Abstract
Cerebral vasospasm determines the prognosis of subarachnoid hemorrhage (SAH). The increased vascular reactiveness has an important role in the development of cerebral vasospasm. This study analyzed the roles of the receptor-mediated signaling and the myofilament Ca2+ sensitivity in the increased vascular reactiveness in SAH, using the basilar artery of a rabbit SAH model. Endothelin-1, thrombin, and phenylephrine induced transient increases in [Ca2+]i, myosin light chain phosphorylation, and contraction in the controls. All these responses were not only enhanced but also became sustained in SAH. In the sequential stimulation of thrombin receptor or α1-adrenoceptor, the second response was substantially attenuated in the controls, whereas it was maintained in SAH. The thrombin-induced contraction in SAH irreversibly persisted even after terminating the thrombin stimulation. This contraction was completely reversed by trypsin and a Gαq inhibitor YM254890, thus suggesting the sustained receptor activity during the sustained contraction. YM254890 also inhibited the endothelin-1- and phenylephrine-induced sustained contraction. Furthermore, the GTPγS-induced transient contraction in the control α-toxin-permeabilized strips was converted to a sustained contraction in SAH. The results provide the first evidence that the feedback inactivation of the receptor activity and the myofilament Ca2+ sensitivity was impaired in SAH, thus contributing to the increased vascular reactiveness.
Keywords: Ca2+ sensitivity, cerebral vasospasm, receptors, smooth muscle, subarachnoid hemorrhage
Introduction
Cerebral vasospasm after aneurysmal subarachnoid hemorrhage (SAH) is characterized by the delayed and prolonged contraction of cerebral arteries, which may cause cerebral ischemia, thereby leading to death or neurological deficit in SAH patients (Kassell et al, 1985). Therefore, the prevention and treatment of vasospasm have an important role in the management of SAH patients. The increased vascular reactiveness has a key role in the pathogenesis of the delayed onset of cerebral vasospasm after SAH (Kai et al, 2008). The contractile reactiveness of the cerebral arteries to various putative spasmogens, including endothelin-1 (Ide et al, 1989; Vatter et al, 2007), thrombin (Kai et al, 2007; Maeda et al, 2007), platelet-derived growth factor (Maeda et al, 2009), and 5-HT (Hansen-Schwartz et al, 2003), have been shown to increase in SAH. Consistent with these increased contractile responses, the expression of ETA receptor, proteinase-activated receptor 1 (PAR1), and 5-HT1B is upregulated in SAH (Hansen-Schwartz et al, 2003; Ide et al, 1989; Kai et al, 2007; Maeda et al, 2007; Vatter et al, 2007). The receptor upregulation therefore has a very important role in the increased vascular reactiveness in SAH. However, this can explain the increased responses of only certain spasmogens that exert an effect on the upregulated receptors. Conversely, the changes in the expression of intracellular signaling proteins, such as RhoA and protein kinase C, have also been shown in SAH, and this could explain the mechanism for the increased responses to various spasmogens (Laher and Zhang, 2001; Miyagi et al, 2000; Sato et al, 2000). However, the changes in the regulatory mechanisms of smooth muscle contraction remain controversial (Maeda et al, 2003; Miyagi et al, 2000; Sato et al, 2000). Therefore, the mechanism underlying the increased vascular reactiveness in SAH still needs to be elucidated.
In many cases, the contractile responses to agonists show diminished responsiveness during the persisted or repeated stimulations. These phenomena are referred to as desensitization or tachyphylaxis, respectively, which represent an important physiological ‘feedback' mechanism that protects against both acute and chronic receptor overstimulation (Ferguson, 2001; Lurie et al, 1985; Miasiro and Paiva, 1990; Trejo, 2003). This feedback regulation could be attributable to not only the inactivation of receptors, but also downregulation of signaling function at the post-receptor level (Ferguson, 2001; Gong et al, 1997). Importantly, the mechanism of receptor desensitization is impaired under various pathological conditions, such as hypoxia, cancer, and diabetes (Booden et al, 2004; Endo et al, 2005; Hinton et al, 2007). The impairment of feedback regulation could thus have an important role in cerebral vasospasm in SAH. White and Robertson (1987) proposed that the desensitization and tachyphylaxis of vascular reactiveness might help to prevent the development of cerebral vasospasm after SAH. However, whether and how such feedback regulation of the vascular reactiveness is altered in SAH still remains to be investigated.
The present study used a rabbit SAH model to examine the changes in the contractile responses of the isolated basilar artery to endothelin-1, thrombin, and phenylephrine, as representatives of putative spasmogens. The analysis was focused on the changes in the smooth muscle reactiveness; therefore, it was conducted in the absence of endothelium. This study evaluated not only the extent of the contractile responses, but also the temporal correlation among cytosolic Ca2+ concentrations ([Ca2+]i), myosin light chain (MLC) phosphorylation, and tension development during the prolonged stimulation as well as the tachyphylaxis of contractile responses. The level of expression of receptors was also evaluated with an immunoblot analysis. Furthermore, any changes in the myofilament Ca2+ sensitivity were evaluated using α-toxin-permeabilized preparations. The result shows, for the first time, that the impairment of negative feedback regulation of receptor signaling and myofilament Ca2+ sensitivity has an important role in the increased vascular reactiveness after SAH.
Materials and methods
Preparation of the Rabbit Subarachnoid Hemorrhage Model
This study protocol was approved by the Animal Care and Use Committee, Kyushu University. Adult male Japanese white rabbits (2.5 to 3.0 kg) were anesthetized with an intramuscular injection of ketamine (40 mg/kg weight) and an intravenous injection of sodium pentobarbital (20 mg/kg weight). On day 0, 0.5 mL of cerebrospinal fluid was aspirated percutaneously from the cisterna magna with use of a 23-gauge butterfly needle, and then 2.5 mL of autologous arterial blood obtained from the middle branch of the ear artery was injected. The animal was then kept in a prone position with the head tilting down at 30° for 30 mins. On day 2, a second injection of autologous blood was similarly performed. The control animals received injections of the same volume of normal physiological salt solution (PSS) instead of the autologous blood.
Preparation of Intact Ring of Basilar Artery
On day 7, the rabbits were heparinized (1,000 U) and then killed by intravenous injection of an overdose of sodium pentobarbital (120 mg/kg weight) and exsanguinated from the common carotid artery. Exposing the brain revealed that the clot formation was observed over the surface of the pons and the basilar artery in SAH. Immediately after the whole brain was excised en bloc and the clot was removed, the narrowing of the basilar artery in SAH was observed under a binocular microscope. The external diameter of the basilar artery was 0.58±0.01 mm in the controls (n=5) and 0.35±0.03 mm in SAH (n=5). The basilar artery of SAH was thus significantly (P<0.001) narrowed to 60.5%±5.1% of the controls.
The basilar artery was then immediately excided and cut into ring preparations measuring 500 μm wide. To remove the endothelium, the internal surface of arteries was rubbed with hair. The removal of endothelium was confirmed by the loss of the relaxant response to acetylcholine. The ring preparations were kept in normal PSS at room temperature until use. These preparations were referred to as ‘intact' preparations, regardless of the presence or absence of endothelium, in contrast to the ‘permeabilized' preparations, which will be described below.
