Abstract
The incidence of infection among stroke patients is alarmingly high and both acute and delayed infections increase morbidity and mortality. Experimental studies support the acute clinical data, but little attention has focused on delayed systemic infections. Here, we investigated the effects of prolonged systemic inflammation either before or 24-h after ischemia. Systemic inflammation was induced by injecting rats with three separate doses of lipopolysaccharide (LPS; 50 μg/kg, i.p.) with core temperature monitoring for 48-h after middle cerebral artery occlusion (MCAo). Lipopolysaccharide injected before MCAo increased injury by ∼30%, whereas delayed injection increased injury by ∼85% (30-day survival). Proinflammatory cytokines assessed repeatedly for 72 h were significantly and persistently elevated with inflammation. This was accompanied by increases in microglia/macrophage and infiltrating leukocyte numbers in delayed LPS-treated animals. Behavioral assessments at 7 and 30 days revealed ∼15% deficit in hindlimb function in animals treated with LPS 24-h after ischemia. Clearly, delayed and prolonged postischemic systemic inflammation has devastating effects on stroke outcome, in the absence of a prolonged febrile response. These findings, together with corroborative clinical data, emphasize the importance of early intervention to counteract the deleterious consequences of stroke-associated inflammation and infection.
Keywords: behavior, cytokines, ischemia, MCAo, neuroinflammation, systemic inflammation
Introduction
Infection in stroke patients, either before or after injury, worsens functional outcome when assessed at delayed time points (Emsley and Hopkins, 2008). It is estimated that ∼30% of ischemic stroke patients present with an antecedent infection, and that infection is a significant risk factor for ischemic stroke (Bova et al, 1996; Grau et al, 1998). A further 30% of patients develop an infection while in hospital, most commonly urinary or respiratory tract infections, which are also associated with poorer functional outcomes (Grau et al, 1999). This poor prognosis may be due to an immunosuppression after stroke, leaving the patient susceptible to developing an infection soon after ischemia (Chamorro et al, 2007; Offner et al, 2006; Prass et al, 2006).
Several experimental studies have examined the effects of acute ischemic inflammation (immediately before or after ischemia), using a low-dose injection of an endotoxin, lipopolysaccharide (LPS) to model the systemic effects of an infection. Spencer et al (2007) injected a single low dose of LPS immediately after global cerebral ischemia and found an acceleration of cell death at 3 days. Further, they found significant elevations of the proinflammatory cytokines tumor necrosis factor α (TNFα) and interleukin (IL)-6 at 4 h after ischemia, independent of significant temperature elevations. This is important because mild hyperthermia worsens outcome in experimental and clinical ischemia (Busto et al, 1987). Additional studies conducted by McColl et al (2007, 2008) showed increased ischemic damage after LPS or IL-1 injection that was related to increased IL-6 and peripheral immune cell infiltration through breakdown of the blood–brain barrier, although postischemic temperature was not assessed. Additional studies have used higher doses of LPS injections at prolonged time points before ischemia (>24 h) and have found equivocal results ranging from neuroprotection (e.g., due to preconditioning) to increased injury (Rosenzweig et al, 2004; Spencer et al, 2006; Tasaki et al, 1997).
Proposed mechanisms for increased neuronal damage after acute ischemic infection include increases in proinflammatory cytokines (TNFα, IL-6, and IL-1β) as well as increases in neuroinflammation as measured by microglial activation, and macrophage, neutrophil and T-cell infiltration (McColl et al, 2007; Shichita et al, 2009; Zheng and Yenari, 2004). Reductions of these cytokines or neuroinflammation lead to reduction in cerebral damage and improved function (Hewlett and Corbett, 2006; McColl et al, 2007). In this study, we evaluated a number of ramifications of concurrent ischemia and systemic inflammation. First, we used more prolonged systemic inflammation than that used by others. Second, we compared the effects of preischemic inflammation to delayed ischemic inflammation, 24 h after middle cerebral artery occlusion (MCAo). Third, we repeatedly assessed serum cytokine levels for 72 h after ischemia instead of just 4, 8, or 24 h after ischemia. Fourth, we assessed long-term functional outcome and both short- and long-term histopathology. On the basis of earlier reports (McColl et al, 2007, 2008; Spencer et al, 2007), we hypothesized that animals exposed to LPS either before or after ischemia would have higher levels of proinflammatory cytokines, increased functional deficits, and increased neuronal injury.
Materials and methods
Subjects
Sixty-three male Sprague-Dawley rats (Charles River, Quebec, Canada) weighing ∼200 to 225 g on arrival were used in this study. Animals were housed in pairs on a 12 h light:dark cycle and were fed food and water ad libitum except during staircase training and testing. All procedures were approved by the Memorial University of Newfoundland Animal Care Committee and conformed to the Canadian Council on Animal Care guidelines.
Surgery
Telemetry core temperature probe implantation
All animals were implanted with a core temperature telemetry probe (TA10TA-F20, Data Sciences International, St Paul, MN, USA) as described earlier (MacLellan et al, 2006). Briefly, animals were anesthetized with Isoflurane (4.0% induction, 2.0% maintenance) in a 30:70 O2/N2O mixture, and a sterile core temperature probe was inserted into the peritoneal cavity using aseptic techniques. Animals were individually housed over a telemetry receiver (RPC-1, Data Sciences International) and temperature recorded every 5 min. Temperature was averaged every hour and the complete 24-h period before surgery served as a baseline. One animal died after telemetry probe surgery.
