Abstract
Hyperbaric oxygen (HBO) and normobaric hyperoxia (NBO) protect the brain parenchyma and the cerebral microcirculation against ischemia. We studied their effect on secondary hemorrhage after thrombolysis in two thromboembolic middle cerebral artery occlusion (MCAO) (tMCAO) models. Beginning 60 minutes after tMCAO with either thrombin-induced thromboemboli (TT) or calcium-induced thromboemboli (CT), spontaneously hypertensive rats (n=96) breathed either air, 100% O2 (NBO), or 100% O2 at 3 bar (HBO) for 1 hour. Immediately thereafter, recombinant tissue plasminogen activator (rt-PA, 9 mg/kg) was injected. Although significant reperfusion was observed after thrombolysis in TT-tMCAO, vascular occlusion persisted in CT-tMCAO. In TT-tMCAO, NBO and HBO significantly reduced diffusion-weighted imaging–magnetic resonance imaging (MRI) lesion volume and postischemic blood–brain barrier (BBB) permeability on postcontrast T1-weighted images. NBO and, significantly more potently, HBO reduced macroscopic hemorrhage on T2* MRI and on corresponding postmortem cryosections. Oxygen therapy lowered hemoglobin content and attenuated activation of matrix metalloproteinases in the ischemic hemisphere. In contrast, NBO and HBO failed to reduce infarct size in CT but both decreased BBB damage and microscopic hemorrhagic transformation. Only HBO reduced hemoglobin extravasation in the ischemic hemisphere. In conclusion, NBO and HBO decrease infarct size after thromboembolic ischemia only if recanalization is successful. As NBO and HBO also reduce postthrombolytic intracerebral hemorrhage, combining the two with thrombolysis seems promising.
Keywords: cerebral ischemia, hemorrhage, hyperbaric oxygen, normobaric hyperoxia, thrombolysis
Introduction
Secondary hemorrhage is a feared complication of thrombolysis in ischemic stroke. In large clinical trials, thrombolysis has been shown to increase the risk of symptomatic intracerebral hemorrhage about sixfold and the mortality associated with symptomatic hemorrhage about 10-fold (Hacke et al, 2004). Accordingly, preventing secondary hemorrhage after thrombolysis has become an important goal of ischemic stroke therapy. In preclinical and clinical studies, ischemic blood–brain barrier (BBB) damage is associated with secondary hemorrhage after thrombolysis (Del Zoppo et al, 1998; Kahles et al, 2005; Kastrup et al, 2008; Wang et al, 2003). Mediators of BBB damage include free radical-induced reperfusion injury, various cytokines, and proteases. The activation and proteolytic activity of matrix metalloproteinases (MMPs), particularly MMP-9, are key factors in the proteolytic disruption of the basal lamina and tight junctions of the BBB (Hawkins and Davis, 2005; Wang et al, 2003).
Oxygen therapy has offered a simple, but plausible therapeutic approach in experimental cerebral ischemia for many years and was shown to be particularly effective when therapy was started early in reperfusion models (Calvert et al, 2007; Helms et al, 2005; Nighoghossian and Trouillas, 1997; Singhal, 2007; Poli and Veltkamp, 2009; Veltkamp et al, 2000; Zhang et al, 2005). Beyond protection of the brain parenchyma, oxygen therapy may also have beneficial effects on the postischemic microcirculation. In previous studies, hyperbaric oxygen (HBO) and normobaric hyperoxia (NBO) attenuated BBB permeability after focal and global ischemia (Mink and Dutka, 1995; Singhal et al, 2002; Veltkamp et al, 2005a). However, the effects of NBO and HBO on hemorrhagic transformation after focal cerebral ischemia are controversial (Liu et al, 2009; Qin et al, 2007; Henninger et al, 2006, 2009). Furthermore, the effects of these two forms of oxygen therapy on secondary hemorrhage and the underlying BBB damage after experimental thrombolysis have not been directly compared to date.
The purpose of this study was to examine the differences in the effectiveness of HBO and NBO in combination with intravenous thrombolysis after thromboembolic middle cerebral artery occlusion (tMCAO). Specifically, we tested the impact of oxygen therapy on postischemic BBB damage and secondary hemorrhage in two thromboembolic stroke models in which thrombolysis recanalizes or fails to reopen the MCA, respectively.
Materials and methods
All experiments were performed on spontaneously hypertensive male rats weighing 300 to 350 g (Janvier, Le Genest St Isle, France). The study and all associated procedures were approved by the governmental animal care authorities (Regierungspraesidium, Karlsruhe, Germany). Using a face mask, anesthesia was induced with 4% halothane in O2 and continued with 0.8% to 1.2% halothane in a 70/30 mixture of nitrous oxide/oxygen under spontaneous respiration. During surgery, rectal temperature was maintained at 37°C with a thermostatically controlled heating pad. The femoral artery and vein were cannulated with PE-50 polyethylene tubing for continuous monitoring of arterial blood pressure and heart rate, to provide samples for blood gas measurements, and to inject the magnetic resonance contrast agent.
Thrombus Generation
Focal cerebral ischemia was induced using the tMCAO model described by Toomey et al (2002) with some modifications. Thrombi were generated in two different ways: for thrombin-induced thromboemboli (TT), 500 μL of fresh arterial blood from a donor rat were drawn into an Eppendorf tube and mixed with 1.0 National Institutes of Health (NIH) units of human thrombin (Sigma Aldrich, St Louis, MO, USA) and 5 μL of 1 mol/L CaCl2 for a final CaCl2 concentration of 10 mmol/L. Within 5 seconds, a small portion of the mixture was drawn into a 15-cm-long PE-50 tube and allowed to coagulate for 2 hours at 37°C. Then, the clot was transferred from the tube into a Petri dish, which was then filled with saline and stored at 4°C for 12 hours. Before MCAO, the clot was incubated in deionized water at room temperature for 5 minutes. Subsequently, the clot was placed into isotonic saline and inspected under a microscope at fivefold magnification. Twelve thrombi—each 0.35 mm in diameter and 1.5 mm in length—were cut under the microscope and then drawn into PE-50 tubing.
