Abstract
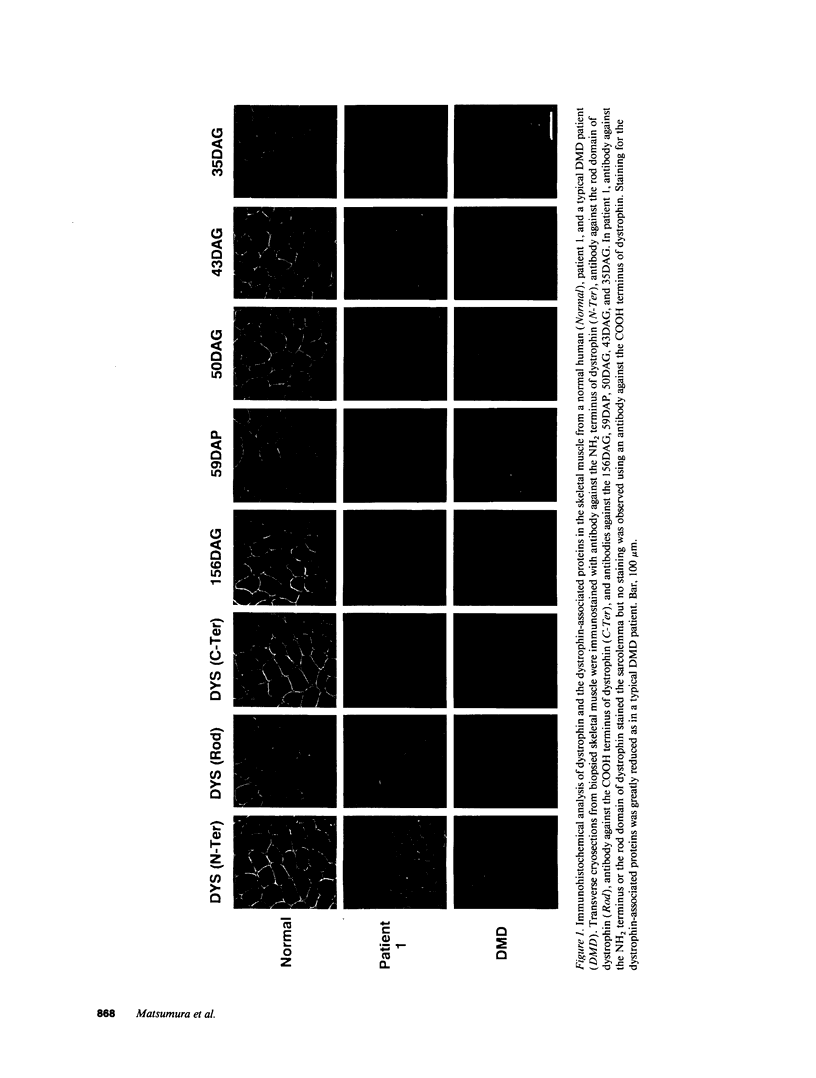
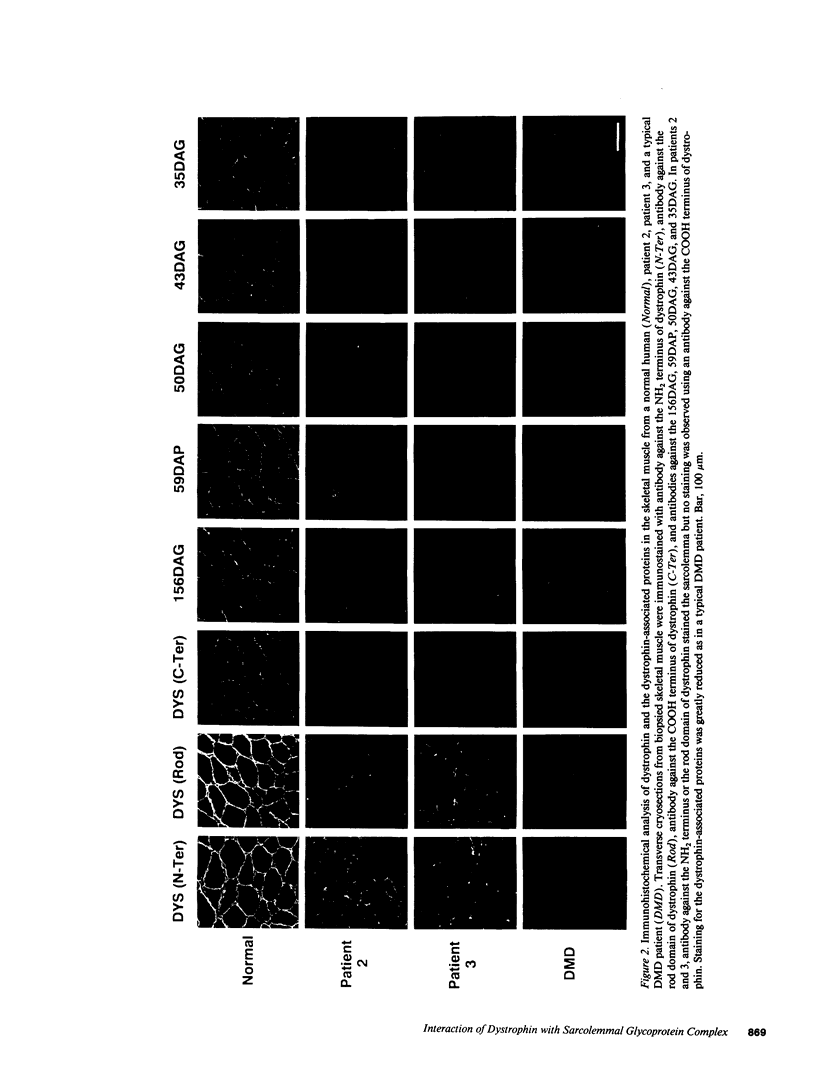
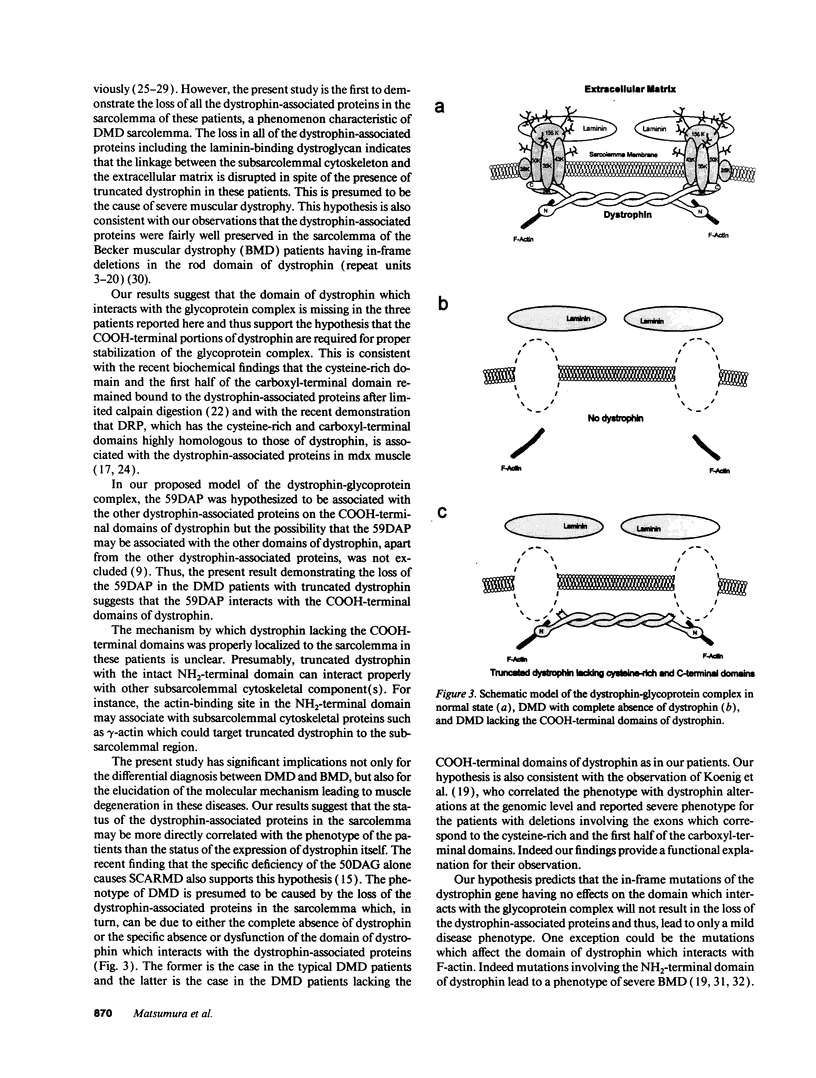
Dystrophin, the protein product of the Duchenne muscular dystrophy (DMD) gene, is a cytoskeletal protein tightly associated with a large oligomeric complex of sarcolemmal glycoproteins including dystroglycan, which provides a linkage to the extracellular matrix component, laminin. In DMD, the absence of dystrophin leads to a drastic reduction in all of the dystrophin-associated proteins, causing the disruption of the linkage between the subsarcolemmal cytoskeleton and the extracellular matrix which, in turn, may render muscle cells susceptible to necrosis. The COOH-terminal domains (cysteine-rich and carboxyl-terminal) of dystrophin have been suggested to interact with the sarcolemmal glycoprotein complex. However, truncated dystrophin lacking these domains was reported to be localized to the sarcolemma in four DMD patients recently. Here we report that all of the dystrophin-associated proteins are drastically reduced in the sarcolemma of three DMD patients in whom dystrophin lacking the COOH-terminal domains was properly localized to the sarcolemma. Our results indicate that the COOH-terminal domains of dystrophin are required for the proper interaction of dystrophin with the dystrophin-associated proteins and also support our hypothesis that the loss of the dystrophin-associated proteins in the sarcolemma leads to severe muscular dystrophy even when truncated dystrophin is present in the subsarcolemmal cytoskeleton.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beggs A. H., Hoffman E. P., Snyder J. R., Arahata K., Specht L., Shapiro F., Angelini C., Sugita H., Kunkel L. M. Exploring the molecular basis for variability among patients with Becker muscular dystrophy: dystrophin gene and protein studies. Am J Hum Genet. 1991 Jul;49(1):54–67. [PMC free article] [PubMed] [Google Scholar]
- Bies R. D., Caskey C. T., Fenwick R. An intact cysteine-rich domain is required for dystrophin function. J Clin Invest. 1992 Aug;90(2):666–672. doi: 10.1172/JCI115909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. P., Kahl S. D. Association of dystrophin and an integral membrane glycoprotein. Nature. 1989 Mar 16;338(6212):259–262. doi: 10.1038/338259a0. [DOI] [PubMed] [Google Scholar]
