Abstract
Blood–brain barrier (BBB) P-glycoprotein activity is rapidly reduced by vascular endothelial growth factor (VEGF) acting via Src and by tumor necrosis factor-α acting via protein kinase C (PKC)β1. To probe underlying mechanism(s), we developed an in vivo, immunoblot-based proteinase K (PK) protection assay to assess the changes in the P-glycoprotein content of the BBB's luminal membrane. Infusion of PK into the brain vasculature selectively cleaved luminal membrane P-glycoprotein, leaving intracellular proteins intact. Intracerebroventricular injection of VEGF partially protected P-glycoprotein from proteolytic cleavage, consistent with transporter internalization. Activation of PKCβ1 did not protect P-glycoprotein. Thus, VEGF and PKCβ1 reduce P-glycoprotein activity by distinct mechanisms.
Keywords: blood–brain barrier, endothelium, pharmacology, physiology, vascular biology
Introduction
The blood–brain barrier (BBB) protects the central nervous system from xenobiotics in part by expression of multispecific efflux pumps including P-glycoprotein in the luminal membrane of brain capillary endothelial cells. Diminished function/expression of P-glycoprotein increases vulnerability to neurotoxicants. However, P-glycoprotein also limits brain accumulation of therapeutic drugs used to treat brain tumors, epilepsy, and HIV encephalitis; thus, reducing P-glycoprotein activity may be one way to overcome central nervous system drug resistance (Miller et al, 2008).
We have identified two distinct signaling pathways in brain capillaries that mediate acute, reversible downregulation of BBB P-glycoprotein transport activity, without changing P-glycoprotein expression. One is a ‘pro-inflammatory' pathway involving tumor necrosis factor-α signaling through endothelin-1, nitric oxide, and protein kinase C (PKC)βI (Rigor et al, 2010). The other is a ‘pro-angiogenic' pathway involving vascular endothelial growth factor (VEGF) signaling though Src kinase (Hawkins et al, 2010). Both pathways are active in isolated rat brain capillaries and in intact rats in vivo. However, it is not clear whether signaling reduces P-glycoprotein activity by removal of transport protein from the membrane, for example, through endocytosis, or by some other mechanism. Moreover, detecting transporter translocation in intact brain capillaries ex vivo or in situ is technically challenging.
We describe here a proteinase K (PK)-based protection assay designed to assess changes in P-glycoprotein exposure to capillary lumens in situ. It is based on the principle that internalization of P-glycoprotein would protect it from cleavage by a protease introduced into the vascular lumen. The PK is a highly reactive and nonspecific protease that can cleave P-glycoprotein (Nuti and Rao, 2002). Using this assay we show that VEGF signaling reduced the amount of P-glycoprotein cleaved by PK, but that PKCβ1 activation was without effect. These findings suggest that VEGF reduces BBB P-glycoprotein activity by removing the transporter from the luminal membrane, but that PKCβ1 activation does not.
Materials and methods
All animal procedures were approved by the Animal Care and Use Committee of the National Institute of Environmental Health Sciences and conform to the guidelines and standards of the National Institutes of Health. Male Sprague-Dawley rats were obtained from Charles River (Raleigh, NC, USA) and maintained under standard conditions. Mouse anti-P-glycoprotein (C219) was purchased from Signet (Dedham, MA, USA). Mouse anti-Mrp2 was purchased from Axxora (San Diego, CA, USA). Mouse anti-Na,K-ATPase α1 was purchased from Millipore (Billerica, MA, USA). Mouse anti-β-actin, VEGF, and proteinase K were purchased from Sigma (St Louis, MO, USA). 12-deoxyphorbol-13-phenylacetate-20-acetate (dPPA) was purchased from Enzo Life Sciences (Plymouth Meeting, PA, USA). Specific activity of PK was confirmed by a kinetic fluorimetric activity assay (Anaspec, San Jose, CA, USA).