Simultaneous Measurement of Changes in the Cytosolic Ca2+ Concentrations and Developed Tension in Intact Ring Preparations of the Basilar Artery
The arterial rings were loaded with the Ca2+ indicator dye, fura-2, by incubation in Dulbecco's modified Eagle's medium containing 25 μmol/L fura-2 acetoxymethyl ester and 5% (v/v) fetal bovine serum for 90 mins at 37°C. The fura-2-loaded rings were washed with normal PSS to remove the dye in the extracellular space, and were then mounted horizontally between two tungsten wires in an organ bath containing 2 mL buffer. One of the wires was connected to the force transducer U gauge (Minebea, Nagano, Japan), whereas the other was fixed. The preparations were equilibrated in normal PSS at 37°C for at least 60 mins before starting the experimental protocol. During the 60-min equilibration period, the rings were stimulated with 118 mmol/L K+ every 15 mins, and the resting load was increased in a stepwise manner. The resting load was finally adjusted to 50 mg, which was the minimal load to give the maximal tension development in response to 118 mmol/L K+. The measurement was then performed at 37°C in PSS aerated with 95% O2 and 5% CO2. The changes in the fura-2 fluorescence intensities obtained with 340 nm (F340) and 380 nm (F380) excitation and their ratio (F340/F380) were monitored using a CAM-230 fluorometer (JASCO, Tokyo, Japan). The data were expressed as a percentage, assigning the values of fluorescence ratio and tension obtained in normal PSS and 118 mmol/L K+ PSS as 0% and 100%, respectively, unless otherwise specified. Loading of fura-2 was confirmed to have no effect on the contractility of ring preparations, because the degree of tension obtained with 118 mmol/L K+ in the fura-2-loaded rings (333.7±30.4 mg; n=6) did not differ significantly from that obtained without fura-2 loading (316.2±55.9 mg; n=5). As specified in the figure legends, tension development was therefore measured without fura-2 loading in some cases. The pH value of the tissues bathed in PSS, in both the presence and absence of agonists, and the contractile response to high K+ depolarization remained unchanged after a 2-h observation period (data not shown), thus suggesting that there was no significant loss in the integrity of the arterial rings.
Tension Measurement in the α -Toxin-Permeabilized Preparations of Basilar Artery
The arterial rings were permeabilized with 5,000 units/mL staphylococcal α-toxin in cytosolic substitution solution (CSS) for 30 mins at 25°C, as previously described (Maeda et al, 2007). The rings were then treated with 10 μmol/L A23187, a Ca2+ ionophore, in Ca2+-free CSS for 15 mins to deplete the intracellular Ca2+ stores. These permeabilized preparations were then mounted between two tungsten wires, as described above, and then were stretched to 1.5-fold their resting length. After obtaining complete relaxation in Ca2+-free CSS, the experimental protocols was started and the tension development was recorded at 25°C.
Analysis of the Myosin Light Chain (MLC) Phosphorylation with Phos-Tag Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
The phosphorylation of MLC was analyzed using a new method based on a Phos-tag technology (Takeya et al, 2008). Phos-tag is a compound that specifically binds to a phosphate group. Therefore, SDS-PAGE containing polyacrylamide-bound Mn2+ Phos-tag (Phos-tag SDS-PAGE) causes a mobility shift in protein, depending on the degree of phosphorylation (Takeya et al, 2008). The samples for analysis were obtained during the measurement of tension in the intact preparations. In brief, at the indicated time points, the bathing buffer was promptly changed to 90% (v/v) acetone, 10% (w/v) trichloroacetic acid, and 10 mmol/L dithiothreitol (DTT) prechilled at −80°C to stop the reaction. The specimens were then transferred to microcentrifuge tubes, and were then extensively washed and stored in acetone containing 10 mmol/L DTT. After the specimens were air-dried to remove acetone, the cellular protein was extracted in the sample buffer (50 mmol/L Tris-hydroxymethyl aminomethane, 2% (w/v) SDS, 5% (v/v) glycerol, 0.01% (w/v) NaN3, 0.01% (w/v) bromophenol blue, 5% (v/v) β-mercaptoethanol, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μmol/ml 4-aminidophenylmethane sulphonyl fluoride, and 5 μmol/L microcystin). The supernatant was heated to 100°C for 5 mins before electrophoresis. Electrophoresis was performed in 0.1% (w/v) SDS, 25 mmol/L Tris-hydroxymethyl aminomethane, and 192 mmol/L glycine at 12 mA constant current/8 cm × 5 cm × 0.75 mm gel for 100 mins. After electrophoresis, the gel was soaked in transfer buffer (25 mmol/L Tris, 192 mmol/L glycine, and 10% (v/v) methanol) containing 2 mmol/L EDTA to remove Mn2+ for 30 mins, and then in transfer buffer without EDTA for 15 mins. Proteins were then transferred to polyvinylidene difluoride membrane (0.2 μm pore size; Bio-Rad, Hercules, CA, USA) in transfer buffer for 2 h at room temperature. The membranes were then washed in phosphate-buffered saline (PBS; 136.9 mmol/L NaCl, 2.7 mmol/L KCl, 8.1 mmol/L Na2HPO4, and 1.47 mmol/L KH2PO4) for 5 mins, and treated with 0.5% (w/v) formaldehyde in PBS for 45 mins (Takeya et al, 2008). After a brief wash in PBS containing 0.1% Tween-20 (T-PBS), the membrane was blocked with 5% (w/v) skimmed milk in T-PBS overnight at 4°C. In the immunoblot detection, all forms of 20 kDa MLCs were detected using a rabbit polyclonal anti-MLC antibody (sc-15370; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (Sigma, St Louis, MO, USA). Primary and secondary antibodies were diluted at 500-fold and 1,000-fold, respectively, in immunoreaction enhancer solution (Can Get Signal; Toyobo, Osaka, Japan). The immune complex was detected using enhanced chemiluminescence technique (ECL plus kit; Amersham, Buckinghamshire, UK). The light emission was detected and analyzed with ChemiDoc XRS-J and the computer program Quantity One (Bio-Rad). The percentage of the phosphorylated forms in total MLCs (sum of unphosphorylated and phosphorylated forms) was calculated to indicate the extent of MLC phosphorylation. The extract from one ring preparation was sufficient to permit the quantification of MLC phosphorylation.
Immunoblot Analysis of the Expression of ETA Receptor, Proteinase-Activated Receptor 1 (PAR1), and α1-Adrenoceptor
The isolated basilar arteries were homogenized in 50 mmol/L HEPES, pH 7.4, 150 mmol/L NaCl, 0.5% (v/v) Nonidet P-40, 1 mmol/L EDTA, 1 mmol/L DTT, 0.5 mmol/L Na3VO4, 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 10 μmol/L 4-aminidophenylmethane sulphonyl fluoride. A total of 5 μg of protein was separated by SDS-PAGE and was then transferred to the polyvinylidene difluoride membrane (Bio-Rad). The membranes were blocked with 5% (w/v) skimmed milk in T-PBS overnight at 4°C. Next, the membrane was incubated for 1 h at room temperature with anti-ETA receptor antibody (sc-33535; Santa Cruz Biotechnology), anti-PAR1 antibody (sc-5605; Santa Cruz Biotechnology), or anti-α1-adrenoceptor antibody (sc-28982; Santa Cruz Biotechnology) diluted 200-fold in Can Get Signal (Toyobo), followed by 1-h incubation with the secondary antibody conjugated with horseradish peroxidase (1,000-fold dilution). The immune complex was detected using an ECL plus kit. The detection and analysis of the chemiluminescence signals were as noted above. After chemiluminescence detection, the membranes were stained with naphthol blue black to visualize the band corresponding to actin. The optical density of the each receptor band was normalized to that of actin.