Middle Cerebral Artery Occlusion
One week after telemetry probe surgery, animals underwent MCAo surgery by infusing 1,200 pmol of the vasoconstrictive peptide, endothelin-1, adjacent to the MCA at coordinates anterioposterior +0.9, mediolateral −5.2, dorsoventral −9.1 from the skull at bregma. Animals were anesthetized with Isoflurane as above. Rectal temperature was maintained at ∼37.0°C throughout surgery using a self-regulating heating blanket (Harvard Apparatus, Holliston, MA, USA). After MCAo surgery, animals were singly housed and temperature was monitored for 48 h. One animal died after MCAo surgery and the resultant data were collected from the remaining 61 animals.
Prolonged Systemic Inflammation
Whereas most researchers use a single large bolus dose of LPS to produce acute inflammation (Marsh et al, 2009; McColl et al, 2007; Rosenzweig et al, 2004), we sought to produce more prolonged, clinically relevant inflammation as might occur in stroke patients. Lipopolysaccharide (Escherichia coli; serotype 026:B6, Sigma, St Louis, MO, USA) was injected intraperitoneally at a concentration of 50 μg/kg so as not to induce a febrile response that may occur at higher concentrations (Spencer et al, 2007). To simulate a more prolonged inflammatory condition, two additional injections of equal concentration were given, separated by 4-h intervals. Animals in the control condition received three injections (separated by 4 h) of the pyrogen-free saline vehicle.
Experimental Conditions
Eighteen animals were used for short-term (3-day survival) cytokine and histological assessments and 43 animals were used for long-term (30 days) functional and histological assessments. All animals underwent both telemetry and MCAo surgeries. Animals were randomized into the following conditions: preischemia inflammation animals (n=6 short-term; n=15 long-term) were administered LPS (50 μg/kg, i.p.) at 8, 4 h, and immediately before MCAo (Pre); delayed postischemia inflammation animals (n=6 short-term; n=14 long-term) were administered LPS (50 μg/kg, i.p.) at 24, 28, and 32 h post-MCAo (Post); and Saline control animals (n=6 short-term; n=14 long-term) received saline injections immediately after MCAo, and at 4 and 8 h after surgery.
Cytokine Assessment
Repeated blood sampling occurred by a tail nick procedure (Canadian Council on Animal Care, 1993) under brief (∼5 mins), light anesthesia (2.0% Isoflurane). Blood sampling was conducted during telemetry surgery (baseline), MCAo surgery (time 0), 12, 24, 36, and 72 h after MCAo. At each time point, 500 μL of blood was collected into a Microtainer (Becton Dickinson, Franklin Lakes, NJ, USA) and allowed to clot for 30 mins. Blood was then centrifuged at 14,000 r.p.m. for 2 mins and serum was decanted and stored at −20°C until further processing. Serum TNFα, IL-6, and IL-1β concentration levels were quantified using standard enzyme-linked immunosorbent assay kits (ELISA; R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
Behavioral Assessments
Three separate motor assessments sensitive to detecting MCAo-induced ischemic injury were used in this study (Windle et al, 2006).
Beam walking test
Animals were trained to traverse a tapered beam (widest portion, 6 cm; narrowest portion, 2 cm), elevated 75 cm from the floor, to enter a dark chamber. Performance was videotaped and the number of errors (forelimb and hindlimb slips) made with each limb was recorded as a percent of the total steps made and averaged over three independent crosses before surgery and at each time point after MCAo.
Staircase test of skilled reaching
Animals were mildly food-restricted (∼90% to 95% of free-feeding body weight) and trained to reach for 45 mg food pellets (TestDiet, Richmond, IN, USA) in the Montoya staircase task before telemetry surgery (Montoya et al, 1991). Training occurred over a 10-day period with animals receiving two 15-mins trials/day until they reached a pre-set criteria of at least 12 out of a possible 21 pellets on an arm with a s.d. ≤2 over a period of eight trials (the average number of pellets successfully reached was 17/21). On postsurgery testing, animals received two trials/day for 2 days at each time point.
Spontaneous limb use (cylinder) test
Animals' forelimb use was calculated before surgery using the cylinder test of limb asymmetry (Schallert, 2006). Briefly, animals were placed in a clear Plexiglas cylinder (20 cm in diameter) on a glass tabletop and videotaped from below. Animals were required to make 20 independent contacts with the cylinder wall and the number of single (ipsilateral or contralateral) or bilateral contacts were calculated. Contralateral forelimb usage was calculated using the equation: [(number of contralateral contacts+½ number bilateral contacts)/(number of ipsilateral+contralateral+bilateral contacts)] × 100 (Schallert and Woodlee, 2005). All animals were assessed before surgery (baseline) on each of the functional assessments and all post-MCAo behavior is presented as a percentage of baseline abilities. Animals were assessed in each behavioral test 7 and 30 days after MCAo.