Proteinase K Infusion, Capillary Isolation, and Immunoblot
Rats were anesthetized with ketamine cocktail and heparinized. The left common carotid artery was exposed to the bifurcation of the internal and external carotid arteries. The external carotid artery was ligated with a 3-0 silk suture, and the common carotid artery was cannulated with PE10 tubing connected to a perfusion circuit. Phosphate-buffered saline was infused by a peristaltic pump at 3.0 mL/min. The left jugular vein was cut to allow for drainage and the left cardiac ventricle was cut to stop the heart. On thorough flushing of the brain (∼1 min), a PK solution (0–60 U/mL in phosphate-buffered saline) was introduced into the circuit and perfused for 5 minutes, followed by flushing with 5 mmol/L phenylmethanesulphonyl fluoride in phosphate-buffered saline for another 5 minutes to arrest proteolysis. The brain was removed and placed into ice-cold phosphate-buffered saline. After removal of the meninges and choroid plexuses, the brain (or hemisphere ipsilateral to intracerebroventricular injection, see below) was homogenized and capillaries were isolated by density centrifugation and purification on a glass bead column as previously described (Hawkins et al, 2010). Capillaries were snap frozen in liquid nitrogen, resuspended in lysis buffer (CelLytic MT, Sigma, St Louis, MO, USA) containing 0.1% sodium dodecyl sulfate, sonicated, and protein was extracted from the supernatants for electrophoresis and Western blot per standard protocols as previously described (Hawkins et al, 2010).
Pretreatment with Vascular Endothelial Growth Factor and 12-Deoxyphorbol-13-Phenylacetate-20-Acetate
We recently reported that both intracerebroventricular injection of VEGF and carotid infusion of the PKCβ activating phorbol ester dPPA increase brain uptake of the P-glycoprotein substrate verapamil (Hawkins et al, 2010; Rigor et al, 2010). To test whether internalization of P-glycoprotein underlies the loss of functional activity observed, we performed these treatments as previously described (Hawkins et al, 2010; Rigor et al, 2010) before infusion of PK. Briefly, rats were placed in a stereotactic frame for injection of either VEGF (500 ng in 2 μL artificial cerebrospinal fluid) or artificial cerebrospinal fluid alone into the lateral ventricle 30 minutes before PK infusion. Alternatively, rats were cannulated as described above and infused with either dPPA in mammalian Ringer or Ringer alone for 20 minutes before infusion of PK.
Results and Discussion
Regulation of P-glycoprotein at the BBB is complex, with multiple signaling pathways modifying protein expression and transport activity (Miller et al, 2008). Recent reports show that rapid loss of P-glycoprotein activity in response to tumor necrosis factor-α/PKCβI or VEGF/Src in vitro and in vivo is posttranslational and dependent on protein phosphorylation-based signaling (Hawkins et al, 2010; Rigor et al, 2010). The events connecting Src-dependent or PKCβI-dependent phosphorylation with loss of P-glycoprotein activity are not understood. The present experiments were designed to test the hypothesis that loss of P-glycoprotein transport activity is accompanied by a shift in transporter protein from the luminal membrane to a compartment where it can no longer function as an efflux transporter.
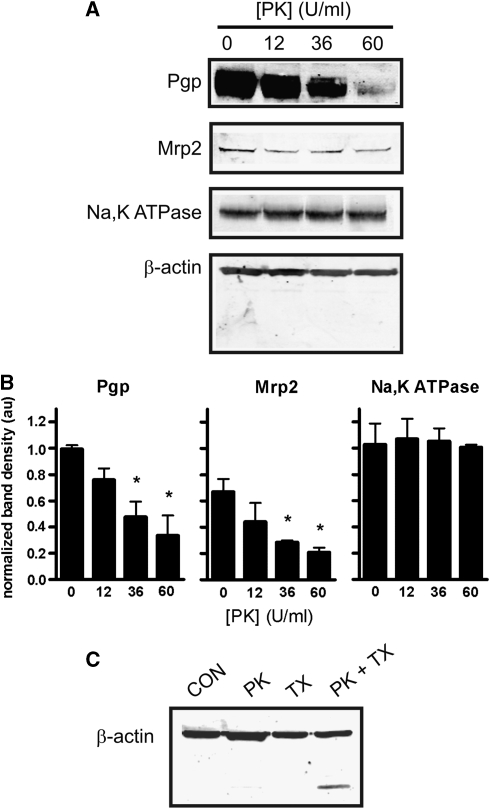
In preliminary experiments, we infused PK into the rat brain vasculature at various concentrations and exposure times, with the goal of finding conditions under which P-glycoprotein would be cleaved but intracellular proteins would not. Infusing PK for 5 minutes caused a concentration-dependent loss of P-glycoprotein immunoreactivity without loss of β-actin (Figures 1A and 1B). Exposure times>10 minutes and PK concentrations>60 U/mL resulted in cleavage of β-actin, indicating proteolysis within the brain capillary endothelial cells. Immunoreactivity for a second luminal transport protein, multidrug resistance-related protein 2 (Mrp2) was also reduced by PK (Figures 1A and 1B). As with actin, immunoreactivity for Na,K-ATPase, a transporter expressed primarily at the abluminal membrane, was unchanged by PK, suggesting that PK-mediated proteolysis was limited to the luminal membrane of the capillary endothelium (Figures 1A and 1B). The lack of actin proteolysis observed was not due to an inability of PK to cleave actin, as permeabalizing the vasculature with Triton X-100 in the presence of PK (60 U/mL) decreased actin immunoreactivity at 44 kDa and induced the appearance of a lower molecular weight band, presumably a degradation product that retained the antigenic epitope (Figure 1C). Thus, in situ infusion of 60 U/mL PK for 5 minutes significantly reduced immunoreactivity of two luminal membrane transport proteins without affecting immunoreactivity of intracellular β-actin or abluminal Na,K-ATPase. We used those infusion conditions for the remaining experiments.