- Clemens P. R., Ward P. A., Caskey C. T., Bulman D. E., Fenwick R. G. Premature chain termination mutation causing Duchenne muscular dystrophy. Neurology. 1992 Sep;42(9):1775–1782. doi: 10.1212/wnl.42.9.1775. [DOI] [PubMed] [Google Scholar]
- Cullen M. J., Walsh J., Nicholson L. V., Harris J. B. Ultrastructural localization of dystrophin in human muscle by using gold immunolabelling. Proc R Soc Lond B Biol Sci. 1990 May 22;240(1297):197–210. doi: 10.1098/rspb.1990.0034. [DOI] [PubMed] [Google Scholar]
- Cullen M. J., Walsh J., Nicholson L. V., Harris J. B., Zubrzycka-Gaarn E. E., Ray P. N., Worton R. G. Immunogold labelling of dystrophin in human muscle, using an antibody to the last 17 amino acids of the C-terminus. Neuromuscul Disord. 1991;1(2):113–119. doi: 10.1016/0960-8966(91)90058-z. [DOI] [PubMed] [Google Scholar]
- Ervasti J. M., Campbell K. P. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991 Sep 20;66(6):1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Ervasti J. M., Kahl S. D., Campbell K. P. Purification of dystrophin from skeletal muscle. J Biol Chem. 1991 May 15;266(14):9161–9165. [PubMed] [Google Scholar]
- Ervasti J. M., Ohlendieck K., Kahl S. D., Gaver M. G., Campbell K. P. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature. 1990 May 24;345(6273):315–319. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- Helliwell T. R., Ellis J. M., Mountford R. C., Appleton R. E., Morris G. E. A truncated dystrophin lacking the C-terminal domains is localized at the muscle membrane. Am J Hum Genet. 1992 Mar;50(3):508–514. [PMC free article] [PubMed] [Google Scholar]
- Hemmings L., Kuhlman P. A., Critchley D. R. Analysis of the actin-binding domain of alpha-actinin by mutagenesis and demonstration that dystrophin contains a functionally homologous domain. J Cell Biol. 1992 Mar;116(6):1369–1380. doi: 10.1083/jcb.116.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Garcia C. A., Chamberlain J. S., Angelini C., Lupski J. R., Fenwick R. Is the carboxyl-terminus of dystrophin required for membrane association? A novel, severe case of Duchenne muscular dystrophy. Ann Neurol. 