Histology and Immunohistochemistry
Animals were deeply anesthetized with 4.0% isoflurane, transcardially perfused with ice-cold heparinized 0.9% saline and 4.0% paraformaldehyde (PFA), and quickly decapitated. The heads were stored in PFA at 4°C for 4 h, the brains removed and then stored overnight in PFA at 4°C. Brains were then transferred to 20% sucrose in phosphate-buffered saline, stored at 4°C until saturated, and then frozen and stored at −20°C until further processing.
Coronal slices were cut at 20 μm using a cryostat and sections were slide mounted for H&E or leukocyte stains, and the immunohistological ED-1 stain for activated microglia as previously described (Langdon et al, 2008). Leukocytes were stained using the naphthol AS-D chloroacetate esterase procedure as outlined by the manufacturer (Sigma-Aldrich, St Louis, MO, USA). ED-1 staining was as follows: sections were washed in PBS, blocked with 1.0% H2O2 for endogenous peroxidase, blocked with normal goat serum (5.0% Jackson Immunoresearch Laboratories, West Grove, PA, USA), incubated overnight with monoclonal mouse anti-rat CD68 (ED-1; 1:1,000, MCA341R; Serotec, Raleigh, NC, USA) at 4°C, exposed to goat anti-mouse biotinylated secondary antibody (1:1,000; Jackson Immunoresearch Laboratories), incubated in 10 μg/mL extravadin (Sigma-Aldrich, Oakville, ON, Canada) and reacted for 5 mins in a 3,3′-diaminobenzidine tablet set (Sigma-Aldrich).
To quantify the infarct volume, the area of contralateral and ipsilateral tissue remaining was calculated from the H&E-stained tissue (ImageJ 1.36b software for Mac, downloaded from the public domain, National Institutes of Health, USA, http://rsb.info.nih.gov/ij/). Tissue was measured at 800-μm intervals throughout the brain, and the volume of intact tissue was calculated as: (average area of intact tissue in each section) × (number of sections analyzed) × (distance between sections (800 μm)). The volume of infarction was calculated as: volume of contralateral hemisphere–volume of intact ipsilateral hemisphere. Infarct volume was corrected for edema at the earlier time point by normalizing the ipsilateral hemispheric volume to the nonaffected contralateral hemisphere and calculating volume of intact tissue as above (Belayev et al, 2005). The average number of activated microglia/macrophages and neutrophils in each brain were estimated using a Leica DMRXE microscope and the Fractionator method of Stereo Investigator (MBF Bioscience, Williston, VT, USA). An average of 25 sampling sites (100 μm × 100 μm) were randomly superimposed on the circumscribed area of damage and positive cells were counted in a 20-μm section, every 800 μm throughout the injury. The average number of cells between each counted section was calculated, and the sum of those averages was multiplied by 40 (800 μm/20 μm) to estimate a total number of cells.
Statistical Analyses
All analyses were conducted using the statistical package for the social sciences (SPSS; v 13.0.0 Grad Pack for Mac OS X, SPSS, Chicago, IL, USA). All data from the cytokine and histological assessments are presented as means±s.e.m. Baseline cytokine and histological data were analyzed with a univariate analysis of variance (ANOVA) and subsequent cytokine data were analyzed using repeated measures ANOVA. Post hoc tests were conducted using the Tukey's honestly significant difference (HSD) test (unequal group sizes) or the least significant difference test (equal group sizes). All data from functional assessments are presented as percentage of baseline and analyzed using repeated measures ANOVA. In cases where the homogeneity of variance or sphericity assumptions were violated, the Brown-Forsythe or Huynh-Feldt correction was used, respectively. Statistical significance was considered at P≤0.05.
Results
Temperature
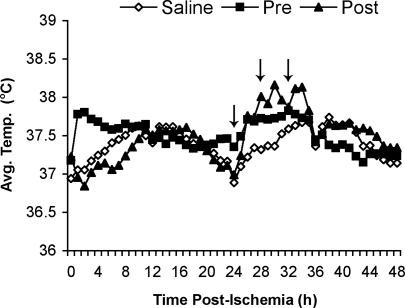
Postischemic core temperature was recorded for 48 h after surgery and averaged over 1 h bins (Figure 1). Over the 8-h period before MCAo surgery, Pre animals' temperature was on average 37.7°C (±0.07°C), but was maintained at ∼37.0°C during surgery. Immediately after MCAo surgery, Pre animals were significantly warmer than Saline animals (P<0.01; 0.45°C) for a period of 4 h. Similarly, at 27-h after ischemia, Post animals exhibited a temperature spike, lasting 8 h, which was significantly higher (P<0.01; 0.47°C) than Saline animals. In both cases, the increases in temperature were mild and sustained for short periods.
Figure 1.
Postischemic temperatures (°C) averaged over 1-h time bins for 48 h. Arrows indicate LPS administration (50 μg/kg, i.p.) in the 24-h Post condition. Pre animals were administered LPS (50 μg/kg, i.p.) 8, 4, and 0 h before MCAo surgery and mean core temperature over this period was ∼37.7°C (data not shown).