For the calcium-rich thromboemboli (CT), whole arterial blood from a donor rat was drawn into a PE-50 tube where it was allowed to coagulate spontaneously for 2 hours at 37°C. Then, the clot was transferred from the tube into a dish where it was exposed to a 20 mmol/L calcium solution for 1 minute. After transfer into saline solution, the clot was dissected as described for the TT.
Surgical Procedure and Experimental Protocol
The right external carotid artery was permanently ligated distally and mobilized as described (Toomey et al, 2002). The PE-50 catheter with 12 clots was inserted into the right external carotid artery proximal to the ligation and advanced through the bifurcation into the proximal internal carotid artery. Ischemia was induced by injecting the 12 clots into the internal carotid artery over a 30-seconds period with 50 μL of saline. After removing the catheter, ligating the proximal external carotid artery and closing the neck, rats were placed into a magnetic resonance imaging (MRI) scanner (Bruker Biospec, 2.35 T, Karlsruhe, Germany).
Perfusion-weighted imaging (PWI) was performed to ensure hypoperfusion in the territory of the occluded MCA in all animals. After PWI, rats were allowed to wake up. Sixty minutes after injecting the thrombus, animals were randomly assigned to one of three groups. Animals breathed either air, 100% O2 at ambient pressure (NBO), or 100% O2 at 3 bar (HBO) for 60 minutes in a pressure chamber. Immediately after oxygen therapy, animals received recombinant tissue plasminogen activator (rt-PA) intravenously (9 mg/kg in 2 mL H2O; Actilyse, Boehringer Ingelheim, Ingelheim am Rhein, Germany). Ten percent of the solution was injected as a bolus; the remainder was infused over a 30-minutes period through the femoral vein. In all groups, a PWI–MRI was performed after the end of the rt-PA infusion (i.e., 2.5 hours after tMCAO) and 24 hours after tMCAO to document the perfusion status after thrombolysis and at the end of the experiment.
Magnetic Resonance Imaging Protocol
For PWI, we used a gradient-echo–echo-planar imaging sequence (repetition time (TR)=1 second, echo time (TE)=15 milliseconds, field of view=4.5 cm × 4.5 cm, 4 slices, thickness=2 mm, 20 repetitions with a time resolution of 1 second/image data set) to monitor the bolus passage of 1 mmol/kg of a paramagnetic contrast agent (Omniscan, Nycomed Amersham, Oslo, Norway). For diffusion-weighted MRI, we acquired a spin-echo–echo-planar imaging sequence (b value=200, 300, 400, 500, 600, and 700 seconds/mm2) 2.5 and 24 hours after tMCAO. The MR protocol also comprised a T1 spin-echo sequence with a TR of 400 milliseconds, TE of 15 milliseconds, a flip angle of 90°, a matrix of 128 × 128, field of view=4 cm × 4 cm, and 6 slices with slice thickness=2 mm; the number of averages was 8. T1-weighted imaging was performed 10 minutes after injecting the contrast agent and again 2.5 and 24 hours after embolization. T2*-weighted imaging was performed 24 hours after embolization with a fast low angle shot sequence (TR=300 milliseconds, TE=20 milliseconds, flip angle=20°C, matrix 128 × 96, field of view=4 cm × 4 cm, number of slices=6, and number of averages=4).
Magnetic Resonance Imaging Data Analysis
For analysis of PWI, the relative cerebral blood volume (rCBV) and the relative mean transit time were calculated in two predefined corresponding regions of interest in the parietal cortex of both hemispheres from the signal–time curve determined from the PWI data set as described earlier (Heiland et al, 1997). Diffusion-weighted imaging (DWI) and T1w data were analyzed by encircling areas of abnormal signal intensity for each MR section using a side-to-side comparison on the screen. Volume of abnormally hyperintense signals on DWI and postcontrast enhancement on T1w was calculated by multiplying the total area with a 2-μm section thickness. Areas of abnormally hypointense signal on T2* reflecting macroscopic hemorrhage were measured by a blinded rater. To validate that the DWI lesion size corresponded to parenchymal histologic damage, we analyzed the infarct area on histologic sections at the level of the bregma 24 hours after tMCAO in a subgroup of animals.
Macroscopic Examination of Secondary Hemorrhage
Twenty-four hours after TT-MCAO, brains (n=10/group) were removed. Unstained coronal cryosections were photographed without magnification. Macroscopic evidence of intracerebral hemorrhage was compared with T2* on corresponding sections.
Spectrophotometric Hemoglobin Assay
The hemoglobin content of brains was quantified with a spectrophotometric assay as described earlier with some modifications (Choudhri et al, 1997). Twenty-four hours after MCAO, rats were deeply anesthetized and transcardially perfused with 100 mL of saline. Brains were rapidly removed, divided into left and right hemisphere, frozen in isopentane, and stored at −80°C. The brain hemispheres were homogenized in 1.0 mL of PBS on ice for 30 seconds, insonated with pulse ultrasound for 1 minute, and centrifuged at 13,000 r.p.m. for 30 minutes. After the hemoglobin-containing supernatant was collected, 120 μL of Drabkin's reagent (Sigma Diagnostics, St Louis, MO, USA; K3Fe(CN)6 200 mg/L, KCN 50 mg/L, NaHCO3 1 g/L, pH 8.6) was added to a 30-μL aliquot and the mixture was allowed to stand for 15 minutes. The optical density was then measured at a wavelength of 540 nm with a spectrophotometer (Synergy 2 Multi-Detection Microplate Reader, BioTec, St Louis, MO, USA). To verify that the measured absorbance after these procedures reflected the amount of hemoglobin, blood was obtained from naive mice by cardiac puncture after anesthesia. Incremental aliquots of this blood (4, 8, 16, 20, 32, and 50 μL) were added to freshly homogenized brain tissue obtained from untreated mice to generate a standard absorbance curve. This curve showed a linear relationship between added blood volume and optical density (Supplementary Figure 1).