Drugs and Solution
The composition of normal PSS was 123 mmol/L NaCl, 4.7 mmol/L KCl, 1.25 mmol/L CaCl2, 1.2 mmol/L MgCl2, 1.2 mmol/L KH2PO4, 15.5 mmol/L NaHCO3, and 11.5 mmol/L -glucose. The PSS was aerated with a mixture of 95% O2 and 5% CO2, with the resulting pH being 7.4. High K+ PSS was prepared by replacing NaCl with equimolar KCl. The composition of Ca2+-free CSS was 100 mmol/L potassium methanesulphonate, 2.2 mmol/L Na2ATP, 3.38 mmol/L MgCl2, 10 mmol/L EGTA, 10 mmol/L creatine phosphate, and 20 mmol/L Tris-malate (pH 6.8). The CSS containing the indicated concentration of free Ca2+ was prepared by adding an appropriate amount of CaCl2, while assuming the Ca2+-EGTA binding constant to be 106 (L/mol) (Maeda et al, 2007). Thrombin (bovine plasma; 1,178 NIH units/mg protein), Staphylococcus aureus α-toxin, GTPγS, phorbol 12,13-dibutyrate (PDBu), phenylephrine, and 4-amidinophenylmethanesulfonyl fluoride were purchased from Sigma. Endothelin-1 was obtained from Peptide Institute (Osaka, Japan). TFLLR-NH2 (PAR1 activating peptide) was obtained from Bachem (Budendorf, Switzerland). Trypsin was purchased from Difco Laboratories (Detroit, MI, USA). Fura-2 acetoxymethyl ester was purchased from Dojindo Laboratories (Kumamoto, Japan). Rabbit polyclonal anti-phospho-MLC (Ser19; no. 3671) and phospho-MLC (Thr18/Ser19; no. 3674) antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). YM254890 was a kind gift from Astellas Pharma (Tokyo, Japan).
Data Analysis
The data are expressed as the mean±s.e.m. of the indicated experimental number. One basilar arterial preparation obtained from one animal was used for each experiment, and therefore the number of experiments (n value) indicates the number of animals. Unpaired Student's t-test was used to determine statistical differences between the two groups. A value of P<0.05 was considered to be statistically significant.
Results
Enhanced Contractile Response of the Rabbit Basilar Artery to Endothelin-1, Thrombin and Phenylephrine after Subarachnoid Hemorrhage (SAH)
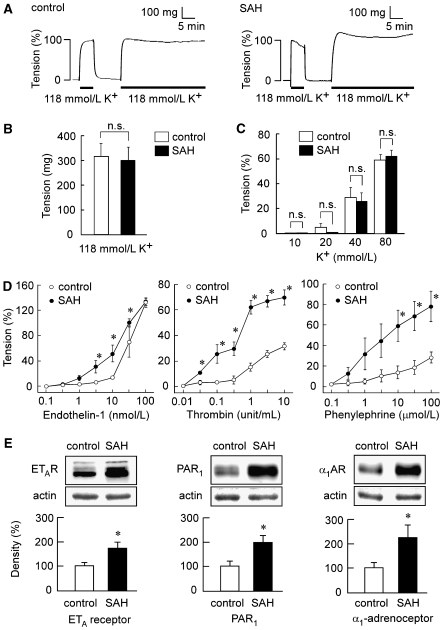
Depolarization with 118 mmol/L K+ induced a sustained increase in tension in the rabbit basilar artery in both the controls and SAH (Figure 1A). The absolute values of the tension induced by 118 mmol/L K+ in SAH did not significantly differ from that observed in the controls (Figure 1B). Therefore, the contractile response to 118 mmol/L K+ was recorded as a reference response at the beginning of each measurement and thus assigned to be 100%. The level of tension obtained with different concentrations of K+ did not significantly differ between the controls and SAH at any concentrations (Figure 1C).
Figure 1.
Contractile responses of the rabbit basilar artery to high K+ depolarization, endothelin-1, thrombin, and phenylephrine and the expression of ETA receptor, proteinase-activated receptor 1 (PAR1), and α1-adrenoceptor in the controls and subarachnoid hemorrhage (SAH). (A) Representative recordings showing the 118 mmol/L K+-induced contractions in the fura-2-unloaded basilar artery of controls and SAH. (B) The level of tension induced by 118 mmol/L K+ depolarization in the controls and SAH, as expressed in absolute value (n=5). (C) Concentration-dependent effect of high K+ depolarization on the tension development in the controls and SAH (n=5). (D) The concentration-response curves of the contraction induced by endotheliln-1, thrombin, and phenylephrine in the basilar artery of the controls and SAH (n=5 to 7). (E) Immunoblot analysis of the expression of ETA receptor, PAR1, and α1-adrenoceptor in the basilar artery of the controls and SAH (n=4 to 5). The level of tension obtained in normal physiological salt solution (PSS) and 118 mmol/L K+ PSS were assigned values of 0% and 100%, respectively. The level of expression observed in the controls was assigned to be 100%. The data represent the mean±s.e.m. n.s., not significantly different; *P<0.05 versus controls.
The evaluation of the concentration-dependent responses to endothelin-1 revealed significant enhancement of contractile responses at 3, 10, and 30 nmol/L in SAH compared with those observed in the controls, whereas the contraction obtained with 100 nmol/L endothelin-1 in SAH was similar to that observed in the controls (Figure 1D). The contractile response to thrombin and phenylephrine was significantly enhanced after SAH at concentrations higher than 0.01 unit/mL and 3 μmol/L, respectively (Figure 1D). An immunoblot analysis of the expression of ETA receptor, PAR1, and α1-adrenoceptor showed the level of expression of all receptors examined to significantly increase in SAH (Figure 1E).
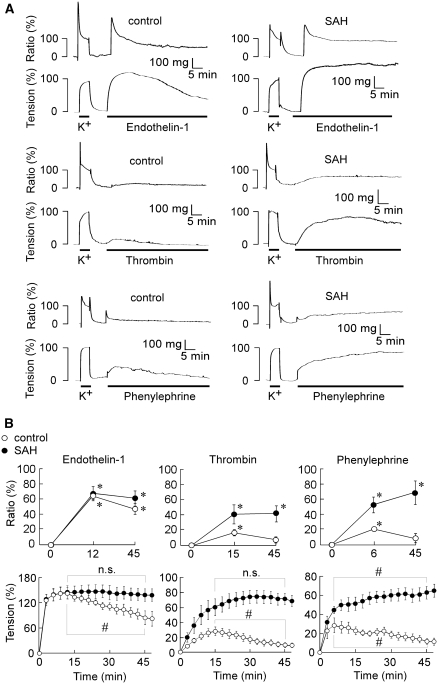
In the controls, 100 nmol/L endothelin-1 induced a contraction, which reached the peak (143.6%±13.8% of the 118 mmol/L K+-induced contraction, n=5) at 12 mins, and thereafter gradually and significantly declined, reaching the level of 85.0%±16.3% (n=5) at 45 mins (Figures 2A and 2B). In SAH, endothelin-1 induced a contraction, which reached a similar peak level with a similar time course as that observed in the controls (Figures 2A and 2B). However, there was no significant decline in the level of tension even at 45 mins (Figures 2A and 2B). Thrombin (1 unit/mL) induced a significant, but small, contraction, which reached a peak at 15 mins and thereafter declined to the significantly lower level at 45 mins in the controls (Figures 2A and 2B). In SAH, thrombin induced not only a greater tension development at 15 mins than that observed in the controls, but also a sustained contraction without a significant decline at 45 mins (Figures 2A and 2B). Phenylephrine (10 μmol/L) induced a transient contraction with a peak at 6 mins in the controls, whereas it induced not only an enhanced, but also a sustained contraction in SAH (Figures 2A and 2B). The time course of the changes in [Ca2+]i correlated with that observed with tension for all agonists (Figures 2A and 2B). Endothelin-1 increased [Ca2+]i to a similar peak level in both controls and SAH. [Ca2+]i thereafter slightly declined in the controls, whereas it remained elevated in SAH. Both thrombin and phenylephrine induced a small transient elevation of [Ca2+]i in the controls, whereas they induced an enhanced and sustained elevation in SAH.