Cytokine Assessment
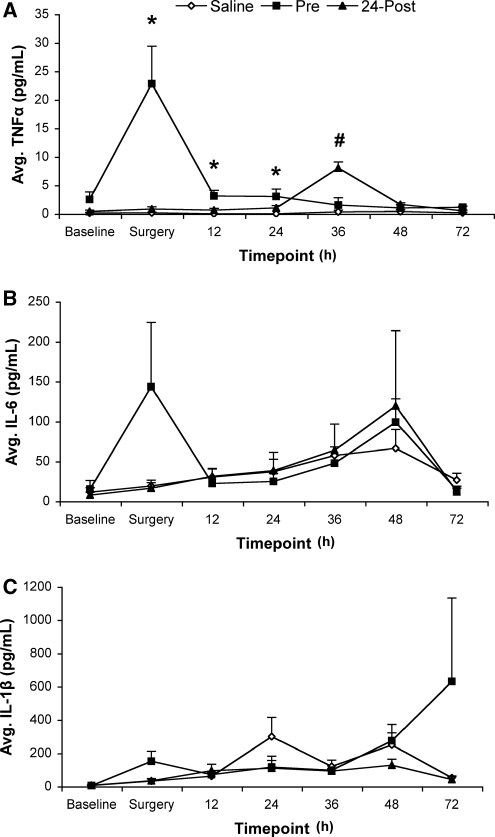
There were no differences among conditions at baseline for any of the cytokines analyzed (P>0.05). All data are presented as means±s.e.m. (Figure 2).
Figure 2.
Repeated sampling of serum proinflammatory cytokines by ELISA measurement. Three separate injections of saline or LPS (50 μg/kg) were administered at 4-h intervals either before MCAo (Pre; 8, 4, and 0 h before MCAo) or 24-h after MCAo (Post; 24, 28, and 32 h after MCAo). Data represent average (mean±s.e.m.) serum concentration of the proinflammatory cytokines at baseline, MCAo surgery, 12, 24, 36, 48, and 72 h after ischemia. (A) Lipopolysacchride administered before MCAo surgery significantly increased the serum concentrations of TNFα at the time of surgery and remained elevated for 24 h after ischemia (*P<0.05 versus Saline and Post). Further, LPS also significantly elevated TNFα levels in the 24-h Post condition for 12 h compared with both Saline and Pre concentrations (#P<0.01). (B) Serum concentrations of IL-6 showed similar trends as TNFα, but there were no significant differences among conditions, nor was there a difference among conditions in IL-1β serum concentrations (C). IL, interleukin; TNFα, tumor necrosis factor α.
Tumor necrosis factor α
Repeated measures ANOVA revealed a significant effect of Time (F1.52,22.77=8.42, P<0.01), Condition (F2,15=15.02, P<0.01) and a significant Time × Condition interaction (F3.04,22.78=10.57, P<0.01) (Figure 2). Follow-up analysis of this interaction showed that TNFα was significantly elevated in the Pre condition (versus Saline and Post) at surgery (P<0.01), 12 and 24 h after ischemia (P<0.05). Further, at 36 h after MCAo, animals in the Post condition had significantly higher plasma TNFα levels than both the Pre and Saline animals (P<0.01).
Interleukin-6
One animal (Pre) was excluded because baseline IL-6 levels were >2 s.d. above the group mean. There was a significant effect of Time (F2.54,35.57=3.01, P=0.05), showing a general increase in plasma IL-6 levels over time after MCAo surgery. There was no effect of Condition and no Time × Condition interaction.
Interleukin-1β
Two animals (one Pre and one Saline) were excluded from further analyses because both were >2 s.d. above group means at baseline measurement. Subsequent analysis of IL-1β showed that there was no effect of Time or Condition and no Time × Condition interaction (P>0.05).
Neuroinflammatory Response
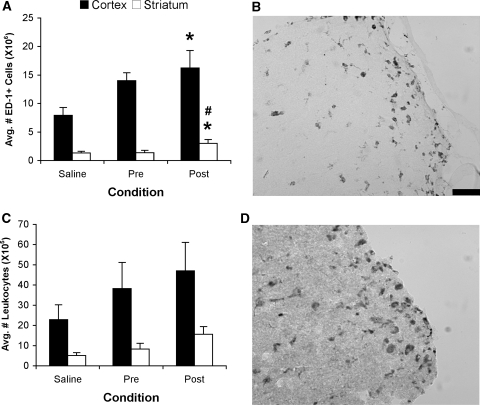
There was a significant difference among conditions in the assessment of the microglial/macrophage response 3 days after MCAo both in the cortex (F2,15=3.81, P<0.05) and striatum (F2,10.6=4.15, P<0.05). There were significantly more ED-1+ cells in the cortex (P<0.02) and striatum of 24-h Post animals than Saline animals (P<0.03) and significantly more positive cells in the striatum of 24-h Post animals than Pre animals (P<0.03; Figures 3A and 3B). There were no differences among conditions with respect to the number of infiltrating leukocytes (F2,15=1.06, P>0.05). However, there was a trend, similar to the microglia/macrophage response where there was ∼100% increase in leukocyte infiltration in the delayed inflammation animals (Post; Figures 3C and 3D).
Figure 3.