Histologic Assessment of Hemorrhagic Transformation
In animals without macroscopically visible hemorrhage on T2*, histologic staining was performed using trichrome. Twenty-four hours after CT-tMCAO, rats were deeply anesthetized and transcardially perfused with 100 mL of saline and fixed with 100 mL 4% paraformaldehyde (PFA). Brains were rapidly removed and postfixed in 1% PFA at 4°C. After embedding in paraffin, 5-μm coronal sections were deparaffinized, hydrated, and treated with Harris hemalaun solution for 5 minutes. Then, sections were incubated with a trichrome solution (pH 3.4, 1.2% chromotrop 2R, 1.2% wolframatophosphoracid, 0.6% fast green FCF, and 1% acetic acid). After rinsing briefly, sections were dehydrated, mounted, coverslipped, and observed under a microscope (Leica, Wetzlar, Germany). To assess the severity of erythrocytic extravasations, a blinded rater used a predefined semiquantitative scale (score 0 to 5, with lower scores representing fewer intraparenchymal erythrocytes, Supplementary Figure 2).
Gelatin Zymography
Corresponding samples of ischemic and nonischemic hemispheres were taken from a series of 20-μm-thick coronal cryosections with 1.2 mm distance and homogenized in ice-cold lysis buffer (50 mmol/L Tris–HCl, pH 7.5, 150 mmol/L NaCl, 5 mmol/L CaCl2, 0.05% Brij-35, 0.02% NaN3, and 1% Triton X-100). After centrifugation, the supernatant was collected and protein concentration of each sample was determined in triplicate using Bradford reagent (Bio-Rad Laboratories GmbH, Munich, Germany). Aliquots of lysates containing 50 μg protein were subjected to electrophoresis on 10% sodium dodecyl sulfate polyacrylamide gel copolymerized with 1 mg/ml gelatin (Sigma Aldrich, Munich, Germany) under nonreducing conditions. After washing in 2.5% Triton-X 100 for 2 hours, gels were incubated in a developing buffer containing 50 mmol/L Tris–HCl, pH 7.5, 150 mmol/L NaCl, 5 mmol/L CaCl2, 0.02% Brij-35, and 0.02% NaN3 for 60 hours. Gels were then stained with 0.125% Coomassie blue R-250 in 10% acetic acid and 50% methanol for 30 minutes before they were destained in a solution containing 5% acetic acid and 25% methanol until clear bands appeared on a dark blue background. After scanning (MCID 7.0, InterFocus GmbH, Mering, Germany) densitometry of bands was performed using the public domain Image J software (National Institutes of Health, Bethesda, MD, USA). A mixture of human MMP-9 and MMP-2 (Chemicon International, Millipore, Schwalbach, Germany) was used as positive control.
Statistical Analysis
All values are expressed as mean±standard deviation. For comparison of physiologic values, infarct volumes, and MRI data, analysis of variance (ANOVA) was used, followed by post hoc Fisher's protected least significant difference test. The scores on the histologic hemorrhage scale were assessed by the Kruskal–Wallis test and then by the Mann–Whitney U-test. All analyses were performed using SPSS analysis software. A P-value <0.05 was considered statistically significant.
Results
Physiologic parameters before tMCAO and 5 minutes after reperfusion did not differ significantly among groups except for arterial pO2 (Table 1). Arterial pO2 in the HBO group could only be measured after opening the pressure chamber.
Table 1. Physiologic parameters.
| Parameter | Air | NBO | HBO*** |
|---|---|---|---|
| MABP (mm Hg) | |||
| 10 minutes before MCAO | 120±11 | 124±10 | 122±8 |
| 120 minutes after MCAO | 122±19 | 130±13 | 124±7 |
| PaO2 (mm Hg) | |||
| 10 minutes before MCAO | 113.9±14.1 | 116.8±13.6 | 121.4±14.0 |
| 120 minutes after MCAO | 126.2±13.7 | 402.5±26.4* | 654.2±29.3** |
| PaCO2 (mm Hg) | |||
| 10 minutes before MCAO | 46.4±4.9 | 45.5±4.5 | 45.4±4.1 |
| 120 minutes after MCAO | 50.2±4.5 | 51.7±4.9 | 46.1±5.0 |
| pH | |||
| 10 minutes before MCAO | 7.40±0.07 | 7.40±0.06 | 7.40±0.06 |
| 120 minutes after MCAO | 7.35±0.03 | 7.33±0.03 | 7.35±0.03 |
HBO, hyperbaric oxygen; MCAO, middle cerebral artery occlusion; NBO, normobaric hyperoxia; MABP, mean arterial blood pressure.
N=30 in each group.
*P<0.05 versus air, **P<0.05 versus air and NBO.
***Parameters were measured after decompression.
On injecting emboli PWI, signal intensity declined in the ischemic hemisphere. The rCBV (ischemic and nonischemic hemisphere) did not differ among groups 20 minutes after tMCAO (P>0.5, ANOVA). Thus, ischemia was equally severe in all groups initially. In TT-tMCAO, thrombolysis improved rCBV in cortex at 2.5 and 24 hours after MCAO in all three groups (P<0.05, ANOVA). Relative CBV changes did not differ between 2.5 and 24 hours after embolism (P>0.5). In contrast, in CT-tMCAO, PWI changes did not reverse after rt-PA infusion (i.e., 2.5 hours after MCAO) and 24 hours after MCAO. No significant differences in rCBV were observed at any time point after CT-tMCAO among all groups (P>0.5, ANOVA). Thus, rt-PA did not induce recanalization in CT-tMCAO (Supplementary Figure 3).