1991 Oct;30(4):605–610. doi: 10.1002/ana.410300414. [DOI] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O., Ervasti J. M., Leveille C. J., Slaughter C. A., Sernett S. W., Campbell K. P. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992 Feb 20;355(6362):696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- Koenig M., Beggs A. H., Moyer M., Scherpf S., Heindrich K., Bettecken T., Meng G., Müller C. R., Lindlöf M., Kaariainen H. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am J Hum Genet. 1989 Oct;45(4):498–506. [PMC free article] [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Lemaire C., Heilig R., Mandel J. L. The chicken dystrophin cDNA: striking conservation of the C-terminal coding and 3' untranslated regions between man and chicken. EMBO J. 1988 Dec 20;7(13):4157–4162. doi: 10.1002/j.1460-2075.1988.tb03311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K., Ervasti J. M., Ohlendieck K., Kahl S. D., Campbell K. P. Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature. 1992 Dec 10;360(6404):588–591. doi: 10.1038/360588a0. [DOI] [PubMed] [Google Scholar]
- Matsumura K., Imoto N. [Two long-living brothers of dystrophin-related muscular dystrophy with an in-frame deletion of exon 3 of the dystrophin gene--clinical features and diagnosis]. Rinsho Shinkeigaku. 1991 Mar;31(3):286–290. [PubMed] [Google Scholar]
- Matsumura K., Nonaka I., Arahata K., Campbell K. P. Partial deficiency of dystrophin-associated proteins in a young girl with sporadic myopathy and normal karyotype. Neurology. 1993 Jun;43(6):1267–1268. doi: 10.1212/wnl.43.6.1267. [DOI] [PubMed] [Google Scholar]
- Matsumura K., Tomé F. M., Collin H., Azibi K., Chaouch M., Kaplan J. C., Fardeau M., Campbell K. P. Deficiency of the 50K dystrophin-associated glycoprotein in severe childhood autosomal recessive muscular dystrophy. Nature. 1992 Sep 24;359(6393):320–322. doi: 10.1038/359320a0. [DOI] [PubMed] [Google Scholar]
- Ohlendieck K., Campbell K. P. Dystrophin constitutes 5% of membrane cytoskeleton in skeletal muscle. FEBS Lett. 1991 Jun 3;283(2):230–234. doi: 10.1016/0014-5793(91)80595-t. [DOI] [PubMed] [Google Scholar]