Figure 1.
Proteinase K (PK) cleaves luminal membrane proteins in situ. (A) Representative blots of capillary proteins isolated from rats perfused with the concentration of PK indicated. (B) Densitometry of blots represented in (A). Immunoreactivity for P-glycoprotein and multidrug resistance-related protein 2 (Mrp2) significantly decreased with increasing concentration of PK. Immunoreactivity for the Na,K-ATPase α1 subunit (expressed primarily on the abluminal membrane) was unchanged. Data are mean relative band densities for the proteins indicated normalized to relative band densities for β-actin±s.e.m. Significance is determined by one-way analysis of variance with a Neuman–Keuls post hoc test. *P<0.05, n=3 for all experiments. (C) Proteinase K cleaves actin in permeabilized brain capillaries. Inclusion of the cell permeabilizing detergent Triton X-100 (TX, 0.1%) in the perfusate together with PK (60 U/mL) facilitates cleavage of actin, as seen by the low molecular weight band recognized by the actin antibody. Note that the lower molecular weight band is not present in any other condition.
To function as an efflux transporter at the BBB, P-glycoprotein must be situated at the luminal surface of capillary endothelium. The extent to which P-glycoprotein localization is polarized in the BBB endothelium is controversial. In studies using immunofluorescence or electron microscopy, localization is reported as primarily luminal (Miller et al, 2000; Virgintino et al, 2002), and both luminal and abluminal (Bendayan et al, 2006). Roberts et al (2008) recently used in vivo biotinylation to nonspecifically label proteins expressed on the luminal membrane of the brain microvasculature. They found that P-glycoprotein immunoreactivity in biotinylated capillaries closely colocalized with Neutravidin-FITC binding. Consistent with most of these findings, we found that under baseline conditions, at least 65%±17% of the P-glycoprotein (and 67%±8% of the Mrp2) expressed in the endothelium is available for proteolysis, that is, exposed on the luminal surface of the endothelium in vivo (Figure 1B).
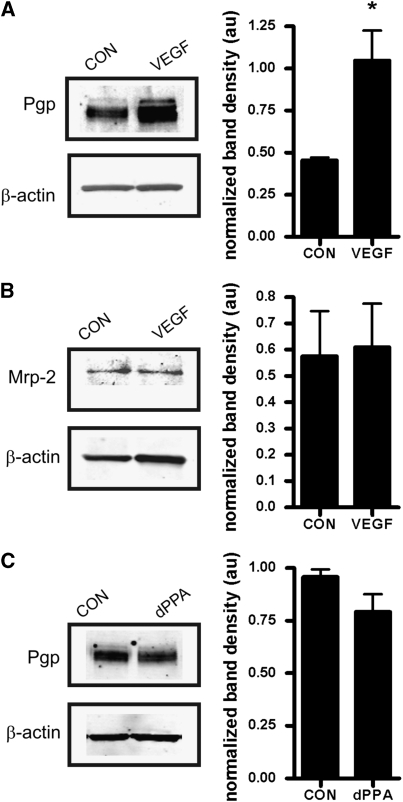
Intracerebroventrivular injection of VEGF at a dose previously shown to significantly increase net transport of P-glycoprotein substrates morphine and verapamil into the brain (Hawkins et al, 2010) protected P-glycoprotein from cleavage by PK. This was indicated by significantly increased immunoreactivity for P-glycoprotein after perfusion with PK in brain capillary lysates from VEGF-injected rats compared with those isolated from controls injected with artificial cerebrospinal fluid (Figure 2A). In a separate group of animals not perfused with PK, VEGF injection did not change total protein expression of P-glycoprotein (data not shown). The VEGF injection also did not protect Mrp2 from cleavage by PK (Figure 2B). This is an important negative control, as we previously showed that VEGF had no effect on Mrp2-mediated transport of Texas Red in isolated brain capillaries (Hawkins et al, 2010). In contrast to VEGF, carotid infusion of dPPA, also shown to increase transport of verapamil into brain (Rigor et al, 2010), did not alter P-glycoprotein immunoreactivity after infusion of PK (Figure 2C).