Oxygen Therapies Reduce Ischemic Lesion in Recanalized but not in Permanent Thrombembolic Middle Cerebral Artery Occlusion
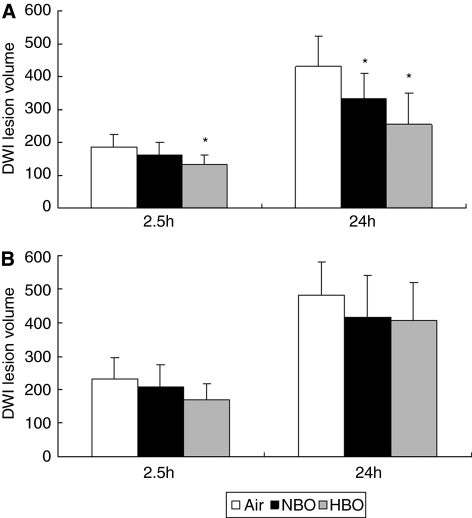
To delineate the brain parenchymal damage, lesion volume was measured on DWI–MRI. Hyperintensity on DWI correlated well with infarct volume on histologic sections (r=0.76, P=0.001), which was similar to the results of an earlier study (Veltkamp et al, 2005a). An abnormally hyperintense signal on DWI was detected already 2.5 hours after MCAO and became more extensive 24 hours after MCAO. In TT-tMCAO, NBO and HBO significantly reduced lesion volume on DWI compared with air (Figure 1). In contrast, in CT-tMCAO, only a transient trend toward reduced lesion volume was detected on DWI–MRI in the HBO group at 2.5 hours but no differences were seen at 24 hours after tMCAO (Figure 1). Hence, oxygen therapy did not reduce lesion volume in permanent tMCAO.
Figure 1.
Hyperintense lesion volumes on magnetic resonance diffusion-weighted images at 2.5 and 24 hours after thromboembolic middle cerebral artery occlusion (tMCAO) (mm3). (A) In thrombin-induced thromboemboli-tMCAO, lesion volume on diffusion-weighted imaging (DWI) was reduced by hyperbaric oxygen (HBO) 2.5 hours after tMCAO and by both oxygen therapies 24 hours after tMCAO (*P<0.05, analysis of variance). (B) In calcium-induced thromboemboli-tMCAO, there were no significant differences among air-, normobaric hyperoxia (NBO)- or HBO-treated animals at these time points.
Normobaric Hyperoxia and Hyperbaric Oxygen Reduce Blood–Brain Barrier Damage
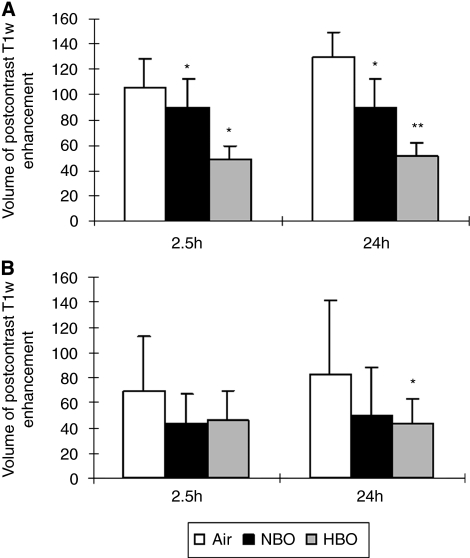
Postischemic microvascular permeability was analyzed on postcontrast T1w images, which correlates well with Evans blue extravasation (data not shown). An abnormally hyperintense signal on postcontrast T1w images was detected 2.5 hours after injecting emboli. In TT-tMCAO, volumes of enhancement on T1w images at this time point were 105.8±21.6 mm3 in the air, 89.5±22.2 mm3 in the NBO, and 48.7±10.9 mm3 in the HBO group (P<0.001, n=12 per group). At 24 hours after tMCAO, volumes of enhancement on T1w images were 129.1±20.2 mm3 in air-, 89.5±22.8 mm3 in NBO-, and 51.3±11.4 mm3 in HBO-treated rats. (Figure 2) Thus, NBO and, more effectively, HBO significantly reduced postischemic BBB damage on T1w images 24 hours after tMCAO with reperfusion (P<0.001, ANOVA). In CT-tMCAO, HBO also significantly reduced postischemic, enhancing T1 volumes 24 hours after tMCAO (P<0.05, ANOVA) whereas NBO failed to attenuate the enhancing volume on T1w images (P=0.18, ANOVA) (Figure 2).
Figure 2.
Volume of enhancement on postcontrast T1w magnetic resonance images 2.5 and 24 hours after thromboembolic middle cerebral artery occlusion (tMCAO) (mm3). (A) In thrombin-induced thromboemboli-tMCAO, normobaric hyperoxia (NBO) and, more effectively, hyperbaric oxygen (HBO) reduced postischemic blood–brain barrier (BBB) damage on T1w images (*significant difference compared with air, **significant difference compared with air and NBO, P<0.05, analysis of variance (ANOVA)). (B) In calcium-induced thromboemboli-tMCAO, HBO reduced BBB permeability significantly compared with air at 24 hours after MCAO, whereas the difference was not significant between the NBO and the air group (*P<0.05 compared with air, ANOVA).