Quantification of the neuroinflammatory response. (A) Number (mean±s.e.m.) of activated microglia/macrophages quantified by ED-1 cell counts in the injured hemisphere. Delayed injection of LPS (50 μg/kg) significantly increased the number of ED-1+ cells compared with saline treatment in both the cortex and striatum (*P<0.03) and compared with Pre infection in the striatum (#P<0.03). (B) Example of cortical ED-1+ staining. (C) Number (mean±s.e.m.) of infiltrating leukocytes in the injured hemisphere. (D) Example of cortical leukocyte staining. Scale bar represents 50 μm.
Functional Assessments
There were no differences among conditions at baseline for any dependent measures of the functional assessments (P>0.05).
Beam
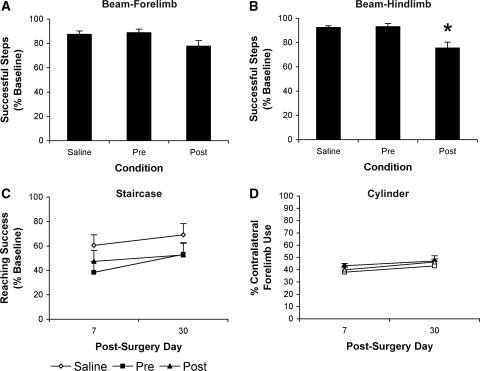
Repeated measures ANOVA of forelimb walking behavior showed that there were no effects of Time or Condition and no Time × Condition interaction (P>0.05; Figure 4A). There was, however, a significant effect of Condition on hindlimb function (F2,40=9.86, P<0.01) but no effect of Time or Time × Condition interaction. Tukey's HSD test showed that animals in the Post condition made significantly more hindlimb foot faults (∼17%) than both Pre and Saline animals P<0.01; Figure 4B).
Figure 4.
Postischemic functional assessments. Forelimb and hindlimb function were assessed at 7- and 30-days postischemia in the (A and B) tapered beam; (C) staircase; and (D) cylinder tasks. There was no effect of Time or Time × Condition interaction on the beam task and thus data collected on days 7 and 30 are represented as the average of both days (A and B). Data are presented as percentage (mean±s.e.m.) of baseline performance. Delayed postischemic administration of LPS (50 μg/kg) significantly increased the percentage of hindlimb paw slips as assessed in the beam walking task when compared with both Saline and Pre animals (B; *P<0.01).
Staircase
Repeated measures ANOVA of skilled forelimb reaching on postischemia days 7 and 30 revealed a significant effect of Time (F1,40=11.52, P<0.01) but no effect of Condition and no Time × Condition interaction (P>0.05; Figure 4C).
Cylinder
Repeated measures ANOVA of forelimb asymmetry revealed a significant effect of Time (F1,40=6.33, P<0.01) but no effect of Condition and no Time × Condition interaction (P>0.05; Figure 4D).
Infarct Assessment
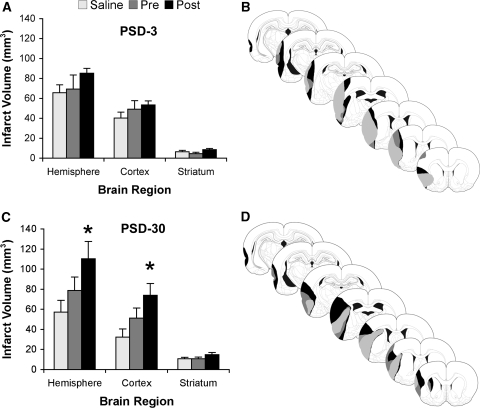
Analysis of postischemia day 3 animals showed no significant differences among conditions with respect to infarct volumes corrected for edema (P>0.05; Figure 5A). There was, however, a trend towards larger infarct volumes in animals in the Post condition compared with the other conditions. At 30 days after ischemia, there was a significant difference among conditions in cortical infarct volume (F2,40=4.15, P<0.03) and hemispheric infarction (F2,40=3.41, P<0.05) but not in the striatum (P>0.05). Tukey's HSD post hoc test revealed that Post animals had significantly larger infarct volumes than Saline animals (Figure 5C). There were no differences among any of the other conditions, although animals treated with LPS before ischemia had ∼30% larger infarcts than Saline animals.
Figure 5.
Assessment of infarct volumes. Animals were killed at either 3 (A and B) or 30 days (C and D) after MCAo surgery and infarct volumes were calculated. (A) At 3 days after MCAo, there was a trend for 24-h Post animals to have larger infarct volumes than both Saline and Pre animals. (B) Representative injury profile of animals at 3 days after MCAo. (C) At 30 days after surgery, the 3-day trend continued and animals in the Post condition had significantly larger infarcts than Saline animals (*P<0.05), most evident in the cortex. Of note, animals in the Pre condition had ∼30% larger infarct volumes than Saline animals at 30 days after MCAo. (D) Representative injury profile of animals at 30 days after MCAo.
Discussion
Here, we describe the differential effects of prolonged pre- and poststroke systemic inflammation on ischemic outcome. This is one of the first studies to assess the effects of delayed and prolonged systemic inflammation after experimental stroke (Prass et al, 2006) and the first to show such detrimental effects on neurological outcome. Short- and long-term effects of systemic inflammation were assessed using multiple outcome measures including histology, behavioral analyses, and cytokine assays. The cytokine profile shown in Figure 2 shows that there were significant changes in serum cytokine levels over time after MCAo and that these changes were further exacerbated by LPS. Delayed systemic inflammation in the Post animals increased functional impairments, which were especially evident in hindlimb function, further indicating the devastating effects of prolonged, delayed inflammation on ischemic outcome. It remains possible that there were also delayed inflammatory events not detected at 3 days that contributed to the increased neuronal damage observed at 30 days but confirmation awaits further studies.
