Abstract
Cortical spreading depression (CSD) is an intense depolarization wave implicated in the pathophysiology of brain injury states and migraine aura. As Cav2.1 channels modulate CSD susceptibility, we tested gabapentin, which inhibits Cav2.1 through high-affinity binding to its α2δ subunit, on CSD susceptibility in anesthetized rats. Gabapentin, 100 or 200 mg/kg, elevated the electrical threshold for CSD and diminished recurrent CSDs evoked by topical KCl, when administered 1 hour before testing. With its favorable safety and tolerability profile, gabapentin may have a role in suppression of injury depolarizations in stroke, intracranial hemorrhage, and traumatic brain injury.
Keywords: cortex, electrophysiology, gabapentin, rat, spreading depression
Introduction
Cortical spreading depression (CSD) is a wave of neuronal and glial depolarization associated with massive K+ and glutamate efflux, and Ca2+ influx, slowly propagating in the brain tissue at a rate of 3 to 4 mm/min by way of gray matter contiguity. Although the massive rise in extracellular K+ is critical for contiguous spread, glutamate adds momentum to sustain the propagation. Among pharmacological inhibitors of CSD are Ca2+ channel blockers, in particular of the Cav2.1 subtype (Kunkler and Kraig, 2004; Richter et al, 2002), possibly by reducing the release of glutamate during CSD. Gabapentin, an adjunct antiepileptic that has shown modest efficacy in migraine prophylaxis, inhibits the Cav2.1 channel by binding with high affinity and specificity to its auxiliary α2δ subunit, which both diminishes the voltage responsiveness of channels and prevents their localization to the neurotransmitter release sites (Gee et al, 1996; Hendrich et al, 2008).
As the Cav2.1 channel is a major regulator of glutamate release, and a modulator of CSD susceptibility, we tested the efficacy of gabapentin to suppress CSD. Our results show a novel acute inhibitory effect of gabapentin on CSD.
Materials and methods
A total of 32 rats (Sprague-Dawley, 200 to 450 g, male) were used to test gabapentin on CSD. Gabapentin doses and treatment protocols were chosen based on published data in other animal models of neurological diseases (Radulovic et al, 1995). Gabapentin was administered as a single 100 or 200 mg/kg intravenous dose (n=7 and 14, respectively; Research Chemical) 60 minutes before CSD testing; intravenous route was chosen because of the saturable oral absorption kinetics, and nonlinear oral bioavailability (Radulovic et al, 1995; Stewart et al, 1993). Saline was used as vehicle control (n=11). A subset of rats in each group was tested blindly.
Procedures
Institutional guidelines for animal care and use for research purposes were strictly followed, and study protocol was approved by institutional review board. Rats were anesthetized (isoflurane 5% induction, 1% maintenance, in 70% N2O/30% O2), paralyzed (pancuronium 0.4 mg/kg), and intubated through a tracheostomy for mechanical ventilation (SAR-830; CWE, Ardmore, PA, USA). Arterial blood gases and pH were measured every 30 minutes and ventilation adjusted to maintain arterial pCO2 between 35 and 45 mmHg (Corning 178; Corning, NY, USA). Continuous measurement of blood pressure (PowerLab; ADInstruments, Colorado Springs, MO, USA) and blood sampling were performed through a femoral artery catheter. Rectal temperature was kept at 37.0°C±0.1°C using a thermostatic heating pad (FHC, Bowdoinham, ME, USA). Level of anesthesia was maintained throughout the experiment to eliminate cardiovascular response to tail pinch. In all treatment groups, systemic physiological parameters were within normal range, although mild and transient blood pressure reductions were observed after 200 mg/kg gabapentin (Table 1).