Figure 2.
The time course of the change in [Ca2+]i and tension induced by endothelin-1, thrombin, and phenylephrine in the rabbit basilar artery of the controls and subarachnoid hemorrhage (SAH). Representative recordings (A) and summaries of the time course (B) of [Ca2+]i and tension induced by 100 nmol/L endothelin-1, 1 unit/mL thrombin, and 10 μmol/L phenylephrine in the fura-2-loaded basilar artery of the controls and SAH. In (B), the time course of [Ca2+]i was evaluated at three time points: just before the stimulation (0 min), at the peak of the tension development observed with each agonist (12, 15, or 6 min), and 45 min after the stimulation. The data represent the mean±s.e.m. (n=5 to 7). The levels of [Ca2+]i and tension obtained at rest and 5 mins after initiating a contraction by 118 mmol/L K+-physiological salt solution (PSS) were assigned values of 0% and 100%, respectively. *P<0.05 versus resting level (0 min); #P<0.05; n.s., not significantly different.
The Phosphorylation of myosin light chains (MLCs) During the Contraction Induced by Endothelin-1, Thrombin, and Phenylephrine in the Rabbit Basilar Artery
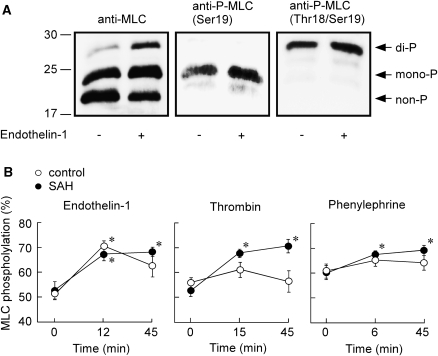
Phos-tag SDS-PAGE followed by immunoblot detection with anti-MLC antibody yielded three bands with an apparent molecular size of 20, 23, and 29 kDa in the extract of the basilar arteries obtained before and after the stimulation with endothelin-1 (Figure 3A). The upper and middle bands were also detected by anti-phospho-MLC (Thr18/Ser19) and phospho-MLC (Ser19) antibodies, respectively (Figure 3A). Conversely, the conventional SDS-PAGE without Phos-tag yielded only a single immunoreactive band with anti-MLC antibody (data not shown). These observations thus indicated the upper, middle, and lower bands to represent di-, mono-, and non-phosphorylated forms of MLC, respectively (Figure 3A).
Figure 3.
Change in myosin light chain (MLC) phosphorylation induced by endothelin-1, thrombin, and phenylephrine in the rabbit basilar artery of the controls and subarachnoid hemorrhage (SAH). (A) Representative immunoblots obtained with anti-MLC, anti-phospho-MLC (Ser19), and anti-phospho-MLC (Thr18/Ser19) antibodies. The samples were obtained before and 12 mins after the stimulation with 100 nmol/L endothelin-1. The upper, middle, and lower bands detected with anti-MLC antibody represent di-, mono-, and non-phosphorylated forms of MLC. (B) Summary of the MLC phosphorylation (percentage of total MLC) induced by 100 nmol/L endothelin-1, 1 unit/mL thrombin, and 10 μmol/L phenylephrine, as evaluated at three time points: just before the stimulation (0 min), at the peak of contraction (12, 15, or 6 min), and 45 mins after the stimulation. The data represent the mean±s.e.m. (n=5). *P<0.05 versus 0 min.
The control basilar arteries contained 39.5%±1.0% and 17.6%±1.5% of mono- and di-phosphorylated forms of MLC before the contractile stimulation (n=15), whereas those of SAH contained 44.7%±2.5% and 15.1%±1.6% (n=15), respectively. There was no significant difference in the resting level of MLC phosphorylation (the sum of di- and mono-phosphorylation) between the controls and SAH. Endothelin-1 (100 nmol/L) significantly increased the MLC phosphorylation to a similar level in the controls and SAH, at the peak of the tension development (12 mins; Figure 3B). The level of MLC phosphorylation thereafter declined in the controls, whereas it remained sustained in SAH (Figure 3B). Thrombin (1 unit/mL) and phenylephrine (10 μmol/L) induced no significant increase in the MLC phosphorylation at any time in the controls, whereas they induced a significant and sustained increase in the MLC phosphorylation in SAH (Figure 3B). As a result, the changes in the agonist-induced MLC phosphorylation correlated with the changes in [Ca2+]i and tension in both the controls and SAH.
As a result, the conversion of a transient response to a sustained response in SAH was common to all agonists examined. Notably, this conversion was observed with all parameters: [Ca2+]i, MLC phosphorylation, and tension.
Changes in the Intracellular Mechanisms of Smooth Muscle Contraction in the Rabbit Basilar Artery After Subarachnoid Hemorrhage (SAH)
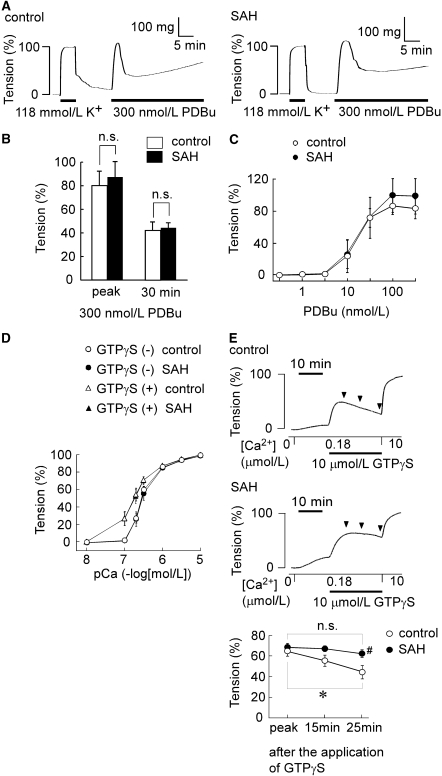
To elucidate the mechanism for enhanced and sustained contractile responses in SAH, any changes in the intracellular mechanisms involved in smooth muscle contraction were evaluated. For this purpose, the contractile response to the direct activation of protein kinase C by PDBu was first examined in intact preparations. In both controls and SAH, 300 nmol/L PDBu induced a precipitous initial contraction, followed by a steady sustained contraction (Figure 4A). There was no significant difference in the level of tension development between the controls and SAH at the initial peak and 30 mins after the initiation of the contraction (Figure 4B). The concentration-dependent effects of PDBu also did not significantly differ between the controls and SAH (Figure 4C).
Figure 4.