Lipopolysaccharide administered before ischemia significantly increased serum TNFα levels for 24 h, whereas LPS administered 24 h after ischemia increased these levels for 12 h. IL-6 levels were significantly increased over 48 h after ischemia, and animals injected with LPS tended to have more elevated serum levels. These changes are similar to those reported in other studies at shorter time points of 4 (Spencer et al, 2007) and 8 h after ischemia (McColl et al, 2007).
Histopathological analysis at 30 days after ischemia showed that delayed inflammation significantly increased brain injury by ∼85%. Animals injected with LPS before ischemia had ∼30% larger infarct volumes than saline-treated animals. After ischemia day 3, infarct volumes showed a similar trend where 24-h Post animals had larger infarct volumes than either Pre or Saline animals. The neuroinflammatory response after LPS injection may partially explain this increased infarction. There was a significant increase in the number of microglia/macrophages at 3 days after MCAo in 24-h Post animals compared with Saline animals, corresponding to the infarct volume difference at 30 days after MCAo. In addition, there were ∼70% more ED-1+ cells in the Pretreated than saline-treated animals, a difference that may have resulted in a further 30% increase in infarct volume at 30 days after ischemia. There was a similar increase in the leukocyte infiltration response. Animals with delayed infection had ∼100% increase in leukocyte counts, corresponding to a similar increase in microglia/macrophage counts, undoubtedly further exacerbating neuronal injury.
The infarct volumes also corresponded to the functional deficits observed in this study, whereby animals in the Post condition had more severe deficits than Pre or Saline animals. Others have shown that LPS increases functional deficits after ischemia, but only at relatively short survival times (e.g., 24 to 72 h after ischemia) (McColl et al, 2007; Spencer et al, 2007), and in these studies it is difficult to know whether injury would have been greater at longer survival times or whether neuronal injury may have been accelerated. Our use of both delayed and prolonged inflammation paradigms, allowed sufficient recovery time before functional assessments began ensuring that we did not assess animals while systemic inflammation was still developing.
In this study, LPS administered 24-h after ischemia significantly impaired hindlimb function as shown in the tapered beam-traversing task. In addition, forelimb function in both the beam and staircase tasks was also compromised, although the differences did not reach statistical significance. This may be because even with the increased injury induced by delayed LPS in this study much of the forelimb motor cortex was spared and two of our three behavioral tests (i.e., staircase and cylinder) are most sensitive for detecting forelimb impairments. Animals with delayed inflammation had forelimb function reduced by ∼15% in both the beam and staircase tasks when compared with animals treated with saline. Animals treated with LPS before MCAo performed similar to saline-treated animals in the beam test with both forelimbs and hindlimbs. Although not statistically different, Pre animals had ∼15% to 20% increased forelimb deficits in the skilled reaching staircase task. Interestingly, these animals also had 30% larger infarct volumes than Saline animals. Despite the increase in infarct size produced by LPS, there was no significant impairment in the staircase and cylinder tasks, likely reflecting compensation resulting from the sparing of forelimb cortex. Nonetheless, there is converging evidence between our short- and long-term histopathological data and functional outcomes in that greater injury was associated with more substantial functional impairments. Delayed inflammation 24 h after ischemia increased the neuroinflammatory response 72 h after surgery, the tissue loss at 30 days after surgery and the functional deficits assessed at both 7 and 30 days after surgery. Further, preischemic inflammation caused a trend for similar effects both at 72 h and 30 days after ischemia.
Postischemic temperature is one of the most important factors in determining stroke outcome (Busto et al, 1987; Colbourne and Corbett, 1994; Kim et al, 1996). As infection is often accompanied by fever, determining whether the increased injury and residual deficits result from the injury, per se, or simply from hyperthermia, is clinically important. In this study, we recorded each animal's postischemic core temperature for 48 h (Figure 1). Animals in the Pre condition had slightly higher temperatures (0.45°C) initially after surgery, but quickly returned to normal within 4 h. Injection of LPS 24-, 28-, and 32-h after MCAo slightly increased core temperature as well, but only by ∼0.5°C, and only for 8 h (27 to 35 h after ischemia). It is unlikely that such brief increases in core temperature for several hours, especially at remote time points after ischemia, would worsen functional outcome and histopathological damage. Indeed, a 24-h postischemic brain temperature of 40°C significantly increased tissue loss, whereas increases to 39°C had no effect (Kim et al, 1996). Those temperature elevations were much greater than in this study. Reports noting detrimental effects of hyperthermia usually involve temperature elevations during or shortly after stroke, not at such prolonged postischemic time points (MacLellan et al, 2009). A more plausible explanation for the increase in damage is that microglia and macrophages recognize LPS, and secrete proinflammatory cytokines such as TNFα and IL-6 when stimulated leading to the production of free radicals and oxidative stress and exacerbation of damage (Bhat et al, 1998; Boje and Arora, 1992).