Table 1. Systemic and electrophysiological parameters.
| Systemic physiology | CSD electrophysiology | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experimental groups | N | pH | pCO2 (mmHg) | pO2 (mmHg) | BP (mmHg) | Amp (mV) | Dur (seconds) | Speed (mm/min) | Propagation failure (1−E2/E1, %) | Electrical threshold (μC (25–75%)) |
| SAL 2.5 ml/kg, i.v. | 11 | 7.43±0.04 | 38±3 | 144±29 | 114±13 | 15±4 | 21±5 | 3.7±0.3 | 33±14 | 300 (150–500) |
| GPT 100 mg/kg, i.v. | 7 | 7.44±0.03 | 35±2 | 141±17 | 102±8 | 17±5 | 21±3 | 3.4±0.6 | 35±26 | 1600 (1000–1800)* |
| GPT 200 mg/kg, i.v. | 14 | 7.41±0.03 | 37±4 | 133±14 | 94±6* | 15±5 | 20±6 | 3.7±0.4 | 33±22 | 800 (600–1200)* |
Amp, DC shift amplitude; CSD, cortical spreading depression; Dur, DC shift duration at half amplitude; E1 and E2, electrodes 1 (proximal) and 2 (distal; see Materials and methods); GPT, gabapentin; SAL, saline control.
The systemic physiological parameters for each rat were calculated by averaging all measurements throughout an experiment. CSD amplitudes are the average amplitude of all CSDs evoked during 1 hour topical KCl application. As CSD duration and propagation speed tended to differ between the first and subsequent CSDs, we measured the duration and propagation speed of only the first CSD after topical KCl application. Propagation failure was assessed during topical KCl-induced repetitive CSDs.
*P<0.05 versus saline.
Rats were placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA) and burr holes were drilled bilaterally under saline cooling at (mm from bregma): (1) posterior 7.0, lateral 2.0 (occipital, 1 mm diameter for KCl application or electrical stimulation); (2) posterior 5.0, lateral 2.0 (frontoparietal, 0.5 mm diameter for electrode 1); (3) posterior 3.0, lateral 2.0 (frontal, 0.5 mm diameter for electrode 2). Dura overlying the occipital cortex was gently removed and care was taken to avoid bleeding. The steady (DC) potential and electrocorticogram were recorded with glass micropipettes filled with 200 mmol/L NaCl, 300 μm below pia (Axoprobe-1A; Axon Instruments, Burlingame, CA, USA). Ag/AgCl reference electrode was placed subcutaneously in the neck. After surgical preparation, cortex was allowed to recover for 15 minutes under saline irrigation. The data were continuously recorded using a data acquisition system for off-line analysis (ADInstruments).
Cortical Spreading Depression Susceptibility
We assessed CSD susceptibility using two independent methods, KCl or electrical stimulation, as described earlier (Ayata et al, 2006). For KCl-induced CSD susceptibility, we placed a cotton ball (1.5 mm diameter) soaked with 1 mol/L KCl on the pial surface and kept it moist by placing 5 μL of the same KCl solution every 15 minutes. The total number of KCl-induced CSDs detected at either recording site during 60 minutes KCl application was counted. In addition, we determined the incidence of propagation failure between the two recording sites in all treatment groups, and expressed this as the number of CSDs failed to appear at electrode 2 as percent of total CSDs recorded at electrode 1. After the end of KCl stimulation, electrical threshold for CSD was determined in the opposite hemisphere by direct cortical stimulation using a stimulator (Grass Instruments, West Warwick, RI, USA), a constant current unit (WPI, Sarasota, FL, USA), and a bipolar stimulation electrode placed on the pial surface (400 μm tip diameter, 1 mm tip separation; FHC). Cathodal square pulses of increasing intensity (100 to 4000 microcoulomb, μC) were applied at 5-minute intervals by adjusting the current and duration of stimulus until a CSD was observed. At 1 mA current, pulses of 100, 200, 300, and 400 milliseconds were applied, followed by 2 mA current of 300, 400, and 500 milliseconds. If CSD did not occur, additional stimuli of 3 mA, 400 milliseconds, and 4 mA, 400, 500, 1000 milliseconds were applied. The logarithmic stepwise escalating stimulation protocol was chosen for its ability to distinguish group differences in CSD threshold based on our prior experience (Ayata et al, 2006). As previously observed, electrical stimulation threshold for CSD showed more variability compared with the frequency of CSDs evoked by topical KCl, which is often attributed to variations in the stimulus current-density geometry between the electrode and the cortex. To achieve sufficient statistical power with higher variability, slightly higher number of rats was studied to determine electrical threshold compared with topical KCl-induced CSDs.