Figure 2.
Intracerebroventricular injection of vascular endothelial growth factor (VEGF) internalizes P-glycoprotein at the blood–brain barrier (BBB). (A) Animals were given an ICV injection of either VEGF or artificial cerebrospinal fluid (CON) before undergoing perfusion with proteinase K (PK). Capillaries were isolated from the hemispheres ipsilateral to the injection site. The VEGF treatment was associated with a significant (P<0.05) increase in immunoreactivity for P-glycoprotein after PK perfusion, indicating that P-glycoprotein was less available for cleavage by PK and implying that P-glycoprotein is internalized in response to VEGF. (B) The VEGF does not induce protection of multidrug resistance-related protein 2 (Mrp2) from proteolysis by PK. (C) Infusion of the protein kinase C (PKC)βI activating phorbol ester 12-deoxyphorbol-13-phenylacetate-20-acetate (dPPA) to the cerebral microvasculature before PK infusion has no effect on P-glycoprotein immunoreactivity. Representative blots are shown, each histogram shows mean normalized band densities±s.e.m. from three separate experiments. Significance is determined by Student's t-test; *P<0.05.
The results of the proteolysis protection assay are consistent with mechanistically distinct signals mediating rapid loss of P-glycoprotein activity by VEGF and dPPA. With VEGF, the increase in P-glycoprotein immunoreactivity could indicate movement of the transporter to a compartment not accessible to luminal PK, either through internalization or induction of a conformational change that protects from PK proteolysis. Our previous work indicated that Src-dependent phosphorylation of caveolin-1 accompanies rapid inhibition of P-glycoprotein in response to VEGF treatment, suggesting a role for caveolin-dependent internalization (Fagerholm et al, 2009; Hawkins et al, 2010). With dPPA, the lack of change in P-glycoprotein immunoreactivity indicates that transport activity was reduced while the transporter was still exposed to luminal PK. We have not observed phosphorylation of caveolin-1 in response to treatment with dPPA (RR Rigor, unpublished data), suggesting that a different phosphorylation-dependent pathway targets P-glycoprotein in response to tumor necrosis factor-α/PKCβI signaling.
On the basis of selective site-directed mutagenesis of P-glycoprotein, putative PKC phosphorylation sites on the P-glycoprotein molecule are not important for PKC control of transport activity (Germann et al, 1996; Goodfellow et al, 1996). Thus, P-glycoprotein inhibition by PKCβI likely involves phosphorylation of an ancillary protein. This could be achieved by recruitment of PKCβI and other binding partners to the adapter protein RBCK1, which is abundant in brain capillaries (Rigor et al, 2010).
P-glycoprotein at the BBB is a major obstacle for delivery of drugs to the brain, especially in diseases with enhanced central nervous system drug resistance. Previous attempts to inhibit P-glycoprotein pharmacologically have caused significant toxicity in combination with chemotherapeutic compounds (Friedenberg et al, 2006; Hubensack et al, 2008). Targeting signal transduction offers an alternative strategy for inhibiting P-glycoprotein selectively at the BBB. Although it is not known whether the same regulatory mechanisms control P-glycoprotein activity at the BBB as in peripheral organs responsible for drug clearance and metabolism (i.e., kidney or liver), a mechanistic understanding of P-glycoprotein regulation is critical to developing this approach to suppress central nervous system drug resistance. Our current work suggests rapid inhibition of P-glycoprotein activity by Src kinase or PKCβI occurs via distinct mechanisms, involving either putative endocytosis of the transporter or modulation within the membrane via accessory proteins, respectively. Targeting one of these processes may prove useful for clinical treatment of drug resistance, depending on desired magnitude and/or duration of P-glycoprotein inhibition.