Contractile responses of the intact artery to phorbol 12,13-dibutyrate (PDBu) and the α-toxin-permeabilized artery to GTPγS in the controls and subarachnoid hemorrhage (SAH). (A–C) The contractile responses to PDBu in the intact basilar artery of the controls and SAH. (A) Representative recordings showing the tension development induced by 300 nmol/L PDBu in the fura-2-unloaded intact basilar artery of the controls and SAH. (B) The levels of tension obtained at the peak and 30 mins after the stimulation and (C) the concentration-response curves for the PDBu-induced tension development in the controls and SAH. The levels of tension obtained at the rest and 5 mins after initiating a contraction by 118 mmol/L K+-physiological salt solution (PSS) were assigned values of 0% and 100%, respectively, in the intact artery. (D, E) The contractile responses to GTPγS in the α-toxin-permeabilized artery of the controls and SAH. (D) The pCa2+-tension curves of the contraction induced by stepwise increment of Ca2+ concentrations in the absence and presence of 10 μmol/L GTPγS, and in the controls and SAH. (E) Representative recordings and summary of the contractions induced by 10 μmol/L GTPγS during the 180 nmol/L Ca2+-induced contractions in the controls and SAH. The level of tension was evaluated at the peak, and at 15 mins and 25 mins after initiating the contraction by GTPγS, as indicated by arrowheads in the traces. The response to 10 μmol/L Ca2+ was recorded as a reference response at the end of each experiment, and this level of tension was assigned a value of 100%, whereas that obtained in Ca2+-free cytosolic substitution solution (CSS) was assigned values of 0% in the permeabilized artery. The data represent the mean±s.e.m. (n=5). n.s., not significantly different; *P<0.05; #P<0.05 versus controls.
Next, any alteration in the Ca2+-dependent contractile mechanism or the myofilament Ca2+ sensitivity after SAH was analyzed by using α-toxin-permeabilized preparations. In this experiment, GTPγS, a nonhydrolyzable GTP analog, which is known to directly activate G proteins by skipping the receptor-mediated activation (Cockcroft and Gomperts, 1985; Gilman, 1984), was used to increase the Ca2+ sensitivity. A stepwise increase in Ca2+ concentrations induced a similar stepwise development of tension in the controls and SAH (Figure 4D). In the presence of 10 μmol/L GTPγS, the pCa2+ tension curves shifted to the left to a similar extent in both the controls and SAH (Figure 4D). During the 180 nmol/L Ca2+-induced sustained contraction, 10 μmol/L GTPγS induced a further development of tension in both the controls and SAH (Figure 4E). The level of peak contraction observed in SAH was similar to that observed in the controls (Figure 4E). In the controls, the tension thereafter gradually decreased and the level observed at 25 mins was significantly lower than that observed at the peak (Figure 4E). In contrast, there was no significant decline in the level of tension in SAH (Figure 4E). As a result, the transient contractile response to GTPγS in the controls was converted to the sustained response in SAH.
Impairment of the Tachyphylaxis of Agonist-Induced Contractile Responses in the Rabbit Basilar Artery After Subarachnoid Hemorrhage (SAH)
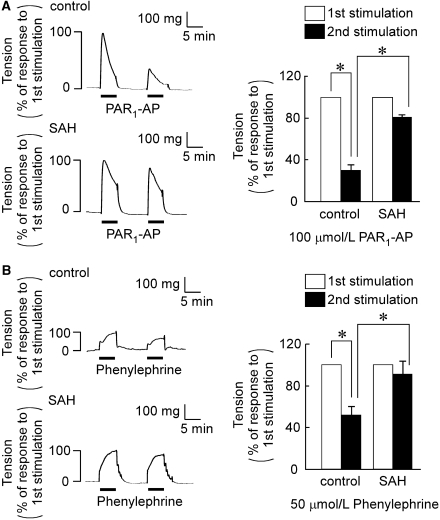
To further elucidate the mechanism of the enhanced and sustained contraction after SAH, the tachyphylaxis of the agonist-induced contractile responses was analyzed by examining the reduction of the second response during the consecutive stimulation of the arteries with the same agonist. For this purpose, 100 μmol/L PAR1-AP and 50 μmol/L phenylephrine was used to induce a significant contraction in the controls. PAR1-AP induced little contraction at 10 μmol/L, whereas it induced an enhanced contraction in SAH (data not shown). However, the contraction induced by 100 μmol/L PAR1-AP in the controls (331±25 mg, n=5) was similar to that observed in SAH (304±37 mg, n=5; Figure 5A). Conversely, the contraction induced by 50 μmol/L phenylephrine in the controls (108.8±11.5 mg, n=5) was significantly enhanced in SAH (183.4±21.8 mg, n=5; Figure 5B). After recording the first response to 100 μmol/L PAR1-AP or 50 μmol/L phenylephrine for 5 mins, the rings were equilibrated in PSS without stimulation for 10 mins, and then they were challenged to the second stimulation with same agonists (Figure 5). In the controls, the second response to PAR1-AP and phenylephrine was significantly reduced to 29.9%±5.1% (n=5) and 51.7%±7.2% (n=5) of the first response, respectively (Figure 5). This reduction of the second response was significantly and substantially attenuated in SAH (Figure 5).
Figure 5.
Contractile response of basilar artery to the consecutive stimulation with PAR1-AP (proteinase-activated receptor 1-activating peptide) and phenylephrine in the controls and subarachnoid hemorrhage (SAH). (A, B) Representative recordings and summary showing the contractile responses to the consecutive stimulation with 100 μmol/L PAR1-AP (A) and 50 μmol/L phenylephrine (B) in the fura-2-unloaded ring preparations. The rings were consecutively stimulated with PAR1-AP or phenylephrine for 5 mins with a 10-min interval of incubation without contractile stimulation. The response to the second stimulation was evaluated by assigning the response to the first stimulation to be 100%. The data represent the mean±s.e.m. (n=5). n.s., not significantly different; *P<0.05.
The Effect of Trypsin on the Thrombin-Induced Sustained Contraction in the Rabbit Basilar Artery After Subarachnoid Hemorrhage (SAH)
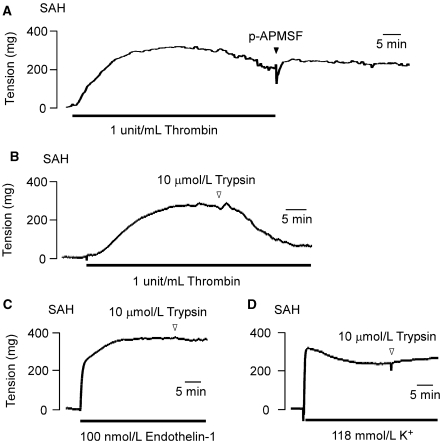
Thrombin activates its receptor by proteolytically cleaving the extracellular domain of PAR1 at the residues 41 to 42, thereby unveiling a new N-terminus that then exerts an effect as a tethered ligand to activate the receptor. Therefore, the thrombin activation of PAR1 is an irreversible process (Macfarlane et al, 2001). The mechanism of feedback regulation helps to terminate its signaling activity (Hollenberg and Compton, 2002; Macfarlane et al, 2001). Otherwise, PAR1 signaling would persist. In SAH, the thrombin-induced contraction persisted even after removing thrombin and adding 10 μmol/L 4-amidinophenylmethanesulfonyl fluoride, a protease inhibitor (Figure 6A). The contribution of the persisted signaling activity of PAR1 to this irreversible contraction was first analyzed by examining the effect of trypsin on the sustained phase of the contraction. Trypsin has been shown to cleave the extracellular domain of PAR1 at the residues 71 to 72 and/or 82 to 83 (in humans), thereby removing the tethered ligand region (Nakayama et al, 2004). The addition of 10 μmol/L trypsin during the thrombin-induced sustained contraction completely inhibited the contraction to the resting level (Figures 6B). However, trypsin had no effect on the sustained contraction induced by 118 mmol/L K+ or endothelin-1 (Figures 6C and 6D).
Figure 6.