In addition, we calculated CSD propagation speed by dividing the distance (millimeters) between the two recording electrodes by the CSD latency (minutes) between these sites. The CSD amplitude and duration at half-maximal amplitude were also measured.
Statistical Analysis
The systemic and electrophysiological data and the number of CSDs after topical KCl were compared using one-way analysis of variance followed by Dunnett's multiple comparisons versus control. Electrical stimulation threshold was analyzed using Kruskal–Wallis one-way analysis of variance on ranks, followed by Dunn's multiple comparisons versus control. Data were expressed as mean±s.d. for systemic physiology, electrophysiology, and number of KCl-induced CSDs, or as median (25% to 75% range) for electrical CSD threshold.
Results
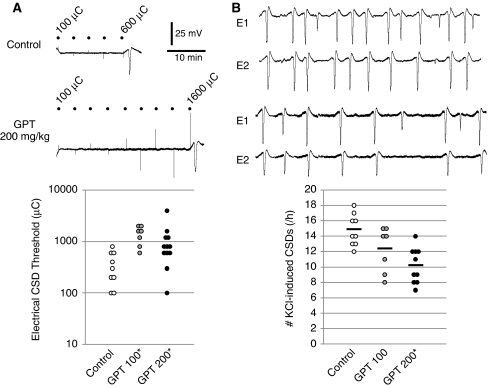
A single intravenous dose of gabapentin (100 or 200 mg/kg) 1 hour before testing elevated the cathodal stimulation threshold for CSD (Figure 1A; Table 1), and dose dependently reduced the frequency of CSDs evoked by topical KCl application by up to 30% compared with saline-injected rats (Figure 1B). Gabapentin did not significantly alter the electrophysiological properties of individual CSDs, including the propagation speed and the incidence of propagation failure between the proximal and distal recording sites (Table 1).
Figure 1.
Effects of gabapentin on cortical spreading depression (CSD) susceptibility assessed by electrical stimulation (A) or topical KCl application (B). (A) Representative tracings showing direct cortical stimulation using cathodal square pulses (dots) of escalating intensities and alternating polarity until a CSD is triggered. Gabapentin significantly elevated the electrical threshold for CSD induction. The complete dataset is shown in the lower panel, where each data point represents one animal. Please note the logarithmic vertical axis scale. See Table 1 for median and interquartile ranges for electrical CSD thresholds. *P<0.05 versus saline. (B) Representative extracellular microelectrode recordings of repetitive CSDs triggered during 1 hour topical KCl application (1 mol/L). E1 and E2 are the proximal and distal recording electrodes, placed in line with the KCl application window, 2 and 4 mm away, respectively. Gabapentin decreased the number of CSDs triggered by KCl. Fewer CSDs were usually detected by the distal electrode than the proximal one, suggesting propagation failure, which did not significantly differ among treatment groups (Table 1). Group means are shown as horizontal lines. Scale bars apply to both A and B. *P<0.05 versus saline. GPT, gabapentin.