One of the most interesting and important findings in our current study is the assessment of a delayed postischemic inflammation. Other studies have modeled acute ischemic infections by injecting LPS either immediately before or after surgery. This represents only ∼50% of the clinical population (Emsley and Hopkins, 2008). The rest develop an infection while in hospital 24 to 48 h after stroke. Our data indicate that delayed postischemic inflammation is more detrimental to long-term function and neuropathology than preischemic inflammation potentially resulting from postischemic immunosuppression (Offner et al, 2006; Prass et al, 2006). Although serum cytokine levels were increased in animals exposed to LPS before MCAo, cerebral blood flow is typically reduced for a number of hours in this model (Biernaskie et al, 2001; Windle et al, 2006), potentially reducing cytokine levels in the brain. In contrast, animals injected with LPS 24-h after MCAo had normalized cerebral blood flow. At this time, TNFα and IL-6 levels were highest in the Post condition, potentially reperfusing significantly higher levels of proinflammatory cytokines to the MCA territory. These data taken together may explain why delayed systemic inflammation significantly increased the early neuroinflammation after MCAo as well as the long-term residual functional deficits and histological damage. It is interesting to note that a recent report has indicated a positive relationship between infarct size and the occurrence of infection (Hug et al, 2009). This creates an even more complex dynamic between stroke and infection, both of which seem to be detrimental to outcome.
In summary, using repeated serum sampling, this study documents significant changes in proinflammatory cytokine levels soon after ischemia and systemic inflammation, which correspond to an increase in neuroinflammation at 3 days after ischemia. Further, we showed that these early effects lead to sustained functional and neuropathological deficits at 30 day survival times. This study also emphasizes the importance of the interaction between the peripheral immune and central nervous systems. After injury, the central nervous system becomes increasingly susceptible to peripheral challenges, such as infection and inflammation, and the resulting consequences may have dramatic effects on both neuronal damage and subsequent function. Peripheral inflammation, independent of a febrile response, which occur at delayed periods after ischemia, significantly increase functional deficits and histopathological damage. Clinicians should vigilantly monitor and aggressively treat patients suffering from a postischemic infection in the hours and days after injury to avoid the devastating results that accompany this condition.
Acknowledgments
The authors thank Garry Chernenko and Shirley Granter-Button for their technical assistance, and Julia Curtis for help with behavioral analyses.
The authors declare no conflict of interest.
References
- Belayev L, Khoutorova L, Zhao W, Vigdorchik A, Belayev A, Busto R, Magal E, Ginsberg MD. Neuroprotective effect of darbepoetin alfa, a novel recombinant erythropoietic protein, in focal cerebral ischemia in rats. Stroke. 2005;36:1071–1076. doi: 10.1161/01.STR.0000160753.36093.da. [DOI] [PubMed] [Google Scholar]
- Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci. 1998;18:1633–1641. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaskie J, Corbett D, Peeling J, Wells J, Lei H. A serial MR study of cerebral blood flow changes and lesion development following endothelin-1-induced ischemia in rats. Magn Reson Med. 2001;46:827–830. doi: 10.1002/mrm.1263. [DOI] [PubMed] [Google Scholar]
- Boje KM, Arora PK. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992;587:250–256. doi: 10.1016/0006-8993(92)91004-x. [DOI] [PubMed] [Google Scholar]
- Bova IY, Bornstein NM, Korczyn AD. Acute infection as a risk factor for ischemic stroke. Stroke. 1996;27:2204–2206. doi: 10.1161/01.str.27.12.2204. [DOI] [PubMed] [Google Scholar]
- Busto R, Dietrich WD, Globus MY, Valdes I, Scheinberg P, Ginsberg MD. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab. 1987;7:729–738. doi: 10.1038/jcbfm.1987.127. [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care . Guide to the Care and Use of Experimental Animals. Ottawa, ON: Canadian Council on Animal Care; 1993. [Google Scholar]
- Chamorro A, Urra X, Planas AM. Infection after acute ischemic stroke: a manifestation of brain-induced immunodepression. Stroke. 2007;38:1097–1103. doi: 10.1161/01.STR.0000258346.68966.9d. [DOI] [PubMed] [Google Scholar]
- Colbourne F, Corbett D. Delayed and prolonged post-ischemic hypothermia is neuroprotective in the gerbil. Brain Res. 1994;654:265–272. doi: 10.1016/0006-8993(94)90488-x. [DOI] [PubMed] [Google Scholar]
- Emsley HC, Hopkins SJ. Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol. 2008;7:341–353. doi: 10.1016/S1474-4422(08)70061-9. [DOI] [PubMed] [Google Scholar]