Macroscopic Evidence of Secondary Hemorrhage
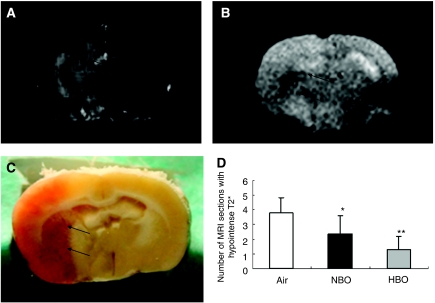
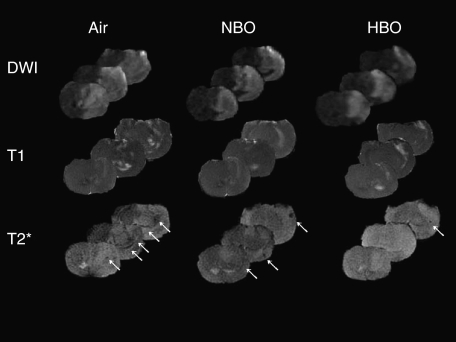
T2* MR imaging was used to detect ‘macroscopic' intracerebral hemorrhage because it corresponded well to hemorrhage on unstained coronal cryosections (Figures 3B and 3C). An abnormally hypointense signal reflecting hemorrhage was observed on T2* MR images 24 hours after embolization (Figure 3). In TT-tMCAO with recanalization, the hypointense T2* signal was found in all animals except for 2 of the 11 HBO-treated rats. (Figures 3B and 3C) The mean number of MRI sections with hypointense T2* signal was 3.8±1.0 in air-, 2.4±1.2 in NBO-, and 1.3±0.9 in HBO-treated rats. Thus, both NBO and HBO induced a significant reduction in macroscopic hemorrhage on T2* MR images (P< 0.01, Kruskal–Wallis test, Mann–Whitney U-test, n=11/group). Moreover, macroscopic hemorrhage was significantly smaller in HBO- than in NBO-treated mice (P=0.037, Mann–Whitney U-test, n=11/group) (Figure 3). Interestingly, the area of hypointense T2* signal at 24 hours after MCAO was located within the area of intense postcontrast enhancement on T1w images at 2.5 hours after MACO (Figure 3). In addition, enhancing T1w lesion at 2.5 hours after MCAO correlated well with the hypointense T2* signal at 24 hours after MACO (r=0.919, P<0.001, Spearman). Thus, a circumscribed, early increase in BBB permeability appeared to indicate a risk for later secondary hemorrhage. Moreover, hypointense T2* signals were mainly observed within areas of the ischemic parenchymal DWI lesion in all the three groups (Figure 4).
Figure 3.
Correspondence of blood–brain barrier damage, infarct lesion, and secondary hemorrhage. (A) Postcontrast enhancement on T1w postcontrast magnetic resonance image (arrow). (B) Corresponding T2* magnetic resonance image showing ‘macroscopic' hemorrhage (arrow). (C) Evidence of hemorrhage on unstained cryosections (arrow). (D) Mean number of magnetic resonance imaging (MRI) sections with hypointense T2* signal (*significant difference compared with air, **significant difference compared with air and normobaric hyperoxia (NBO), P<0.05, analysis of variance). HBO, hyperbaric oxygen.
Figure 4.
Multimodal magnetic resonance imaging images showing the topography of the parenchymal infarct (diffusion-weighted imaging (DWI) at 24 hours), blood–brain barrier permeability (postcontrast T1w at 2.5 hours), and hemorrhage (T2* at 24 hours) in three representative rats receiving either air, normobaric hyperoxia (NBO), or hyperbaric oxygen (HBO).
In permanent CT-tMCAO, only 3/10 animals in the air group and none of the NBO- or HBO-treated rats showed such ‘macroscopic' hemorrhage on T2* MRI, despite thrombolysis.
Oxygen Therapy Reduces Postischemic Hemoglobin Extravasation
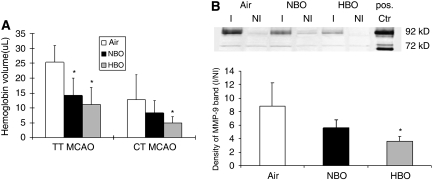
To quantify hemorrhagic transformation, total brain hemoglobin contents were analyzed 24 hours after embolization using a spectrophotometric assay. On the basis of the standard absorbance curve, the measured absorption of optical density was converted to hemoglobin volume. A pronounced increase in hemoglobin content in the ischemic hemisphere was observed as compared with the contralateral hemisphere in the air-treated rats. In groups with recanalized MCA, both NBO (14.2±5.9 μL) and HBO (11.2±5.7 μL) significantly decreased the mean hemoglobin volume as compared with air in the ischemic hemisphere (25.4±5.6 μL, P<0.05, n=5 per group, ANOVA). Again, there was less hemorrhagic transformation in permanent than in recanalized tMCAO. In CT-tMCAO, only HBO (4.9±2.2 μL) reduced hemoglobin volume as compared with air (12.8±8.3 μL, P<0.05, n=10, ANOVA). No significant differences in mean hemoglobin contents were found between NBO (8.3±4.1 μL) and air (12.8±8.3 μL, P=0.32) or between NBO and HBO (P=0.79) (Figure 5).
Figure 5.
Hemoglobin spectrophotometry of perfused ischemic brain hemisphere and expression of matrix metalloproteinase (MMP)-2 and MMP-9 24 hours after thromboembolic middle cerebral artery occlusion (tMCAO). (A) In thrombin-induced thromboemboli (TT)-MCAO, both forms of oxygen therapy significantly decreased hemoglobin volume compared with air (*significant difference compared with air, P<0.05, analysis of variance (ANOVA)). In calcium-induced thromboemboli (CT)-MCAO, a significant reduction in hemoglobin volume was only observed in hyperbaric oxygen (HBO)-treated rats (*P<0.05, ANOVA). (B) Upper panel: representative gelatin zymography showing MMP-9 and MMP-2 activity in ischemic (I) and nonischemic (NI) brain hemishperes 24 hours after tMCAO. Pos. Ctr: positive control. Ischemia resulted in strong activation of MMP-9, but not MMP-2. Note the clearly reduced MMP-9 bands in samples of the ischemic hemisphere treated with HBO as compared with normobaric hyperoxia (NBO) or air-treated mice. Lower panel: ratio of MMP-9 bands intensity from ischemic divided by nonischemic side. A significant reduction of MMP-9 activity protein was observed in the HBO group (*P<0.05, ANOVA, n=5).
Normobaric Hyperoxia and Hyperbaric Oxygen Attenuate Erythrocytic Extravasation
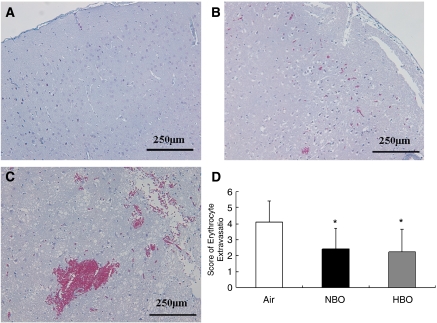
As ‘macroscopic' hemorrhage was rarely found after CT-tMCAO, the extent of hemorrhagic transformation was also quantified on trichrome sections at the level of the bregma +0.26 mm (commissura anterior) using a predefined semiquantitative scale. No erythrocytic extravasation was found in the contralateral hemisphere (Figure 6). In contrast, a leakage of erythrocytes into brain tissue was observed in cortex and subcortex throughout the ischemic tissue (Figure 6). However, dense accumulation of erythrocytes developed predominantly in the striatum and in the ventral cortex (Figure 6). Mean scores of erythrocytic extravasation were 4.1±1.3 in the air, 2.4±1.3 in the NBO, and 2.3±1.4 in the HBO group (P<0.05 for HBO and NBO versus air; Kruskal–Wallis test, Mann–Whitney U-test, n=9/group).