The effects of trypsin on the sustained contractions induced by thrombin in the rabbit basilar artery with subarachnoid hemorrhage (SAH). Representative recordings of at least three independent experiments, showing the effect of the removal of thrombin on the subsequent time course of the thrombin-induced contraction (A) and the effect of 10 μmol/L trypsin on the contraction induced by 1 unit/mL thrombin (B), 100 nmol/L endothelin-1 (C), and 118 mmol/L K+ (D) in the fura-2-unloaded ring preparations of SAH. In panel (A), when thrombin was washed out, 10 μmol/L 4-amidinophenylmethanesulfonyl fluoride was added to the bathing buffer to ensure the complete inhibition of any residual thrombin activity.
The Effect of YM254890 on the Agonist-Induced Contraction in the Rabbit Basilar Artery After Subarachnoid Hemorrhage (SAH)
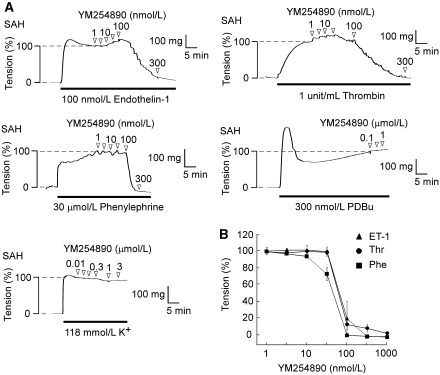
Next, the persistent receptor activity during the sustained contraction was analyzed by examining the effect of YM254890, a selective Gαq inhibitor, on the sustained phase of the contraction. YM254890 inhibited the thrombin-induced sustained contraction, with complete inhibition at 100 nmol/L (Figures 7A and 7B). YM254890 also completely inhibited the contraction induced by 100 nmol/L endothelin-1 and 30 μmol/L phenylephrine at 100 nmol/L (Figures 7A and 7B). Furthermore, the [Ca2+]i elevations induced by these agonists were completely inhibited by 100 nmol/L YM254890 (data not shown). The IC50 values of YM254890 for the inhibition of the contraction induced by thrombin, endothelin-1, and phenylephrine were 69.8, 69.7, and 84.9 nmol/L, respectively. Conversely, YM254890, even at 1 μmol/L, had no significant effect on the level of [Ca2+]i (data not shown) and tension (Figure 7A) during the contraction induced by 300 nmol/L PDBu or 118 mmol/L K+.
Figure 7.
The effects of YM254890 on the contractions induced by thrombin, endothelin-1, phenylephrine, phorbol 12,13-dibutyrate (PDBu), and 118 mmol/L K+ depolarization in the rabbit basilar artery with subarachnoid hemorrhage (SAH). (A) Representative recordings showing the effect of YM254890 on the contraction induced by 100 nmol/L endothelin-1, 1 unit/mL thrombin, 30 μmol/L phenylephrine, 300 nmol/L PDBu, and 118 mmol/L K+ in the fura-2-unloaded ring preparations of SAH. (B) Concentration-dependent effects of YM254890 on the contraction induced by 1 unit/mL thrombin, 100 nmol/L endothelin-1, and 30 μmol/L phenylephrine in SAH. The relaxant effect of YM254890 was evaluated by assigning the level of tension obtained just before the application of YM254890 and that obtained in the normal physiological salt solution (PSS) before initiating the pre-contraction to be 100% and 0%, respectively. The data represent the mean±s.e.m. (n=5).
Discussion
The most noticeable observation of this study is that the contractile response of the isolated basilar artery to three G protein-coupled receptor agonists was not only enhanced but also sustained after SAH. In the controls, the contractions induced by endothelin-1, thrombin, and phenylephrine declined more or less after reaching the peak of contraction despite the continuous presence of the receptor stimulation. In SAH, these contractions sustained with no significant decline. In addition, the experiments of the consecutive stimulations showed that the responsiveness to the second stimulation was substantially attenuated in the control artery, whereas it was maintained in SAH. These observations therefore suggest that some negative feedback regulation of the contractile response was impaired in SAH, thus converting the transient response to the sustained response and also maintaining the responsiveness to the second stimulation. In this respect, it is noteworthy that the conversion of the transient response to the sustained response was observed with not only tension but also with [Ca2+]i and MLC phosphorylation. This observation therefore suggests that the mechanism that was impaired in SAH reside at the upstream of the Ca2+ signal, and presumably at a receptor level. In addition, the GTPγS-induced contraction observed at the fixed Ca2+ concentration in the permeabilized rings also declined in the control artery, whereas it remained sustained in SAH. This observation thus suggests another feedback regulation to reside at a level of regulation of the myofilament Ca2+ sensitivity, which was also impaired in SAH. As a result, this study suggests that the impaired feedback regulation of the receptor activity and the myofilament Ca2+ sensitivity have a critical role in the increased vascular reactiveness in SAH.
The mechanism for cerebral vasospasm can be attributable to either increased production of spasmogens or increased vascular reactiveness (Kai et al, 2008). The increase in the vascular reactiveness may result from either endothelial dysfunction (Sasaki et al, 1985; Sasaki et al, 1986) or an increase in the smooth muscle contractility (Kassell et al, 1985). This study proposes the impairment of the feedback regulation of the contractile response as a novel mechanism contributing to the increased vascular reactiveness in SAH. The receptor upregulation contributes to the increased contractile responses in SAH (Hansen-Schwartz et al, 2003; Itoh et al, 1994; Kai et al, 2007; Maeda et al, 2007; Vatter et al, 2007). However, this could explain the increased responses to certain spasmogens. In contrast, the impaired feedback regulation of the receptor activity seems to affect a broad range of G protein-coupled receptors, thus contributing to the reported increase in the contractile response to various spasmogens (Hansen-Schwartz et al, 2003; Itoh et al, 1994; Kai et al, 2007; Kai et al, 2008; Maeda et al, 2009; Maeda et al, 2007; Vatter et al, 2007).
The impairment of the feedback regulation of the receptor activity in SAH is convincingly suggested for PAR1. The thrombin activation of PAR1 is an irreversible process (Macfarlane et al, 2001). The subsequent receptor phosphorylation, β-arrestin binding, endocytotic internalization, and lysosomal degradation thus help to terminate the signaling activity of PAR1 (Hollenberg and Compton, 2002; Trejo, 2003). Otherwise, PAR1 signaling would persist even after the termination of thrombin stimulation, as previously reported in Sf9 cells or metastatic breast cancer cells (Booden et al, 2004; Chen et al, 1996; Trejo, 2003). The observation that the thrombin-induced contraction irreversibly persisted after terminating the thrombin stimulation therefore suggests the impairment of the receptor inactivation. Furthermore, the observations with trypsin and YM254890 support the persistent signaling activity of PAR1 during the thrombin-induced contraction. The removal of the tethered ligand region of PAR1 by proteinases converts PAR1 to the inactive conformation, thereby terminating the irreversibly persisted signals (Holinstat et al, 2009). Trypsin is one such proteinase and it removes the tethered ligand region of PAR1 (Nakayama et al, 2004; Nakayama et al, 2003). The inhibition of the thrombin-induced sustained contraction by trypsin thus suggests the requirement of the active conformation of PAR1 for the thrombin-induced sustained contraction. Because the contractile responses to endothelin-1 and phenylephrine were also converted from the transient response in the controls to the sustained response in SAH, and YM254890 also inhibited the sustained phase of these contractions, the feedback regulation of ETA receptor and α1-adrenoceptor is also suggested to be impaired after SAH.