- Grau AJ, Buggle F, Becher H, Zimmermann E, Spiel M, Fent T, Maiwald M, Werle E, Zorn M, Hengel H, Hacke W. Recent bacterial and viral infection is a risk factor for cerebrovascular ischemia: clinical and biochemical studies. Neurology. 1998;50:196–203. doi: 10.1212/wnl.50.1.196. [DOI] [PubMed] [Google Scholar]
- Grau AJ, Buggle F, Schnitzler P, Spiel M, Lichy C, Hacke W. Fever and infection early after ischemic stroke. J Neurol Sci. 1999;171:115–120. doi: 10.1016/s0022-510x(99)00261-0. [DOI] [PubMed] [Google Scholar]
- Hewlett KA, Corbett D. Delayed minocycline treatment reduces long-term functional deficits and histological injury in a rodent model of focal ischemia. Neuroscience. 2006;141:27–33. doi: 10.1016/j.neuroscience.2006.03.071. [DOI] [PubMed] [Google Scholar]
- Hug A, Dalpke A, Wieczorek N, Giese T, Lorenz A, Auffarth G, Liesz A, Veltkamp R. Infarct volume is a major determiner of post-stroke immune cell function and susceptibility to infection. Stroke. 2009;40:3226–3232. doi: 10.1161/STROKEAHA.109.557967. [DOI] [PubMed] [Google Scholar]
- Kim Y, Busto R, Dietrich WD, Kraydieh S, Ginsberg MD. Delayed postischemic hyperthermia in awake rats worsens the histopathological outcome of transient focal cerebral ischemia. Stroke. 1996;27:2274–2280. doi: 10.1161/01.str.27.12.2274. [DOI] [PubMed] [Google Scholar]
- Langdon KD, Granter-Button S, Corbett D. Persistent behavioral impairments and neuroinflammation following global ischemia in the rat. Eur J Neurosci. 2008;28:2310–2318. doi: 10.1111/j.1460-9568.2008.06513.x. [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Clark DL, Silasi G, Colbourne F. Use of prolonged hypothermia to treat ischemic and hemorrhagic stroke. J Neurotrauma. 2009;26:313–323. doi: 10.1089/neu.2008.0580. [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Davies LM, Fingas MS, Colbourne F. The influence of hypothermia on outcome after intracerebral hemorrhage in rats. Stroke. 2006;37:1266–1270. doi: 10.1161/01.STR.0000217268.81963.78. [DOI] [PubMed] [Google Scholar]
- Marsh B, Stevens SL, Packard AE, Gopalan B, Hunter B, Leung PY, Harrington CA, Stenzel-Poore MP. Systemic lipopolysaccharide protects the brain from ischemic injury by reprogramming the response of the brain to stroke: a critical role for IRF3. J Neurosci. 2009;29:9839–9849. doi: 10.1523/JNEUROSCI.2496-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci. 2008;28:9451–9462. doi: 10.1523/JNEUROSCI.2674-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya CP, Campbell-Hope LJ, Pemberton KD, Dunnett SB. The ‘staircase test': a measure of independent forelimb reaching and grasping abilities in rats. J Neurosci Methods. 1991;36:219–228. doi: 10.1016/0165-0270(91)90048-5. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Prass K, Braun JS, Dirnagl U, Meisel C, Meisel A. Stroke propagates bacterial aspiration to pneumonia in a model of cerebral ischemia. Stroke. 2006;37:2607–2612. doi: 10.1161/01.STR.0000240409.68739.2b. [DOI] [PubMed] [Google Scholar]
- Rosenzweig HL, Lessov NS, Henshall DC, Minami M, Simon RP, Stenzel-Poore MP. Endotoxin preconditioning prevents cellular inflammatory response during ischemic neuroprotection in mice. Stroke. 2004;35:2576–2581. doi: 10.1161/01.STR.0000143450.04438.ae. [DOI] [PubMed] [Google Scholar]
- Schallert T. Behavioral tests for preclinical intervention assessment. NeuroRx. 2006;3:497–504. doi: 10.1016/j.nurx.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T, Woodlee MT.2005Orienting and placing The Behavior of the Laboratory Rat: a Handbook with Tests(Whishaw IQ, Kolb B, eds),New York, NY: Oxford University Press; 129–140. [Google Scholar]
- Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, Iwakura Y, Yoshimura A. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Auer RN, Pittman QJ. Rat neonatal immune challenge alters adult responses to cerebral ischaemia. J Cereb Blood Flow Metab. 2006;26:456–467. doi: 10.1038/sj.jcbfm.9600206. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Mouihate A, Pittman QJ. Peripheral inflammation exacerbates damage after global ischemia independently of temperature and acute brain inflammation. Stroke. 2007;38:1570–1577. doi: 10.1161/STROKEAHA.106.476507. [DOI] [PubMed] [Google Scholar]
- Tasaki K, Ruetzler CA, Ohtsuki T, Martin D, Nawashiro H, Hallenbeck JM. Lipopolysaccharide pre-treatment induces resistance against subsequent focal cerebral ischemic damage in spontaneously hypertensive rats. Brain Res. 1997;748:267–270. doi: 10.1016/s0006-8993(96)01383-2. [DOI] [PubMed] [Google Scholar]
- Windle V, Szymanska A, Granter-Button S, White C, Buist R, Peeling J, Corbett D. An analysis of four different methods of producing focal cerebral ischemia with endothelin-1 in the rat. Exp Neurol. 2006;201:324–334. doi: 10.1016/j.expneurol.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Yenari MA. Post-ischemic inflammation: molecular mechanisms and therapeutic implications. Neurol Res. 2004;26:884–892. doi: 10.1179/016164104X2357. [DOI] [PubMed] [Google Scholar]