;107:121–127. doi: 10.3171/JNS-07/07/0121. [DOI] [PubMed] [Google Scholar]
- Venance L, Stella N, Glowinski J, Giaume C. Mechanism involved in initiation and propagation of receptor-induced intercellular calcium signaling in cultured rat astrocytes. J Neurosci. 1997;17:1981–1992. doi: 10.1523/JNEUROSCI.17-06-01981.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windmüller O, Lindauer U, Foddis M, Einhaupl KM, Dirnagl U, Heinemann U, Dreier JP. Ion changes in spreading ischaemia induce rat middle cerebral artery constriction in the absence of NO. Brain. 2005;128:2042–2051. doi: 10.1093/brain/awh545. [DOI] [PubMed] [Google Scholar]
- Xie A, Aihara Y, Bouryi VA, Nikitina E, Jahromi BS, Zhang ZD, Takahashi M, Macdonald RL. Novel mechanism of endothelin-1-induced vasospasm after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2007;27:1692–1701. doi: 10.1038/sj.jcbfm.9600471. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yanamoto H, Miyamoto S, Tohnai N, Nagata I, Xue JH, Nakano Y, Nakajo Y, Kikuchi H. Induced spreading depression activates persistent neurogenesis in the subventricular zone, generating cells with markers for divided and early committed neurons in the caudate putamen and cortex. Stroke. 2005;36:1544–1550. doi: 10.1161/01.STR.0000169903.09253.c7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.