Discussion
These data show a novel inhibitory effect of gabapentin on CSD susceptibility using two independent but complementary experimental paradigms. Importantly, CSD suppression appeared within 1 hour after a single intravenous dose, suggesting that gabapentin may suppress propagating waves of injury depolarizations akin to CSD in stroke, subarachnoid or intracerebral hemorrhage, and traumatic brain injury patients in the acute neurocritical care setting. Injury depolarizations worsen tissue outcome presumably through hemodynamic and metabolic mechanisms exacerbating the energy supply-demand mismatch (Hashemi et al, 2009; Shin et al, 2006; Strong et al, 2007). Suppression of injury depolarizations using drugs that inhibit CSD has been beneficial in animal models of stroke, and anecdotally in the clinical setting (Sakowitz et al, 2009; Shin et al, 2006). However, drugs that acutely inhibit CSD (e.g., N-methyl--aspartate receptor antagonists) often have neurological side effects that may limit their clinical usefulness. In that respect, gabapentin, widely used as an analgesic, adjunct antiepileptic and migraine prophylactic drug, offers an advantage with its highly favorable safety and tolerability profile. Importantly, however, intravenous administration may be required to rapidly achieve therapeutic plasma levels and CSD suppression.
Gabapentin attenuates both the stimulated presynaptic Ca2+ influx and the neurotransmitter release in synaptosomal, brain slice, and neuromuscular junction preparations (Dooley et al, 2007). Importantly, attenuation of neurotransmitter release appears to be more marked when release is evoked by intense stimulation, such as high [K+]e, rather than the more physiological stimulation paradigms. During CSD, massive elevations in [K+]e lead to large uncontrolled glutamate release, which augments CSD induction and propagation. Hence, gabapentin may suppress glutamate release during CSD more potently than during normal neurotransmission, and by this way reduce CSD susceptibility.
Gabapentin doses used in this study are clinically relevant, because they are well within the 50 to 300 mg/kg range that show efficacy in most models of epilepsy and pain in rats, and only two- to fourfold higher than the usual human doses. The plasma half-life of gabapentin is ∼100 minutes in rats compared with ∼6 hours in humans (Radulovic et al, 1995). Importantly, the inhibitory effect of gabapentin on presynaptic Ca2+ influx and neurotransmitter release becomes manifest rapidly within 30 minutes of drug application (van Hooft et al, 2002). Therapeutically, maximal antiepileptic effect is achieved within 120 minutes after a single intravenous dose (Welty et al, 1993), whereas maximal analgesic effect is attained within 30 to 60 minutes after a single intraperitoneal dose in rodents (Hunter et al, 1997).
Recent data implicated CSD suppression as a final common mechanism shared by five seemingly unrelated migraine prophylactic drugs (Ayata et al, 2006). Here, we provide evidence suggesting that gabapentin may also use this mechanism. Unlike previously tested drugs, however, gabapentin did not require chronic treatment to suppress CSD. Clinical evidence, albeit limited, supports modest efficacy for gabapentin in migraine prophylaxis. A gradual buildup of clinical efficacy has not been reported for gabapentin in migraine, the obvious constraints of trial design and reporting to assess onset of clinical efficacy notwithstanding. Nevertheless, in preliminary studies, we found that a lower dose of gabapentin (50 mg/kg, orally twice a day) for 5 weeks did not achieve CSD suppression, suggesting that chronic treatment does not augment gabapentin efficacy (data not shown).
In summary, gabapentin suppressed CSD susceptibility after a single intravenous dose. This may be a potential mechanism of action of gabapentin in migraine with aura shared by other migraine prophylactic drugs, and suggest that gabapentin may have efficacy in brain injury states where spreading depolarizations worsen tissue outcome (Williams et al, 2006).
The authors declare no conflict of interest.