Figure 6.
Erythrocytic extravasation on trichrome-stained coronal brain sections at the level of the bregma +0.26 mm (anterior commissure) 24 hours after calcium-induced thromboemboli-middle cerebral artery occlusion without recanalization. (A) Contralateral cortex without erythrocytic extravasation. (B) Ischemic cortex with a single aggregate of erythrocytes (score 2). (C) Ischemic striatum with large aggregates of erythrocytes (score 4). (D) Effect of oxygen therapy on erythrocytic extravasation. Both hyperbaric oxygen (HBO) and normobaric hyperoxia (NBO) reduced the semiquantitative score of erythrocytic extravasation (*significant difference compared with air, P<0.05, analysis of variance).
Hyperbaric Oxygen Attenuates Ischemia-Induced Matrix Metalloproteinase-9
To analyze the effect of oxygen therapy on MMP activity, protein extracts from brains collected 24 hours after TT-tMCAO were analyzed for MMP activity. Both MMP-9 (92 kDa) and MMP-2 (72 kDa) were observed as clear bands (Figure 5). At 24 hours after TT-tMCAO, MMP-2 levels did not differ between ischemic and nonischemic hemispheres in any group. However, MMP-9 levels in the ischemic hemisphere were distinctly higher than in the nonischemic hemisphere in all groups. Remarkably, when comparing the MMP-9 levels in the ischemic hemisphere among groups, HBO treatment resulted in a significant reduction in the MMP-9 band in the ischemic hemisphere in comparison to the air and NBO groups whereas NBO only tended to reduce MMP-9 levels. No differences were noted between MMP-9 bands after tMCAO in the nonischemic hemispheres among air-, NBO-, and HBO-treated mice (Figure 5).
Discussion
This study provides several major new findings. (1) Oxygen therapy in combination with thrombolytic therapy only affects infarct size if recanalization is successful. (2) Both NBO and, more effectively, HBO reduce size and frequency of gross parenchymal hemorrhage after thrombolysis-induced reperfusion. (3) HBO and NBO reduce early BBB permeability after tMCAO, which is a marker for subsequent hemorrhagic complications of thrombolysis. (4) Oxygen therapy improves microvascular integrity even in regions that undergo parenchymal infarction.
Secondary hemorrhage in ischemic stroke patients is stronger and occurs more frequently if the occluded cerebral artery is recanalized (Molina et al, 2001). Therefore, when experimentally testing the effect of an adjunctive therapy on cerebral hemorrhage after thrombolysis, it is desirable to control the recanalization effect of rt-PA. To appropriately model the variable effect of thrombolysis in patients in our study, two different clots were injected for tMCAO, and reperfusion was monitored using repetitive PW–MRI. Although rt-PA successfully recanalized the MCA in TT-tMCAO, vascular occlusion persisted despite thrombolysis in CT-tMCAO. Intriguingly, the effects of oxygen therapy in combination with rt-PA differed substantially between these two models. First, oxygen therapy reduced parenchymal damage in TT- but not in CT-tMCAO. This is consistent with most previous studies showing a cerebroprotective effect of early oxygen therapy in transient cerebral ischemia (Singhal et al, 2002; Veltkamp et al, 2005a, 2005b) but limited or no infarct size reduction after permanent MCAO (for review, see Helms et al, 2005; Poli and Veltkamp, 2009; Singhal, 2007). A limitation of this study is that neurologic function was not assessed.
The primary goal of this study was to examine the effect of oxygen therapy on secondary hemorrhage after thrombolysis. In line with findings from Qin et al (2007), who reported a reduction of hemorrhagic transformation in rats treated with HBO compared with air after filament-induced MCAO, HBO reduced both macroscopic parenchymal hemorrhage in recanalized and and hemorrhagic transformation in permanent thromboembolic ischemia, respectively. Interestingly, NBO also reduced secondary hemorrhage in TT-tMCAO although its effect was less powerful and less consistent than that of HBO. In a previous experimental study similar to ours in which NBO was also administered before thrombolysis, NBO tended to reduce hemorrhage volume (Henninger et al, 2006, 2009). In another recent study, NBO coadministered with thrombolysis failed to decrease secondary hemorrhage after thromboembolic MCAO (Fujiwara et al, 2009). Different timing of thrombolysis and oxygen treatment may underlie the discrepancy in these results. A strength of our study is that our experimental setup frequently induced parenchymal hemorrhage after TT-MCAO, which is clinically more relevant than hemorrhagic transfomation. NBO tended to increase hemorrhagic transformation in a clinical pilot study (Singhal et al, 2005a) but this increase in petechial-type ‘asymptomatic' secondary hemorrhage was related to a higher rate of reperfusion.
Our findings confirm a clear association between the effects of oxygen therapy on postischemic BBB permeability and on hemorrhage after thrombolysis. Topographic analysis of multimodal MRI showed that more than 95% of the hypointense T2* signal 24 hours after tMCAO was located within the area of intense postcontrast enhancement on T1w images at 2.5 hours after tMCAO. This is in accordance with previous experimental and clinical findings that early appearance of a circumscribed contrast enhancement on T1w shows tissue at risk for subsequent secondary hemorrhage (Kastrup et al, 2008; Neumann-Haefelin et al, 2002). Although an attenuation of postischemic BBB permeability in animals treated with NBO or HBO has been reported earlier (Liu et al, 2009; Qin et al, 2007; Veltkamp et al, 2005a), it was unclear as to whether this reflected a specific protective effect on cerebral microvessels or was proportional to overall parenchymal protection. As 95% of macroscopic hemorrhage on T2* MRI 24 hours after TT-tMCAO was located within the infarcted tissue (i.e., hyperintense area on DWI) in our experiments, oxygen therapy reduced secondary hemorrhage within the infarcted area. Thus, oxygen therapy had a beneficial effect on microvascular integrity despite its failing to protect the parenchyma. This protective effect on the microvasculature is further supported by our findings in permanent CT-tMCAO where oxygen therapy (in particular HBO) decreased histologic hemorrhagic transformation and hemoglobin content but did not reduce infarct size.