In summary, we developed a novel in situ proteolysis protection assay for determining localization of key luminal membrane proteins at the BBB. In addition to efflux transporters, this method may prove useful for studying regulation of other luminal surface proteins, including receptors involved in signaling and carrier-mediated endocytosis (e.g., insulin and transferrin receptors) for which internalization is critical to normal function. Further, we have used this method to establish that VEGF and PKCβ1 rapidly reduce P-glycoprotein activity by distinct mechanisms, one of which may involve removal of the transporter from the luminal membrane.
The authors declare no conflict of interest.
References
- Bendayan R, Ronaldson PT, Gingras D, Bendayan M. In situ localization of P-glycoprotein (ABCB1) in human and rat brain. J Histochem Cytochem. 2006;54:1159–1167. doi: 10.1369/jhc.5A6870.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerholm S, Ortegren U, Karlsson M, Ruishalme I, Strålfors P. Rapid insulin-dependent endocytosis of the insulin receptor by caveolae in primary adipocytes. PLoS One. 2009;4:e5985. doi: 10.1371/journal.pone.0005985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenberg WR, Rue M, Blood EA, Dalton WS, Shustik C, Larson RA, Sonneveld P, Greipp PR. Phase III study of PSC-833 (valspodar) in combination with vincristine, doxorubicin, and dexamethasone (valspodar/VAD) versus VAD alone in patients with recurring or refractory multiple myeloma (E1A95): a trial of the Eastern Cooperative Oncology Group. Cancer. 2006;106:830–838. doi: 10.1002/cncr.21666. [DOI] [PubMed] [Google Scholar]
- Germann UA, Chambers TC, Ambudkar SV, Licht T, Cardarelli CO, Pastan I, Gottesman MM. Characterization of phosphorylation-defective mutants of human P-glycoprotein expressed in mammalian cells. J Biol Chem. 1996;217:1708–1716. doi: 10.1074/jbc.271.3.1708. [DOI] [PubMed] [Google Scholar]
- Goodfellow HR, Sardini A, Ruetz S, Callaghan R, Gros P, McNaughton PA, Higgins CF. Protein kinase C-mediated phosphorylation does not regulate drug transport by the human multidrug resistance P-glycoprotein. J Biol Chem. 1996;271:13668–13674. doi: 10.1074/jbc.271.23.13668. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Sykes DB, Miller DS. Rapid, reversible modulation of blood-brain barrier P-glycoprotein transport activity by vascular endothelial growth factor. J Neurosci. 2010;30:1417–1425. doi: 10.1523/JNEUROSCI.5103-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubensack M, Müller C, Höcherl P, Fellner S, Spruss T, Bernhardt G, Buschauer A. Effect of the ABCB1 modulators elacridar and tariquidar on the distribution of paclitaxel in nude mice. J Cancer Res Clin Oncol. 2008;134:597–607. doi: 10.1007/s00432-007-0323-9. [DOI] [PubMed] [Google Scholar]
- Miller DS, Bauer B, Hartz AM. Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008;60:196–209. doi: 10.1124/pr.107.07109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DS, Nobmann SN, Gutmann H, Toeroek M, Drewe J, Fricker G. Xenobiotic transport across isolated brain microvessels studied by confocal microscopy. Mol Pharmacol. 2000;58:1357–1367. doi: 10.1124/mol.58.6.1357. [DOI] [PubMed] [Google Scholar]
- Nuti SL, Rao US. Proteolytic cleavage of the linker region of the human P-glycoprotein modulates its ATPase function. J Biol Chem. 2002;277:29417–29423. doi: 10.1074/jbc.M204054200. [DOI] [PubMed] [Google Scholar]
- Rigor RR, Hawkins BT, Miller DS. Activation of PKC isoform βI at the blood-brain barrier rapidly decreases P-glycoprotein activity and enhances drug delivery to the brain. J Cereb Blood Flow Metab. 2010;30:1373–1383. doi: 10.1038/jcbfm.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LM, Black DS, Raman C, Woodford K, Zhou M, Haggerty JE, Yan AT, Cwirla SE, Grindstaff KK. Subcellular localization of transporters along the rat blood-brain barrier and blood-cerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience. 2008;155:423–438. doi: 10.1016/j.neuroscience.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Virgintino D, Robertson D, Errede M, Benagiano V, Girolamo F, Maiorano E, Roncali L, Bertossi M. Expression of P-glycoprotein in human cerebral cortex microvessels. J Histochem Cytochem. 2002;50:1671–1676. doi: 10.1177/002215540205001212. [DOI] [PubMed] [Google Scholar]