References
- Ayata C, Jin H, Kudo C, Dalkara T, Moskowitz MA. Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol. 2006;59:652–661. doi: 10.1002/ana.20778. [DOI] [PubMed] [Google Scholar]
- Dooley DJ, Taylor CP, Donevan S, Feltner D. Ca2+ channel alpha2delta ligands: novel modulators of neurotransmission. Trends Pharmacol Sci. 2007;28:75–82. doi: 10.1016/j.tips.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- Hashemi P, Bhatia R, Nakamura H, Dreier JP, Graf R, Strong AJ, Boutelle MG. Persisting depletion of brain glucose following cortical spreading depression, despite apparent hyperaemia: evidence for risk of an adverse effect of Leao's spreading depression. J Cereb Blood Flow Metab. 2009;29:166–175. doi: 10.1038/jcbfm.2008.108. [DOI] [PubMed] [Google Scholar]
- Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc Natl Acad Sci USA. 2008;105:3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JC, Gogas KR, Hedley LR, Jacobson LO, Kassotakis L, Thompson J, Fontana DJ. The effect of novel anti-epileptic drugs in rat experimental models of acute and chronic pain. Eur J Pharmacol. 1997;324:153–160. doi: 10.1016/s0014-2999(97)00070-8. [DOI] [PubMed] [Google Scholar]
- Kunkler PE, Kraig RP. P/Q Ca2+ channel blockade stops spreading depression and related pyramidal neuronal Ca2+ rise in hippocampal organ culture. Hippocampus. 2004;14:356–367. doi: 10.1002/hipo.10181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic LL, Turck D, von Hodenberg A, Vollmer KO, McNally WP, DeHart PD, Hanson BJ, Bockbrader HN, Chang T. Disposition of gabapentin (neurontin) in mice, rats, dogs, and monkeys. Drug Metab Dispos. 1995;23:441–448. [PubMed] [Google Scholar]
- Richter F, Ebersberger A, Schaible HG. Blockade of voltage-gated calcium channels in rat inhibits repetitive cortical spreading depression. Neurosci Lett. 2002;334:123–126. doi: 10.1016/s0304-3940(02)01120-5. [DOI] [PubMed] [Google Scholar]
- Sakowitz OW, Kiening KL, Krajewski KL, Sarrafzadeh AS, Fabricius M, Strong AJ, Unterberg AW, Dreier JP. Preliminary evidence that ketamine inhibits spreading depolarizations in acute human brain injury. Stroke. 2009;40:e519–e522. doi: 10.1161/STROKEAHA.109.549303. [DOI] [PubMed] [Google Scholar]
- Shin HK, Dunn AK, Jones PB, Boas DA, Moskowitz MA, Ayata C. Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J Cereb Blood Flow Metab. 2006;26:1018–1030. doi: 10.1038/sj.jcbfm.9600252. [DOI] [PubMed] [Google Scholar]
- Stewart BH, Kugler AR, Thompson PR, Bockbrader HN. A saturable transport mechanism in the intestinal absorption of gabapentin is the underlying cause of the lack of proportionality between increasing dose and drug levels in plasma. Pharm Res. 1993;10:276–281. doi: 10.1023/a:1018951214146. [DOI] [PubMed] [Google Scholar]
- Strong AJ, Anderson PJ, Watts HR, Virley DJ, Lloyd A, Irving EA, Nagafuji T, Ninomiya M, Nakamura H, Dunn AK, Graf R. Peri-infarct depolarizations lead to loss of perfusion in ischaemic gyrencephalic cerebral cortex. Brain. 2007;130:995–1008. doi: 10.1093/brain/awl392. [DOI] [PubMed] [Google Scholar]
- van Hooft JA, Dougherty JJ, Endeman D, Nichols RA, Wadman WJ. Gabapentin inhibits presynaptic Ca(2+) influx and synaptic transmission in rat hippocampus and neocortex. Eur J Pharmacol. 2002;449:221–228. doi: 10.1016/s0014-2999(02)02044-7. [DOI] [PubMed] [Google Scholar]
- Welty DF, Schielke GP, Vartanian MG, Taylor CP. Gabapentin anticonvulsant action in rats: disequilibrium with peak drug concentrations in plasma and brain microdialysate. Epilepsy Res. 1993;16:175–181. doi: 10.1016/0920-1211(93)90078-l. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Bautista CC, Chen RW, Dave JR, Lu XC, Tortella FC, Hartings JA. Evaluation of gabapentin and ethosuximide for treatment of acute nonconvulsive seizures following ischemic brain injury in rats. J Pharmacol Exp Ther. 2006;318:947–955. doi: 10.1124/jpet.106.105999. [DOI] [PubMed] [Google Scholar]