The general impairment of the receptor inactivation may be consistent with previous reports showing the enhanced contractile response to various receptor ligands (Hansen-Schwartz et al, 2003; Itoh et al, 1994; Kai et al, 2007; Kai et al, 2008; Maeda et al, 2009; Maeda et al, 2007; Vatter et al, 2007). However, the impaired receptor inactivation exerts a significant effect especially on the activity of ETA receptor and PAR1. The affinity of ETA receptor for endothelin-1 is extremely high, and therefore their binding is practically irreversible under physiological conditions (Endo et al, 2005; Freedman et al, 1997). Conversely, PAR1 activation is an irreversible process. The impaired receptor inactivation therefore has a greater effect on the signaling activity of PAR1 than other receptors. The massive hemorrhage around cerebral arteries is the fundamental pathology of SAH, which causes the extensive production of thrombin. The increased reactiveness of the basilar artery to thrombin is characteristic of SAH. Thrombin and its receptor PAR1 are therefore suggested to have an important role in the development of cerebral vasospasm.
The mechanism for the impairment of the receptor inactivation in SAH still remains to be elucidated, with regard to how SAH causes such impairment and which step of the feedback regulation is impaired. The phosphorylation of receptor by G protein-coupled receptor kinases and the binding of β-arrestin to receptor contribute to the rapid termination of receptor signaling, while the endocytotic internalization and the lysosomal degradation also contribute to the subsequent termination (Drake et al, 2006; Moore et al, 2007). The relative contribution of each step and precise molecular mechanism varies depending on the type of receptor (Abe et al, 2000; Chalothorn et al, 2002; Freedman et al, 1997). Therefore, the step common to ETA receptor, PAR1, and α1-adrenoceptor is considered to be impaired in SAH, and the molecule(s) positively or negatively involved in such a step may be up- or down-regulated in the cerebral artery after SAH. Those molecules still remain to be identified.
The present study also suggests the impairment of the feedback regulation at the step regulating the myofilament Ca2+ sensitivity in SAH. However, the importance of this impairment may vary with the contractile stimulation, depending on the degree of contribution of the myofilament Ca2+ sensitization to the contractile response. The evaluation of the [Ca2+]i–tension relationship based on the observations in Figure 2 suggests that Ca2+ sensitization makes a relatively greater contribution to the endothelin-1-induced contractions than other contractions. Rho kinase and protein kinase C are two major signaling molecules known to contribute to the myofilament Ca2+ sensitization (Hirano, 2007). The activation of protein kinase C by PDBu induced a sustained contraction in cerebral artery. However, this contractile response remained unchanged after SAH, thus ruling out the major contribution of protein kinase C to the feedback regulation of the myofilament Ca2+ sensitivity. Conversely, the activity of Rho kinase is upregulated in SAH (Miyagi et al, 2000; Sato et al, 2000). The increased Rho kinase activity may therefore contribute to the persistent increase in the myofilament Ca2+ sensitivity in SAH, especially in the endothelin-induced contraction.
In SAH, the maximal contractile responses to thrombin and phenylephrine were greatly enhanced, whereas the concentration-response curve of endothlin-1 was shifted to the left. This study showed the upregulation of receptors for all these agonists in SAH. The upregulation of ETA receptor and PAR1 is consistent with the observations of the previous reports (Ide et al, 1989; Itoh et al, 1994; Maeda et al, 2007; Vatter et al, 2007), whereas no study has previously reported the upregulation of α1-adrenoceptor in SAH. The upregulation of PAR1 and α1-adrenoceptor may be consistent with the enhancement of the maximal response. However, the upregulation of ETA receptor was not associated with the enhancement of the maximal response to endothelin-1. The reason for this, however, remains unknown. It may be related to a large population of the spare receptor for ETA receptor or a relatively lower degree of the upregulation in comparison to that observed with PAR1 and α1-adrenoceptor (Figure 1E).
In conclusion, this study provides the first evidence that the feedback regulation of the smooth muscle contraction is impaired in SAH. The impairment of this feedback regulation is suggested to contribute to the persistent receptor signaling and the sustained increase in the myofilament Ca2+ sensitivity. The impaired feedback regulation is thus suggested to contribute to the increased vascular reactiveness, and therefore the pathogenesis of cerebral vasospasm.
Acknowledgments
The authors thank Mr Brain Quinn for linguistic comments and help with the manuscript.
The authors declared no conflict of interest
References
- Abe Y, Nakayama K, Yamanaka A, Sakurai T, Goto K. Subtype-specific trafficking of endothelin receptors. J Biol Chem. 2000;275:8664–8671. doi: 10.1074/jbc.275.12.8664. [DOI] [PubMed] [Google Scholar]
- Booden MA, Eckert LB, Der CJ, Trejo J. Persistent signaling by dysregulated thrombin receptor trafficking promotes breast carcinoma cell invasion. Mol Cell Biol. 2004;24:1990–1999. doi: 10.1128/MCB.24.5.1990-1999.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalothorn D, McCune DF, Edelmann SE, Garcia-Cazarin ML, Tsujimoto G, Piascik MT. Differences in the cellular localization and agonist-mediated internalization properties of the α1-adrenoceptor subtypes. Mol Pharmacol. 2002;61:1008–1016. doi: 10.1124/mol.61.5.1008. [DOI] [PubMed] [Google Scholar]
- Chen X, Earley K, Luo W, Lin SH, Schilling WP. Functional expression of a human thrombin receptor in Sf9 insect cells: evidence for an active tethered ligand. Biochem J. 1996;314:603–611. doi: 10.1042/bj3140603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S, Gomperts BD. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985;314:534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Drake MT, Shenoy SK, Lefkowitz RJ. Trafficking of G protein-coupled receptors. Circ Res. 2006;99:570–582. doi: 10.1161/01.RES.0000242563.47507.ce. [DOI] [PubMed] [Google Scholar]
- Endo K, Matsumoto T, Kobayashi T, Kasuya Y, Kamata K. Diabetes-related changes in contractile responses of stomach fundus to endothelin-1 in streptozotocin-induced diabetic rats. J Smooth Muscle Res. 2005;41:35–47. doi: 10.1540/jsmr.41.35. [DOI] [PubMed] [Google Scholar]
- Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- Freedman NJ, Ament AS, Oppermann M, Stoffel RH, Exum ST, Lefkowitz RJ. Phosphorylation and desensitization of human endothelin A and B receptors. Evidence for G protein-coupled receptor kinase specificity. J Biol Chem. 1997;272:17734–17743. doi: 10.1074/jbc.272.28.17734. [DOI] [PubMed] [Google Scholar]