- Ohlendieck K., Campbell K. P. Dystrophin-associated proteins are greatly reduced in skeletal muscle from mdx mice. J Cell Biol. 1991 Dec;115(6):1685–1694. doi: 10.1083/jcb.115.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlendieck K., Ervasti J. M., Matsumura K., Kahl S. D., Leveille C. J., Campbell K. P. Dystrophin-related protein is localized to neuromuscular junctions of adult skeletal muscle. Neuron. 1991 Sep;7(3):499–508. doi: 10.1016/0896-6273(91)90301-f. [DOI] [PubMed] [Google Scholar]
- Ohlendieck K., Ervasti J. M., Snook J. B., Campbell K. P. Dystrophin-glycoprotein complex is highly enriched in isolated skeletal muscle sarcolemma. J Cell Biol. 1991 Jan;112(1):135–148. doi: 10.1083/jcb.112.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlendieck K., Matsumura K., Ionasescu V. V., Towbin J. A., Bosch E. P., Weinstein S. L., Sernett S. W., Campbell K. P. Duchenne muscular dystrophy: deficiency of dystrophin-associated proteins in the sarcolemma. Neurology. 1993 Apr;43(4):795–800. doi: 10.1212/wnl.43.4.795. [DOI] [PubMed] [Google Scholar]
- Récan D., Chafey P., Leturcq F., Hugnot J. P., Vincent N., Tomé F., Collin H., Simon D., Czernichow P., Nicholson L. V. Are cysteine-rich and COOH-terminal domains of dystrophin critical for sarcolemmal localization? J Clin Invest. 1992 Feb;89(2):712–716. doi: 10.1172/JCI115640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Yoshida M., Yamamoto H., Ozawa E. Glycoprotein-binding site of dystrophin is confined to the cysteine-rich domain and the first half of the carboxy-terminal domain. FEBS Lett. 1992 Aug 17;308(2):154–160. doi: 10.1016/0014-5793(92)81265-n. [DOI] [PubMed] [Google Scholar]
- Tinsley J. M., Blake D. J., Roche A., Fairbrother U., Riss J., Byth B. C., Knight A. E., Kendrick-Jones J., Suthers G. K., Love D. R. Primary structure of dystrophin-related protein. Nature. 1992 Dec 10;360(6404):591–593. doi: 10.1038/360591a0. [DOI] [PubMed] [Google Scholar]
- Way M., Pope B., Cross R. A., Kendrick-Jones J., Weeds A. G. Expression of the N-terminal domain of dystrophin in E. coli and demonstration of binding to F-actin. FEBS Lett. 1992 Apr 27;301(3):243–245. doi: 10.1016/0014-5793(92)80249-g. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Ozawa E. Glycoprotein complex anchoring dystrophin to sarcolemma. J Biochem. 1990 Nov;108(5):748–752. doi: 10.1093/oxfordjournals.jbchem.a123276. [DOI] [PubMed] [Google Scholar]