The molecular mechanisms underlying the effects of oxygen therapy on the cerebral microvessels remain to be fully elucidated. Our findings are consistent with previous reports showing that oxygen therapy attenuates the activation of MMPs and reduces the digestion of basal lamina components and tight junction proteins (Kim et al, 2005; Liu et al, 2009; Singhal et al, 2002; Veltkamp et al, 2006b). We already showed that HBO reduces tissue hypoxia, and attenuates induction of hypoxia-inducible factor-1 and one of its target gene, the vascular permeability factor vascular endothelial growth factor (Sun et al, 2008). Furthermore, we recently showed that hypoxia-induced edema formation in the brain is mediated by MMP-9-dependent rearrangement and gap formation of tight junction proteins through a vascular endothelial growth factor-dependent mechanism (Bauer et al, 2009). Thus, our data suggest that HBO therapy can attenuate the activation of this hypoxia-permeability axis.
In conclusion, our findings are of profound relevance for translational studies analyzing the usefulness of oxygen therapy in acute ischemic stroke. Whether oxygen therapy can reduce an ischemic brain lesion depends largely on successful, subsequent recanalization. As oxygen therapy also attenuates secondary hemorrhage within infarcted tissue, the most dreaded complication of recanalization therapy, combining it with thrombolytic therapy early on appears particularly promising. Despite some differences in efficacy, NBO and HBO could be viewed as complementary treatment strategies in the clinical setting (e.g., prehospital NBO followed by inhospital HBO).
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (VE 196/2-2) and the GEMI fund. RV is supported by an Else-Kröner-Memorial Scholarship.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow and Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Bauer AT, Bürgers HF, Rabie T, Marti HH. Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J Cereb Blood Flow Metab. 2009;30:837–848. doi: 10.1038/jcbfm.2009.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert JW, Cahill J, Zhang JH. Hyperbaric oxygen and cerebral physiology. Neurol Res. 2007;29:134–141. doi: 10.1179/016164107X174156. [DOI] [PubMed] [Google Scholar]
- Choudhri TF, Hoh BL, Solomon RA, Connolly ES, Jr, Pinsky DJ. Use of a spectrophotometric hemoglobin assay to objectively quantify intracerebral hemorrhage in mice. Stroke. 1997;28:2296–2302. doi: 10.1161/01.str.28.11.2296. [DOI] [PubMed] [Google Scholar]
- Del Zoppo GJ, von Kummer R, Hamann GF. Ischemic damage of brain microvessels: inherent risks for thrombolytic treatment in stroke. J Neurol Neurosurg Psychiatry. 1998;65:1–9. doi: 10.1136/jnnp.65.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara N, Murata Y, Arai K, Egi Y, Lu J, Wu O, Singhal AB, Lo EH. Combination therapy with normobaric oxygen (NBO) plus thrombolysis in experimental ischemic stroke. BMC Neurosci. 2009;10:79. doi: 10.1186/1471-2202-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, Brott T, Frankel M, Grotta JC, Haley EC, Jr, Kwiatkowski T, Levine SR, Lewandowski C, Lu M, Lyden P, Marler JR, Patel S, Tilley BC, Alber G, Bluhmki E, Wilhelm M, Hamilton S. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trails. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neuro vascular unit in health and disease. Pharmacol Rev. 2005;57:173–85. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Heiland S, Benner T, Reith W, Forsting M, Sartor K. Perfusion-weighted MRI using gadobutrol asa contrast agent in a rat stroke model. J Magn Reson Imaging. 1997;7:1109–1115. doi: 10.1002/jmri.1880070625. [DOI] [PubMed] [Google Scholar]
- Helms AK, Whelan HT, Torbey MT. Hyperbaric oxygen therapy of cerebral ischemia. Cerebrovasc Dis. 2005;20:417–426. doi: 10.1159/000088979. [DOI] [PubMed] [Google Scholar]
- Henninger N, Küppers-Tiedt L, Sicard KM, Günther A, Schneider D, Schwab S. Neuroprotective effect of hyperbaric oxygen therapy monitored by MR-imaging after embolic stroke in rats. Exp Neurol. 2006;201:316–323. doi: 10.1016/j.expneurol.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Henninger N, Bratane BT, Bastan B, Bouley J, Fischer M. Normaobaric hyperoxia and delayed tPA treatment in a rat embolic stroke model. J Cereb Blood Flow Metab. 2009;29:119–129. doi: 10.1038/jcbfm.2008.104. [DOI] [PubMed] [Google Scholar]
- Kahles T, Foerch C, Sitzer M, Schroeter M, Steinmetz H, Rami A, Neumann-Haefelin T. Tissue plasminogen activator mediated blood-brain barrier damage in transient focal cerebral ischemia in rats: relevance of interactions between thrombotic material and thrombolytic agent. Vascul Pharmacol. 2005;43:254–259. doi: 10.1016/j.vph.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Kastrup A, Groeschel K, Ringer TM, Redecker C, Cordesmeyer R, Witte OW, Terborg C. Early disruption of the blood-brain barrier after thrombolytic therapy predicts hemorrhage in patients with acute stroke. Stroke. 2008;39:2385–2387. doi: 10.1161/STROKEAHA.107.505420. [DOI] [PubMed] [Google Scholar]