- Gilman AG. G proteins and dual control of adenylate cyclase. Cell. 1984;36:577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Gong MC, Fujihara H, Walker LA, Somlyo AV, Somlyo AP. Down-regulation of G-protein-mediated Ca2+ sensitization in smooth muscle. Mol Biol Cell. 1997;8:279–286. doi: 10.1091/mbc.8.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen-Schwartz J, Hoel NL, Xu CB, Svendgaard NA, Edvinsson L. Subarachnoid hemorrhage-induced upregulation of the 5-HT1B receptor in cerebral arteries in rats. J Neurosurg. 2003;99:115–120. doi: 10.3171/jns.2003.99.1.0115. [DOI] [PubMed] [Google Scholar]
- Hinton M, Gutsol A, Dakshinamurti S. Thromboxane hypersensitivity in hypoxic pulmonary artery myocytes: altered TP receptor localization and kinetics. Am J Physiol Lung Cell Mol Physiol. 2007;292:L654–L663. doi: 10.1152/ajplung.00229.2006. [DOI] [PubMed] [Google Scholar]
- Hirano K. Current topics in the regulatory mechanism underlying the Ca2+ sensitization of the contractile apparatus in vascular smooth muscle. J Pharmacol Sci. 2007;104:109–115. doi: 10.1254/jphs.cp0070027. [DOI] [PubMed] [Google Scholar]
- Holinstat M, Preininger AM, Milne SB, Hudson WJ, Brown HA, Hamm HE. Irreversible platelet activation requires protease-activated receptor 1-mediated signaling to phosphatidylinositol phosphates. Mol Pharmacol. 2009;76:301–313. doi: 10.1124/mol.109.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg MD, Compton SJ. Proteinase-activated receptors. Pharmacol Rev. 2002;54:203–217. doi: 10.1124/pr.54.2.203. [DOI] [PubMed] [Google Scholar]
- Ide K, Yamakawa K, Nakagomi T, Sasaki T, Saito I, Kurihara H, Yosizumi M, Yazaki Y, Takakura K. The role of endothelin in the pathogenesis of vasospasm following subarachnoid haemorrhage. Neurol Res. 1989;11:101–104. doi: 10.1080/01616412.1989.11739870. [DOI] [PubMed] [Google Scholar]
- Itoh S, Sasaki T, Asai A, Kuchino Y. Prevention of delayed vasospasm by an endothelin ETA receptor antagonist, BQ-123: change of ETA receptor mRNA expression in a canine subarachnoid hemorrhage model. J Neurosurg. 1994;81:759–764. doi: 10.3171/jns.1994.81.5.0759. [DOI] [PubMed] [Google Scholar]
- Kai Y, Hirano K, Maeda Y, Nishimura J, Sasaki T, Kanaide H. Prevention of the hypercontractile response to thrombin by proteinase-activated receptor-1 antagonist in subarachnoid hemorrhage. Stroke. 2007;38:3259–3265. doi: 10.1161/STROKEAHA.107.487769. [DOI] [PubMed] [Google Scholar]
- Kai Y, Maeda Y, Sasaki T, Kanaide H, Hirano K. Basic and translational research on proteinase-activated receptors: the role of thrombin receptor in cerebral vasospasm in subarachnoid hemorrhage. J Pharmacol Sci. 2008;108:426–432. doi: 10.1254/jphs.08r11fm. [DOI] [PubMed] [Google Scholar]
- Kassell NF, Sasaki T, Colohan AR, Nazar G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985;16:562–572. doi: 10.1161/01.str.16.4.562. [DOI] [PubMed] [Google Scholar]
- Laher I, Zhang JH. Protein kinase C and cerebral vasospasm. J Cereb Blood Flow Metab. 2001;21:887–906. doi: 10.1097/00004647-200108000-00001. [DOI] [PubMed] [Google Scholar]
- Lurie KG, Tsujimoto G, Hoffman BB. Desensitization of alpha-1 adrenergic receptor-mediated vascular smooth muscle contraction. J Pharmacol Exp Ther. 1985;234:147–152. [PubMed] [Google Scholar]
- Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- Maeda Y, Hirano K, Hirano M, Kikkawa Y, Kameda K, Sasaki T, Kanaide H. Enhanced contractile response of the basilar artery to platelet-derived growth factor in subarachnoid hemorrhage. Stroke. 2009;40:591–596. doi: 10.1161/STROKEAHA.108.530196. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Hirano K, Kai Y, Hirano M, Suzuki SO, Sasaki T, Kanaide H. Up-regulation of proteinase-activated receptor 1 and increased contractile responses to thrombin after subarachnoid haemorrhage. Br J Pharmacol. 2007;152:1131–1139. doi: 10.1038/sj.bjp.0707435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Hirano K, Nishimura J, Sasaki T, Kanaide H. Rho-kinase inhibitor inhibits both myosin phosphorylation-dependent and -independent enhancement of myofilament Ca2+ sensitivity in the bovine middle cerebral artery. Br J Pharmacol. 2003;140:871–880. doi: 10.1038/sj.bjp.0705487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasiro N, Paiva AC. Homologous desensitization of the effects of endothelin on rabbit aorta rings and on cultured rat aorta smooth muscle cells. Eur J Pharmacol. 1990;179:151–158. doi: 10.1016/0014-2999(90)90412-y. [DOI] [PubMed] [Google Scholar]
- Miyagi Y, Carpenter RC, Meguro T, Parent AD, Zhang JH. Upregulation of rho A and rho kinase messenger RNAs in the basilar artery of a rat model of subarachnoid hemorrhage. J Neurosurg. 2000;93:471–476. doi: 10.3171/jns.2000.93.3.0471. [DOI] [PubMed] [Google Scholar]
- Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Hirano K, Hirano M, Nishimura J, Kuga H, Nakamura K, Takahashi S, Kanaide H. Inactivation of protease-activated receptor-1 by proteolytic removal of the ligand region in vascular endothelial cells. Biochem Pharmacol. 2004;68:23–32. doi: 10.1016/j.bcp.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Hirano K, Shintani Y, Nishimura J, Nakatsuka A, Kuga H, Takahashi S, Kanaide H. Unproductive cleavage and the inactivation of protease-activated receptor-1 by trypsin in vascular endothelial cells. Br J Pharmacol. 2003;138:121–130. doi: 10.1038/sj.bjp.0705008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Kassell NF, Yamashita M, Fujiwara S, Zuccarello M. Barrier disruption in the major cerebral arteries following experimental subarachnoid hemorrhage. J Neurosurg. 1985;63:433–440. doi: 10.3171/jns.1985.63.3.0433. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kassell NF, Zuccarello M, Nakagomi T, Fijiwara S, Colohan AR, Lehman M. Barrier disruption in the major cerebral arteries during the acute stage after experimental subarachnoid hemorrhage. Neurosurgery. 1986;19:177–184. doi: 10.1227/00006123-198608000-00002. [DOI] [PubMed] [Google Scholar]
- Sato M, Tani E, Fujikawa H, Kaibuchi K. Involvement of Rho-kinase-mediated phosphorylation of myosin light chain in enhancement of cerebral vasospasm. Circ Res. 2000;87:195–200. doi: 10.1161/01.res.87.3.195. [DOI] [PubMed] [Google Scholar]
- Takeya K, Loutzenhiser K, Shiraishi M, Loutzenhiser R, Walsh MP. A highly sensitive technique to measure myosin regulatory light chain phosphorylation: the first quantification in renal arterioles. Am J Physiol Renal Physiol. 2008;294:F1487–F1492. doi: 10.1152/ajprenal.00060.2008. [DOI] [PubMed] [Google Scholar]
- Trejo J. Protease-activated receptors: new concepts in regulation of G protein-coupled receptor signaling and trafficking. J Pharmacol Exp Ther. 2003;307:437–442. doi: 10.1124/jpet.103.052100. [DOI] [PubMed] [Google Scholar]
- Vatter H, Konczalla J, Weidauer S, Preibisch C, Zimmermann M, Raabe A, Seifert V. Effect of delayed cerebral vasospasm on cerebrovascular endothelin A receptor expression and function. J Neurosurg. 2007;107:121–127. doi: 10.3171/JNS-07/07/0121. [DOI] [PubMed] [Google Scholar]
- White RP, Robertson JT. Pharmacodynamic evaluation of human cerebral arteries in the genesis of vasospasm. Neurosurgery. 1987;21:523–531. doi: 10.1227/00006123-198710000-00014. [DOI] [PubMed] [Google Scholar]