- Kim HY, Singhal AB, Lo EH. Normaobaric hyperoxia extends the reperfusion window in focal crerbral ischemia. Ann Neurol. 2005;57:571–575. doi: 10.1002/ana.20430. [DOI] [PubMed] [Google Scholar]
- Liu WL, Hendren J, Qin XJ, Liu KJ. Normobaric hyperoxia reduces the neurovascular complication associated with delayed tissue plasminogen activator treatment in a rat model of focal cerebral ischemia. Stroke. 2009;40:2526–2531. doi: 10.1161/STROKEAHA.108.545483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink RB, Dutka AJ. Hyperbaric oxygen after global cerebral ischemia in rabits reduces brain vascular permeability and blood flow. Stroke. 1995;26:2307–2312. doi: 10.1161/01.str.26.12.2307. [DOI] [PubMed] [Google Scholar]
- Molina CA, Montaner J, Abilleira S, Ibarra B, Romero F, Arenaillas JF, Alvares-Sabin J. Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke. 2001;32:1079–1084. doi: 10.1161/01.str.32.5.1079. [DOI] [PubMed] [Google Scholar]
- Nighoghossian N, Trouillas P. Hyperbaric oxygen in the treatment of acute ischemic stroke: an unsettled issue. J Neurol Sci. 1997;150:27–31. doi: 10.1016/s0022-510x(97)05398-7. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin C, Brinker G, Uhlenküken U, Pillekamp F, Hossmann KA, Hoehn M. Prediction of hemorrhagic transformation after thrombolytic therapy of clot embolism: an MRI investigation in rat brain. Stroke. 2002;33:1392–1398. doi: 10.1161/01.str.0000014619.59851.65. [DOI] [PubMed] [Google Scholar]
- Poli S, Veltkamp R. Oxygen therapy in acute ischemic stroke experimental efficacy and molecular mechanisms. Curr Mol Med. 2009;9:227–241. doi: 10.2174/156652409787581619. [DOI] [PubMed] [Google Scholar]
- Qin ZH, Karabiyikoblu M, Hua Y, Silbergleit R, He Y, Keep RF, Xi G. Hyperbaric oxygen-induced attenuation of hemorrhagic transformation after experimental focal transient cerebral ischemia. Stroke. 2007;38:1362–1367. doi: 10.1161/01.STR.0000259660.62865.eb. [DOI] [PubMed] [Google Scholar]
- Singhal AB. A review of oxygen therapy in ischemic stroke. Neurol Res. 2007;29:173–183. doi: 10.1179/016164107X181815. [DOI] [PubMed] [Google Scholar]
- Singhal AB, Benner T, Roccatagliata L, Koroschetz WJ, Schaefer PW, Lo EH, Buonanno FS, Gonzalez RG, Sorensen AG. A pilot study of normobaric oxygen therapy in acute ischemic stroke. Stroke. 2005a;36:797–802. doi: 10.1161/01.STR.0000158914.66827.2e. [DOI] [PubMed] [Google Scholar]
- Singhal AB, Dijikhuizen RM, Rosen BR, Lo EH. Normaobaric hyperoxia reduces MRI diffusion abnormalities and infarct size in experimental stroke. Neurology. 2005b;58:945–952. doi: 10.1212/wnl.58.6.945. [DOI] [PubMed] [Google Scholar]
- Singhal AB, Wang X, Sumii T. Effect of normobaric hyperoxia in a rat model of focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2002;22:861–868. doi: 10.1097/00004647-200207000-00011. [DOI] [PubMed] [Google Scholar]
- Sun L, Marti HH, Veltkamp R. Hyperbaric oxygen reduces tissue hypoxia and hypoxia-inducible factor-1 alpha expression in focal cerebral ischemia. Stroke. 2008;39:1000–1006. doi: 10.1161/STROKEAHA.107.490599. [DOI] [PubMed] [Google Scholar]
- Toomey JR, Valocik RE, Koster PF, Gabriel MA, McVey M, Hart TK, Ohlstein EH, Parsons AA, Barone FC. Inhibition of factor IX(a) is protective in a rat model of thromboembolic stroke. Stroke. 2002;33:578–585. doi: 10.1161/hs0202.102950. [DOI] [PubMed] [Google Scholar]
- Veltkamp R, Bieber K, Wagner S, Beynon C, Siebing DA, Veltkamp C, Schwaninger M, Marti HH. Hyperbaric oxygen reduces basal lamina degradation after transient focal cerebral ischemia in rat. Brain Res. 2006b;1076:231–237. doi: 10.1016/j.brainres.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Veltkamp R, Siebing DA, Heiland S. Hyperbaric oxygen induces rapid protection against focal cerebral ischemia. Brain Res. 2005b;1037:134–138. doi: 10.1016/j.brainres.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Veltkamp R, Siebing DA, Sun L. Hyperbaric oxygen reduces blood-brain barrier damage and edema after transient focal cerebral ischemia. Stroke. 2005a;36:1679–1683. doi: 10.1161/01.STR.0000173408.94728.79. [DOI] [PubMed] [Google Scholar]
- Veltkamp R, Sun L, Hermann O, Wolferts G, Hagmann S, Siebing DA, Marti HH, Veltkamp C, Schwaninger M. Oxygen therapy in permanent brain ischemia: potential and limitations. Brain Res. 2006a;1107:185–191. doi: 10.1016/j.brainres.2006.05.108. [DOI] [PubMed] [Google Scholar]
- Veltkamp R, Warner DS, Domoki F, Brinkhous AD, Toole JF, Busija DW. Hyperbaric oxygen decreases infarct size and behavioral deficit after transient focal cerebral ischemia in rats. Brain Res. 2000;853:68–73. doi: 10.1016/s0006-8993(99)02250-7. [DOI] [PubMed] [Google Scholar]
- Wang X, Lee SR, Arai K, Lee SR, Tsuji K, Rebeck GW, Lo EH. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003;10:1038. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Lo T, Mychaskiw G, Colohan A. Mechanisms of hyperbaric oxygen and neuroprotection in stroke. Pathophysiology. 2005;12:63–77. doi: 10.1016/j.pathophys.2005.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.